The study of disparities in trastuzumab use has been complicated by the lack of population‐based cohorts. Real‐world research is lacking. This study used real‐world data from a multicenter study in China to explore disparities in trastuzumab use and to investigate the survival effects of trastuzumab on early stage and metastatic breast cancer.
Keywords: Human epidermal growth receptor 2 positive breast cancer, Medical resource disparity, Real‐world research, Survival
Abstract
Background.
Trastuzumab is a key component of therapy for human epidermal growth receptor 2 (HER2) positive breast cancer. Because real‐world data are lacking, the present research was conducted to evaluate the actual use of and the effectiveness of trastuzumab in the real world in China.
Methods.
Inpatients with HER2 positive invasive breast cancer from 13 hospitals in Eastern China (2010–2015, n = 1,139) were included in this study. We aimed to assess the actual use of trastuzumab and to evaluate potential efficacy from trastuzumab in real‐world research.
Results.
Of 1,017 patients with early stage breast cancer (EBC), 40.5% (412/1,017) received trastuzumab therapy. Patients with EBC in resource‐abundant regions (gross domestic product per capita >$15,000 and trastuzumab included in Medicare) are more likely to receive trastuzumab than those in resource‐limited regions (37.3% vs. 13.0%, p < .05). After metastasis, 50.8% (366/720) patients received trastuzumab as their first‐line therapy. More than 10% of patients with metastatic breast cancer (MBC) continued trastuzumab therapy after twice progression in resource‐abundant regions, whereas more than 40% of patients never received any trastuzumab therapy during the whole course of therapy in resource‐limited regions. Overall, the improvement in survival for trastuzumab versus non‐trastuzumab was substantial in EBC (hazard ratio [HR] = 0.609, 95% confidence interval [CI]: 0.505–0.744) and in MBC (HR = 0.541, 95% CI: 0.418–0.606). This association was greater for patients with MBC who had never received trastuzumab (HR = 0.493, 95% CI: 0.372–0.576) than for those who had received adequate trastuzumab therapy in EBC stage (HR = 0.878, 95% CI: 0.506–1.431).
Conclusion.
This study showed great disparities in trastuzumab use in different regions and different treatment stages. Both EBC and MBC patients can benefit from trastuzumab, as the survival data show; however, when trastuzumab is adequate in the early stage, a further trastuzumab‐based therapy in first‐line treatment of MBC will be ineffective, especially for those with short disease‐free survival, and a second line of anti‐HER2 therapy will be recommended. (Research number: CSCO‐BC RWS 15001).
Implications for Practice.
This article explores the disparities in the rates of trastuzumab use due to the inequitable allocation of medical resources in China. The irrational use can be found both in resource‐abundant regions and in resource‐limited regions. Although trastuzumab‐based therapy improved survival, the actual use of trastuzumab in the early stage of breast cancer may influence the subsequent therapeutic effect after metastasis. These findings from real‐world research could help to optimize HER2 therapy after metastasis, especially in regions with limited access to these expensive targeted drugs.
摘要
背景.曲妥珠单抗是用于人类表皮生长因子受体2(HER2)阳性乳腺癌治疗的重要组成部分。由于缺少真实世界数据, 本研究旨在评价曲妥珠单抗在中国真实世界中的实际应用和疗效。
方法.本研究纳入了华东地区13个医院(2010‐2015年, n=1 139)的HER2阳性浸润性乳腺癌住院患者。我们旨在评估曲妥珠单抗在真实世界研究中的实际应用和潜在疗效。
结果.在1 017例早期乳腺癌患者(EBC)中, 40.5%(412/1 017)接受了曲妥珠单抗治疗。资源丰富地区的EBC患者(人均国民生产总值>15 000美元, 曲妥珠单抗被纳入医保)比资源有限地区更有可能接受曲妥珠单抗治疗(37.3% vs. 13.0%, p <0.05)。转移后, 50.8%(366/720)患者接受了作为一线治疗的曲妥珠单抗治疗。在资源丰富的地区, 两次进展后超过10%的转移性乳腺癌患者(MBC)继续接受曲妥珠单抗治疗, 而在资源有限的地区, 超过40%的患者在整个治疗过程中从未接受过任何曲妥珠单抗治疗。总体而言, 在EBC患者中, 与非曲妥珠单抗组相比, 曲妥珠单抗组患者的生存期显著改善[风险比(HR)=0.609, 95%置信区间(CI):0.505‐0.744], 在MBC患者中, 与非曲妥珠单抗组相比, 曲妥珠单抗组患者的生存期显著改善(HR=0.541, 95%CI:0.418‐0.606)。从未接受过曲妥珠单抗治疗的MBC患者(HR=0.493, 95%CI:0.372‐0.576)的这种关联优于在EBC期接受了足够曲妥珠单抗治疗的患者(HR=0.878, 95%CI: 0.506–1.431)。
结论.本研究显示曲妥珠单抗在不同地区和不同治疗阶段使用的差异很大。生存期数据显示, EBC和MBC患者均可从曲妥珠单抗治疗中获益;然而, 当在早期接受了足够的曲妥珠单抗治疗时, 在MBC的一线治疗中进一步接受基于曲妥珠单抗的治疗将是无效的, 尤其是对于无病生存期较短的患者, 因此建议采用二线抗HER2治疗。(研究编号: CSCO‐BC RWS 15001)。
Introduction
Breast cancer was the most frequently diagnosed cancer in women in the past 5 years [1]. Approximately 15%–20% of breast cancers overexpress human epidermal growth receptor 2 (HER2), and overexpression of HER2 is associated with high recurrence rates and poor outcomes [2]. To date, several randomized controlled trials have shown that trastuzumab‐based systemic therapy is effective in prolonging disease‐free survival (DFS) [3], [4], [5] and progression‐free survival (PFS) [6], [7] and were the basis for international treatment guidelines [8], [9].
Since its approval for breast cancer in China, trastuzumab has been rapidly adopted as a standard therapy [10]. Given the contradiction between the high price of anti‐HER2 drugs and the low disposable income per capita in China, until 2015, 10 provinces offered Medicaid coverage for trastuzumab to lighten the financial burden of patients [11]. The patient assistance program (PAP) founded by the Cancer Foundation of China [12] also provides support for patients who cannot afford the high price of targeted therapy. However, breast cancer health disparities exist worldwide, and the association between breast cancer and socioeconomic status has been well established [13], [14]. The actual use of trastuzumab in community settings and the extent to which trastuzumab is used or underused in Chinese populations, particularly among resource‐limited regions, are unknown [15].
The study of disparities in trastuzumab use has been complicated by the lack of population‐based cohorts. There is still a lack of real‐world research (RWR) to provide decision makers with a useful basis to confirm whether the outcomes of randomized controlled trials (RCTs) are applicable to real‐world clinical practice, or to understand how and why they differ [16], [17].
Hence, using real‐world data from a multicenter study in China, we aimed to explore the disparities of trastuzumab use in different regions and periods and to evaluate the irrational use of trastuzumab. We also investigated the survival effects of trastuzumab on early stage breast cancer (EBC) and metastatic breast cancer (MBC) in RWR.
Methods
Study Population and Data Collection
We used data from 13 hospitals in 11 cities for the period from 2010 to 2015. These 11 cities are all located in Eastern China. Data elements included demographic characteristics, timing and type of cancer diagnosis, and treatment course. Our preliminary samples included HER2 positive invasive breast cancer patients who received hospitalized therapy between January 1, 2010, and October 30, 2015, whatever their diagnosis time. Human epidermal growth receptor 2 status can be assigned by combined available results from immunohistochemistry and fluorescence in situ hybridization tests [18].
Patients were required to receive at least four cycles of systemic therapy after diagnosis. Patients enrolled in the EBC group were required to receive a primary breast cancer surgery (either breast‐conserving surgery or mastectomy). Patients with borderline, unknown, or missing information for treatment were excluded.
Outcome Assessment
The primary outcome was receipt of trastuzumab, defined as at least two cycles for trastuzumab in EBC or in MBC after diagnosis. Trastuzumab exposure could occur at any time after diagnosis. Only one cycle of trastuzumab was defined as no receipt of targeted therapy due to the ineffectiveness of anti‐HER2 therapy. One cycle of trastuzumab therapy was defined as 6 mg/kg every 3 weeks.
The secondary outcomes were DFS and PFS of first‐line therapy. We calculated DFS as the interval from surgery to the earliest occurrence of disease progression resulting in inoperability, locoregional recurrence (after neoadjuvant therapy), distant metastases, or death from any cause [19]. Progression‐free survival of first line after metastasis was calculated from date of salvage therapy to progression. Patients who were alive without an event as of the analysis cutoff date were censored at the last study follow‐up date. Date of trastuzumab use was calculated as the interval from the first time used to the last time used, in EBC or MBC, respectively.
Statistical Analysis
We compared trastuzumab use with different periods and regions using Pearson's test. We also used Kaplan‐Meier and Cox proportional hazards regression to estimate hazard ratio (HR) and 95% confidence interval (CI) for the relationship between trastuzumab and DFS or PFS in first line. Results were considered significant at p < .05. Statistical analyses were performed using version 9.4 of the SAS System for Windows (SAS Institute, Cary, NC, https://www.sas.com/en_us/home.html).
Results
Demographic Characteristics
A total of 1,139 patients who were diagnosed with HER2 positive breast cancer between 1996 and 2015 were included in the analysis. Table 1 shows clinical and demographic characteristics of patients. Less than half of patients (41.9%) were younger than 45 years; 72.2% of patients had tumors <5 cm, and 34.9% had node‐negative disease, which reflected a relatively mild biology of HER2 positive cancer in China. Meanwhile, 42.2% of patients received chemotherapy, and 57.8% of patients received surgery after initial diagnosis. All EBC patients would receive both surgery and adjuvant therapy, if necessary. Approximately 732 patients underwent metastasis, including the 122 patients who were initially diagnosed as stage IV metastatic breast cancer (M1); however, only 720 patients received salvage therapies, among whom 598 patients underwent metastasis sometime after their early stage therapies (M0), and the remaining 122 were M1 patients.
Table 1. Demographic characteristics of 1,139 patients.
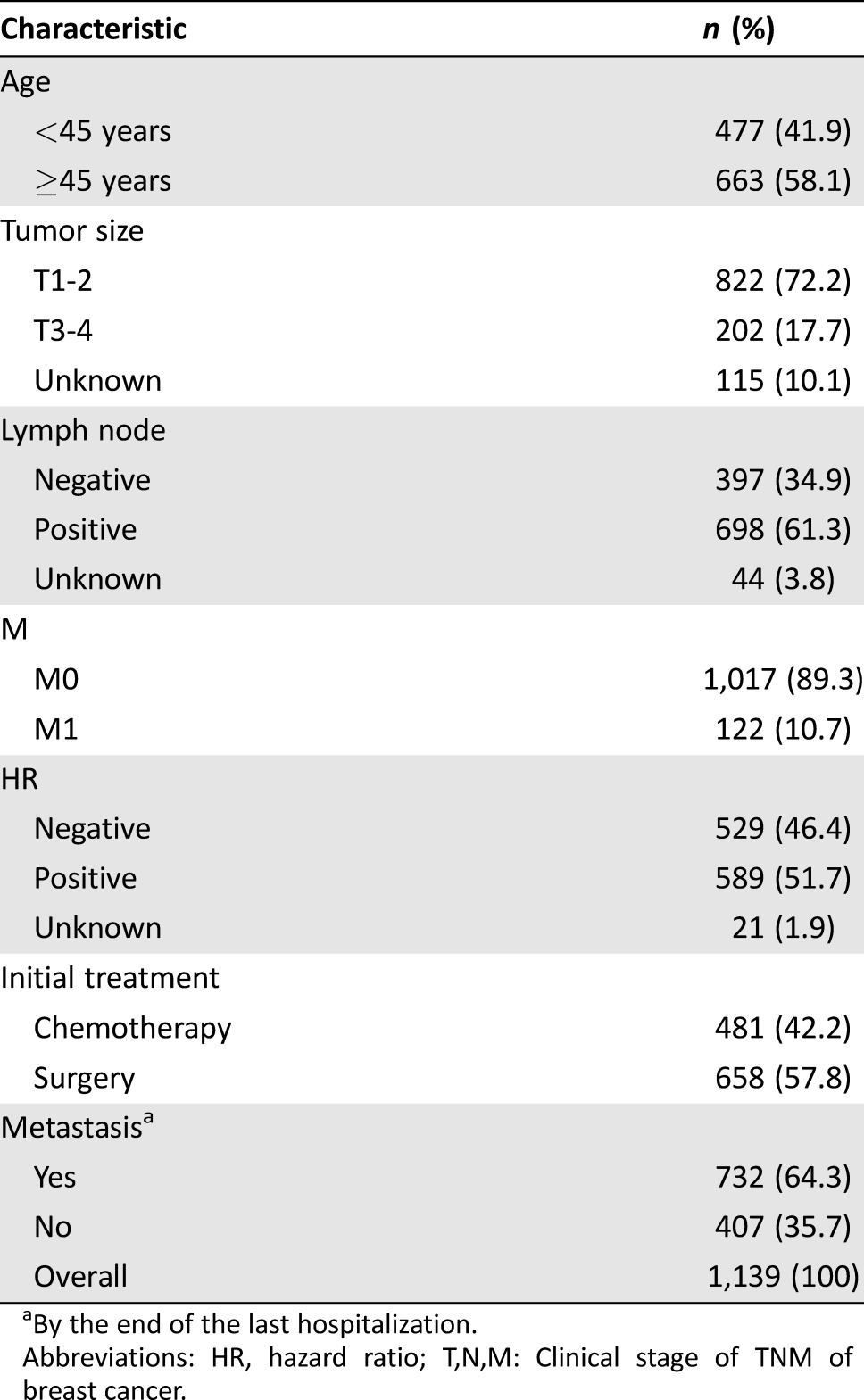
By the end of the last hospitalization.
Abbreviations: HR, hazard ratio; T,N,M: Clinical stage of TNM of breast cancer.
Proportion of Trastuzumab Use
In the EBC group, trastuzumab was indicated in 412 patients (40.5%; Table 2); 54.9% (226/412) of patients had stopped trastuzumab, and the median time of target‐drug use was 12 months (supplemental online Table 1). In the MBC group, the overall rate of trastuzumab use was 72.9%. The median time to trastuzumab use for these patients was 10 months.
Table 2. Proportions for trastuzumab use in EBC and MBC.
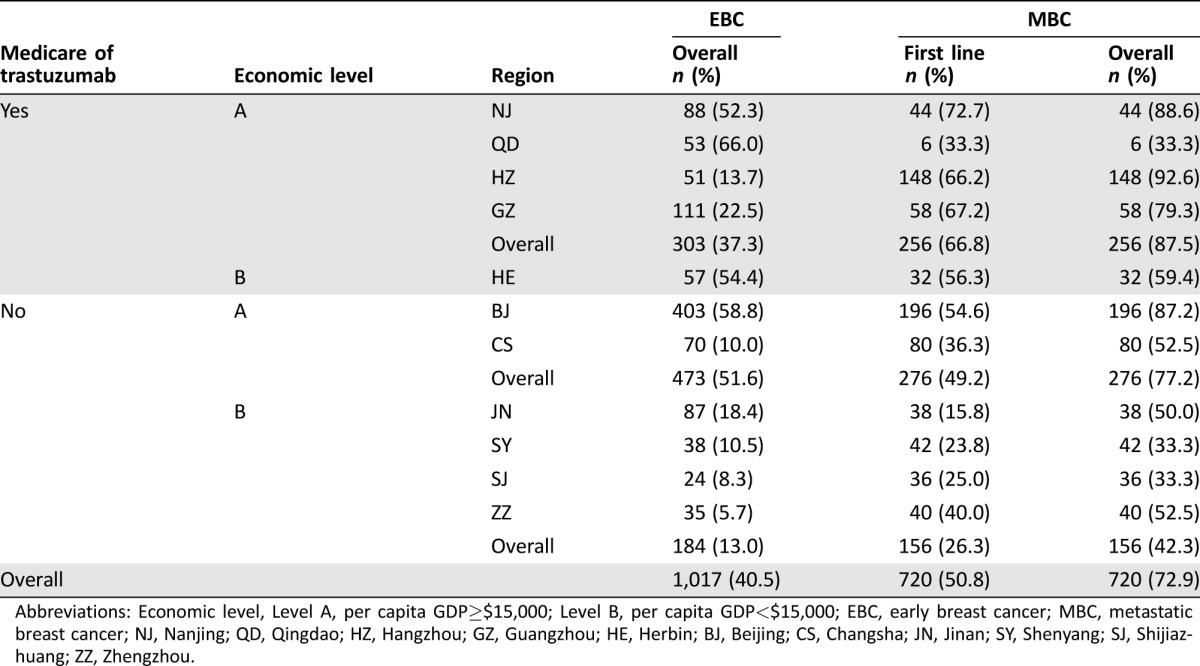
Abbreviations: Economic level, Level A, per capita GDP≥$15,000; Level B, per capita GDP<$15,000; EBC, early breast cancer; MBC, metastatic breast cancer; NJ, Nanjing; QD, Qingdao; HZ, Hangzhou; GZ, Guangzhou; HE, Herbin; BJ, Beijing; CS, Changsha; JN, Jinan; SY, Shenyang; SJ, Shijiazhuang; ZZ, Zhengzhou.
To address the concern that Medicare eligibility and economic status may exert a stronger effect on the likelihood of trastuzumab receipt, we classified these regions of research into four levels. Patients in resource‐abundant regions (gross domestic product per capita >$15,000 or trastuzumab included in Medicare) were more likely to receive trastuzumab than those in resource‐limited regions during their early stage (37.3% vs. 13.0%) and metastatic stage (87.5% vs. 42.3%). There were still huge disparities even in the resource‐abundant regions. In the EBC group, the highest rate for trastuzumab use in the resource‐abundant region was 66.0% (35/53), which was about 4.8 times higher than the lowest rate (13.7%, 7/51).
Irrational Trastuzumab Use in MBC
We performed descriptive analyses to find the irrational use after metastasis for patients who received trastuzumab. Irrational trastuzumab use was defined as no trastuzumab use during all therapeutic courses or a continuous use of trastuzumab after twice progression.
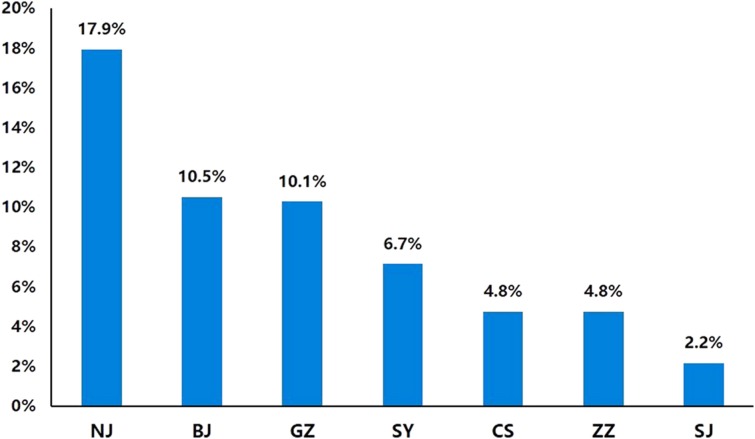
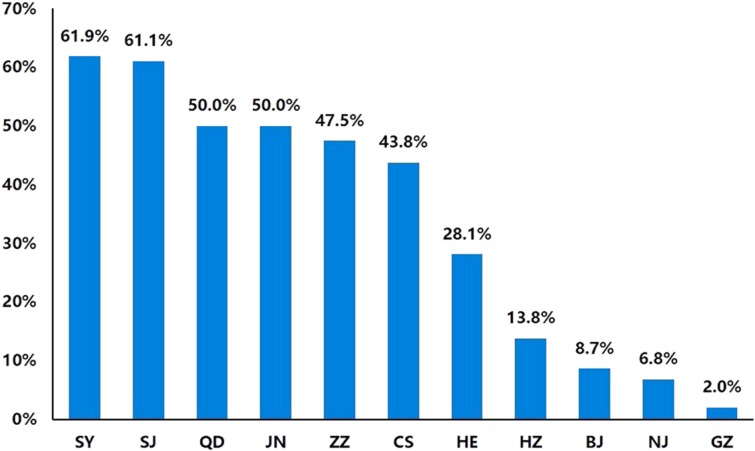
Patients in resource‐abundant regions (Guangzhou, Nanjing, Beijing) were more likely to use trastuzumab even after twice progression, and the highest proportion was 17.9% (7/39; Fig. 1). In these regions, less than 10% of patients never received any trastuzumab therapy during their whole course of therapy (Fig. 2). On the contrary, the proportion rose to more than 40% in resource‐limited regions (Changsha, Zhengzhou, Shenyang), and less than 10% of patients used trastuzumab at the same time. In the region of Hangzhou due to the short time that trastuzumab was accepted in Medicare, 13.8% (8/58) of patients never used trastuzumab, and only 2.2% (1/46) of patients continued trastuzumab after twice progression.
Figure 1.
Continuous use of trastuzumab after twice progression in metastatic breast cancer.
Abbreviations: BJ, Beijing; CS, Changsha; GZ, Guangzhou; NJ, Nanjing; SY, Shenyang; SJ, Shijiazhuang; ZZ, Zhengzhou.
Figure 2.
Proportions of metastatic breast cancer patients who never used trastuzumab during their whole therapeutic courses. The whole therapeutic courses include their early stage and metastatic stage.
Abbreviations: BJ, Beijing; CS, Changsha; GZ, Guangzhou; HE, Herbin; HZ, Hangzhou; JN, Jinan; NJ, Nanjing; QD, Qingdao; SJ, Shijiazhuang; SY, Shenyang; ZZ, Zhengzhou.
DFS and PFS for HER2 Positive Patients
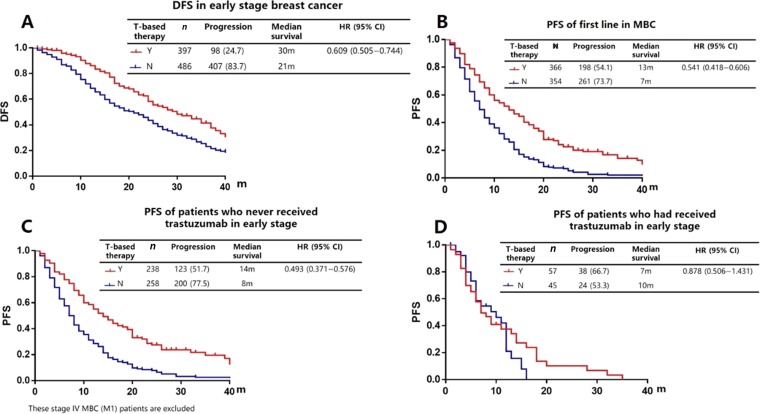
We analyzed the association between the effect of trastuzumab and long‐term outcomes in EBC and in first‐line salvage therapy from 2008 to 2015, among those for whom trastuzumab was available. In EBC, we recorded 505 progressions in 883 patients. Treatment with trastuzumab contributed to a significant improvement in disease‐free progression compared with no targeted therapy (median survival 30 months vs. 21 months, HR = 0.609, 95% CI: 0.505–0.744; Fig. 3A). After metastasis, a comparison of PFS in first‐line salvage therapy also showed significant association between therapeutic regimens (13 months vs. 7 months, HR = 0.541, 95% CI: 0.418–0.606; Fig. 3B).
Figure 3.
Survival for human epidermal growth receptor 2 positive patients in real world. (A): DFS in early stage breast cancer. (B): PFS of first line in metastatic breast cancer. (C): PFS of patients who never received trastuzumab in early stage. (D): PFS of patients who had received trastuzumab in early stage.
Abbreviations: CI, confidence interval; DFS, disease‐free survival; HR, hazard ratio; m, months; N, no; PFS, progression‐free survival; T, trastuzumab; Y, yes.
We also analyzed the 598 M0 patients who were initially diagnosed as EBC but then eventually developed into MBC. For patients who received no trastuzumab therapy in the early stage (n = 496), median time for DFS was 24 months. The initial use of trastuzumab after metastasis was highly associated with the improvement of PFS in the first‐line (14 months vs. 8 months, HR = 0.493, 95% CI: 0.372–0.576; Fig. 3C). For patients who had received trastuzumab‐based therapy in the EBC stage (n = 122), median time for trastuzumab use was 12 months, and median DFS was 17 months. After metastasis, the reuse of trastuzumab was unable to improve PFS in first‐line therapy (HR = 0.878, 95% CI: 0.506–1.431; Fig. 3D).
Discussion
Randomized controlled trials are the cornerstone of evidence development in oncology; however, the applicability of the evidence generated from RCTs to real‐life populations will depend on the degree of alignment between the research and target population in terms of clinical and molecular characteristics [20]. Although not a substitute for RCTs, RWR does offer the potential to supplement knowledge gaps and address questions that cannot be solved by clinical trials [21]. Thus, our results provide important information to facilitate treatment decisions [22].
To our knowledge, this is the first RWR to systematically analyze the reality of treatment conditions of HER2 positive breast cancer in China. Our results have several troubling implications. First, although patients in resource‐abundant regions had a higher proportion of targeted therapy than those in resource‐limited regions, it does not mitigate the overall pattern of treatment disparity observed among different regions. There are still huge disparities even in the same level of region. The low overall rates of use raise concerns for widespread underuse of trastuzumab in China or some other similar countries. Second, the irrational use of trastuzumab suggests that huge disparities exist in different regions; targeted therapy is more likely to be overused in resource‐abundant regions and more likely to be underused in resource‐limited regions. Finally, patients with HER2 positive breast cancer who were given trastuzumab showed substantial improvements in DFS and PFS outcomes compared with those who were given no trastuzumab. Meanwhile, the use of trastuzumab in the early stage is a key factor affecting the subsequent treatment options. Patients who had received adequate trastuzumab‐based therapy in EBC did not benefit from trastuzumab again in their first‐line therapy; a switch to anti‐HER2 therapy after trastuzumab is strongly recommended.
Although the health expenditure per person in China increased from $54 to $420 between 2002 and 2014 [23], out‐of‐pocket expenditure as a percentage of private health expenditure (78.8%) remains higher than that of high‐income countries such as the U.S. (20.9%) and the U.K. (53.1%) [24]. Hence, drug reimbursement policies and disposable income of residents in China strongly affect the availability of optimum systemic therapies [25], [26]. We found that patients in resource‐limited regions were less likely to receive trastuzumab‐based therapy. In some cases, such variation may be appropriate, because patients with poor financial status must save precious resources to continue their costly and lasting therapies [27]. However, given the benefit of trastuzumab use and the aggressive biology of this subtype, the hypothesis that economic status is used as an index to withhold trastuzumab‐based therapy deserves further discussion.
Several aspects may contribute to the underuse of trastuzumab in resource‐limited regions and, sometimes, in the resource‐abundant regions. First, the small gains in DFS or PFS from trastuzumab‐based therapy may be attenuated or lost due to the low correlation between the actual efficacy of a new drug and its price [28]. Second, trastuzumab is a costly and long‐term therapy. A lack of well‐established follow‐up will contribute to the reduction of trastuzumab use. Third, despite the guarantee of payment from Medicare, varying benefits and coverage of medical insurance policies still greatly influence trastuzumab use [29], which contributes to the disparities of trastuzumab use for patients treated in the same city but at a different therapeutic stage.
Because of the development of PAP, the implementation of health insurance policy, and the promotion of anti‐HER2 therapy, a rapid growth in trastuzumab use has been witnessed in both the EBC and the MBC groups. Yet, even for insured patients, infused therapy of trastuzumab in MBC is costly, and there is less of a probability that MBC patients can tolerate this intensive and lengthy course of treatment. This protracted treatment course may be a disincentive for patients with limited resources, which explains why patients in nonreimbursement regions are more likely to underuse trastuzumab. In contrast, in resource‐abundant regions, over 80% of patients received trastuzumab‐based therapy. Additionally, over 10% of patients who received trastuzumab therapy did not withhold or switch to another anti‐HER2 therapy even if they had twice experienced disease progression. The aim of the PAP, or of medical insurance, is to guarantee the regular access to trastuzumab, not to advocate a waste of medical resources. A new resource allocation model in China will be the key to solving such variation. Our cohort offers insight into the opportunity of drug use or withdrawal and offers useful strategies for future medical insurance policymaking or randomized controlled trial designing.
Although there was a huge disparity of trastuzumab use in different regions and a wide difference in the treatment course between the real world and the standard recommended guidelines [30], we still found that DFS or PFS results for patients treated with trastuzumab in the real world are comparable to those in the pivotal randomized controlled trials. And the HR in the EBC group was similar to that in clinical trial [31]. These results bring reassurance that eligibility and treatment results of clinical trials can be translated to the real‐world setting, and the results confirm that implementation in the real world has been successful. Additional information in first‐line salvage therapy also indicated that trastuzumab in the early stage would influence the curative effect after metastasis. Only patients who received no trastuzumab in the EBC setting would benefit from targeted therapy in their first‐line salvage therapy. For patients who had used adequate trastuzumab in the early stage, a reuse of trastuzumab after metastasis would have no effect, ascribed to the poor response (short time of DFS) to trastuzumab. Because of the difficulty in designing such randomized clinical trials, there is no evidence or consensus to guide us to choose an appropriate anti‐HER2 therapy in first‐line salvage therapy after an adequate trastuzumab‐based therapy in the early stage; hence, for patients with HER2 positive breast cancer, we recommend using trastuzumab as early as possible. If it has been used in the early stage, especially for patients with short DFS, a second line of anti‐HER2 therapy is recommended after metastasis.
There were several important limitations of our study. First, with an observational design, it was inevitable that some missing data could not be retrieved. Second, we cannot ignore the selection bias; only 11 hospitals, representing the top level of provinces, were identified, and so the results may not reflect the whole situation of targeted therapy in China. Meanwhile, as only inpatients were included, there were reductions of DFS and PFS compared with those of patients in RCTs. Finally, the number of patients in some subgroups became too small to draw firm conclusions.
Conclusion
The importance of real‐world research should not be overlooked. In future studies, we will expand the sample size and cooperate with more hospitals to have a better understanding of anti‐HER2 therapy in China. Meanwhile, we are going to optimize the scheme of anti‐HER2 therapy after trastuzumab and explore when to refer to other HER2‐directed therapies.
See http://www.TheOncologist.com for supplemental material available online.
Supplementary Material
Author Contributions
Conception/design: Zefei Jiang
Provision of study material or patients: Shusen Wang, Yongsheng Wang, Xiaojia Wang, Haibo Wang, Jifeng Feng, Qingyuan Zhang, Tao Sun, Quchang Ouyang, Yongmei Yin, Yinhua Liu, Cuizhi Geng, Min Yan, Zefei Jiang
Collection and/or assembly of data: Jianbin Li, Shusen Wang, Yongsheng Wang, Xiaojia Wang, Haibo Wang, Jifeng Feng, Qingyuan Zhang, Tao Sun, Quchang Ouyang, Yongmei Yin, Yinhua Liu, Cuizhi Geng, Min Yan
Data analysis and interpretation: Jianbin Li
Manuscript writing: Jianbin Li
Final approval of manuscript: Zefei Jiang
Disclosures
The authors indicated no financial relationships.
References
- 1. Chen W, Zheng R, Baade PD et al. Cancer statistics in China, 2015. CA Cancer J Clin 2016;66:115–132. [DOI] [PubMed] [Google Scholar]
- 2. Parkinson B, Viney R, Haas M et al. Real‐world evidence: A comparison of the Australian Herceptin program and clinical trials of trastuzumab for HER2‐positive metastatic breast cancer. Pharmacoeconomics 2016;34:1039–1050. [DOI] [PubMed] [Google Scholar]
- 3. Piccart‐Gebhart MJ, Procter M, Leyland‐Jones B et al. Trastuzumab after adjuvant chemotherapy in HER2‐positive breast cancer. N Engl J Med 2005;353:1659–1672. [DOI] [PubMed] [Google Scholar]
- 4. Smith I, Procter M, Gelber RD et al. 2‐year follow‐up of trastuzumab after adjuvant chemotherapy in HER2‐positive breast cancer: A randomised controlled trial. Lancet 2007;369:29–36. [DOI] [PubMed] [Google Scholar]
- 5. Gianni L, Eiermann W, Semiglazov V et al. Neoadjuvant and adjuvant trastuzumab in patients with HER2‐positive locally advanced breast cancer (NOAH): Follow‐up of a randomised controlled superiority trial with a parallel HER2‐negative cohort. Lancet Oncol 2014;15:640–647. [DOI] [PubMed] [Google Scholar]
- 6. Slamon DJ, Leyland‐Jones B, Shak S et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med 2001;344:783–792. [DOI] [PubMed] [Google Scholar]
- 7. Andersson M, Lidbrink E, Bjerre K et al. Phase III randomized study comparing docetaxel plus trastuzumab with vinorelbine plus trastuzumab as first‐line therapy of metastatic or locally advanced human epidermal growth factor receptor 2‐positive breast cancer: The HERNATA study. J Clin Oncol 2011;29:264–271. [DOI] [PubMed] [Google Scholar]
- 8. Coates AS, Winer EP, Goldhirsch A et al. Tailoring therapies—Improving the management of early breast cancer: St Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2015. Ann Oncol 2015;26:1533–1546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.NCCN . Clinical practice guidelines in oncology (NCCN guidelines), breast cancer. Available at https://www.nccn.org/professionals/physician_gls/f_guidelines.asp#breast. Accessed August 2, 2017.
- 10. Jiang Z, Shao Z, Xu B. Expert consensus of clinical diagnosis and treatment for human epidermal growth factor receptor 2 positive breast cancer [in Chinese]. National Medical Journal of China 2016;96:1091–1096. [Google Scholar]
- 11. Yip WC, Hsiao WC, Chen W et al. Early appraisal of China's huge and complex health‐care reforms. Lancet 2012;379:833–842. [DOI] [PubMed] [Google Scholar]
- 12.Cancer foundation of China . Available at http://www.cfchina.org.cn. Accessed August 2, 2017.
- 13. Fei X, Wu J, Kong Z et al. Urban‐rural disparity of breast cancer and socioeconomic risk factors in China. PLoS One 2015;10:e0117572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Smith EC, Ziogas A, Anton‐Culver H. Delay in surgical treatment and survival after breast cancer diagnosis in young women by race/ethnicity. JAMA Surg 2013;148:516–523. [DOI] [PubMed] [Google Scholar]
- 15. Reeder‐Hayes K, Peacock Hinton S, Meng K et al. Disparities in use of human epidermal growth hormone receptor 2‐targeted therapy for early‐stage breast cancer. J Clin Oncol 2016;34:2003–2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Prince RM, Atenafu EG, Krzyzanowska MK. Hospitalizations during systemic therapy for metastatic lung cancer: A systematic review of real world vs clinical trial outcomes. JAMA Oncol 2015;1:1333–1339. [DOI] [PubMed] [Google Scholar]
- 17. Sherman RE, Anderson SA, Dal Pan GJ et al. Real‐world evidence—What is it and what can it tell us? N Engl J Med 2016;375:2293–2297. [DOI] [PubMed] [Google Scholar]
- 18. Wolff AC, Hammond ME, Hicks DG et al. Recommendations for human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists Clinical Practice Guideline update. J Clin Oncol 2013;31:3997–4013. [DOI] [PubMed] [Google Scholar]
- 19. Cortazar P, Zhang L, Untch M et al. Pathological complete response and long‐term clinical benefit in breast cancer: The CTNeoBC pooled analysis. Lancet 2014;384:164–172. [DOI] [PubMed] [Google Scholar]
- 20. Lewis JR, Kerridge I, Lipworth W. Coverage with evidence development and managed entry in the funding of personalized medicine: Practical and ethical challenges for oncology. J Clin Oncol 2015;33:4112–4117. [DOI] [PubMed] [Google Scholar]
- 21. Booth CM, Tannock IF. Randomised controlled trials and population‐based observational research: Partners in the evolution of medical evidence. Br J Cancer 2014;110:551–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Galsky MD, Stensland KD, Moshier E et al. Effectiveness of adjuvant chemotherapy for locally advanced bladder cancer. J Clin Oncol 2016;34:825–832. [DOI] [PubMed] [Google Scholar]
- 23. Bank W. Health expenditure per capita. Available at http://data.worldbank.org/topic/health?locations=DJ-ZA-8S-SS. Accessed August 2, 2017.
- 24. Bank W. Out‐of‐pocket health expenditure. Available at http://data.worldbank.org/topic/health?locations=DJ-ZA-8S-SS. Accessed August 2, 2017.
- 25. Meng Q, Fang H, Liu X et al. Consolidating the social health insurance schemes in China: Towards an equitable and efficient health system. Lancet 2015;386:1484–1492. [DOI] [PubMed] [Google Scholar]
- 26. Fan L, Strasser‐Weippl K, Li JJ et al. Breast cancer in China. Lancet Oncol 2014;15:e279–e289. [DOI] [PubMed] [Google Scholar]
- 27. Goss PE, Strasser‐Weippl K, Lee‐Bychkovsky BL et al. Challenges to effective cancer control in China, India, and Russia. Lancet Oncol 2014;15:489–538. [DOI] [PubMed] [Google Scholar]
- 28. Kantarjian HM, Fojo T, Mathisen M et al. Cancer drugs in the United States: Justum pretium—The just price. J Clin Oncol 2013;31:3600–3604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Wu M, Lu P, Shi L et al. Traditional Chinese patent medicines for cancer treatment in China: A nationwide medical insurance data analysis. Oncotarget 2015;6:38283–38295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.CSCO . Chinese society of clinical oncology guidelines for the diagnosis and treatment of breast cancer (version 1, 2017) [M]. Beijing: People's Medical Publishing House, 2017. [Google Scholar]
- 31. Goldhirsch A, Gelber RD, Piccart‐Gebhart MJ et al. 2 years versus 1 year of adjuvant trastuzumab for HER2‐positive breast cancer (HERA): An open‐label, randomised controlled trial. Lancet 2013;382:1021–1028. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.