Abstract
Introduction The petrous apex poses a challenge for surgical intervention due to poor access. As intraoperative image guidance and surgical instrumentation improve, newer endoscopic approaches are increasingly favored. This study aims to provide normative data on the anatomy of the lateral sphenoid sinus recess and petrous apex. These normative data could assist in determining the efficacy of a transnasal transsphenoidal approach to lesions of the anteroinferior petrous apex.
Methods This is a retrospective study investigating normative data on all maxillofacial computed tomography (CT) scans performed at a level I trauma center over a 6-month period. All appropriate images had the pneumatization pattern of the petrous apex and lateral recess of the sphenoid sinus reviewed by a single otologist and graded bilaterally. These were then analyzed in SPSS; Pearson correlation analyses and χ 2 test were used.
Results A total of 481 patients were identified, yielding a total of 962 temporal bones and sphenoid sinuses for analysis. Eighty-eight percent of sides analyzed had a nonpneumatized lateral recess. The petrous apex was nonpneumatized in 54% of sides analyzed. There was a correlation noted between the degree of pneumatization of the petrous apex and pneumatization of the lateral recess of the sphenoid.
Conclusion This study is the first to provide normative data comparing pneumatization of the petrous apex and sphenoid sinus. These data may support future work evaluating the utility of an endonasal approach to the petrous apex.
Keywords: petrous apex, sphenoid sinus, skull base, pneumatization
Introduction
As the most medial portion of the temporal bone, the petrous apex is a challenge for surgical intervention. It is best described as a pyramid with an anteromedial apex and posterolateral base; the otic capsule forms the base of the petrous apex, and the apex itself is bordered anteriorly by the foramen lacerum and posteriorly by the inferior petrosal sinus. 1 Current approaches to the petrous apex include transmastoid, translabyrinthine, infracochlear, middle fossa, infratemporal fossa, and transnasal techniques. 2 Transosseous endonasal approaches to the anteroinferior petrous apex have grown in popularity; the choice of surgical approach depends on several factors, including status of the inner ear, cranial nerve function, location of the lesion, histopathology of the lesion, and surgeon experience. 3 Endoscopic approaches are gaining favor with improved intraoperative image guidance and surgical instrumentation.
Cholesterol granuloma is the most common petrous apex lesion and can be treated endoscopically by establishing a drainage path into the sphenoid sinus. 4 Other lesions of the petrous apex include epidermoid/cholesteatoma, mucocele, and benign or malignant tumors. 2 There have been numerous descriptions of transnasal endoscopic approaches to cholesterol granulomas of the petrous apex in the literature 5 as well as articles proposing criteria for favorable anatomy for choosing endoscopic intranasal approaches. 6 Anatomical features promoted as favorable for intranasal approaches include pneumatization of the concha, 7 lateral pneumatization beyond the vidian nerve, 8 and wide area between the petrous carotid artery and optic nerve. 9 Some have proposed that endoscopic techniques are better tolerated, with decreased complications and morbidity compared with other traditional approaches, but little data exist to support this claim. 10
The choice of transnasal versus lateral approaches to the petrous apex is highly variable. Factors such as poor pneumatization of the sphenoid sinus can make a transnasal approach significantly more challenging. The relatively short distance to the petrous apex contributes to the ease of an endoscopic approach, whereas the need to drill through densely underpneumatized or marrow-laden bone can make the lateral approach very challenging. This study aims to provide normative data on the pneumatization of the lateral recess of the sphenoid sinus and petrous apex, the assumption being that a high degree of pneumatization makes for easier access to this region. These normative data could prove useful for surgeons in determining the favorability of transnasal transsphenoidal approaches to lesions of the petrous apex.
Methods
This is an Institutional Review Board approved, retrospective study performed at a single tertiary teaching hospital. All maxillofacial computed tomography (CT) scans performed at a single institution from July 2012 to December 2012 were reviewed by a single board certified otologist (K. P. B) and scored for pneumatization of the sphenoid sinus and petrous apex. Images were obtained using a multislice CT scanner at a slice thickness of 2 mm and were reviewed using the Tampa General Hospital PACS system (McKesson Horizon Medical Imaging, Vancouver, British Columbia, Canada). Inclusion criteria included patient age greater or equal to 18 years and complete visualization of the sphenoid sinus and bilateral temporal bones on CT scan. Exclusion criteria included pathology located within the petrous apex. Images were predominantly performed in the emergency room as part of a trauma evaluation. This consecutive hospital-based series was used to avoid possible overrepresentation of sinus and/or otological disease in the study cohort. Axial bone window images parallel to the C3 segment of the carotid artery were analyzed.
Grading of the petrous apex and sphenoid sinus were performed separately and bilaterally for each patient. Scans were assessed for pneumatization of the lateral recess of the sphenoid sinus and scored. A binary scale was used, with 0 representing a nonpneumatized lateral recess and 1 representing pneumatization of the lateral recess of the sphenoid sinus. Pneumatization of the lateral recess was defined as pneumatization extending laterally into the greater wing of the sphenoid and/or pterygoid processes. 9 11 Grading the petrous apex pneumatization was based on the presence and location of petrous apex air cells as described in the literature. 12 Briefly, the following definitions were used: grade 0, no pneumatization in the petrous apex; grade 1, a single irregular outpouching of the Eustachian tube; grade 2, pneumatization in the petrous apex lateral to the bony canal of the carotid artery; and grade 3, petrous apex pneumatization medial to the carotid artery. Fig. 1 demonstrates a grade 0 sphenoid sinus and grade 0 petrous apex. Fig. 2 demonstrates a grade 1 sphenoid sinus and a grade 3 petrous apex.
Fig. 1.
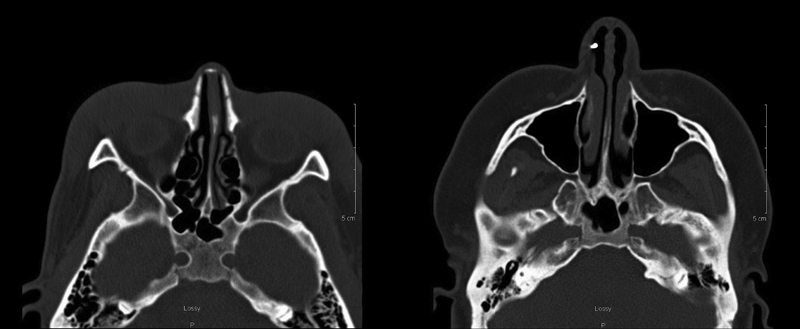
Bilateral grade 0 petrous apex and grade 0 sphenoid sinus.
Fig. 2.
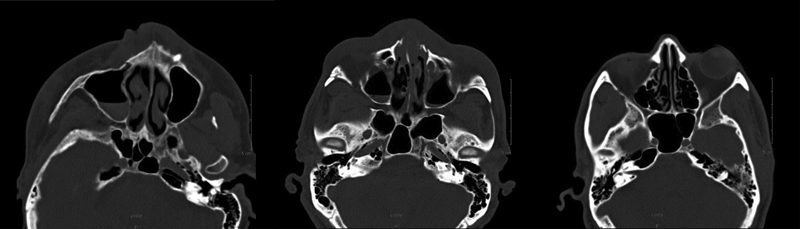
Bilateral grade 3 petrous apex and grade 1 sphenoid sinus.
Statistical Analysis
All analyses were completed using SPSS 21.0 (IBM, Armonk, New York, United States). A χ 2 test was used to analyze the relationship of pneumatization of contralateral sides and of the petrous apex and sphenoid sinus. Correlations were evaluated using Pearson's r. All tests were considered significant at α < 0.05 and were two-tailed.
Results
In total, 481 patients met the inclusion criteria, yielding a total of 962 temporal bones and sphenoid sinuses that were analyzed; no cases were excluded due to disease within the petrous apex itself. There were 295 (61.3%) males and 186 (38.7%) females; mean age was 44.2 years (range: 18–95). Table 1 illustrates the distribution of pneumatization grades for both areas. There was no correlation between gender or age, and the petrous apex pneumatization or pneumatization of the lateral recess of the sphenoid sinus. Age was negatively correlated with petrous apex pneumatization (Pearson's r = –0.112; p = 0.014).
Table 1. Pneumatization patterns observed.
| Location and grade | |
|---|---|
| Sphenoid sinus | |
| 0 | 846 |
| 1 | 116 |
| Petrous apex | |
| 0 | 521 |
| 1 | 157 |
| 2 | 77 |
| 3 | 207 |
For the petrous apex, bilateral nonpneumatized bone (Grade 0) was the most common variant, identified in 221 patients (46%). Seventy-four (15%) patients had bilateral pneumatization medial to the carotid artery (grade 3). The degree of pneumatization encountered along one side of the petrous apex and the degree of pneumatization along the contralateral side were significant related (χ 2 = 360.15; p < 0.001).
Three hundred and ninety eight (83%) patients had bilateral nonpneumatized lateral recesses of the sphenoid sinus, 50 (10%) had a unilateral pneumatized recess, and 33 (7%) patients had bilateral pneumatization. Presence of pneumatization along one side of the lateral sphenoid recess and pneumatization along the contralateral side were significantly related (χ 2 = 125.10; p < 0.001).
Of 116 sides with a grade 1 sphenoid, 53 (46%) were associated with a grade 3 petrous apex. Eleven (2%) patients had bilateral grade 3 petrous apices and grade 1 sphenoid sinuses. Overall pneumatization of the petrous apex was significantly related to overall pneumatization of the lateral recess of the sphenoid sinus (χ 2 = 72.129; p < 0.001).
Discussion
As transnasal approaches for lesions of the petrous apex become more widely utilized, the need for normative data regarding pertinent anatomy has arisen. Normative data have proved useful for other transnasal approaches and may contribute to both surgeon education and the development of new exposure-enhancing tools and techniques. 13 Small series evaluating the transnasal approach to the petrous apex have provided an initial groundwork on key anatomical landmarks of interest for investigating in the population at large. 14 15
This series identified some degree of pneumatization of the petrous apex in 54% of the patients. Previously published reports have noted a 30 to 35% incidence of petrous apex pneumatization. 12 16 17 18 A likely reason for the elevated prevalence of pneumatization in our study compared with prior studies is the classification scheme used, as grade 1 pneumatization in other studies might not routinely be classified as petrous apex pneumatization. Recalculation of the pneumatization data and elimination of grade 1 pneumatization reduces the prevalence of pneumatization of the petrous apex in our study to just below 35%.
Factors influencing pneumatization of the temporal bone have been extensively studied given the importance of pneumatization patterns on otological pathology and surgery. Developmentally, the pneumatization pattern of the petrous portion of the mastoid, as well as the anterior epitympanic recess, are formed from the saccus medius, whereas the pneumatization pattern of the squamous portion of mastoid is formed from the saccus superioris. 19 There is some evidence to suggest that development of the anterior epitympanic space plays a role in petrous apex pneumatization. 20 21 The degree of mastoid pneumatization is well-known to affect middle ear pressure, and poor pneumatization predisposes one to retraction pockets and cholesteatoma. 22 23 The pneumatization pattern of the mastoid is influenced by both environmental and genetic factors, and early surgical intervention to correct negative middle ear pressure can improve mastoid pneumatization. 24 Proper aeration influences the pneumatization patterns of all bones and is one of the most important environmental factors that can be modified (e.g., with the use of pressure equalization tubes). 25 The role that the size of the Eustachian tube lumen plays in pneumatization of the mastoid is unclear, with various conflicting studies within the literature, though dilation of the Eustachian tube has been shown to improve negative middle ear pressure. 26 27 The high degree of pneumatization commonly observed in cholesterol granulomas of the petrous apex may indicate a predisposition for certain pneumatization patterns to be more commonly seen compared with the general population. 28
The importance of sphenoid sinus pneumatization has been recognized since the earliest endoscopic techniques for approaching the petrous apex. 29 Only 17% of total patients, or 12% of sides analyzed, demonstrated sphenoid sinus pneumatization extending into the lateral recess. This is on the low end of the reported ranges, but there is a great variation in the literature (16–72% of sides analyzed). 8 9 11 30 31 These differences could be due to several factors. First, sphenoid sinus pneumatization varies in different ethnic and geographical locations. 30 32 Second, the indication for imaging in our study was predominantly related to trauma. We chose a hospital-based cohort to avoid selection bias related to the use of patients with otolaryngological disease. However, conversely, the presence of pneumatization may be related to trauma. Additionally, the type of CT scanner and resolution of images could have affected the final reading, as finer cuts have been proposed to allow for better detection of subtle variances in anatomical patterns, but a midcarotid cut was used both in this and in previous papers and was available in the images we analyzed. 33
Factors that may affect pneumatization patterns of the sphenoid bone have not been as thoroughly investigated compared with those of the temporal bone due to the relative recent rise of endoscopic sinus surgery as well as appropriate imaging modalities for investigation. Paranasal sinus pneumatization was historically attributed to expansion of the ethmoid air cells into surrounding bony structure, but this process has come into question, and there is some evidence that the sinuses may develop independently from the ethmoid air cells through fat involution. 34 Failure of this process can lead to the equivalent of the “cue ball,” or hypoplastic sphenoid sinus. 35 Hypothetically, increased air flow through the nasal cavity increases pneumatization of the paranasal sinuses, but obstruction by adenoidal hypertrophy has been demonstrated to have no effect on sinonasal pneumatization patterns, including that of the sphenoid sinus. 36 Furthermore, certain disease processes that affect respiratory mucosa, such as cystic fibrosis, have been demonstrated to have a negative effect on paranasal sinus pneumatization. 37 This appears to be unrelated to the presence of sinus disease with the nasal cavities, but the pathophysiology of this is not clearly understood.
There are conflicting reports regarding the correlation between mastoid and sphenoid sinus pneumatization patterns. 8 38 39 40 The postnatal growth patterns between the mastoid and paranasal sinuses are different, suggesting different developmental factors. 38 One interesting finding of note is that cystic fibrosis is associated with increased pneumatization of the temporal bone 41 42 43 and decreased pneumatization of the sphenoid sinus. 44 Septal deviations may also play a role in mastoid pneumatization, and there are reports of septoplasty improving chronic middle ear disease in select patients. 45 46 47 This is theoretically due to decreased airflow through the Eustachian tube ipsilateral to the deviation, though further work is needed to substantiate this claim. Whether pneumatization of the lateral recess of the sphenoid sinus is influenced differently than the other paranasal sinuses is not known, and further evaluation of lateral recess pneumatization among cystic fibrosis patients may yield additional valuable information.
It is worth noting that this anatomical study was performed in patients without known petrous apex pathology, and in the case of known pathology, remodeling of surrounding bone by expansile processes may increase the utility of an endoscopic approach. As an example, we have included pre- and postoperative imaging from a case where an endoscopic approach was taken to a petrous apex epidermoid cyst. As illustrated in the preoperative image ( Fig. 3A ), significant bony remodeling occurred with lateral displacement of the carotid, thus improving access to the petrous apex portion of the lesion. The intraoperative photo ( Fig. 3B ) demonstrates excellent visualization into the petrous apex lesion, with adequate drainage attained, as demonstrated by the postoperative imaging ( Fig. 3C ).
Fig. 3.
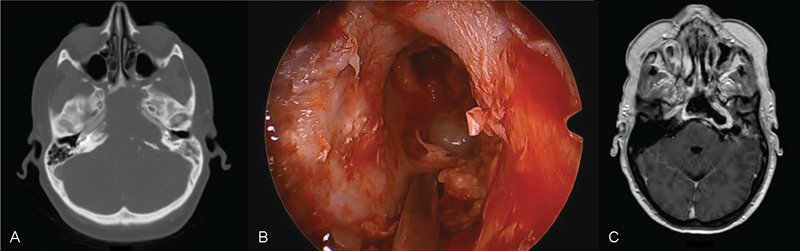
Sample case of an epidermoid cyst removed using an endoscopic approach illustrating how anatomical favorability may be altered by pathology. Preoperative imaging ( A ) demonstrates a lateralized internal carotid artery. Intraoperative photo ( B ) shows excellent visualization, with the site being left open to drain as demonstrated in the postoperative MRI (magnetic resonance imaging) ( C ).
In describing a consistent radiological landmark for the endonasal approach to the petrous apex, Shoman et al proposed the petrous angle, which is derived from the medial aspect of the proximal C3 segment of the internal carotid artery, the vomer, and the external occipital protuberance. 48 They further noted that pneumatization of the petrous apex and/or sphenoid sinus was an additional factor that could affect this angle. This study is the first, to our knowledge, to provide normative data comparing pneumatization of the petrous apex and sphenoid sinus. Further evaluation of factors predicting the favorability of an endonasal approach to the petrous apex is warranted.
Conclusion
This anatomical study demonstrates a subset of patients with temporal bone pneumatization patterns on CT scan that are likely favorable for an endoscopic approach. Bilateral nonpneumatization of the petrous apex and lateral recess of the sphenoid was the most common variant identified; however, a portion of patients who demonstrated well-pneumatized lateral recess of the sphenoid had favorable petrous apex pneumatization. Determining the genetic and environmental factors affecting pneumatization patterns and whether favorable anatomy is associated with lesions of the petrous apex warrants further investigation.
Note
Paper presented at the 24th Annual Meeting North American Skull Base Society, San Diego, California, United States.
References
- 1.Chole R A. Petrous apicitis: surgical anatomy. Ann Otol Rhinol Laryngol. 1985;94(03):251–257. [PubMed] [Google Scholar]
- 2.Isaacson B, Kutz J W, Roland P S.Lesions of the petrous apex: diagnosis and management Otolaryngol Clin North Am 20074003479–519., viii [DOI] [PubMed] [Google Scholar]
- 3.Meneses M S, Moreira A L, Bordignon K C, Pedrozo A A, Ramina R, Nikoski J G.Surgical approaches to the petrous apex: distances and relations with cranial morphology Skull Base 200414019–19., discussion 19–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kassam A B, Gardner P, Snyderman C, Mintz A, Carrau R. Expanded endonasal approach: fully endoscopic, completely transnasal approach to the middle third of the clivus, petrous bone, middle cranial fossa, and infratemporal fossa. Neurosurg Focus. 2005;19(01):E6. [PubMed] [Google Scholar]
- 5.Zanation A M, Snyderman C H, Carrau R L, Gardner P A, Prevedello D M, Kassam A B. Endoscopic endonasal surgery for petrous apex lesions. Laryngoscope. 2009;119(01):19–25. doi: 10.1002/lary.20027. [DOI] [PubMed] [Google Scholar]
- 6.Van Gompel J J, Alikhani P, Tabor M H et al. Anterior inferior petrosectomy: defining the role of endonasal endoscopic techniques for petrous apex approaches. J Neurosurg. 2014;120(06):1321–1325. doi: 10.3171/2014.2.JNS131773. [DOI] [PubMed] [Google Scholar]
- 7.Hamid O, El Fiky L, Hassan O, Kotb A, El Fiky S. Anatomic Variations of the Sphenoid Sinus and Their Impact on Trans-sphenoid Pituitary Surgery. Skull Base. 2008;18(01):9–15. doi: 10.1055/s-2007-992764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Iseli T A, Yahng J, Leung R, Briggs R J, King J A, Phal P M. Anatomic comparison of nasal versus lateral surgical access to the petrous apex. Clin Anat. 2013;26(06):682–687. doi: 10.1002/ca.22113. [DOI] [PubMed] [Google Scholar]
- 9.Wang J, Bidari S, Inoue K, Yang H, Rhoton A., Jr Extensions of the sphenoid sinus: a new classification. Neurosurgery. 2010;66(04):797–816. doi: 10.1227/01.NEU.0000367619.24800.B1. [DOI] [PubMed] [Google Scholar]
- 10.Wachter D, Behm T, Gilsbach J M, Rohde V. Neurosurgical strategies and operative results in the treatment of tumors of or extending to the petrous apex. Minim Invasive Neurosurg. 2011;54(02):55–60. doi: 10.1055/s-0031-1275290. [DOI] [PubMed] [Google Scholar]
- 11.Tomovic S, Esmaeili A, Chan N J et al. High-resolution computed tomography analysis of variations of the sphenoid sinus. J Neurol Surg B Skull Base. 2013;74(02):82–90. doi: 10.1055/s-0033-1333619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jen A, Sanelli P C, Banthia V, Victor J D, Selesnick S H. Relationship of petrous temporal bone pneumatization to the eustachian tube lumen. Laryngoscope. 2004;114(04):656–660. doi: 10.1097/00005537-200404000-00011. [DOI] [PubMed] [Google Scholar]
- 13.Lazaridis N, Natsis K, Koebke J, Themelis C. Nasal, sellar, and sphenoid sinus measurements in relation to pituitary surgery. Clin Anat. 2010;23(06):629–636. doi: 10.1002/ca.20984. [DOI] [PubMed] [Google Scholar]
- 14.Scopel T F, Fernandez-Miranda J C, Pinheiro-Neto C D et al. Petrous apex cholesterol granulomas: endonasal versus infracochlear approach. Laryngoscope. 2012;122(04):751–761. doi: 10.1002/lary.22448. [DOI] [PubMed] [Google Scholar]
- 15.Chatrath P, Nouraei S A, De Cordova J, Patel M, Saleh H A. Endonasal endoscopic approach to the petrous apex: an image-guided quantitative anatomical study. Clin Otolaryngol. 2007;32(04):255–260. doi: 10.1111/j.1365-2273.2007.01465.x. [DOI] [PubMed] [Google Scholar]
- 16.Brackmann D E, Toh E H. Surgical management of petrous apex cholesterol granulomas. Otol Neurotol. 2002;23(04):529–533. doi: 10.1097/00129492-200207000-00023. [DOI] [PubMed] [Google Scholar]
- 17.Virapongse C, Sarwar M, Bhimani S, Sasaki C, Shapiro R. Computed tomography of temporal bone pneumatization: 1. Normal pattern and morphology. AJR Am J Roentgenol. 1985;145(03):473–481. doi: 10.2214/ajr.145.3.473. [DOI] [PubMed] [Google Scholar]
- 18.Yamakami I, Uchino Y, Kobayashi E, Yamaura A.Computed tomography evaluation of air cells in the petrous bone--relationship with postoperative cerebrospinal fluid rhinorrhea Neurol Med Chir (Tokyo) 20034307334–338., discussion 339 [DOI] [PubMed] [Google Scholar]
- 19.Gulya A J, Gulya A J. New York, NY: Informa Healthcare; 2007. Gulya and Schuknecht's Anatomy of the Temporal Bone with Surgical Implications. 3rd ed. [Google Scholar]
- 20.Tono T, Schachern P A, Morizono T, Paparella M M, Morimitsu T. Developmental anatomy of the supratubal recess in temporal bones from fetuses and children. Am J Otol. 1996;17(01):99–107. [PubMed] [Google Scholar]
- 21.Lee D H, Kim M J, Lee S, Choi H. Anatomical factors influencing pneumatization of the petrous apex. Clin Exp Otorhinolaryngol. 2015;8(04):339–344. doi: 10.3342/ceo.2015.8.4.339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sadé J, Fuchs C. A comparison of mastoid pneumatization in adults and children with cholesteatoma. Eur Arch Otorhinolaryngol. 1994;251(04):191–195. doi: 10.1007/BF00628421. [DOI] [PubMed] [Google Scholar]
- 23.Sadé J, Fuchs C, Luntz M. The pars flaccida middle ear pressure and mastoid pneumatization index. Acta Otolaryngol. 1996;116(02):284–287. doi: 10.3109/00016489609137842. [DOI] [PubMed] [Google Scholar]
- 24.Valtonen H J, Dietz A, Qvarnberg Y H, Nuutinen J. Development of mastoid air cell system in children treated with ventilation tubes for early-onset otitis media: a prospective radiographic 5-year follow-up study. Laryngoscope. 2005;115(02):268–273. doi: 10.1097/01.mlg.0000154731.08410.b8. [DOI] [PubMed] [Google Scholar]
- 25.Palva T, Ramsay H. Fate of the mesenchyme in the process of pneumatization. Otol Neurotol. 2002;23(02):192–199. doi: 10.1097/00129492-200203000-00015. [DOI] [PubMed] [Google Scholar]
- 26.Silvola J, Kivekäs I, Poe D S. Balloon dilation of the cartilaginous portion of the Eustachian tube. Otolaryngol Head Neck Surg. 2014;151(01):125–130. doi: 10.1177/0194599814529538. [DOI] [PubMed] [Google Scholar]
- 27.Daniel H J, Schmidt R T, Fulghum R S, Ruckriegal L.Otitis media: a problem for the physical anthropologist Am J Phys Anthropol 198831(S9):143–167. [Google Scholar]
- 28.Jackler R K, Cho M.A new theory to explain the genesis of petrous apex cholesterol granuloma Otol Neurotol 2003240196–106., discussion 106 [DOI] [PubMed] [Google Scholar]
- 29.Fucci M J, Alford E L, Lowry L D, Keane W M, Sataloff R T. Endoscopic management of a giant cholesterol cyst of the petrous apex. Skull Base Surg. 1994;4(01):52–58. doi: 10.1055/s-2008-1058990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lu Y, Pan J, Qi S, Shi J, Zhang X, Wu K. Pneumatization of the sphenoid sinus in Chinese: the differences from Caucasian and its application in the extended transsphenoidal approach. J Anat. 2011;219(02):132–142. doi: 10.1111/j.1469-7580.2011.01380.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Arslan H, Aydinlioğlu A, Bozkurt M, Egeli E. Anatomic variations of the paranasal sinuses: CT examination for endoscopic sinus surgery. Auris Nasus Larynx. 1999;26(01):39–48. doi: 10.1016/s0385-8146(98)00024-8. [DOI] [PubMed] [Google Scholar]
- 32.Mato D, Yokota H, Hirono S, Martino J, Saeki N. The vidian canal: radiological features in Japanese population and clinical implications. Neurol Med Chir (Tokyo) 2015;55(01):71–76. doi: 10.2176/nmc.oa.2014-0173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lee W T, Kuhn F A, Citardi M J. 3D computed tomographic analysis of frontal recess anatomy in patients without frontal sinusitis. Otolaryngol Head Neck Surg. 2004;131(03):164–173. doi: 10.1016/j.otohns.2004.04.012. [DOI] [PubMed] [Google Scholar]
- 34.Kuntzler S, Jankowski R. Arrested pneumatization: witness of paranasal sinuses development? Eur Ann Otorhinolaryngol Head Neck Dis. 2014;131(03):167–170. doi: 10.1016/j.anorl.2013.01.008. [DOI] [PubMed] [Google Scholar]
- 35.Prabhu A V, Branstetter B F., IVThe CT prevalence of arrested pneumatization of the sphenoid sinus in patients with sickle cell diseaseAJNR Am J Neuroradiol2016(e-pub ahead of print) [DOI] [PMC free article] [PubMed]
- 36.Apuhan T, Yıldırım Y S, Özaslan H. The developmental relation between adenoid tissue and paranasal sinus volumes in 3-dimensional computed tomography assessment. Otolaryngol Head Neck Surg. 2011;144(06):964–971. doi: 10.1177/0194599811399712. [DOI] [PubMed] [Google Scholar]
- 37.Kim H J, Friedman E M, Sulek M, Duncan N O, McCluggage C. Paranasal sinus development in chronic sinusitis, cystic fibrosis, and normal comparison population: a computerized tomography correlation study. Am J Rhinol. 1997;11(04):275–281. doi: 10.2500/105065897781446676. [DOI] [PubMed] [Google Scholar]
- 38.Lee D H, Shin J H, Lee D C. Three-dimensional morphometric analysis of paranasal sinuses and mastoid air cell system using computed tomography in pediatric population. Int J Pediatr Otorhinolaryngol. 2012;76(11):1642–1646. doi: 10.1016/j.ijporl.2012.07.037. [DOI] [PubMed] [Google Scholar]
- 39.Hindi K, Alazzawi S, Raman R, Prepageran N, Rahmat K. Pneumatization of mastoid air cells, temporal bone, ethmoid and sphenoid sinuses. Any correlation? Indian J Otolaryngol Head Neck Surg. 2014;66(04):429–436. doi: 10.1007/s12070-014-0745-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kim J, Song S W, Cho J H, Chang K H, Jun B C. Comparative study of the pneumatization of the mastoid air cells and paranasal sinuses using three-dimensional reconstruction of computed tomography scans. Surg Radiol Anat. 2010;32(06):593–599. doi: 10.1007/s00276-009-0618-4. [DOI] [PubMed] [Google Scholar]
- 41.Todd N W, Martin W S. Temporal bone pneumatization in cystic fibrosis patients. Laryngoscope. 1988;98(10):1046–1049. doi: 10.1288/00005537-198810000-00004. [DOI] [PubMed] [Google Scholar]
- 42.Yildirim N, Sone M, Mutlu C, Schachern P A, Paparella M M, Le C T. Histopathologic features of the temporal bone in patients with cystic fibrosis. Arch Otolaryngol Head Neck Surg. 2000;126(01):75–78. doi: 10.1001/archotol.126.1.75. [DOI] [PubMed] [Google Scholar]
- 43.Berkhout M C, van Rooden C J, Aalbers R C et al. Temporal bone pneumatization in cystic fibrosis: a correlation with genotype? Laryngoscope. 2014;124(07):1682–1686. doi: 10.1002/lary.24575. [DOI] [PubMed] [Google Scholar]
- 44.Eggesbø H B, Eken T, Eiklid K, Kolmannskog F. Hypoplasia of the sphenoid sinuses as a diagnostic tool in cystic fibrosis. Acta Radiol. 1999;40(05):479–485. doi: 10.3109/02841859909175571. [DOI] [PubMed] [Google Scholar]
- 45.Koch U, Herberhold C, Opitz H J. Middle ear pressure after rhinoplasty surgery (author's transl) [in German] Laryngol Rhinol Otol (Stuttg) 1977;56(08):657–661. [PubMed] [Google Scholar]
- 46.Gencer Z K, Özkiriş M, Okur A, Karaçavus S, Saydam L. The possible associations of septal deviation on mastoid pneumatization and chronic otitis. Otol Neurotol. 2013;34(06):1052–1057. doi: 10.1097/MAO.0b013e3182908d7e. [DOI] [PubMed] [Google Scholar]
- 47.Lee D H, Jin K S. Effect of nasal septal deviation on pneumatization of the mastoid air cell system: 3D morphometric analysis of computed tomographic images in a pediatric population. J Int Adv Otol. 2015;10(03):251–255. [Google Scholar]
- 48.Shoman N, Donaldson A M, Ksiazek J, Pensak M L, Zimmer L A. First stage in predicative measure for transnasal transsphenoidal approach to petrous apex cholesterol granuloma. Laryngoscope. 2013;123(03):581–583. doi: 10.1002/lary.23754. [DOI] [PubMed] [Google Scholar]


