Readouts from the thrombin generation (TG) assay (TGA) have been used to predict the effects of hemostatic drugs in hemophilic patients [1]. There is better correlation between an individual’s bleeding tendency and his or her TG capacity compared with factor levels measured using routine assays [2,3]. However, the clinical utility of TGA has not yet been validated in multicenter studies because of lack of standardization. This report aims to highlight variables that may impact directly on TGA reliability and steps to reduce TGA variability within and between laboratories to improve precision of results. There are two semi-automated (CAT, Stago, Asniéres, France; Technothrombin-TGA, Technoclone, Vienna, Austria) and two fully-automated (CEVERON® alpha w. TGAm, Technoclone; ST-Genesia, Stago) instruments that use fluorogenic thrombin substrates. Three instruments on the market use chromogenic thrombin substrates: HemoScan Thrombin Generation Assay (HemoScan, Groningen, the Netherlands), Pefakit in-TDT (Pentapharm, Aesch, Switzerland) and Innovance ETP (Siemens Healthcare SAS, Erlangen, Deutschland).
Recommendations formulated here are valid for all the above-mentioned methods and are based on objective data of the current literature and expert opinion.
Pre-analytical variables
Venous blood collection
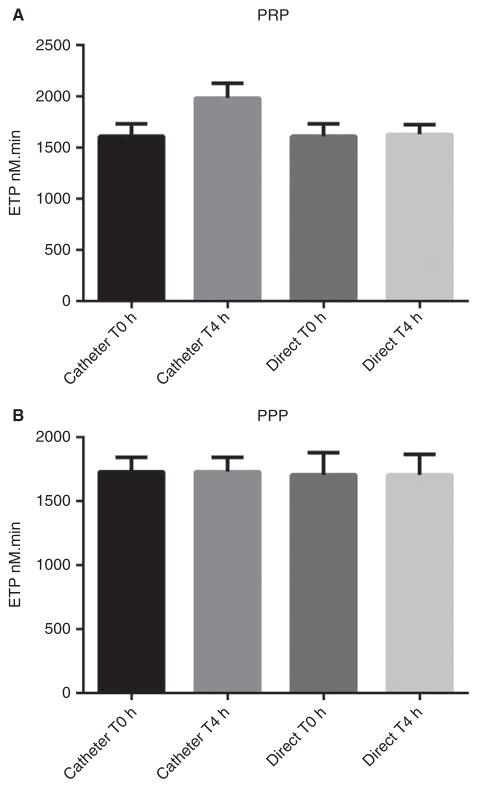
Blood should be drawn by direct venipuncture into a citrate anticoagulated tube. Blood sampling through venous catheters modifies TG in platelet-rich plasma (PRP), but not in platelet-poor plasma (PPP) (Fig. 1).
Fig. 1.
(A) Thrombin generation measured in platelet-rich plasma (PRP) from six healthy volunteers, using tissue factor (TF) 1 pM. Blood samples were obtained by direct venepuncture and from a venous catheter at time 0 and 4 h after catheter introduction. Thrombin generation was significantly higher in samples drawn from catheters compared with direct venipuncture (P = 0.0043; Mann–Whitney U-test). (B) Similar experiment in platelet-poor plasma (PPP) from the same volunteers using TF 1 pM and phospholipids 4 μM. Thrombin generation was not significantly different in samples drawn from catheters compared with direct venipuncture (P > 0.05; Mann–Whitney U-test). ETP, endogenous thrombin potential.
Data comparing different blood-drawing systems for TGA in PPP showed no significant difference between Monovette-syringe tubes (Sarstedt, Orsay, France) and Vacutainer vacuum tubes (Becton Dickinson, Meylan, France) containing sodium citrate 106 mM with negative air pressure inside. However, when TG was measured in PRP from Vacutainer tubes, peak and endogenous thrombin potential (ETP) were overestimated by 35% and 29.5%, respectively, compared with those obtained from Monovette tubes [4]. Needles used for venipuncture (19– 21 gauge) showed no difference in TGA values [4]. The tourniquet should be applied only long enough to locate a vein; ideally 60 s or less [5]. Prolonged stasis, as may occur with long application of the tourniquet, may result in changes in coagulation assays [5].
Contact system inhibition for TG testing
The need for contact pathway inhibition such as corn trypsin inhibitor (CTI) for blood sampling is still debatable [6–9]. The presence of such inhibitors ensures the TGA proceeds via tissue factor (TF) activity added to trigger the assay. The maximal effect of CTI occurs when blood is collected into tubes preloaded with CTI [6]. CTI also eliminates the need for immediate sample preparation and analysis for both PPP and PRP samples [10]. However, because the cost of CTI is a barrier to its routine use, the need for CTI has been questioned [8,11,12]. Advantages of CTI used at the final concentration of 1.45 μM have been confirmed [13,14], and an official communication of the ISTH subcommittee on the control of anticoagulation also emphasized the advantages of CTI for TGA triggered by low TF concentrations (≤ 1 pM) [15]. Limited availability of tubes preloaded with CTI on the market is a real limitation for clinical use.
Transport of blood samples to the laboratory
A recent study evaluating the effects of pneumatic tube transport on TGA showed that all TGA parameters are significantly modified in both PPP and PRP samples compared with hand-carrier transport [16]. Thus, pneumatic tubes should not be used prior to TGA. Whole blood samples should be kept at room temperature (RT) [5].
PPP and PRP preparation for TG measurements
One of the most critical preanalytical steps is plasma sample preparation. Samples should be processed as quickly as possible (ideally within 1 h of collection) [5].
PPP
We recommend double centrifugation [17,18]. With this approach, whole blood samples (5 mL) are centrifuged (2500 × g, 15 min, RT) to obtain PPP. As soon as centrifugation is complete, 1.5 mL of supernatant is collected, ensuring that the top surface of the plasma where some light platelets may be found is not aspirated, and not too close to the platelet layer, taking care not to disturb the buffy coat. The collected plasma is then recentrifuged (2500 × g, 15 min, RT) and 1200 μL of supernatant is then collected and frozen at −80 °C immediately after collection. In frozen plasma at −80 °C, coagulation proteins are stable for a minimum of 2 years [19]. Frozen plasma should be thawed at 37 °C before testing; we recommend not using plasma that has been freezethawed more than once.
The preanalytical issue of remaining platelets and/or white cells may lead to incorrect estimation of TG [4]. The quality of PPP samples can be evaluated for each sample by measuring TG without any triggering agent. If plasma contains CTI, no TG should be observed in the absence of TF and phospholipids.
PRP
PRP is usually obtained by a single centrifugation (150 × g, 10 min, RT), and by collecting only the upper half of the PRP fraction [20]. The platelet count is then adjusted to 150 × 109/L with autologous PPP [17]. We recommend using fresh PRP prepared within 30 min after venipuncture and not freezing samples.
A DVD demonstrating key steps that may interfere with TGA results has been evaluated [14]. It significantly improved reproducibility by operators who watched it and is available upon request.
Analytical variables
Reagents for TGA in patients with bleeding disorders
TGA is typically triggered by addition of TF and TGA results are highly dependent on the TF concentration [17]. A major cause of inter-laboratory variation is the source and concentration of TF used [9,21]. The TF source (e.g. plasma or placenta-derived human TF, recombinant human TF, rabbit TF and also the content of phospholipids in TF reagents) might be a significant source of variability between homemade reagents [21]. Use of a standardized trigger reagent can significantly reduce inter-center variability [21]. The absence of an international reference standard for TF is a major problem hampering standardization; however, reagents with labeled TF may improve consistency of results. We recommend commercially available, standardized reagents. Sensitivity of the assay to low TF concentrations (i.e. ≤ 1 pM) is increased in plasmas prepared from blood collected into contact pathway inhibitors [10–21]. Use of a low TF concentration (≤ 1 pM) can detect hypocoagulability induced by factor VIII or IX deficiency and monitor hemostatic therapies in hemophilia patients [18,22,23]. Other bleeding disorders may require different conditions: in factor XI deficiency, correlation between TG and clinical bleeding risk was observed only when coagulation was triggered by very low TF levels (0.5 pM) in the presence of platelets [24,25].
Temperature of plasma samples
The importance of plate temperature for TGA was reported by De Smedt et al. [26]. A recent international standardization study for TGA showed 29% higher area-under-the-curve (AUC) values when plates were not pre-heated, compared with plates heated at 37 °C before TGA. Differences were a result of decreased thrombin inactivation at lower temperatures [27], suggesting antithrombin anticoagulant activity is not sufficiently detected without preheating. The use of non-preheated plates might therefore be a source of error when bleeding risk is evaluated.
The need for thermostability in the fluorometer for reproducible TG measurement was previously highlighted [28]. Although stability of the temperature in the device cannot be controlled, investigators should be aware that this may induce inter-center variability.
The main source of variability remains the difference between the temperature of the plate as it enters the fluorometer and the temperature in the fluorometer. If results are normalized against a reference plasma, temperature is less important.
The use of a reference plasma to reduce inter-center variability of TGA
Normalization of TGA results against a reference plasma run in the same experiment significantly improved intercenter variability of TGA [21]. Choice of the reference plasma should be carefully determined, and batch-to-batch variability of reference plasmas considered [14].
Interpretation of the results
Abnormal AUC and peak values were reported in both hemophilia and factor XI deficiency [18,24,25]; abnormal time-to-peak was also observed in hemophilia [18]. When AUC is not automatically calculated by the dedicated software, operators can manually determine the tail start point, which can be a major source of variability. The best way to tackle this is to analyze fluorescence curves by hand in an Excel sheet [29].
Variation of the method
Assay precision has substantially improved in the last decade. Multicenter standardization exercises between expert laboratories reported intra- and inter-assay variability < 10% [13].
Standardized test conditions may improve inter-center variability [21]. However, a recent survey on TGA performed in ‘real-life’ conditions reported acceptable intercenter variability: ≤ 10% for normal and hypercoagulable plasma samples, whereas variability was still high (56%) for heparinized plasma samples [30]. New generation fully-automated devices for TGA reduce variability by offering thermostability, and may improve results. However, such devices may not overcome variability caused by pre-analytical assay steps that are crucial for reliable, comparable results.
Final remarks
In addition to pre-analytical and analytical variables, certain aspects of TGA may cause variability between centers.
Use of different methods and fluorometers for measuring TG can induce variability between centers.
Use of standardized, commercially available reagents is preferable to home-made reagents. Low TF concentrations (i.e. ≤ 1 pM) are suitable for TG measurement in both PPP and PRP.
Taking great care during plasma preparation can reduce assay imprecision.
Normalization of results against a standardized reference plasma significantly reduces inter-center variability and is highly suitable for multicenter clinical trials
Acknowledgments
A. S. Wolberg receives funding from the National Institutes of Health (R01HL126974). The study was unfunded.
Footnotes
Addendum
Y. Dargaud designed and performed several studies on standardization of TGA for hemophilia and wrote the manuscript. A. S. Wolberg, E. Gray and C. Negrier critically revised the manuscript. H. C. Hemker critically revised the manuscript. All authors approved the final draft.
Disclosure of Conflict of Interests
H. C. Hemker is a consultant to Diagnostica Stago (Asnieres, France).
References
- 1.Dargaud Y, Lienhart A, Negrier C. Prospective assessment of thrombin generation test for dose monitoring of bypassing therapy in hemophilia patients with inhibitors undergoing elective surgery. Blood. 2010;116:5734–7. doi: 10.1182/blood-2010-06-291906. [DOI] [PubMed] [Google Scholar]
- 2.Santagostino E, Mancuso ME, Tripodi A, Chantarangkul V, Clerici M, Garagiola I, Mannucci PM. Severe hemophilia with mild bleeding phenotype: molecular characterization and global coagulation profile. J Thromb Haemost. 2010;8:737–43. doi: 10.1111/j.1538-7836.2010.03767.x. [DOI] [PubMed] [Google Scholar]
- 3.Trossaert M, Lienhart A, Nougier C, Fretigny M, Sigaud M, Meunier S, Fouassier M, Ternisien C, Negrier C, Dargaud Y. Diagnosis and management challenges in patients with mild haemophilia A and discrepant FVIII measurements. Haemophilia. 2014;20:550–8. doi: 10.1111/hae.12381. [DOI] [PubMed] [Google Scholar]
- 4.Dargaud Y, Negrier C. Thrombin generation testing in haemophilia comprehensive care centres. Haemophilia. 2010;16:223–30. doi: 10.1111/j.1365-2516.2009.02082.x. [DOI] [PubMed] [Google Scholar]
- 5.Adcock DM, Favoloro EJ, Lippi G. Critical pre-analytical variables in the hemostasis laboratory and their quality indicators. Clin Biochem. 2016;49:1315–20. doi: 10.1016/j.clinbiochem.2016.08.022. [DOI] [PubMed] [Google Scholar]
- 6.Luddington R, Baglin T. Clinical measurement of thrombin generation by calibrated automated thrombography requires contact factor inhibition. J Thromb Haemost. 2004;2:1954–9. doi: 10.1111/j.1538-7836.2004.00964.x. [DOI] [PubMed] [Google Scholar]
- 7.Dargaud Y, Luddington R, Baglin T. Platelet-dependent thrombography: a method for diagnostic laboratories. Br J Haematol. 2006;134:323–5. doi: 10.1111/j.1365-2141.2006.06188.x. [DOI] [PubMed] [Google Scholar]
- 8.Spronk H, Dielis A, PanovaNoeva M, van Oerle R, GoversRiemslag J, Hamulyak K, Falanga A, Ten Cate H. Monitoring thrombin generation: is addition of corn trypsin inhibitor needed? Thromb Haemost. 2009;101:1156–62. [PubMed] [Google Scholar]
- 9.vanVeen JJ, Gatt A, Cooper PC, Kitchen S, Bowyer AE, Makris M. Corn trypsin inhibitor in fluorogenic thrombin generation measurements is only necessary at low tissue factor concentrations and influences the relationship between FVIII:C and thrombogram parameters. Blood Coagul Fibrinolysis. 2008;19:183–9. doi: 10.1097/MBC.0b013e3282f4bb47. [DOI] [PubMed] [Google Scholar]
- 10.Dargaud Y, Luddington R, Baglin T. Elimination of contact factor activation improves measurement of platelet dependent thrombin generation by calibrated automated thrombography at low concentration tissue factor. J Thromb Haemost. 2006;4:1160–1. doi: 10.1111/j.1538-7836.2006.01905.x. [DOI] [PubMed] [Google Scholar]
- 11.Tripodi A, Chantarangkul V, Santagostino E. Failure of corn trypsin inhibitor to affect the thrombin generation assay in plasma from severe haemophiliacs: comment. J Thromb Haemost. 2015;13:163–4. doi: 10.1111/jth.12769. [DOI] [PubMed] [Google Scholar]
- 12.Mohammed BM, Martin EJ, Salinas V, Carmona R, Young G, Brophy DF. Failure of corn trypsin inhibitorto affect the thrombin generation assay in plasma from severe hemophiliacs. J Thromb Haemost. 2014;12:1558–61. doi: 10.1111/jth.12659. [DOI] [PubMed] [Google Scholar]
- 13.Dargaud Y, Luddington R, Gray E, Lecompte T, Siegemund T, Baglin T, Hogwood J, Regnault V, Siegemund A, Negrier C. Standardisation of thrombin generation test – which reference plasma for thrombin generation test?: an international multicentre study. Thromb Res. 2010;125:353–6. doi: 10.1016/j.thromres.2009.11.012. [DOI] [PubMed] [Google Scholar]
- 14.Dargaud Y, Wolberg AS, Luddington R, Regnault V, Spronk H, Bagli T, Lecompte T, Ten Cate H, Negrier C. Evaluation of a standardized protocol for thrombin generation measurement using the Calibrated Automated Thrombogram: an international multicentre study. Thromb Res. 2012;130:929–34. doi: 10.1016/j.thromres.2012.07.017. [DOI] [PubMed] [Google Scholar]
- 15.Baglin T, Besser M, Catatneo M, Dargaud Y, Gray E, Key NS, Luddington R, Petros S, Siegemund T, Wolberg AS. Towards a recommendation for the standardization of the measurement of platelet-dependent thrombin generation. J Thromb Haemost. 2011;9:1859–61. doi: 10.1111/j.1538-7836.2011.04427.x. [DOI] [PubMed] [Google Scholar]
- 16.Le Quellec S, Paris M, Nougier C, Sobas F, Girard S, Bordet JC, Negrier C, Dargaud Y. Pre-analytical effects of pneumatic tube system transport on routine haematology tests, global coagulation assays and platelet function aggregation. Thromb Res. 2016;116:638–50. doi: 10.1016/j.thromres.2016.12.022. [DOI] [PubMed] [Google Scholar]
- 17.Hemker HC, Giesen P, Al Dieri R, Regnault V, de Smedt E, Wagenvoord R, Lecompte T, Beguin S. Calibrated automated thrombin generation measurement in clotting plasma. Pathophysiol Haemost Thromb. 2003;33:4–15. doi: 10.1159/000071636. [DOI] [PubMed] [Google Scholar]
- 18.Dargaud Y, Beguin S, Lienhart A, Al Dieri R, Trzeciak C, Bordet JC, Hemker HC, Negrier C. Evaluation of thrombin generating capacity in plasma from patients with haemophilia A and B. Thromb Haemos. 2005;93:475–80. doi: 10.1160/TH04-10-0706. [DOI] [PubMed] [Google Scholar]
- 19.Woodhams B, Girardot O, Blano MJ, Colesse G, Gourmelin Y. Stability of coagulation proteins in frozen plasma. Blood Coagul Fibrinolysis. 2001;12:229–36. doi: 10.1097/00001721-200106000-00002. [DOI] [PubMed] [Google Scholar]
- 20.Hemker HC, Giesen PLA, Ramjee M, Wagenvoord R, Béguin S. Thethrombogram: monitoring thrombin generation in platelet rich plasma. Thromb Haemost. 2000;83:589–91. [PubMed] [Google Scholar]
- 21.Dargaud Y, Luddington R, Gray E, Negrier C, Lecompte T, Petros S, Hogwood J, Bordet JC, Regnault V, Siegemund A, Baglin T. Effect of standardisation and normalisation on imprecision of Calibrated automated thrombography (CAT): an international multicentre study. Br J Haematol. 2007;139:303–9. doi: 10.1111/j.1365-2141.2007.06785.x. [DOI] [PubMed] [Google Scholar]
- 22.Lewis SJ, Stefens E, Florou G, Macartney NJ, Hathaway LS, Knipping J, Collins PW. Measurement of global haemostasis in severe haemophilia A following FVIII infusion. Br J Haematol. 2007;138:775–82. doi: 10.1111/j.1365-2141.2007.06722.x. [DOI] [PubMed] [Google Scholar]
- 23.vanVeen JJ, Gatt A, Bowyer AE, Cooper PC, Kitchen S, Makris M. Calibrated automated thrombin generation and modified thromboelastometry in haemophilia A. Thromb Res. 2009;123:895–901. doi: 10.1016/j.thromres.2008.09.011. [DOI] [PubMed] [Google Scholar]
- 24.Rugeri L, Quelin F, Chatard B, De Mazancourt P, Negrier C, Daragud Y. Thrombin generation in patients with FXI deficiency and clinical bleeding risk. Haemophilia. 2010;16:771–7. doi: 10.1111/j.1365-2516.2010.02246.x. [DOI] [PubMed] [Google Scholar]
- 25.Pike GN, Cumming AM, Hay CR, Bolton-Maggs PH, Burthem J. Sample conditions determine the ability of thrombin generation parameters to identify bleeding phenotype in FXI deficiency. Blood. 2015;126:397–405. doi: 10.1182/blood-2014-12-616565. [DOI] [PubMed] [Google Scholar]
- 26.De Smedt E, Hemker HC. Thrombin generation is extremely sensitive to preheating conditions. J ThrombHaemos. 2011;9:233–4. doi: 10.1111/j.1538-7836.2010.04136.x. [DOI] [PubMed] [Google Scholar]
- 27.Hemker HC, De Smedt E, Hemker PW. During coagulation, thrombin generation shifts from chemical to diffusional control. J Thromb Haemost. 2005;3:2399–400. doi: 10.1111/j.1538-7836.2005.01565.x. [DOI] [PubMed] [Google Scholar]
- 28.Hemker HC. The application of thrombin generation in real life clinical situations. Thromb Res. 2015;136:3–4. doi: 10.1016/j.thromres.2015.04.010. [DOI] [PubMed] [Google Scholar]
- 29.Hemker HC, Kremers R. Data management in TG. Thromb Res. 2013;131:3–11. doi: 10.1016/j.thromres.2012.10.011. [DOI] [PubMed] [Google Scholar]
- 30.Perrin J, Depasse F, Lecompte T French-speaking CAT group and under the aegis of GEHT; French-speaking CAT group (all in France unless otherwise stated); French-speaking CAT group all in France unless otherwise stated. Large external quality assessment survey on thrombin generation with CAT: further evidence for the usefulness of normalisation with an external reference plasma. Thromb Res. 2015;136:125–30. doi: 10.1016/j.thromres.2014.12.015. [DOI] [PubMed] [Google Scholar]