Summary
The role of macrophage infiltrates in oral mucosal acute graft‐versus‐host disease (AGVHD) remains unclear, although clinical studies suggest that macrophage infiltration correlates directly with the severity of AGVHD. In this study, we investigated the role of M1 macrophage infiltration in the oral mucosa of rats with AGVHD. Lewis rat spleen cells were injected into (Lewis × Brown Norway) F1 rats to induce systemic GVHD. Tongue samples were evaluated using histology, immunohistochemistry, dual immunofluorescence, real‐time reverse transcription–polymerase chain reaction, Transwell migration assays and Stamper–Woodruff binding assays. At the onset of oral mucosal AGVHD, dual immunofluorescence and migration assays revealed that M1 macrophages had accumulated in the basement membrane (BM) region via the laminin/CD29 β1 integrin pathway. Macrophage‐secreted matrix metalloproteinase‐2 was related to BM degradation. The adhesion of macrophages to the oral epithelium could be inhibited by pretreating macrophages with a CC chemokine receptor 2 (CCR2) antibody and/or pretreating lesion sections with monocyte chemoattractant protein‐1 (MCP‐1) antibody. Our data show that the migration and adhesion of M1 macrophages are associated with oral mucosal AGVHD, which is mediated in part by both laminin/CD29 β 1 intern and MCP‐1/CCR2 pathways. Therefore, our study provides additional support for the contribution of macrophage infiltrate to the development of oral mucosal AGVHD.
Keywords: acute graft‐versus‐host disease, CC chemokine receptor 2, CD29 β 1 integrin, M1 macrophages, monocyte chemoattractant protein‐1
Introduction
Graft‐versus‐host disease (GVHD) remains a major complication following allogeneic stem cell transplantation. Mucocutaneous organs, including the oral mucosa, are some of the tissues affected by acute GVHD (AGVHD) 1. Oral mucosal AGVHD is characterized pathologically by the migration and infiltration of effector cells, particularly CD8‐positive T cells, into the surface epithelium of the oral mucosa. This results in satellitosis, in which lymphocytes form clusters around dyskeratotic and/or necrotic epithelial keratinocytes (KCs). Our previous studies demonstrated that the infiltration and adhesion of CD8‐positive cells were mediated by the intercellular adhesion molecule‐1 (ICAM‐1)/lymphocyte adhesion function‐associated antigen‐1 (LFA‐1) and the mannose‐binding protein/mannose pathways 2, 3. We suggest that the up‐regulation of these pathways are responsible for the onset and progression of oral mucosal AGVHD.
Macrophages are tissue‐resident professional phagocytes and antigen‐presenting cells, which differentiate from circulating peripheral blood monocytes. They are divided into two major polarizations, M1 and M2, based on in‐vitro data 4, 5, 6, 7. The M1 subtype comprises classically activated macrophages stimulated by interferon (IFN)‐γ and lipopolysaccharide (LPS), and the M2 subtype comprises alternatively activated macrophages following stimulation with interleukin (IL)‐4 and IL‐13 8. M1 macrophages comprise immune effector cells with an acute inflammatory phenotype and exhibit an enhanced microbicidal capacity with the ability to secrete high levels of proinflammatory cytokines [e.g. tumour necrosis factor (TNF)‐α, IL‐1, IL‐6 and IL‐23)] 9. Although macrophage involvement in GVHD has been thought to be described principally in terms of their innate immune function (e.g. the production of TNF‐α in response to IFN‐γ and LPS) 10, 11, a recent study has implied that macrophages contribute to the activation and proliferation of CD8‐positive cells by antigen presentation and cytokine secretion in AGVHD 12. Furthermore, clinical studies have reported that dermal macrophage infiltration was correlated directly with AGVHD severity in the skin 13, 14. These reports prompted us to examine whether macrophages contribute actively to the destructive processes of target tissues in AGVHD. However, the involvement of macrophages in the onset and development of AGVHD remains undetermined.
An interaction between macrophages and surface epithelium, the target of oral mucosal lesions, is important to elucidate whether or not activated macrophages contribute to the development of oral mucosal AGVHD. Activated macrophage migration to a basement membrane (BM) zone seems to be the first step of lesion onset. Integrins are one of the adhesion molecules critical for the recruitment of immunocompetent cells, including macrophages, from the blood into target tissues during the inflammatory process. The β1subunit (CD29) of the integrin family can bind extracellular matrix (ECM), including collagen IV and laminin of the BM components, and is found on macrophages 15. A relationship between β1 integrin and ECM of BM is required for epithelial migration of macrophages. Activated macrophages in inflamed tissues can secrete to various factors. Among these factors, matrix metalloproteinases (MMPs) are a family of secreted and membrane‐associated zinc‐requiring neutral endopeptidases cleaving most matrix and BM proteins 16. In the inflammation and malignant tumour condition, MMPs contribute to tissue degradation 17, 18. Therefore, the BM degradation by MMPs is necessary for the progression of the oral mucosal AGVHD, resulting in direct interaction between activated macrophages and epithelial KCs.
A direct reaction of macrophages to the epithelium may be related to monocyte chemoattractant protein‐1 (MCP‐1), which is considered to be one of the most important chemokines regulating the migration and infiltration of macrophages. MCP‐1 belongs to a CC chemokine subfamily and its levels are increased in various inflammatory diseases 19. In experimental models of intestinal disease, macrophages produce MCP‐1 to recruit monocytes and neutrophils into the muscle layer 20. MCP‐1 expression in the gut, liver, skin and lung is consistent with the increase of inflammatory cytokines during the development of AGVHD 21, 22. The chemokine receptor–ligand pairs of CC chemokine receptor 2 (CCR2) and its main ligand MCP‐1 control leucocyte migration and adhesion during inflammatory processes 23. CCR2 belongs to the class of hepta‐helical G‐protein‐coupled transmembrane CC chemokine receptors and is expressed in several haematopoietic cell types, including macrophages 24. Furthermore, CCR2‐absent leucocytes exhibit reduced migration towards MCP‐1 and decreased adhesion to the microvascular endothelium 25, 26. These findings suggest that the MCP‐1/CCR2 pathway may be important for the recruitment and adhesion of macrophages to epithelial KCs during the development of oral mucosal AGVHD.
These studies have led to the suggestion that determining changes in macrophage dynamics will aid in understanding the pathogenesis of the oral mucosal AGVHD. Our approach to this premise has been to focus upon macrophage migration to the oral epithelium by various factors related to macrophage migration and adhesion. This study had three main goals: (i) to determine whether M1 macrophages accumulate at the surface epithelium during the development of oral mucosal AGVHD; (ii) to investigate whether BM degradation is mediated by MMP‐producing activated macrophages that migrate to BM via β1 integrin/ECM; and (iii) to determine whether the direct interaction of activated macrophages with epithelial KCs is mediated by the MCP‐1/CCR2 adhesive pathway.
Methods
Materials
A high pure RNA isolation kit and FastStart Universal Probe Master were purchased from Roche Diagnostics (Basel, Switzerland). A ReverTra Ace quantitative reverse transcription–polymerase chain reaction (qRT–PCR) kit was obtained from Tokyobo Co., Ltd (Osaka, Japan). Alkaline phosphatase‐conjugated anti‐mouse antibody and the 5‐bromo‐4‐chloro‐3‐indolyl phosphate/nitro blue tetrazolium chloride solution (BCIP/NBT) were purchased from Dako Japan (Tokyo, Japan). Anti‐mouse or anti‐rabbit immunoglobulin (Ig)G antibody conjugated with Alexa Fluor 488 or 568 were obtained from ThermoFisher Scientific (Molecular Probes, Eugene, OR, USA). Diff‐Quick was purchased from Sysmex Corp. (Hyogo, Japan). Rat laminin‐5 (CC145) was purchased from Merk Millipore (Billerica, MA, USA). Mouse monoclonal anti‐rat CD8 (clone 0X8), CD68 (clone ED1), CD54 (ICAM‐1; clone 1A29) and CD163 (clone ED2) and a rabbit polyclonal anti‐rat MCP‐1 were obtained from Bio‐Rad (Serotec, Tokyo, Japan). Rabbit polyclonal anti‐laminin, MMP‐2 and CCR2 were purchased from Abcam (Tokyo, Japan). A rabbit anti‐MCP‐1/CCL2 polyclonal antibody was obtained from Bioss Inc. (Boston, MA, USA). Anti‐mouse and rat CD29 (integrin beta1; clone HMB‐1) were obtained from eBioscience, Inc. (San Diego, CA, USA). Miltenyi microbeads were obtained from Miltenyi Biotec (Tokyo, Japan). Isoflurane was purchased from Abbott Laboratories (Abbott Park, IL, USA).
Animals
This study was performed in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health. Animal studies were conducted in accordance with the protocols approved by the Animal Care and Use Committee of Fukuoka Dental College (no. 13009). We used a total of 65 adult female (6–8 weeks) inbred Lewis (LEW, RT1l; n = 25) and LEW × Brown Norway F1 hybrid (LBNF1, RTll/n; n = 40) rats, weighing 250–350 g, purchased from Kyudo Co. (Saga, Japan). The rats were housed in micro‐isolator cages and received food and drinking water ad libitum. Euthanasia was performed using 5% isoflurane, and the isoflurane flow rate was controlled using an anaesthesia gas machine (anaesthesia machine SF‐B01; MR Technology, Inc., Tsukuba, Ibaraki, Japan).
Induction and assessment of AGVHD
Induction of AGVHD was performed using the P‐F1 semi‐allogeneic model, as described previously 2, 3..Briefly, the suspension of splenocytes at 3 × 108 cells isolated from LEW rats was injected intraperitoneally into LBNF1 rats. Untreated LBNF1 rats and LBNF1 rats injected with an equal number of syngeneic LBNF1 splenocytes were used as controls. All rats were weighed daily and a clinical assessment of AGVHD was determined by weight loss and the development of cutaneous or mucosal erythema. The rats were scored for clinical evidence of AGVHD by assessment of (i) changes in skin (alopecia and inflamed or scaly skin), (ii) inflammation of the eyes and (iii) diarrhoea. Each parameter was scored as follows: 0 = normal, 1 = mild, 2 = moderate and 3 = severe; eye inflammation was scored as follows: 4 = eyes very severely inflamed and 5 = eyes very severely inflamed and closed; maximum total score = 11. The spleen weights were determined at autopsy to help support the subsequent immunological assessment of AGVHD.
Tissue preparation
Whole tongues were excised 6–22 days post‐injection for three animals from each treatment group. Normal or control tongues were collected from the phosphate‐buffered saline (PBS)‐treated or the syngeneic group. Half the tongue specimens were fixed in 4% paraformaldehyde in PBS and embedded in paraffin. Paraffin sections (4 µm) were then stained with haematoxylin and eosin (H&E) to help visualize any histopathological changes. The other specimens were frozen immediately in liquid nitrogen, and serial frozen sections were used for immunostaining, the extraction of total RNA and in‐vitro adhesion assays.
Immunohistochemistry
Acetone‐fixed frozen sections were first incubated with normal rabbit or goat serum to reduce non‐specific binding and then reacted with one of the following antibodies: anti‐CD8 monoclonal antibody (mAb) (1 : 100 dilution, OX8) or anti‐CD54 (ICAM‐1) mAb (1 : 100 dilution, 1A29). The sections were then incubated with the alkaline phosphatase‐conjugated anti‐mouse antibody (1 : 150 dilution). Immune complexes were visualized using BCIP/NBT. Controls for the immunohistochemical analysis included substitution of PBS for primary antibodies and replacement of primary antibodies by non‐immune mouse immunoglobulin.
Single and dual immunofluorescence
Single and dual immunofluorescence assays were performed on acetone‐fixed frozen sections. In the single immunofluorescence, the frozen sections were reacted with primary antibodies (ED1 and MMP‐2, 1 : 100), followed by a reaction with an anti‐mouse or rabbit immunoglobulin IgG antibody conjugated with Alexa Fluor 488. We performed four types of double immunofluorescent staining simultaneously on the frozen sections comprising mixtures of two primary antibodies: (i) laminin and CD29; (ii) laminin and inducible nitric oxide synthase (iNOS); (iii) MMP‐2 and iNOS; and (iv) iNOS and MCP‐1. After an incubation of primary antibodies, sections were incubated in a mixture of anti‐mouse IgG antibodies conjugated with Alexa Fluor 488 or 568 and anti‐rabbit IgG antibody conjugated with Alexa Fluor 488 or 568 (1 : 200). Immunostained sections were then counterstained with Hoechst 33342 (5 µg/ml). Controls for the immunohistochemical analysis included substitution of PBS for primary antibodies and replacement of primary antibodies by a mixture of mouse and rabbit non‐immune immunoglobulins.
qRT–PCR
The total RNA derived from the tongue was isolated from the untreated and AGVHD‐mediated rats using a high pure RNA isolation kit, according to the manufacturer's instructions. The total RNA (1 µg) was transcribed into cDNA using random oligo(dT) primers and reverse transcriptase in a total volume of 10 µl (ReverTra Ace RT–qPCR kit). Reverse transcription was performed at 37°C for 15 min, at 50°C for 5 min and then at 98°C for 5 min. The resulting templates were amplified in a LighCycler Nano RT‐PCR system (Roche Diagnostics), according to the manufacturer's protocol. G3PDH was used as an internal control. The relative mRNA expression was determined to be the ratio of MMP‐2, MCP‐1 or CCR2 to G3PDH mRNA. The sequence of the specific primers and Universal Probe Library (Roche Diagnostics) probe numbers used for the qRT–PCR analysis are listed in Table 2. FastStart Universal Probe Master was used for the preparation of PCR master mixes. All reactions were run in quadroplicate. The results are expressed as fold increases in mRNA expression (normalized to that of G3PHD mRNA) and compared with the results from the tongues of the untreated rats.
Table 2.
Primers and probes used in this study
| Gene | Forward and reverse primers (5′→3′) | Probe no. |
|---|---|---|
| G3PDH | ATGATGAAGGTCGGTGTGAAT | 92 |
| GCAGAGAAGGTAGCCCTGGT | ||
| MMP‐2 | CAGTACATGCCCTGGCTTC | 115 |
| TCTGGAAAGGAGGTGGGATT | ||
| MCP‐1 | CGTGCTGTCTCAGCCAGAT | 62 |
| GGATCATCTTGCCAGTGAATG | ||
| CCR2 | CAGGGGTCAAGAAGATGAC | 89 |
| TTCAATTAGCCTTGCTCCATTT |
G3PHD, glyceraldehyde 3‐phosphate dehydrogenase; MMP‐2, matrix metallo‐proteinase‐2; MCP‐1, monocyte chemotactic protein‐1; CCR2, C‐C chemokine receptor type
Isolation of ED1‐positive cells from the spleens and tongues of GVHD‐mediated rats
ED1‐positive macrophages from GVHD rats were used in Transwell migration and Stamper–Woodruff binding assays (SWBA). Macrophages were isolated from the spleens and tongues exhibiting splenomegaly and ICAM‐1 expression in the tongue epithelium from AGVHD rats and resuspended in RPMI‐1640 medium at 4°C. The cells were dispersed by rapid, in–out pipetting, and the cell clumps were removed by passage through a 70‐µm pore size nylon mesh. The resultant single‐cell suspension was washed three times in the same medium and resuspended at a concentration of 3 × 107 mononuclear cells/ml. Using a positive selection, ED1‐positive cells from the suspension were isolated twice by magnetic bead purification using Mitenyi microbeads labelled with an ED1 antibody, according to the manufacturer's protocol. Cell purity of ED1‐positive macrophages from the spleens and tongues of AGVHD rats was 86·7 ± 9·8% and 90·2 ± 10·2%, respectively, assessed using the flow cytometry analysis.
Transwell macrophage migration
Migration of ED1‐positive macrophages from the spleens and tongues of AGVHD rats was evaluated in a Transwell insert system. ED1‐positive macrophages, pre‐incubated or not with anti‐CD29 antibody (50 µg/ml), were seeded at a density of 5 × 104 cells/well into 8‐µm Transwell inserts. The lower chamber was filled with 500 µl RPMI medium only or medium containing laminin‐5 (0·5 µg/ml). The cells were allowed to migrate for 18 h at 37°C in a 5% CO2 humidified atmosphere. Six hours prior to the completion of the migration assay, the cells were stained with Diff‐Quick. Assays were stopped by removing cells from the upper surface of the polycarbonate membrane by wiping the surface twice with a cotton‐tipped applicator. The migration activity was evaluated as the percentage migration of cells from the upper chamber of the Transwell insert into the lower chamber in three high‐power fields (×100) per well. The experiment was performed in quintuplicate.
SWBA
The SWBA was performed as described previously 2, 3. Briefly, aliquots containing 2·5 × 105 isolated ED1‐positive macrophages from spleens and tongues of AGVHD‐mediated rats in 200 µl of RPMI‐1640 medium were added to freshly cut (6 µm) frozen sections of the tongue obtained from rats in both the untreated and AGVHD groups. The sections were agitated on a rotary shaker (4g) for 60 min at room temperature. Next, the cell suspension was decanted carefully and the cells adhering to the section were fixed in 2·5% glutaraldehyde in PBS for 5 min. The slides were then washed in PBS and stained with 0·5% toluidine blue. The number of adherent ED1‐positive macrophages was determined by a light microscopy examination of fields at ×200 magnification (each high‐power field represented approximately 400 µm of the oral epithelium). The number of ED1‐positive cells that were attached directly over KCs (but not in the cornified layer) was enumerated. Several blocking experiments were performed as follows: (i) ED1‐positive macrophages were pre‐incubated with 50 µg/ml of an anti‐iNOS or CCR2 antibody for 30 min at room temperature before the entire reaction mixture was transferred into frozen sections of the AGVHD tongues; (ii) frozen tongue sections from AGVHD rats were incubated with 50 µg/ml of anti‐MCP‐1 antibody for 30 min at room temperature following a brief wash with PBS, and was then added to the macrophages; (iii) the two approaches were used simultaneously (i.e. ED1‐positive cells were treated with an anti‐iNOS or CCR2 antibody and the tissue sections were treated with an anti‐MCP‐1 antobody). Antibodies used in the inhibition test were same as those for immunohistochemical studies. The results were calculated as the percentage of binding relative to the untreated macrophages and tissue sections exposed to the buffer alone.
Statistical analysis
A statistical analysis used statview (statview for Windows, version 5). The analysis was performed with two‐way analysis of variance (anova) and Scheffé's multiple comparison test or Student's t‐test to determine the statistical differences among the samples. Data are presented as the mean ± standard deviation (s.d.) and P‐values < 0·05 were considered to be statistically significant.
Results
Establishment of oral mucosal AGVHD
To determine whether the P‐F1 semi‐allogeneic transplantation model could induce AGVHD, we monitored all rats for clinical signs of AGVHD. Table 1 shows changes in the GVHD score of each group. By day 12, the maximum mean GVHD score of 9·2 ± 1·2 was reached in the semi‐allogeneic rats. In contrast, both syngeneic and PBS‐treated control rats remained in a normal‐appearing condition. Figure 1a shows the average weight changes of the groups. Syngeneic and semi‐allogeneic groups showed initial weight loss. All syngeneic groups later recovered their weight. The maximum weight loss of the semi‐allogeneic rats was significantly greater than those of the control and syngeneic groups (P < 0·05 on days 10–16 post‐inoculation). These clinical changes indicate that the P‐F1 model induced systemic AGVHD.
Table 1.
Acute graft‐versus‐host disease (AGVHD) score
| Mean score | |||
|---|---|---|---|
| Days after inoculation | Semi‐allogeneic | Syngeneic | Control |
| 2 | 3·2 ± 0·4 | 3·4 ± 0·5 | 3·0 |
| 8 | 5·8 ± 1·3 | 3·6 ± 0·5 | 3·0 |
| 10 | 7·4 ± 1·5 | 3·4 ± 0·5 | 3·0 |
| 12 | 9·2 ± 1·2 | 3·2 ± 0·4 | 3·0 |
| 14 | 9·2 ± 0·7 | 3·2 ± 0·4 | 3·0 |
| 16 | 9·0 ± 0·9 | 3·0 | 3·0 |
| 18 | 8·6 ± 0·8 | 3·6 ± 0·5 | 3·0 |
| 20 | 8·4 ± 1·0 | 3·0 | 3·0 |
| 22 | 8·2 ± 0·7 | 3·2 ± 0·4 | 3·0 |
Figure 1.
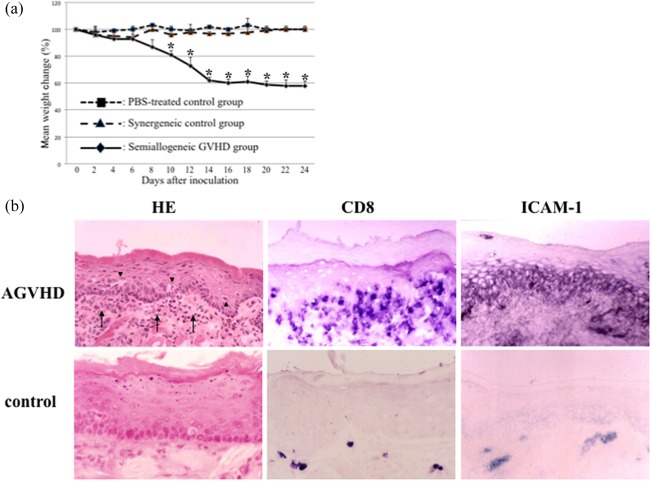
Assessment of oral mucosal acute graft‐versus‐host disease (AGVHD). (a) Weight curve in rats developing AGVHD. Rats were divided into three groups; control, phosphate‐buffered saline (PBS)‐treated rats; syngeneic, matched donor transplant, no AGVHD rats; and semi‐allogeneic, unmatched donor transplant, AGVHD rats. Weights are represented as the percentage of the mean ± standard deviation (s.d.) for each day compared with baseline for that group (weight on day 0). *Significantly different at P < 0·05 compared with control and syngeneic groups [analysis of variance (anova) followed by Scheffé's test]. Animal experiments performed in triplicate. (b) Histopathological and immunohistochemical assessments of oral mucosal AGVHD. Sections of both AGVHD and control tongues were stained with haematoxylin and eosin (H&E) and anti‐CD8 and anti‐ intercellular adhesion molecule‐1 (ICAM‐1) antibodies. Reactive products for immunostaining were developed using 5‐bromo‐4‐chloro‐3‐indolyl phosphate/nitro blue tetrazolium chloride solution (BCIP/NBT) solution. Arrows, mononuclear cells; arrow heads, epithelial vacuolation. Original magnification, ×200. Histological experiments performed in quadruplicate.
The mucocutaneous organs are one of the targets. We examined whether the oral mucosa was affected histologically and immunohistochemically by the systemic AGVHD model (Fig. 1b). Histological analysis of the tongue showed that mononuclear cells had infiltrated into the perivascular interstitium of the superficial lamina propria and surface epithelium of rats with AGVHD. Intra‐epithelial infiltration of mononuclear cells resulted in vacuolar degeneration. In the control tongue, no histological changes were observed. CD8‐positive cells, known as effector cells of AGVHD, were observed immunohistochemically in both the upper lamina propria and surface epithelium. Only a few CD8‐positive cells were observed in the control tongue. ICAM‐1 expression was up‐regulated in the basal to upper spinous KCs, whereas vascular slits expressed ICAM‐1 in the control tongue. ICAM‐1 expression was applied to distinguish between the early and advanced oral mucosal AGVHD as described previously 3. In the early lesion, ICAM‐1 expression was localized in basal to lower spinous layer of the epithelium. The advanced lesion showed that ICAM‐1 was reacted in almost all epithelial cells without keratinized cells. These results suggest clearly that the oral mucosal lesions display the characteristics of mucocutaneous AGVHD as selective epithelial inflammation 1, 27.
Activated macrophages accumulate and persist in the oral mucosa of AGVHD
Recent clinical studies revealed that an infiltrate of activated macrophages correlated directly with disease severity of skin AGVHD 27. We first examined the intensity and distribution of macrophages via immunohistochemistry on frozen sections of oral mucosal AGVHD. Figure 2a shows the macrophage dynamics using an ED1 (CD68) antibody during the development of early AGVHD in the oral mucosa. In the oral mucosa of the controls, very few ED1‐positive cells were observed in the lamina propria and submucosal tissues. These cells were thought to be resident macrophages in the oral mucosa, because the resident macrophages are known to express the ED1 antigen partially and weakly, although they usually express the ED2 antigen. In contrast, in the rat AGVHD model at 8–10 days, numerous ED1‐positive, ramified‐shaped cells were seen mainly in the lamina propria. In particular, macrophages tended to accumulate and attach to the basement membrane zone. The number of ED1‐positive cells in oral mucosal AGVHD were increased by approximately sixfold compared with the control. In the dual immunofluorescence staining assay, the majority of the ED1‐positive macrophages co‐expressed iNOS (Fig. 2b), indicating that the infiltrating cells are activated M1 macrophages.
Figure 2.
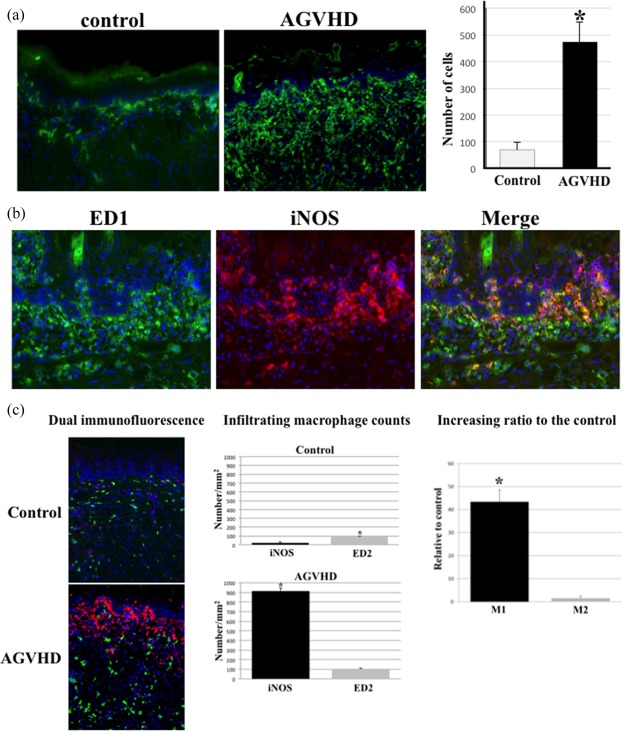
Activated macrophages accumulate and persist in oral mucosal acute graft‐versus‐host disease (AGVHD). (a) Immunofluorescent images of ED1‐positive macrophages in the tongue from the control and AGVHD‐mediated rats. Original magnification, ×200. For the quantitative analysis of macrophage numbers, 10 areas 50 µm2 each in the tongue were selected randomly from each of the five rats and the total number of ED1‐positive cells was counted. The mean number of ED1‐positive cells per 10 areas ± standard deviation (s.d.) is shown for each group. *Significant difference at P < 0·01 (Student's t‐test). (b) Dual immunofluorescent images of ED1 (green) and inducible nitric oxide synthase (iNOS) (red) in oral mucosal AGVHD. The nucleus was stained with Hoechst 33324 (blue). Original magnification, ×200. (c) Distribution of M1 and M2 macrophages using iNOS (red) and ED2 (green) antibodies, respectively. Original magnification, ×200. Infiltration of iNOS‐ and ED2‐positive macrophages in the tongues from the AGVHD and control rats. Infiltrating macrophages in the lamina propria were counted. Cell numbers/mm2 as mean ± standard deviation (s.d.). *Significantly different at P < 0·05 compared with iNOS‐positive cells in the control tongue and P < 0·01 compared with ED2 in the AGVHD tongue (Student's t‐test). Increasing ratio of M1 or M2 macrophages in the AGVHD‐tongue, relative to those in the control tongue. *Significantly different at P < 0·01(Student's t‐test). All experiments performed in quadruplicate.
Macrophages of different phenotypes have been classified into M1 and M2 based on in‐vitro data 4, 6. As the role of both macrophage types is unknown during the development of oral mucosal AGVHD, we performed dual immunofluorescence staining for the combinations of M1 and M2 activation markers in oral mucosal AGVHD. Figure 2c shows the intensity and distribution of iNOS and ED2 (CD163), known to be M2 markers 28, in the oral mucosa of both the control and experimental rats. In the control mucosa, ED2‐positive ramified‐shaped cells were observed to be scattered throughout the entire mucosa, while there were few iNOS‐positive cells. In the lamina propria of the control oral mucosa, a number of ED2‐positive cells (89·8 ± 10·6) was greater than that of iNOS‐positive cells (21 ± 8·6). These results indicate that the ED2‐positive resident macrophages exist in the oral mucosa under normal conditions. In contrast, the number of iNOS‐positive cells (914 ± 32·6) was increased in oral mucosal AGVHD. An increasing ratio to the control of iNOS‐positive cells against the control was 27‐fold higher than that of the ED2‐positive cells. These data suggest that M1 macrophages are associated with the development of the oral mucosal AGVHD.
Macrophage migration is mediated by the laminin/CD29 pathway
We first studied the potential immunohistochemical interaction between the laminin, one of the BM components, and CD29, integrin β1. Figure 3a shows the dual immunofluorescence staining of laminin and CD29 in the frozen sections of early oral mucosal AGVHD. The oral epithelium of the lesional mucosa showed a continuous and linear expression of laminin in the BM region. Laminin was also expressed on the vascular slits. CD29‐positive ramified‐shaped macrophages infiltrated into the upper lamina propria. Dual staining revealed that some of the CD29‐positive macrophages were attached to the laminin‐expressing BM. The immunohistochemical results speculate that macrophage migration to the BM region may be mediated by the laminin/integrin pathway.
Figure 3.
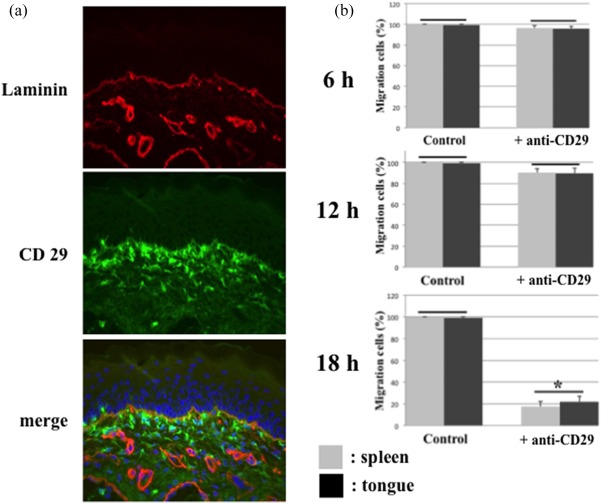
Induction of laminin to activated macrophage migration. (a) Dual immunofluorescent images of laminin (red) and CD29 (green). Original magnification, ×200. (b) CD29‐positive cell migration was examined by a Transwell migration assay. Laminin was placed into the lower chamber well. The wells of the upper chamber received isolated ED1‐positive cells from the spleens and tongues of acute graft‐versus‐host disease (AGVHD) rats, pretreated with or without (control) an anti‐CD29 antibody. The cells were allowed to migrate towards laminin placed into the lower chamber of the Transwell system for 6, 12 and 18 h (h) at 37°C in a 5% CO2 humidified atmosphere. The figure represents the results of five different experiments expressed as the mean ± standard deviation (s.d.). There are no significant differences in groups jointed to horizon bar. *Significantly different at P < 0·05 [analysis of variance (anova) followed by Scheffé's test]. All experiments performed in quintuplicate.
We next performed a macrophage migration assay to elucidate whether CD29‐positive macrophages could migrate towards laminin in a Transwell system. In these experiments, laminin (50 µg/ml) was placed in the lower chamber and migration was initiated by adding ED1‐positive cells from AGVHD spleens and tongues to the upper chamber. Migration was allowed to proceed for 18h at 37°C, at which time the number of cells adhering to the underside of the filter coated with laminin was determined (Fig. 3b). ED1‐positive macrophages migrated efficiently towards laminin. To examine the contribution of CD29 to migration, cells were pre‐incubated with 2 µg/ml of anti‐CD29 functional blocking antibodies prior to the addition to the upper chamber of the Transwell system. Migration of cells from the AGVHD‐spleens and tongues treated with the anti‐CD29 antibody was inhibited by approximately 17 and 22%, respectively (Fig. 3b). Thus, these results suggest that the migration of macrophages from oral mucosal AGVHD towards the BM region was mediated in part via the laminin/integrin β1 pathway.
BM degradation in advanced oral mucosal AGVHD
Mucocutaneous AGVHD is characterized pathologically by satellitosis, epithelial destruction caused by the infiltration of effector cells into the surface epithelium. The degradation of the BM seems to be the first target tissue of oral mucosal AGVHD. We first examined whether macrophage infiltration around the BM region contributes to BM discontinuity during the development of oral mucosal AGVHD. Figure 4a presents the dual immunofluorescence of laminin and iNOS in frozen sections from both early and advanced oral mucosal AGVHD. Linear and continuous expression of laminin on BM was disconnected partially in the early lesion. iNOS‐positive M1 macrophages infiltrated into the discontinuous region of BM observed by double staining. Laminin expression was completely absent in BM in advanced AGVHD, in association with dense infiltrate of iNOS‐positive macrophages. These results suggest that active macrophage infiltration is related to the loss of BM during the development of the oral mucosal AGVHD.
Figure 4.
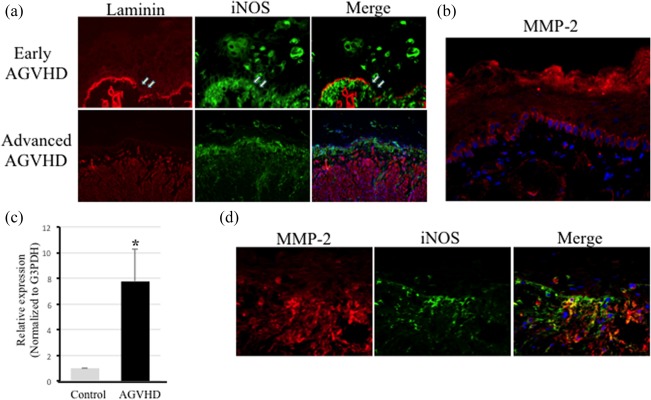
Loss of the epithelial basement membrane components mediated by MMP‐2. (a) Dual immunofluorescent images of laminin (red) and inducible nitric oxide synthase (iNOS) (green) in both early and advanced acute graft‐versus‐host disease (AGVHD) of the oral mucosa. Arrows indicate a loss of laminin expression on the basement membrane. Original magnification, ×200. (b) Basal immunofluorescence of matrix metalloproteinase (MMP)‐2 in the oral mucosa of untreated rats. The nucleus was stained with Hoechst 33324 (blue). (c) Using quantitative reverse transcription–polymerase chain reaction (qRT–PCR) analyses, the tissue expression of MMP‐2 in tongues from untreated (grey) and AGVHD‐mediated (black) rats. Results are expressed as fold increases in mRNA expression (normalized to that of G3PDH mRNA) and compared with results for the oral mucosa from untreated rats. The results are presented as the mean ± standard deviation (s.d.) of five independent experiments. *Significantly different at P < 0·05 compared with the control rats (Student's t‐test). G3PDH = glyceraldehyde 3‐phosphate dehydrogenase. All experiments performed in quintuplicate. (d) Dual immunofluorescent images of MMP‐2 (red) and iNOS (green) in the AGVHD‐tongue. Nuclei were stained with Hoechst 33324 (blue). Original magnification, ×200. All experiments performed in quintuplicate.
In various inflammatory diseases, MMPs contribute to tissue degradation 16. Among the MMP family members, MMP‐2 (gelatinase A) cleaves laminin‐5 and collagen type IV of the BM components 29, 30. Thus, we next studied whether MMP‐2 expression participates in BM degradation. Immunohistochemical expression of MMP‐2 was seen in the basal cells of the oral epithelia in the control untreated oral mucosa (Fig. 4b). Figure 4c shows the mRNA expression of MMP‐2 in the advanced lesion of oral mucosal AGVHD. MMP‐2 expression (normalized to G3PDH mRNA expression) was up‐regulated 7.8‐fold, compared with that in the normal, untreated oral mucosa. Immunohistochemical expression of MMP‐2 showed that the basal expression of the oral epithelium observed in the control had disappeared, and a positive reaction was observed in infiltrated cells beneath the surface epithelium in the advanced lesions (Fig. 4d, left). From the double staining, most of the infiltrating cells expressing MMP‐2 also expressed iNOS (Fig. 4d, right). However, a few MMP‐2 cells were negative for iNOS expression. These data reveal that co‐expression of MMP‐2 and iNOS suggest that laminin degradation in BM may be responsible for MMP‐2 produced by M1 macrophages in oral mucosal AGVHD.
The MCP‐1 and CCR2 pathways mediate the adhesion of activated macrophages to epithelial KCs
Our previous studies have shown that the effector CD8‐positive cells were bound to lesional KCs by the ICAM‐1/LFA‐1 or mannose binding protein (MBP)/Lens culinaris lectin (LCA) pathways 2, 3. We investigated whether activated macrophages can bind to lesional KCs via the MCP‐1 and CCR2 pathways, because MCP‐1 is considered to be one of the important chemokines regulating the migration and infiltration of macrophages and its effects are mediated by CCR2 19. Figure 5a shows the dual immunofluorescence of iNOS and MCP‐1 in the frozen sections from advanced oral mucosal AGVHD. iNOS‐positive M1 macrophages tended to infiltrate the surface epithelium (Fig. 5a, left), and some infiltrated the upper regions of the epithelium. No MCP‐1 expression was observed in the normal oral epithelium (data not shown). In the advanced lesions, MCP‐1 expression was observed on the surface epithelium, extending to the middle of the spinous layers (Fig. 5a, middle). MCP‐1‐positive immunofluorescence showed a cell surface‐binding pattern of KCs. Dual staining revealed that iNOS‐positive macrophages tended to be localized to MCP‐1‐positive KCs (Fig. 5a, right). Immunohistochemical studies of the lesional sections suggest that intra‐epithelial migration of activated macrophages is mediated by MCP‐1‐positive KCs.
Figure 5.
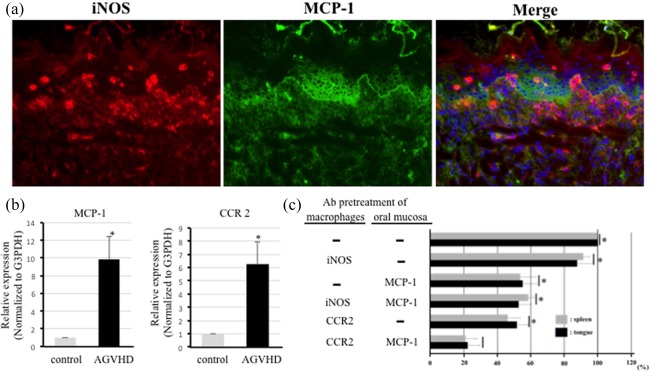
Intra‐epithelial infiltration of activated macrophages mediated by the monocyte chemoattractant protein‐1 (MCP‐1) and C‐C chemokine receptor type 2 (CCR2) pathway. (a) Dual immunofluorescent images of iNOS (red) and MCP‐1 (green) in oral mucosal acute graft‐versus‐host disease (AGVHD). The nucleus was stained with Hoechst 33324. Original magnification, ×200. (b) Quantitative reverse transcription–polymerase chain reaction (qRT–PCR) results for MCP‐1 and CCR2 mRNA expression in the oral mucosa from the untreated (grey) and AGVHD‐mediated (black). Results are the mRNA fold increases (normalized to G3PDH mRNA) in the oral mucosa from the AGVHD rats and compared with results from the untreated rats. Vertical lines represent the means ± standard deviation (s.d.) of five independent experiments, each performed in triplicate. *Significantly different at P < 0·05 compared with the control rats (Student's t‐test). G3PDH = glyceraldehyde 3‐phosphate dehydrogenase. All experiments performed in quintuplicate. (c) Adhesion of activated macrophages taken from spleens and tongues of AGVHD‐mediated rats to the oral epithelial cells by Stamper–Woodruff binding assay (SWBA). The pretreatment of macrophages examined the inhibition of macrophage binding to an anti‐iNOS or CCR2 antibody, as well as the pretreatment of oral epithelia with the anti‐MCP‐1 antibody. Results are mean ± standard deviation (s.d.) for five independent experiments. There are no significant differences in groups jointed to vertical bar. *Significantly different at P < 0·05 [analysis of variance (anova) followed by Scheffé's test). All experiments performed in quintuplicate.
We next examined the tissue expression of both MCP‐1 and CCR2 mRNA by a qRT–PCR assay in the advanced lesions of oral mucosal AGVHD. The mRNA expression of both MCP‐1 and CCR2 (normalized to G3PDH mRNA expression) was up‐regulated by 9.9‐ and 6.3‐fold, respectively, compared with that in the normal oral mucosa. Up‐regulation of CCR2 mRNA expression is related to an increased number of activated macrophages in the advanced lesions of oral mucosal AGVHD.
To provide evidence of a direct role of the MCP‐1/CCR2‐binding pathway in the binding of activated macrophages to epithelial KCs, we performed SWBA using macrophages from spleens and tongues and the oral mucosa taken from rats with advanced AGVHD (Fig. 5c). When macrophages from spleens and tongues of advanced AGVHD were pre‐incubated with an anti‐iNOS antibody, macrophage adhesion to epithelial KCs was 91·4 ± 3·7% (from spleens) and 87·7 ± 8·9% (from tongue) of the control value. Pretreatment of the frozen tissue sections was prepared from the oral mucosa with an anti‐MCP‐1 antibody, and macrophage adhesion was 53·6 ± 9·5% (from spleens) and 55 ± 8·6% (from tongues). When the macrophages were treated with an anti‐iNOS antibody and the tissue sections were treated with anti‐MCP‐1 antibody, macrophage adhesion was 58·4 ± 3·6% (from spleens) and 52·6 ± 7·4% (from tongues) of the control value. Pretreatment with an anti‐CCR2 antibody revealed 46 ± 7·4% (from spleens) and 51·6 ± 6·4% (from tongues) macrophage adhesion to the lesional tissue section. Finally, when the two approaches were applied simultaneously (i.e. when the macrophages were treated with an anti‐CCR2 antibody and the tissue sections were treated with anti‐MCP‐1 antibody), macrophage adhesion (from both spleens and tongues) was reduced dramatically to less than 25% of the control value. These results indicate that the adhesion of activated macrophages from oral mucosal AGVHD to lesional KCs is mediated in part by the MCP‐1/CCR2‐binding pathway.
Discussion
In the present study we used the haploidentical allogenic F1 hybrid rat model to investigate the macrophage dynamics in oral mucosal AGVHD. This model provides a convenient and genetically well‐defined system from which to gain insights into the mechanisms underlying immune‐mediated tissue damages to host epithelial tissue by studying the in‐vivo interactions between KCs and effector cells, even though it does not represent clinical haematopoietic cell transplantation directly in humans 2, 3, 31. Here, we present three lines of evidence to support the conclusion that activated macrophage infiltrates are associated with the oral mucosal AGVHD: (i) immunohistochemical approaches of M1 macrophage infiltration around the BM region was one of the characteristic events to occur during lesion development; (ii) both immunohistochemical and migration assays suggested that the discontinuity of BM was mediated by MMP‐2‐producing activated macrophages from oral mucosal AGVHD that had migrated to BM via the laminin/CD29 pathway; and (iii) activated macrophage (from oral mucosal AGVHD) infiltration of the mucosal surface epithelium was mediated by the MCP‐1/CCR2 adhesive pathway, as revealed using SWBA.
The immunohistochemical results of iNOS expression presented here indicate that activated M1 macrophage infiltration is a pathological characteristic of the development of oral mucosal AGVHD. There is a considerable number of resident macrophages in the lamina propria and submucosal tissue of normal oral mucosa. Those cells were reactive with ED2, the scavenger receptor CD163 as a marker of the M2 macrophage phenotype in rats 32. M2 macrophages were stable in their intensity and distribution during the development of oral mucosa AGVHD. In contrast, the lesional oral mucosa exhibited an increase in the number of iNOS‐positive M1 macrophages, indicating an onset of inflammation. Accumulation of M1 macrophages in the BM region indicates that BM is the initial target of the oral mucosal lesions in AGVHD. Therefore, the persistence of M1 macrophage infiltrate is responsible for the perpetuation of inflammation in the oral mucosa, in association with the suppression of the anti‐inflammatory effects of M2 macrophages.
The loss of BM is mediated by activated macrophages producing MMP‐2, which migrate to BM via the laminin/CD29 pathway. Dual immunostaining and a Transwell migration assay revealed that the accumulation of activated macrophages was mediated by the laminin/CD29 pathway in the early lesions of oral mucosal AGVHD. Many human diseases have been associated with altered integrin‐mediated adhesion and migration. CD29 is one of the β1 integrin members that play a central role in the inflammatory response by mediating the adhesive reactions of leucocytes during their migration from the peripheral blood to extravascular sites of injury and are up‐regulated strongly on inflammatory macrophages 15, 33. The β1 integrins are capable of adhesion to various EMC proteins, including fibronectin, vitronectin, fibrinogen, collagen and laminin 15. Laminin is a heterotrimeric glycoprotein component of the anchoring filaments in the skin, intestine and gingiva BM regions 34. In the early lesions of oral mucosal AGVHD, a continuous linear expression of laminin was retained on both epithelial BM regions and vascular slits. Dual staining of laminin and CD29 revealed that CD29‐positive cells tended to attach to the laminin‐expressing BM. The role of CD29 β1 integrin as a migratory receptor is supported by our data with an anti‐CD29 blocking antibody, which invariably inhibited the migration of activated macrophages from oral mucosal AGVHD to laminin protein via a Transwell migration assay. These findings suggest that the migration and accumulation of M1 macrophages in BM, one of the pathological characteristics of the early lesion of oral mucosal AGVHD, is mediated in part by the laminin/β1 integrin pathway.
Dual immunostaining of laminin and iNOS revealed that the discontinuous BM areas are extended during the development of oral mucosal AGVHD. In advanced lesions, a complete loss of laminin expression was observed in the BM region. In pathophysiological processes, including inflammatory disease, dysregulated MMPs often cause tissue damage 16, 35. Our immunohistochemical results revealed that MMP‐2 expression was observed in the basal cells of the oral epithelia in the controls. In addition, MMP‐2 is expressed in human oral epithelial cells and cultured human KCs 36, 37. These studies suggest that MMP‐2 may contribute to the migration of oral mucosal and skin KCs into the subepithelial connective tissue under inflammatory conditions. In contrast, during the development of oral AGVHD, the tissue expression of MMP‐2 mRNA increased and co‐expression of MMP‐2 and iNOS was observed in M1 macrophages that had accumulated in the BM regions. Our dual immunofluorescence results demonstrated a small number of cells expressed by MMP‐2 only. These cells may be T helper type 1 (Th1) lymphocytes because recent study revealed that Th1 cells were related actively to MMP‐2 secretion 38. These results indicate that M1 macrophages secrete MMP‐2 predominantly in the BM region. MMP‐2 is reported as a family of proteinases that play an important role in the degeneration of extracellular matrices (e.g. collagen, laminin‐5 and elastin) 17. Therefore, M1 macrophage‐derived MMP‐2, capable of cleaving laminin‐5 and type IV collagen, could participate in the BM degradation of oral mucosal AGVHD. From these findings, an interaction between M1 macrophages and BM via macrophage‐derived MMP‐2 may be responsible for the progression of oral mucosal AGVHD.
Following the BM degradation, activated macrophages attach to KCs in an MCP‐1/CCR2 adhesive manner. The MCP‐1/CCR2 pathway is involved in the development of inflammation in the skin 39. Furthermore, MCP‐1/CCR2 interactions play a critical role in the development of GVHD 21, 22. Our SWBA results demonstrated that macrophage adhesion to the oral mucosal epithelium was reduced dramatically when macrophages from the tongues of AGVHD rats were treated with an anti‐CCR2 antibody and the tissue sections were treated with anti‐MCP‐1 antibody. These findings provide important evidence that the MCP‐1/CCR pathway is associated directly with the migration and adhesion of activated macrophages to lesional KCs in the advanced stages of oral mucosal AGVHD.
We acknowledge there are two possible limitations in this study. First, this study may be limited by the lack of direct evidence as to whether the inhibition of macrophage migration in the epithelium can prevent the occurrence or alleviate the severity of oral mucosal AGVHD. However, our data show that M1 macrophage infiltrates are associated with MMP‐2 secretion and the MCP‐1/CCR2 signalling axis which may contribute to the epithelial destruction in the oral mucosal AGVHD. Secondly, in this study we could not examine whether an innate immune response is associated with oral mucosal AGVHD. Although the pathophysiology of GVHD depends upon aspects of adaptive immunity, recent studies have revealed that components of the innate immune response are also important 40, 41, 42. Particularly, neutrophils could amplify tissue damage caused by conditioning regimens during the development of GVHD 40. However, the AGVHD model used in this study was established by the P‐F1 semi‐allogeneic immune response without the conditioning regimens. As tissue damage caused by treatment with the conditioning regimens is not induced in the oral mucosa, components of the innate immune response may be inactive during the development of AGVHD. This study may shed additional light on the role of macrophages during the development of oral mucosal AGVHD.
In summary, we have demonstrated that, in the oral lesions of the rat AGVHD model, a process of macrophage‐mediated epithelial changes is divided into three steps: (i) the accumulation of macrophages in the BM region via the laminin/CD29 β1 intern pathway; (ii) the BM degradation mediated by macrophage‐related MMP‐2; and (iii) the migration and adhesion of macrophages from oral mucosal AGVHD to epithelial KCs via the interaction of MCP‐1/CCR2. Our data show that the migration and adhesion of M1 macrophages are associated with oral mucosal AGVHD, which is mediated in part by both the laminin/CD29 β1 integrin and MCP‐1/CCR2 pathways. Therefore, our study provides additional support for the contribution of macrophage infiltrate to the development of oral mucosal AGVHD.
Disclosure
The authors declare that they have no competing interests.
Acknowledgements
The authors would like to thank Enago (www.enago.jp) for the English language review. This work was supported by JSPS KAKENHI grant numbers 4390422 (J. O.), 25670802 (J. O.) and 26463203 (T. H).
References
- 1. Woo SB, Lee SJ, Schubert MM. Graft‐vs.‐host disease. Crit Rev Oral Biol Med 1997; 8:201–16. [DOI] [PubMed] [Google Scholar]
- 2. Hanada H, Ohno J, Seno K, Ota N, Taniguchi K. Dynamic changes in cell‐surface expression of mannose in the oral epithelium during the development of graft‐versus‐host disease of the oral mucosa in rats. BMC Oral Health 2014; 14:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ohno J, Iwahashi T, Ehara M, Hanada H, Funakoshi T, Taniguchi K. Induction of epithelial migration of lymphocytes by intercellular adhesion molecule‐1 in a rat model of oral mucosal graft‐versus‐host disease. Histol Histopathol 2011; 26:725–33. [DOI] [PubMed] [Google Scholar]
- 4. Gordon S, Taylor PR. Monocyte and macrophage heterogeneity. Nat Rev Immunol 2005; 5:953–64. [DOI] [PubMed] [Google Scholar]
- 5. Mosser DM, Edwards JP. Exploring the full spectrum of macrophage activation. Nat Rev Immunol 2008; 8:958–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Mantovani A, Sica A, Sozzani S, Allavena P, Vecchi A, Locati M. The chemokine system in diverse forms of macrophage activation and polarization. Trends Immunol 2004; 25:677–86. [DOI] [PubMed] [Google Scholar]
- 7. Goerdt S, Orfanos CE. Other functions, other genes: alternative activation of antigen‐presenting cells. Immunity 1999; 10:137–42. [DOI] [PubMed] [Google Scholar]
- 8. Gordon S. Alternative activation of macrophages. Nat Rev Immunol 2003; 3:23–35. [DOI] [PubMed] [Google Scholar]
- 9. Dale DC, Boxer L, Liles WC. The phagocytes: neutrophils and monocytes. Blood 2008; 112:935–45. [DOI] [PubMed] [Google Scholar]
- 10. Hill GR, Crawford JM, Cooke KR, Brinson YS, Pan L, Ferrara JL. Total body irradiation and acute graft‐versus‐host disease: the role of gastrointestinal damage and inflammatory cytokines. Blood 1997; 90:3204–13. [PubMed] [Google Scholar]
- 11. Nestel FP, Price KS, Seemayer TA, Lapp WS. Macrophage priming and lipopolysaccharide‐triggered release of tumor necrosis factor alpha during graft‐versus‐host disease. J Exp Med 1992; 175:405–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Haniffa M, Ginhoux F, Wang XN et al Differential rates of replacement of human dermal dendritic cells and macrophages during hematopoietic stem cell transplantation. J Exp Med 2009; 206:371–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Nishiwaki S, Nakayama T, Murata M et al Dexamethasone palmitate ameliorates macrophages‐rich graft‐versus‐host disease by inhibiting macrophage functions. PLOS ONE 2014; 9:e96252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Terakura S, Martin PJ, Shulman HM, Storer BE. Cutaneous macrophage infiltration in acute GvHD. Bone Marrow Transplant 2015; 50:1135–7. [DOI] [PubMed] [Google Scholar]
- 15. Hynes RO. Integrins: bidirectional, allosteric signaling machines. Cell 2002; 110:673–87. [DOI] [PubMed] [Google Scholar]
- 16. Shapiro SD. Matrix metalloproteinase degradation of extracellular matrix: biological consequences. Curr Opin Cell Biol 1998; 10:602–8. [DOI] [PubMed] [Google Scholar]
- 17. Pirila E, Ramamurthy NS, Sorsa T, Sato T, Hietanen J, Maisi P. Gelatinase A (MMP‐2), collagenase‐2 (MMP‐8), and laminin‐5 gamma2‐chain expression in murine inflammatory bowel disease (ulcerative colitis). Dig Dis Sci 2003; 48:93–8. [DOI] [PubMed] [Google Scholar]
- 18. Kessenbrock K, Plaks V, Werb Z. Matrix metalloproteinases: regulators of the tumor microenvironment. Cell 2010; 141:52–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Rollins BJ. Chemokines. Blood 1997; 90:909–28. [PubMed] [Google Scholar]
- 20. Turler A, Schwarz NT, Turler E, Kalff JC, Bauer AJ. MCP‐1 causes leukocyte recruitment and subsequently endotoxemic ileus in rat. Am J Physiol Gastrointest Liver Physiol 2002; 282:G145–55. [DOI] [PubMed] [Google Scholar]
- 21. Terwey TH, Kim TD, Kochman AA et al CCR2 is required for CD8‐induced graft‐versus‐host disease. Blood 2005; 106:3322–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hildebrandt GC, Duffner UA, Olkiewicz KM et al A critical role for CCR2/MCP‐1 interactions in the development of idiopathic pneumonia syndrome after allogeneic bone marrow transplantation. Blood 2004; 103:2417–26. [DOI] [PubMed] [Google Scholar]
- 23. Gerard C, Rollins BJ. Chemokines and disease. Nat Immunol 2001; 2:108–15. [DOI] [PubMed] [Google Scholar]
- 24. Mack M, Chihak J, Simonis C et al Expression and characterization of the chemokine receptors CCR2 and CCR5 in mice. J Immunol 2001; 166:4697–704. [DOI] [PubMed] [Google Scholar]
- 25. Yopp AC, Fu S, Honig SM et al FTY720‐enhanced T cell homing is dependent on CCR2, CCR5, CCR7, and CXCR4: evidence for distinct chemokine compartments. J Immunol 2004; 173:855–65. [DOI] [PubMed] [Google Scholar]
- 26. Kurihara T, Warr G, Loy J, Bravo R. Defects in macrophage recruitment and host defense in mice lacking the CCR2 chemokine receptor. J Exp Med 1997; 186:1757–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Aractingi S, Chosidow O. Cutaneous graft‐versus‐host disease. Arch Dermatol 1998; 134:602–12. [DOI] [PubMed] [Google Scholar]
- 28. Nishiwaki S, Terakura S, Ito M et al Impact of macrophage infiltration of skin lesions on survival after allogeneic stem cell transplantation: a clue to refractory graft‐versus‐host disease. Blood 2009; 114:3113–6. [DOI] [PubMed] [Google Scholar]
- 29. Giannelli G, Falk‐Marzillier J, Schiraldi O, Sterler‐Stevenson WG, Quaranta V. Induction of cell migration by matrix metalloprotease‐2 cleavage of laminin‐5. Science 1997; 277:225–8. [DOI] [PubMed] [Google Scholar]
- 30. Stetler‐Stevenson WG, Krutzsh HC, Wacher MP, Margulies IM, Liotta LA. The activation of human type IV collagenase proenzyme. Sequence identification of the major conversion product following organomercurial activation. J Biol Chem 1989; 264:1353–6. [PubMed] [Google Scholar]
- 31. Thomas DW, Matthews JB, Prime SS. Mucosal cell‐mediated immunological changes associated with experimental graft‐versus‐host disease. J Oral Pathol Med 1996; 25:145–50. [DOI] [PubMed] [Google Scholar]
- 32. Polfliet MM, Fabriek BO, Daniels WP, Dijkstra CD, van den Berg TK. The rat macrophage scavenger receptor CD163: expression, regulation and role in inflammatory mediator production. Immunobiology 2006; 211:419–25. [DOI] [PubMed] [Google Scholar]
- 33. Becker HM, Rullo J, Chen M et al Alpha1 beta1 integrin‐mediated adhesion inhibits macrophage exit from a peripheral inflammatory lesion. J Immunol 2013; 190:4305–14. [DOI] [PubMed] [Google Scholar]
- 34. Colognatao H, Yurchenco PD. Form and function: the laminin family of heterotrimers. Dev Dyn 2000; 218:213–34. [DOI] [PubMed] [Google Scholar]
- 35. Baugh MD, Perry MJ, Hollander AP et al Matrix metalloproteinase levels are elevated in inflammatory bowel disease. Gastroenterology 1999; 117:814–22. [DOI] [PubMed] [Google Scholar]
- 36. Bachmeier BE, Boukamp P, Lichtinghagen R, Fusenig NE, Fink E. Matrix metalloproteinases‐2,‐3,‐7,‐9 and‐10, but not MMP‐11, are differentially expressed in normal, benign tumorigenic and malignant human keratinocyte cell lines. Biol Chem 2000; 381:497–507. [DOI] [PubMed] [Google Scholar]
- 37. Makela M, Larjava H, Pirila E et al Matrix metalloproteinase 2 (gelatinase A) is related to migration of keratinocytes. Exp Cell Res 1999; 251:67–78. [DOI] [PubMed] [Google Scholar]
- 38. Oviedo‐Orta E, Bermudez‐Fajardo A, Karanam S, Benhow U, Newby A. Comparison of MMP‐2 and MMP‐9 secretion from T helper 0, 1 and 2 lymphocytes alone and in culture with macrophages. Immunology 2007; 124:42–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Vestergaard C, Just H, Baumgartner Nielsen J, Thestrup‐Pedersen K, Deleuran M. Expression of CCR2 on monocytes and macrophages in chronically inflamed skin in atopic dermatitis and psoriasis. Acta Derm Venereol 2004; 84:353–8. [DOI] [PubMed] [Google Scholar]
- 40. Schwab L, Goroncy L, Palaniyandi S et al Neutrophil granulocytes recruited upon translocation of intestinal bacterial enhance graft‐versus‐host disease via tissue damage. Nat Med 2014; 20:648–54. [DOI] [PubMed] [Google Scholar]
- 41. Klambt V, Wohlfeil SA, Schwab L et al A novel function for P2Y2 in myeloid recipient‐derived cells during graft‐versus‐host disease. J Immunol 2015; 195:5795–804. [DOI] [PubMed] [Google Scholar]
- 42. Fischer JC, Wintges A, Haas T, Poeck H. Assessment of mucosal integrity by quantifying neutrophil granulocyte influx in murine models of acute intestinal injury. Cell Immunol 2017; 316:70–6. [DOI] [PubMed] [Google Scholar]


