Summary
B‐cell memory was long characterized as isotype‐switched, somatically mutated and germinal centre (GC)‐derived. However, it is now clear that the memory pool is a complex mixture that includes unswitched and unmutated cells. Further, expression of CD73, CD80 and CD273 has allowed the categorization of B‐cell memory into multiple subsets, with combinatorial expression of the markers increasing with GC progression, isotype‐switching and acquisition of somatic mutations. We have extended these findings to determine whether these markers can be used to identify IgM memory phenotypically as arising from T‐dependent versus T‐independent responses. We report that CD73 expression identifies a subset of antigen‐experienced IgM+ cells that share attributes of functional B‐cell memory. This subset is reduced in the spleens of T‐cell‐deficient and CD40‐deficient mice and in mixed marrow chimeras made with mutant and wild‐type marrow, the proportion of CD73+ IgM memory is restored in the T‐cell‐deficient donor compartment but not in the CD40‐deficient donor compartment, indicating that CD40 ligation is involved in its generation. We also report that CD40 signalling supports optimal expression of CD73 on splenic T cells and age‐associated B cells (ABCs), but not on other immune cells such as neutrophils, marginal zone B cells, peritoneal cavity B‐1 B cells and regulatory T and B cells. Our data indicate that in addition to promoting GC‐associated memory generation during B‐cell differentiation, CD40‐signalling can influence the composition of the unswitched memory B‐cell pool. They also raise the possibility that a fraction of ABCs may represent T‐cell‐dependent IgM memory.
Keywords: age‐associated B cells, CD40, CD73, IgM, memory, T cells
Abbreviations
- ABC
age‐associated B cell
- APC
allophycocyanin
- ASC
antibody‐secreting cell
- BCL6
B lymphoma 6 protein
- BCR
B‐cell receptor
- BM
bone marrow
- Breg
regulatory B cell
- GC
germinal centre
- KO
knockout
- MZ
marginal zone
- nMZ
nodal marginal zone
- NP
4‐hydroxy‐3‐nitrophenylacetyl
- PE
phycoerythrin
- TCR
T‐cell receptor
- TD
T‐dependent
- Tfh
T follicular helper
- TI
T‐independent
- Treg
regulatory T cell
- WT
wild‐type
Introduction
Humoral protection against re‐encountered pathogens relies on long‐lived plasma cells and memory B cells, and both are generated predominantly in germinal centre (GC) reactions associated with the primary response.1, 2, 3, 4 Long‐lived plasma cells serve as a source of pre‐existing protective antibodies in the host, but memory B cells contribute by undergoing rapid activation and differentiation into plasma cells secreting relatively high‐affinity antibodies. Naive antigen‐specific B cells can also contribute in secondary responses – either by initiating a fresh primary response, which leads to the generation of short‐lived plasma cells that contribute antibodies for immediate protection, or by participating in the induction of fresh GCs.5, 6 Hence, the humoral response to antigen challenge includes IgM and switched antibodies with germline and mutated specificities, and represents the output of primary and secondary B‐cell stimulation in spatially separated areas such as foci and GCs.7
There is evidence to indicate that B‐cell memory may be GC‐independent and unmutated.8, 9, 10 Conditional ablation of B lymphoma 6 (Bcl6) leads to the generation of IgG1 memory cells without mutations, indicating that they may be GC‐independent.11 A population of ‘pre‐GC’ cells that are CD38+ GL7+ has been identified early in the phycoerythrin (PE) ‐specific response and shown to differentiate directly into IgM or switched memory cells in a GC‐independent but CD40‐signalling‐dependent process9 or to progress to ‘true’ BCL6+ GC cells that give rise primarily to switched memory cells. Although GCs are a characteristic feature of T‐dependent (TD) responses, they may also form in response to T‐independent (TI) antigens, especially if B‐cell receptor (BCR) crosslinking is extensive and the frequency of antigen‐specific B cells is high.12 Such GC‐like structures, which support isotype switching to IgG3, as well as low levels of somatic hypermutation, have been shown to be dependent on CD40 expression but independent of CD154 (CD40L)‐mediated signalling, and may involve presentation of immune/complement‐complexed antigens on follicular dendritic cells.13, 14, 15 Switched antibodies and GCs are also seen in the spleen and Peyer's patches of T‐cell receptor (TCR) ‐α −/− mice in the absence of overt immunization.16, 17 Further, memory cells and GCs can appear simultaneously3, 18 and although not all TD memory cells show mutations, a low level of somatic hypermutation has been observed following immunization with the TI antigen 4‐hydroxy‐3‐nitrophenylacetyl (NP)‐Ficoll.10, 13, 19, 20 Hence, the memory B‐cell pool in mice responding to environmental antigens should include IgM and switched memory cells arising from TI and TD responses, and generated either within or outside GCs.
In this study, we tried to determine whether the markers CD73, CD80 and CD273 could be used to identify the provenance of IgM memory cells in unimmunized mice as arising from TD responses to environmental antigens. Murine IgM memory has been difficult to characterize phenotypically as the cells do not express specific markers such as CD27, which has been useful in the identification of human memory B cells. However, CD73, CD80 and CD273 have been used to characterize ‘less‐mature’ and ‘more‐mature’ memory B‐cell populations, with combinatorial expression of these markers increasing as the immune response matures and accumulates mutations and switched receptors.10, 21, 22, 23 We reasoned that IgM memory cells arising from TD responses might resemble the ‘more mature’ population and show preferential acquisition of one or more of these markers compared with IgM memory cells arising from TI responses. Hence, we compared expression of these markers on IgM memory cells in the spleens of wild‐type (WT) mice and mice lacking either T‐cell help (TCR‐β −/− and TCR‐β −/− δ −/−) or the ability to form GCs (CD40−/−). We report that CD40 signalling influences the size of the CD73+ IgM memory B‐cell pool in the spleen of unimmunized mice. It also affects the expression of this marker on splenic age‐associated B cells (ABCs) and T cells, but not on neutrophils, marginal zone (MZ) B cells, regulatory T (Treg) cells, regulatory B (Breg) cells or peritoneal B‐1 B cells.
Materials and methods
Mice
BALB/cByJ (BALB/c), C57BL/6ByJ (B6), B6.SJL‐PtprcaPepcb/BoyJ (B6.SJL), CNCr.129P2‐Cd40 tm1Kik/J (CD40−/−), B6.129P2‐Tcrb tm1Mom/J (TCR‐β −/−) and B6.129P2‐Tcrd tm1Mom/J (TCR‐δ −/−) and B6.129S2‐Ighm tm1Cgn/J (μMT) mice were obtained from the Jackson Laboratories (Bar Harbor, ME) and maintained in the Small Animal Facility of the National Institute of Immunology. TCR‐β −/− δ −/− mice were bred in‐house from the single mutants. All mouse protocols were carried out in accordance with the Institutional Animal Ethics Committee guidelines.
Mixed bone marrow chimeras and adoptive transfer
BALB/c: CD40−/− and B6.SJL: TCR‐β −/− δ −/−mixed bone marrow (BM) chimeras were generated by transferring BM from the two strains in a 1 : 1 ratio (3 × 107 cells/mouse) intravenously into lethally irradiated (9 Gy) BALB/c or B6.SJL mice, respectively. Approximately 1 : 1 chimerism was confirmed by staining peripheral blood mononuclear cells for CD45.1 and CD45.2 8 weeks after reconstitution. For adoptive transfer experiments, 3 × 107 spleen cells from B6.SJL mice were transferred intravenously into non‐irradiated TCR‐β −/− δ −/− mice.
Flow cytometry
The following reagents were used for flow cytometry: FITC/PE‐TexasRed®/PE‐CF594/Pacific Blue™/V450 anti‐mouseB220 (clone RA3‐6B2), PE‐CF594 anti‐mouse CD40 (clone 3/23), FITC anti‐mouse CR1/CR2 (CD21/35, clone 7G6), FITC/PE anti‐mouse IgD (clone 11‐26c.2a), PE‐Cy7 anti‐mouse CD23 (clone B3B4), PE/Biotin anti‐mouse CD80 (clone 16‐10A1), V450 anti‐mouse CD45.2 (clone 104), allophycocyanin (APC)/PE‐Cy7 anti‐mouse CD45.1 (clone A20), FITC/APC‐Cy7 anti‐mouse CD90.2 (clone 53‐2.1), PE/APC anti‐mouse CD4 (clone RM4‐5), V450 anti‐mouse CD8 (clone 53.6.7), APC anti‐mouse CD279 clone (J43), PE anti‐mouse CXCR5 (clone 2G8), PE anti‐mouse Gr1 (clone RB6‐8C5), FITC anti‐mouse CD11b (clone M1/70), PE‐Cy7 anti‐mouse CD19 (clone 1D3) (all from BD Biosciences, San Jose, CA). In addition, FITC/APC anti‐mouse CD93 (clone AA4.1), eFluor450® anti‐mouse CD21/35 (eBio4E3), Peridinin chlorophyll protein (PerCP)‐eFluor710® anti‐mouse IgM (clone II/41), PE/PE‐Cy7/Biotin anti‐mouse CD73 (clone eBioTY/11.8), PE/Biotin anti‐mouse CD273 (clone 122), FITC anti‐mouse CD19 (clone eBio1D3), APC anti‐mouse CD5 (clone 53‐7.3), APC‐Cy7 anti‐mouse CD25 (clone PC61.5), FITC anti‐FoxP3 (clone FJK‐16s), V450 anti‐mouse CD4 (clone GK1.5), PerCP5.5 anti‐mouse CD4 (clone RM4‐5), APC anti‐mouse CD90.2 (clone 53‐2.1) (all from Thermo Fisher Scientific, Waltham, MA), Pacific‐Blue™ anti‐mouse CD1d (clone 1B1; Biolegend, San Diego, CA). PE/APC/APC‐Cy7‐streptavidin (BD Biosciences, San Jose, CA) was used as a secondary reagent. For intracellular FoxP3 staining, cells were fixed and permeabilized with FoxP3/transcription factor staining buffer set (Thermo Fisher) as per the manufacturer's instructions. Samples were run on FACS Canto II or FACSAria III (BD Biosciences) and data were analysed using flowjo (Tree Star, Ashland, OR).
Identification of cell subsets
Antigen‐experienced B cells were gated as B220+ IgD− CD93− CD21/35− CD23+ and separated into unswitched (IgM+) and switched (IgM−) subsets. Peritoneal B‐1 B cells were identified as CD19hi B220lo. Splenic MZ B cells were identified as B220+ IgMhi CD93− CD23− CD21/35+. ABCs were identified as B220+ CD93− CD21/35− CD23−. Treg cells were identified as CD4+ CD25+ FoxP3+. B reg cells were identified as B220+ CD90− CD1dhi CD5+. T follicular helper (Tfh) cells were identified CD4+ FoxP3− CD279+ CXCR5+. Blood neutrophils were identified as B220− CD90− Gr1+ CDllb+.
B‐cell stimulation
IgM+ and IgM– fractions of antigen‐experienced splenic cells from BALB/c mice were gated as above, sorted on a FACSAria III, plated at 2 × 104 cells/well in 96‐well round‐bottomed plates (Costar, Corning, NY) and stimulated with 10 μg/ml of lipopolysaccharide (LPS; Sigma Aldrich, St. Louis, MO) for 72 hr in the presence or absence of 1 μg/ml of aphidicolin (Sigma) in RPMI‐1640 medium (Biological Industries, Cromwell, CT) supplemented with fetal bovine serum (Thermo Fisher) antibiotics (HiMedia, Mumbai, India) and β‐mercaptoethanol (Sigma). Secreted immunoglobulin was estimated by ELISA on plates (Costar) coated with goat anti‐mouse immunoglobulin (Southern Biotechnology, Birmingham, AL) and detected using goat anti‐mouse immunoglobulin‐horseradish peroxidase (Southern Biotechnology). Immunoglobulin concentrations were calculated from a standard curve run in parallel with purified mouse immunoglobulin. Plasma cells were scored as antibody secreting cells (ASCs) on multiscreen filter plates (MultiScreenHTS IP Filter Plates; Merck‐Millipore, Billerika, MA) activated with 70% ethanol, using the same coating and detection reagents. Spots were quantified on an ELISPOT reader (AID, GmBH, Straßberg, Germany). Cells were titrated down from 50 000 to 200 cells and the number of antigen‐secreting cells/105 input cells was calculated.
Real‐time RT‐PCR
Cells were suspended in TRI‐Reagent (Sigma), and RNA was isolated by chloroform extraction and precipitation. cDNA was prepared from 180 ng of RNA by reverse transcription (Promega, Madison, WI) and Power SYBR Green master mix was used for amplifications (AB Systems, Waltham, MA). The following primers were used: Blimp‐1, 5′‐TGAGTGCCAGGTCTGCCA‐3′ and 5′‐CTGGGCACACTTGTGAGG‐3′; Bcl6, 5′‐CATCTGCGCATCCACACAGGA‐3′ and 5′‐CGAGGAACACTCCATGCTTCA‐3′; Bmi1, 5′‐ATGAGTCACCAGAGGGATGG‐3′ and 5′‐AAGAGGTGGAGGGAACACCT‐3′; Klf2, 5′‐GCCTGTGGGTTCGCTATAAA‐3′ and 5′‐TTTCCCACTTGGGATACAGG‐3′; Ski, 5′‐AAAAGCCCTCCGCTCTAGTC‐3′ and 5′‐GACGTCAGGGCTTAGCAGTC‐3; Tcf4, 5′‐CACAACGGAGCGATGGGTA‐3′ and 5′‐GGGTGGGTTCAAGTCAGG‐3′; Gapdh, 5′‐ATGGCCTTCCGTGTTCCTA‐3′ and 5′‐TGAAGTCGCAGGAGACAACCT‐3′. Amplification was initiated by denaturation at 95° for 10 min and followed by 40 cycles of 95° for 30 seconds, 55° for 30 seconds, and 72° for 30 seconds. All reactions were carried out on a 7500 Real Time PCR system (AB Systems). Relative RNA expression was determined as described previously.24
Statistical analysis
P values were determined by two‐tailed unpaired Student's t‐test for samples of unequal variance.
Results
Unswitched antigen‐experienced B‐cell subsets can be identified in unimmunized mice
We first determined whether antigen‐experienced IgM+ and switched cells, generated in response to environmental antigens, could be reliably identified in unimmunized mice by flow cytometry, and whether they differed in the relative expression of CD73, CD80 and CD273 as reported for memory cells arising in response to immunization. Hence, splenocytes were first gated as B220+ IgD– CD93− CD21/35− CD23+ and then separated into unswitched and switched fractions based on the expression of IgM (Fig. 1a). The combination of these markers reliably excludes naive, MZ, transitional and B‐1 B cells, ABCs and plasmacytoid dendritic cells.25, 26, 27, 28 The two antigen‐experienced B‐cell populations were then analysed for expression of CD73, CD80 and CD273. Our analysis was restricted to determining the frequencies of cells expressing individual markers due to the unavailability of reagents for simultaneous detection of all three.21 We found that CD73+, CD80+ and CD273+ cells were readily detectable in both B‐cell pools in unimmunized mice, and that higher frequencies of these markers were seen on switched cells, as reported earlier in immunization experiments (Fig. 1b,c).
Figure 1.
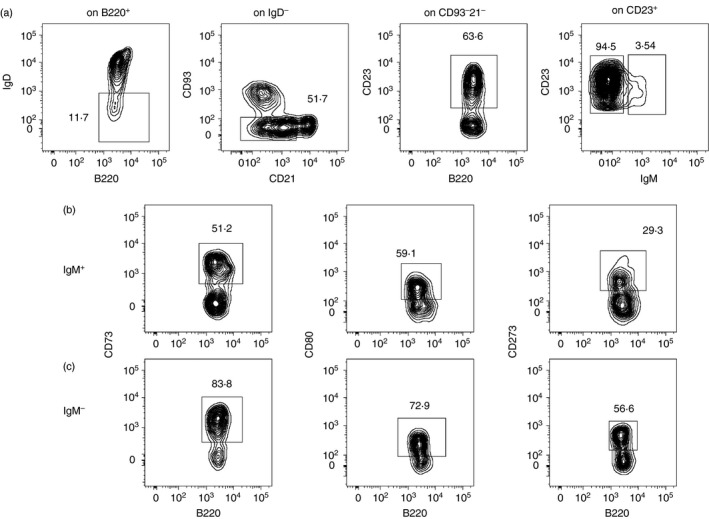
Gating strategy for identification of antigen‐experienced B cells in unimmunized mice. B220+ cells in the spleen were sequentially gated as IgD– CD21− CD93− CD23+ to identify total antigen‐experienced cells and then as IgM+ and IgM– for identification of the unswitched and switched components, respectively (a). Gating for identification of CD73+, CD80+ and CD273+ cells on IgM+ (b) and IgM– (c) antigen‐experienced cells.
IgM+ antigen‐experienced cells in unimmunized mice share attributes of functional B‐cell memory
To determine whether antigen‐experienced cells identified phenotypically could be classified as memory B cells, we first looked for the relative expression of transcripts for Blimp‐1, Bcl‐6, Tcf4, Bmi1, Ski and Klf2, which are reported to be differentially expressed in GC B cells, plasma cells and memory cells in NP‐immunized mice.29 Hence, IgM+ and IgM– antigen‐experienced cells from unimmunized mice, identified as in Fig. 1, were sorted and amounts of the various transcripts in these cells were compared with those in B cells stimulated with LPS for 24 hr (to serve as a pool of recently activated B cells) or for 96 hr (to serve as a pool of plasmablasts and plasma cells). We found that Blimp‐1 transcript amounts were very low in both sorted cell populations compared with the plasma cell pool (relative expression of 0·08 and 0·01 in the IgM+ and IgM– pools, respectively). However, transcript amounts were similar to those in 24‐hr blasts (Fig. 2a). On the other hand, Tcf4, Bmi1, Ski and Klf2 transcripts were higher in both sorted populations compared with the 24‐hr blasts, as reported for memory cells versus GC B cells in the microarray,29 although all four transcripts were more abundant in the IgM– pool than in the IgM+ pool (Fig. 2a). The most striking increase over 24‐hr blasts was in Klf2 transcript amounts and these data also fit in with the microarray data. Bcl‐6 transcripts were reported in the microarray to be lower in memory cells than in GC B cells but these were higher in our sorted cells. Hence, B cells that have responded to environmental antigens in mice share transcript profiles that differentiate antigen‐specific memory B cells from recently activated cells and plasmablasts in primed mice.
Figure 2.
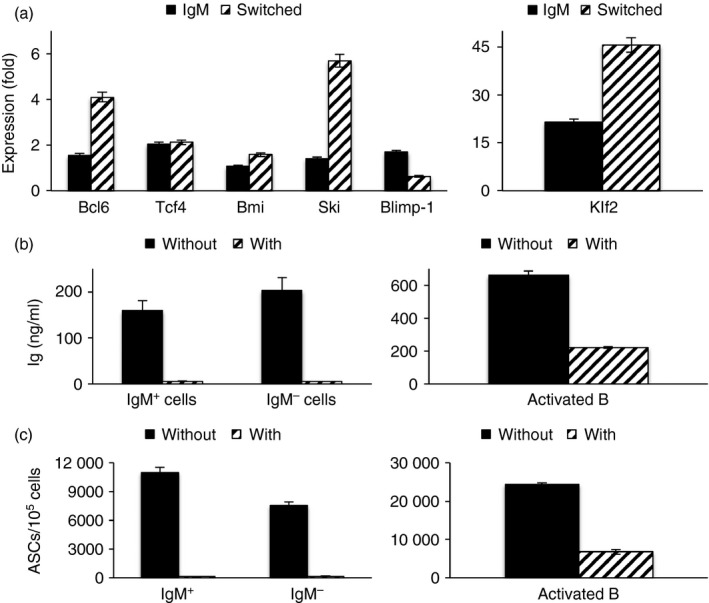
IgM+ antigen‐experienced cells share features of memory. Expression of transcripts (as labelLed) in sorted IgM+ and IgM– antigen‐experienced cells from unimmunized mice relative to 24‐hr lipopolysaccharide (LPS) blasts (a). Immunoglobulin in supernatants of sorted cells stimulated with 10 μg/ml LPS in the absence (without) or presence (with) of 1 μg/ml aphidicolin for 84 hr. Activated B cells are spleen cells pre‐stimulated with LPS for 72 hr and re‐stimulated with or without aphidicolin for 48 hr (b). (ASCs)/105 cells that were stimulated with LPS ± aphidicolin for 72 hr. Activated B cells are spleen cells pre‐stimulated with LPS for 72 hr and re‐stimulated with or without aphidicolin for 48 hr (c). Data are shown as mean ± SD of replicates (a), mean ± SEM of triplicate cultures (b), and mean ± SEM of triplicate cultures, with cells from each culture loaded onto six wells each for ASC assay (c).
To determine whether these cells shared functional attributes of B‐cell memory, we determined whether they could undergo division‐linked differentiation. It has been shown previously that NP‐specific B cells from prime‐boosted mice can differentiate into plasma cells upon stimulation with LPS for 5 days.30 It has also been shown that pre‐plasmablasts, but not memory cells, secrete immunoglobulin when stimulated in TD cultures even if cell division is blocked with aphidicolin.31 Hence, IgM+ and IgM– populations were sorted as above, cultured with LPS ± aphidicolin for 84 hr, and secreted immunoglobulin was estimated. Spleen cells that had been pre‐activated with LPS to serve as a source of plasmablasts/plasma cells were also plated with/without aphidicolin. We found that both IgM+ and IgM– antigen‐experienced cells could be stimulated with LPS to secrete immunoglobulin and also that neither population did so in the presence of aphidicolin (Fig. 2b). Similar results were obtained in experiments where supernatants were harvested at 48 or 72 hr (data not shown). As expected, the pre‐activated cells secreted immunoglobulin even in the presence of aphidicolin. Similar results were obtained when ASCs were estimated at 72 hr (Fig. 2c). Together, the data indicate that the IgM+ antigen‐experienced cells identified by phenotypic markers probably represent quiescent IgM memory cells.
The CD73+ subset of IgM memory is reduced in mice lacking T cells or CD40
IgM memory cells have been reported to form in response to both TD and TI antigens, to be important in recall responses and to enter GCs upon antigenic challenge.9, 10, 11, 22, 32, 33 However, the factors required for the generation of IgM memory remain poorly understood and murine IgM memory cells have been difficult to characterize due to a lack of specific surface markers for identification. As expression of CD73, CD80 and CD273 appears to correlate with mature memory or mutated memory,9, 21, 22 we reasoned that higher expression of one or more of these markers may help to identify IgM memory arising from TD responses. Hence, we compared expression of these markers on IgM memory cells in the spleens of WT mice and mice deficient either in T‐cell help (TCR‐β −/−) or in the ability to form GCs (CD40−/−). As expected, we found that frequencies of switched memory B cells were significantly lower in the spleen of both knockout (KO) strains, and that within the switched pool the proportion of cells expressing CD73, CD80 or CD273 was also significantly lower (Fig. 3). When we analysed unswitched memory, we found that TCR‐β −/− mice had similar frequencies as WT mice, but that fewer cells expressed CD73. In the CD40−/− mice, IgM memory frequencies were lower, and there was a significant reduction in the proportion of cells expressing CD73 and CD273 (Fig. 4). In TCR‐δ −/− mice, however, neither the frequency of IgM memory cells nor the proportion of cells that expressed any of the three markers was affected (Fig. 4). Hence, the CD73+ fraction of IgM memory appears to be dependent on classical T‐cell–B‐cell interaction.
Figure 3.
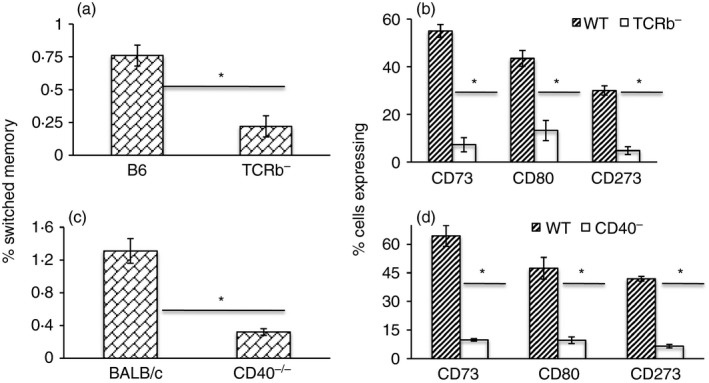
Switched memory subsets are reduced in mice lacking T cells and CD40. Frequencies of switched memory cells, and the proportion of CD73+, CD80+ and CD273+ subsets in the spleens of B6 and TCR‐β −/− (TCRb–) mice (a, b) and of BALB/c and CD40−/− (CD40–) mice (c, d). Data are shown as mean ± SEM of 8–12 mice from three or four experiments. *P ≤ 8·8 × 10−4.
Figure 4.
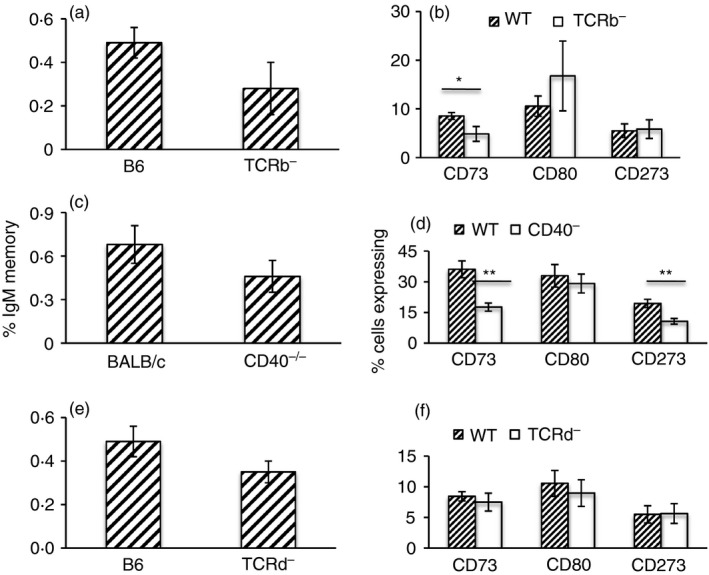
IgM memory subsets are reduced in TCR‐β −/− and CD40−/− mice. Frequencies of IgM memory cells and the proportion of CD73+, CD80+ and CD273+ subsets in the spleens of B6 and TCR‐β −/− (TCRb–) mice (a, b), BALB/c and CD40−/− (CD40–) mice (c, d) and B6 and TCR‐δ −/− (TCRd–) mice. Data are shown as mean ± SEM of 8–12 mice from three or four experiments. *P = 0·03, **P ≤ 0·002.
The CD73+ subset of IgM memory is CD40 dependent
To confirm that conventional T‐cell help, and CD40 signalling in particular, was necessary for generation of the CD73+ memory pool, we made mixed marrow chimeras in irradiated WT mice with B6.SJL and TCR‐β −/− δ −/− cells on the one hand and BALB/c and CD40−/− BM cells on the other. We restricted our analysis to IgM memory, as the frequency of switched memory was reduced in both KO strains. The CD73+ and CD273+ populations were reduced in the IgM memory pool in both CD40−/− and TCR‐β −/− δ −/− mice. However, the presence of WT cells in vivo led to restoration of both populations in the TCR‐β −/− δ −/− donor pool but not in the CD40−/− donor pool (Fig. 5). In another approach, we determined whether CD73+ memory B‐cell frequencies in TCR‐β −/− δ −/− mice could be increased by provision of peripheral WT T cells. Hence, congenic (CD45.1+) spleen cells were adoptively transferred into the KO mice and memory subsets were assessed on gated recipient (CD45.2+) B cells 6 weeks later. As seen in Fig. 6, provision of T‐cell help in the periphery led to an increase in the frequencies of CD73+ and CD273+ subsets of unswitched memory cells, and to an increase in expression of all three markers in switched memory cells in the KO mice.
Figure 5.
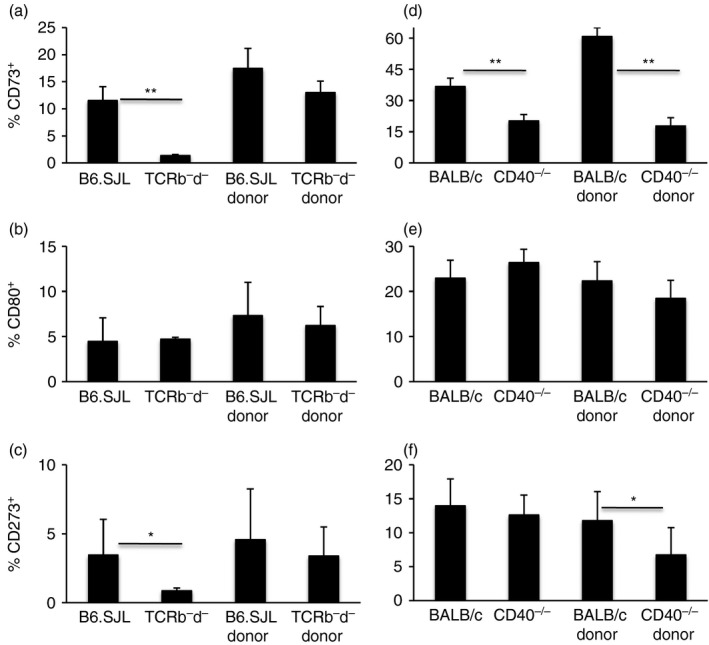
The CD73+ subset of IgM memory requires CD40 ligation. Frequencies of CD73+, CD80+ and CD273+ subsets of IgM memory in the spleens of bone marrow (BM) chimeras made with a 1 : 1 mixture of BM from wild‐type (WT; B6.SJL) and TCR‐β – δ ‐– (TCRb–d–) mice (a–c) and from WT (BALB/c) and CD40−/− (CD40−/−) mice (d–f). The donors were identified as B220+ CD45.1+ and B220+ CD45.2+ (a–c) and as B220+ CD40+ and B220+ CD40− (d–f). Data are shown as mean ± SEM of 10 chimeras (WT/TCRb–d–) and six chimeras (WT/CD40−/−) from two experiments. *P ≤ 0·03, **P ≤ 1·2 × 10−5.
Figure 6.
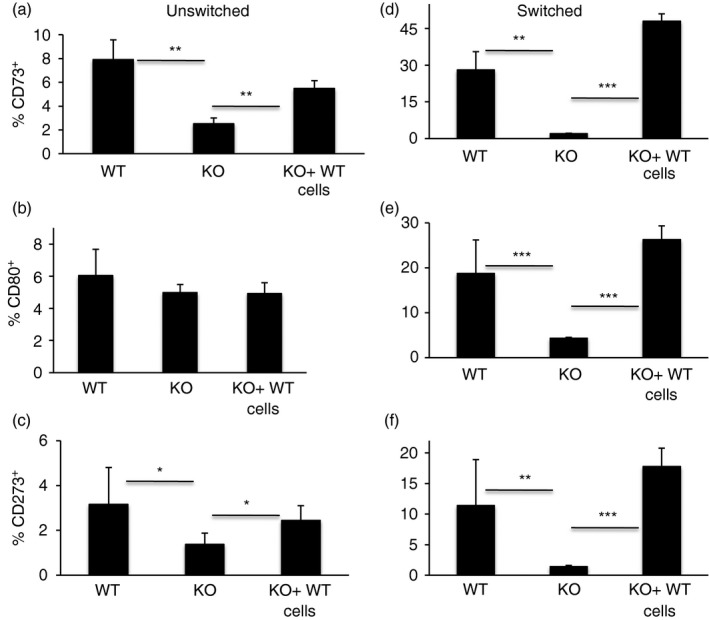
Transfer of peripheral T cells rescues the CD73+ subset of IgM memory in T‐cell‐deficient mice. Frequencies of IgM memory subsets (a–c) and switched memory subsets (d–f) on gated CD45.2 cells in the spleen of TCR‐β – δ – mice 6 weeks after adoptive transfer of congenic wild‐type (WT) CD45.1 spleen cells. Data are shown as mean ± SEM of 10 recipients from two experiments. *P ≤ 0·04, **P ≤ 0·01, ***P ≤ 0.009.
CD40 deficiency affects CD73 expression on ABCs and T‐cell subsets
A number of other cells of the immune system have been reported to express CD73. These include neutrophils, Treg cells, nodal marginal zone (nMZ) B cells, ABCs, Breg cells, B‐1 B cells, CD11c+ B cells and Tfh cells.28, 34, 35, 36, 37, 38, 39 We found equivalent expression of CD73 on blood neutrophils, peritoneal cavity B‐1 B cells and splenic Treg cells, Breg cells and MZ B cells from WT and CD40−/− mice (Fig. 7). In the mature follicular B‐cell pool, almost all the CD73+ cells were IgD‐negative (data not shown), indicating that its expression was restricted largely to memory cells. In the unimmunized mice used in this study, the frequencies of nMZ B cells and CD11c+ B cells were too low to allow for accurate scoring. Peyer's patches were sampled as the tissue most likely to have a significant Tfh population in unimmunized mice, and we found that although Peyer's patches of CD40−/− mice had fewer cells (around 25% of WT) and they contained fewer Tfh cells (32·6 ± 3·58 versus 7·77 ± 2·7), almost all Tfh cells in both strains expressed CD73 (Fig. 7). Interestingly, fewer ABCs in the CD40−/− mice expressed CD73. Representative gating for the cells analysed is shown in the Supplementary material (Figs S1 and S2).
Figure 7.
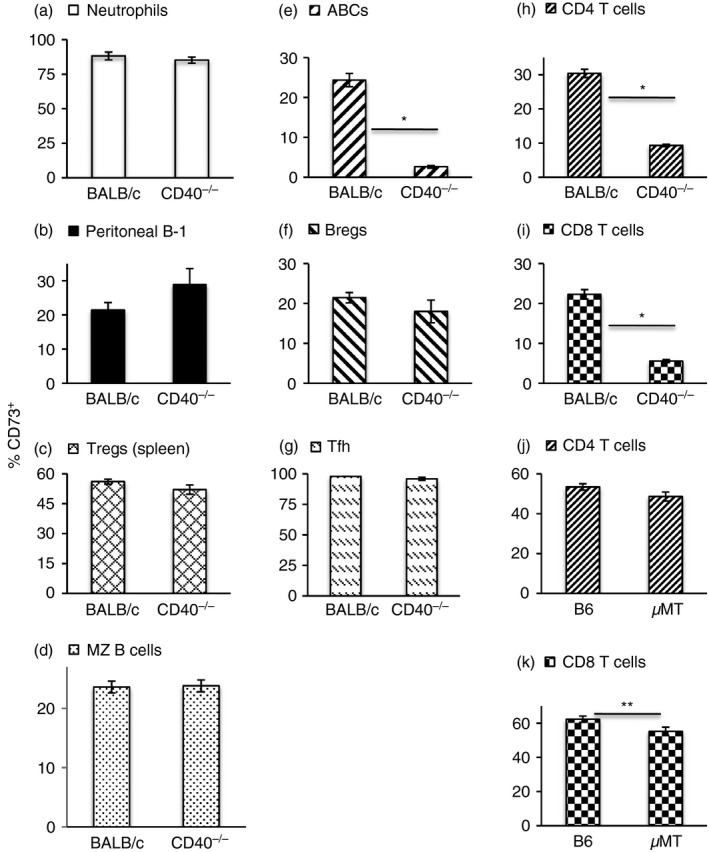
CD40 deficiency affects CD73 expression on age‐associated B cells (ABCs) and peripheral T cells. Frequency of CD73+ cells on gated blood neutrophils (a, n = 10), peritoneal cavity B‐1 B cells (b, n = 10), splenic regulatory T (Treg) cells (c, n = 10), marginal zone (MZ) B cells (d, n = 14), ABCs (e, n = 15), B regulatory (Breg) cells (f, n = 12), Peyer's patch follicular helper T (Tfh) (g, n = 8) and splenic CD4+ T (h, n = 9) and CD8+ T cells (i, n = 9) in BALB/c and CD40−/− mice. (j, k) Frequency of CD73+ cells on gated CD4+ and CD8+ (n = 8) T cells in B6 and μMT mice. Data are shown as mean ± SEM of 8–15 mice, as indicated, from two to five experiments. *P ≤ 2·4 × 10−9, **P = 0·01.
CD73 has also been used to identify a subset of primed but cytokine‐uncommitted CD4+ T helper cells that can be identified as CD4+ CD44+ Sca‐1– CD73+. We did not look for these cells because the CD40−/− mice used in this study are on the BALB/c background and such T helper primed precursor cells cannot be identified in this strain with this marker combination.40 However, we did find that fewer CD4+ and CD8+ cells in the spleens of CD40−/− mice expressed CD73. Hence, CD40 signalling can also support the induction of CD73 on peripheral T cells. Notably, specific B‐cell–T‐cell interactions are not apparently required for this, as T cells in μMT mice were not significantly deficient in CD73 expression (Fig. 7).
Discussion
The generation of memory B cells has classically been ascribed to interactions between follicular B cells and T cells in GCs. As cells bearing isotype‐switched BCRs can readily be identified as belonging to the memory pool, much of our understanding of memory B‐cell biology has come from studies on switched cells. However, immunization of mice with TD antigens such as PE and experiments using NP‐specific transgenic B cells have revealed that the memory B‐cell pool includes both IgM and switched populations, and that IgM memory is functional and long lived, with a half‐life similar to that of switched memory.10, 21, 22, 41 They may outnumber switched memory cells in vivo, and contribute to GC reactions in secondary responses.10, 22 IgM memory has also been reported to contribute adoptive protection against Borrelia hermsii infection41 and to mount a rapid recall response to malaria challenge.42 There are conflicting reports about the ability of IgM memory B cells against nominal antigens to respond to a boost. An early challenge of sheep red blood cell‐primed mice with homologous antigen indicated that they do22 whereas a late challenge of PE‐immunized mice indicated that they do not.10 The difference between the two immunogens is the maintenance of a sustained GC reaction following sheep red blood cell immunization and it has been suggested that IgM memory cells may be recruited into secondary responses if GCs are present.43
Recent efforts from several groups have attempted to characterize B‐cell memory into phenotypic and functional subsets, and these studies have revealed that relative expression of the markers CD73, CD80 and CD273 can be used to define at least five subsets of memory.20, 21, 44, 45 CD73 is expressed on a proportion of IgG and IgM memory and marks GC‐derived, mutated memory.9, 11, 22, 46 CD80+ memory cells are also mutated, and adoptive transfer experiments show that they may represent a significant fraction of long‐lived memory.47 Expression of CD80 and CD273 correlates with sustained GCs and with survival of GC B cells,23, 48 indicating that signalling through these receptors contributes to response maturation. Cells co‐expressing CD80 and CD273 are present in IgG as well as IgM memory pools, and they differentiate rapidly into plasma cells upon challenge, as opposed to double‐negative cells that preferentially seed GCs.44 Hence, the combinatorial expression of the three markers increases with response maturation.
The aim of this study was to determine whether IgM memory to environmental antigens could be identified as arising from TD or TI responses based on the relative expression of CD73, CD80 and CD273. Multi‐colour flow cytometry enabled identification of IgM+ and IgM– antigen‐experienced follicular cells, and the relative expression of Blimp‐1, Tcf4, Bmi1, Ski and Klf2 in these cells, as well as their ability to undergo division‐linked differentiation into immunoglobulin‐secreting cells when re‐stimulated, indicated that they share attributes of functional memory. In the two WT mouse strains used (B6 and BALB/c), IgM memory was found to be less abundant than switched memory (Figs 3 and 4) and it is possible that this reflects inclusion of all subclasses of IgG, and possibly other isotypes, in the switched pool. CD73 expression was also lower in the unswitched pool (10–40% of the IgM+ population versus 55–60% of the IgM– population). CD73 expression has been shown to increase progressively on GC B cells34 and it is possible, therefore, that IgM memory cells exit the GC relatively early.
As expected, switched memory cell frequencies, as well as expression of all three markers, were lower in TCR‐β −/− mice and CD40−/− mice when compared with their WT controls Interestingly, the CD73+ subset was especially affected in the unswitched pool in both KO strains (Fig. 4). IgM memory was unaltered in the absence of TCR‐γδ T cells, but we did find that switched memory (frequencies and phenotype) was compromised (data not shown). Our gating strategy includes the total B‐cell memory pool in unimmunized mice, and the data indicate that in the absence of TCR‐γδ cells, B‐cell responses to environmental antigens or their differentiation outcomes may be disturbed, as has been reported for the development of natural resistance to Eimeria vermiformis in mice49 and for control of mycobacterial and viral infections.50, 51, 52
A specific role for CD40 signalling for optimal generation of the CD73+ IgM memory pool came from irradiation BM chimera experiments, which showed re‐establishment of CD73+ IgM memory frequencies in the TCR−/− donor pool but not in the CD40−/− donor pool in the respective chimeras. Transfer of congenic WT spleen cells into non‐irradiated TCR‐deficient mice for provision of peripheral T‐cell help also led to restoration of IgM and switched memory subsets within 6 weeks (Figs 5 and 6). Together, our data indicate that CD73 expression identifies a subset of IgM memory in mice that is TD and CD40 signalling dependent. The population is, however, quite small and whether their small numbers can mediate significant biological effects remains to be determined.
CD73 is a 5′ ectonucleotidase that converts extracellular 5′‐AMP into adenosine and its activity has multiple consequences for inflammation and infection, including inhibition of cytokine release from endothelial cells, promotion of lymphocyte transmigration and attenuation of neutrophil accumulation at vascular surfaces.53, 54 It is expressed on a number of immune cell types and we sought to determine whether CD40 deficiency affected CD73 expression on some of these cells. In WT and CD40−/− mice, expression of CD73 was similar on neutrophils, peritoneal B‐1 B cells and Treg cells, although CD40‐null mice had fewer Treg cells, as noted earlier.55 It has been reported that IgM memory may be generated from splenic MZ B cells56 and hence we included MZ B cells in our analysis. No effect of CD40 deficiency was observed. We did find that CD73 expression was lower on gated CD4+ and CD8+ T cells in CD40−/− mice. However, frequencies were similar in μMT mice, indicating that CD73 expression on T cells can be maintained by interactions with other CD40‐expressing cells in vivo. CD73 signalling by adenosine through the adenosine receptor A2A has been shown to support the survival of naive T cells responding to tonic signalling by preventing the down‐regulation of interleukin‐7 receptor57 and also to arrest the terminal differentiation of CD8 T cells.58 Our data indicate that constitutive CD40 signalling may be involved in T‐cell homeostasis. We also found that Peyer's patches of CD40−/− mice had fewer Tfh cells and our results are in keeping with reports indicating that B‐cell–T‐cell interactions involving CD40 signalling promote Tfh cell differentiation.59, 60
Another subset of cells in CD40−/− mice that showed relatively low expression of CD73 was the ABC subset. ABCs are reported to be antigen‐experienced cells, mostly unswitched, that have been generated following BCR and Toll‐like receptor engagement in the response to intracellular pathogens like viruses.27 It is possible, therefore, that the CD73+ fraction of ABCs may also represent TD IgM memory. CD73 has also been shown to promote isotype switching autonomously in human B cells stimulated coordinately by BCR and Toll‐like receptor ligation by participating in the conversion of ATP released from Ca2+ sensitive vesicles during B‐cell activation.61 It is possible, therefore, that the IgM memory pool that has been identified as preferential entrants into GCs in secondary responses10, 22, 43 may well be the CD73+ population.
Disclosures
The authors declare no conflict of interest.
Supporting information
Figures S1 and S2. Gating strategy for cells, as labelled.
Acknowledgements
LD and SLG carried out experiments, analysed data, and contributed equally to the manuscript. VB, SR and AG designed the experiments and supervised the study. AG helped with experiments, wrote the manuscript and drafted the figures. The work was supported by grants from the Department of Biotechnology, Ministry of Science and Technology, Government of India and Science and Engineering Research Board, Ministry of Science and Technology, Government of India (AG, SR VB). The National Institute of Immunology also received core funding from the Department of Biotechnology, Ministry of Science and Technology, Government of India. The authors acknowledge Dr P Nagarajan for providing mice, Ms Sarojini Minj for mouse screening, Mr Inderjit Singh for maintenance of mice and Mr K Rajesh Kumar for help in flow cytometry.
References
- 1. Slifka MK, Ahmed R. Long‐lived plasma cells: a mechanism for maintaining persistent antibody production. Curr Opin Immunol 1998; 10:252–8. [DOI] [PubMed] [Google Scholar]
- 2. McHeyzer‐Williams MG, Ahmed R. B cell memory and the long‐lived plasma cell. Curr Opin Immunol 1999; 11:172–9. [DOI] [PubMed] [Google Scholar]
- 3. Blink EJ, Light A, Kallies A, Nutt SL, Hodgkin PD, Tarlinton DM. Early appearance of germinal center‐derived memory B cells and plasma cells in blood after primary immunization. J Exp Med 2005; 201:545–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Nutt SL, Hodgkin PD, Tarlinton DM, Corcoran LM. The generation of antibody‐secreting plasma cells. Nat Rev Immunol 2015; 15:160–71. [DOI] [PubMed] [Google Scholar]
- 5. Ho F, Lortan JE, MacLennan IC, Khan M. Distinct short‐lived and long‐lived antibody‐producing cell populations. Eur J Immunol 1986; 16:1297–301. [DOI] [PubMed] [Google Scholar]
- 6. McHeyzer‐Williams LJ, Milpied PJ, Okitsu SL, McHeyzer‐Williams MG. Class‐switched memory B cells remodel BCRs within secondary germinal centers. Nat Immunol 2015; 16:296–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Jacob J, Kassir R, Kelsoe G. In situ studies of the primary immune response to (4‐hydroxy‐3‐nitrophenyl)acetyl. I. The architecture and dynamics of responding cell populations. J Exp Med 1991; 173:1165–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Toyama H, Okada S, Hatano M, Takahashi Y, Takeda N, Ichii H et al Memory B cells without somatic hypermutation are generated from Bcl6‐deficient B cells. Immunity 2002; 17:329–39. [DOI] [PubMed] [Google Scholar]
- 9. Taylor JJ, Pape KA, Jenkins MK. A germinal center‐independent pathway generates unswitched memory B cells early in the primary response. J Exp Med 2012; 209:597–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Pape KA, Taylor JJ, Maul RW, Gearhart PJ, Jenkins MK. Different B cell populations mediate early and late memory during an endogenous immune response. Science 2011; 331:1203–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kaji T, Ishige A, Hikida M, Taka J, Hijikata A, Kubo M et al Distinct cellular pathways select germline‐encoded and somatically mutated antibodies into immunological memory. J Exp Med 2012; 209:2079–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. de Vinuesa CG, Cook MC, Ball J, Drew M, Sunners Y, Cascalho M et al Germinal centers without T cells. J Exp Med 2000; 191:485–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Toellner K‐M, Jenkinson WE, Taylor DR, Khan M, Sze DM‐Y, Sansom DM et al Low‐level hypermutation in T cell‐independent germinal centers compared with high mutation rates associated with T cell‐dependent germinal centers. J Exp Med 2002; 195:383–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Gaspal FMC, McConnell FM, Kim M‐Y, Gray D, Kosco‐Vilbois MH, Raykundalia CR et al The generation of thymus‐independent germinal centers depends on CD40 but not on CD154, the T cell‐derived CD40‐ligand. Eur J Immunol 2006; 36:1665–73. [DOI] [PubMed] [Google Scholar]
- 15. El Shikh MEM, El Sayed RM, Szakal AK, Tew JG. T‐independent antibody responses to T‐dependent antigens: a novel follicular dendritic cell‐dependent activity. J Immunol Baltim Md 1950 2009; 182:3482–91. [DOI] [PubMed] [Google Scholar]
- 16. Dianda L, Gulbranson‐Judge A, Pao W, Hayday AC, MacLennan IC, Owen MJ. Germinal center formation in mice lacking αβ T cells. Eur J Immunol 1996; 26:1603–7. [DOI] [PubMed] [Google Scholar]
- 17. Wen L, Pao W, Wong FS, Peng Q, Craft J, Zheng B et al Germinal center formation, immunoglobulin class switching, and autoantibody production driven by “non α/β” T cells. J Exp Med 1996; 183:2271–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Chan TD, Gatto D, Wood K, Camidge T, Basten A, Brink R. Antigen affinity controls rapid T‐dependent antibody production by driving the expansion rather than the differentiation or extrafollicular migration of early plasmablasts. J Immunol Baltim Md 1950 2009; 183:3139–49. [DOI] [PubMed] [Google Scholar]
- 19. Schittek B, Rajewsky K. Natural occurrence and origin of somatically mutated memory B cells in mice. J Exp Med 1992; 176:427–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Anderson SM, Tomayko MM, Ahuja A, Haberman AM, Shlomchik MJ. New markers for murine memory B cells that define mutated and unmutated subsets. J Exp Med 2007; 204:2103–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Tomayko MM, Steinel NC, Anderson SM, Shlomchik MJ. Cutting edge: hierarchy of maturity of murine memory B cell subsets. J Immunol Baltim Md 1950 2010; 185:7146–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Dogan I, Bertocci B, Vilmont V, Delbos F, Mégret J, Storck S et al Multiple layers of B cell memory with different effector functions. Nat Immunol 2009; 10:1292–9. [DOI] [PubMed] [Google Scholar]
- 23. Good‐Jacobson KL, Szumilas CG, Chen L, Sharpe AH, Tomayko MM, Shlomchik MJ. PD‐1 regulates germinal center B cell survival and the formation and affinity of long‐lived plasma cells. Nat Immunol 2010; 11:535–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Chang S, Chen W, Yang J. Another formula for calculating the gene change rate in real‐time RT‐PCR. Mol Biol Rep 2009; 36:2165–8. [DOI] [PubMed] [Google Scholar]
- 25. Allman D, Pillai S. Peripheral B cell subsets. Curr Opin Immunol 2008; 20:149–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Nakano H, Yanagita M, Gunn MD. CD11c+ B220+ Gr‐1+ cells in mouse lymph nodes and spleen display characteristics of plasmacytoid dendritic cells. J Exp Med 2001; 194:1171–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Naradikian MS, Hao Y, Cancro MP. Age‐associated B cells: key mediators of both protective and autoreactive humoral responses. Immunol Rev 2016; 269:118–29. [DOI] [PubMed] [Google Scholar]
- 28. Kaku H, Cheng KF, Al‐Abed Y, Rothstein TL. A novel mechanism of B cell‐mediated immune suppression through CD73 expression and adenosine production. J Immunol Baltim Md 1950 2014; 193:5904–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Bhattacharya D, Cheah MT, Franco CB, Hosen N, Pin CL, Sha WC et al Transcriptional profiling of antigen‐dependent murine B cell differentiation and memory formation. J Immunol Baltim Md 1950 2007; 179:6808–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Richard K, Pierce SK, Song W. The agonists of TLR4 and 9 are sufficient to activate memory B cells to differentiate into plasma cells in vitro but not in vivo . J Immunol Baltim Md 1950 2008; 181:1746–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. George A, Cebra JJ. Responses of single germinal‐center B cells in T‐cell‐dependent microculture. Proc Natl Acad Sci U S A. 1991; 88:11–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Obukhanych TV, Nussenzweig MC. T‐independent type II immune responses generate memory B cells. J Exp Med 2006; 203:305–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Yates JL, Racine R, McBride KM, Winslow GM. T cell‐dependent IgM memory B cells generated during bacterial infection are required for IgG responses to antigen challenge. J Immunol Baltim Md 1950 2013; 191:1240–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Conter LJ, Song E, Shlomchik MJ, Tomayko MM. CD73 expression is dynamically regulated in the germinal center and bone marrow plasma cells are diminished in its absence. PLoS ONE 2014; 9:e92009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Deaglio S, Dwyer KM, Gao W, Friedman D, Usheva A, Erat A et al Adenosine generation catalyzed by CD39 and CD73 expressed on regulatory T cells mediates immune suppression. J Exp Med 2007; 204:1257–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Mori DN, Shen H, Galan A, Goldstein DR. Aged B cells alter immune regulation of allografts in mice. Eur J Immunol 2016; 46:2650–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Palm A‐KE, Friedrich HC, Kleinau S. Nodal marginal zone B cells in mice: a novel subset with dormant self‐reactivity. Sci Rep 2016; 6:27687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Iyer SS, Latner DR, Zilliox MJ, McCausland M, Akondy RS, Penaloza‐Macmaster P et al Identification of novel markers for mouse CD4+ T follicular helper cells. Eur J Immunol 2013; 43:3219–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Yanaba K, Bouaziz J‐D, Matsushita T, Tsubata T, Tedder TF. The development and function of regulatory B cells expressing IL‐10 (B10 cells) requires antigen receptor diversity and TLR signals. J Immunol Baltim Md 1950 2009; 182:7459–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Yang L, Kobie JJ, Mosmann TR. CD73 and Ly‐6A/E distinguish in vivo primed but uncommitted mouse CD4 T cells from type 1 or type 2 effector cells. J Immunol Baltim Md 1950 2005; 175:6458–64. [DOI] [PubMed] [Google Scholar]
- 41. Alugupalli KR, Leong JM, Woodland RT, Muramatsu M, Honjo T, Gerstein RM. B1b lymphocytes confer T cell‐independent long‐lasting immunity. Immunity 2004; 21:379–90. [DOI] [PubMed] [Google Scholar]
- 42. Krishnamurty AT, Thouvenel CD, Portugal S, Keitany GJ, Kim KS, Holder A et al Somatically hypermutated plasmodium‐specific IgM+ memory B cells are rapid, plastic, early responders upon malaria rechallenge. Immunity 2016; 45:402–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Weill J‐C, Le Gallou S, Hao Y, Reynaud C‐A. Multiple players in mouse B cell memory. Curr Opin Immunol 2013; 25:334–8. [DOI] [PubMed] [Google Scholar]
- 44. Zuccarino‐Catania GV, Sadanand S, Weisel FJ, Tomayko MM, Meng H, Kleinstein SH et al CD80 and PD‐L2 define functionally distinct memory B cell subsets that are independent of antibody isotype. Nat Immunol 2014; 15:631–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Good‐Jacobson KL, Shlomchik MJ. Plasticity and heterogeneity in the generation of memory B cells and long‐lived plasma cells: the influence of germinal center interactions and dynamics. J Immunol Baltim Md 1950 2010; 185:3117–25. [DOI] [PubMed] [Google Scholar]
- 46. Yamashita Y, Hooker SW, Jiang H, Laurent AB, Resta R, Khare K et al CD73 expression and fyn‐dependent signaling on murine lymphocytes. Eur J Immunol 1998; 28:2981–90. [DOI] [PubMed] [Google Scholar]
- 47. Bemark M, Bergqvist P, Stensson A, Holmberg A, Mattsson J, Lycke NY. A unique role of the cholera toxin A1‐DD adjuvant for long‐term plasma and memory B cell development. J Immunol Baltim Md 1950 2011; 186:1399–410. [DOI] [PubMed] [Google Scholar]
- 48. Good‐Jacobson KL, Song E, Anderson S, Sharpe AH, Shlomchik MJ. CD80 expression on B cells regulates murine T follicular helper development, germinal center B cell survival, and plasma cell generation. J Immunol Baltim Md 1950 2012; 188:4217–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Ramsburg E, Tigelaar R, Craft J, Hayday A. Age‐dependent requirement for γδ T cells in the primary but not secondary protective immune response against an intestinal parasite. J Exp Med 2003; 198:1403–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Déchanet J, Merville P, Lim A, Retière C, Pitard V, Lafarge X et al Implication of γδ T cells in the human immune response to cytomegalovirus. J Clin Invest 1999; 103:1437–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Sciammas R, Bluestone JA. TCR‐γδ cells and viruses. Microbes Infect 1999; 1:203–12. [DOI] [PubMed] [Google Scholar]
- 52. Dieli F, Troye‐Blomberg M, Farouk SE, Sireci G, Salerno A. Biology of γδ T cells in tuberculosis and malaria. Curr Mol Med 2001; 1:437–46. [DOI] [PubMed] [Google Scholar]
- 53. Airas L, Niemelä J, Jalkanen S. CD73 engagement promotes lymphocyte binding to endothelial cells via a lymphocyte function‐associated antigen‐1‐dependent mechanism. J Immunol Baltim Md 1950 2000; 165:5411–7. [DOI] [PubMed] [Google Scholar]
- 54. Eltzschig HK, Thompson LF, Karhausen J, Cotta RJ, Ibla JC, Robson SC et al Endogenous adenosine produced during hypoxia attenuates neutrophil accumulation: coordination by extracellular nucleotide metabolism. Blood 2004; 104:3986–92. [DOI] [PubMed] [Google Scholar]
- 55. Guiducci C, Valzasina B, Dislich H, Colombo MP. CD40/CD40L interaction regulates CD4+ CD25+ T reg homeostasis through dendritic cell‐produced IL‐2. Eur J Immunol 2005; 35:557–67. [DOI] [PubMed] [Google Scholar]
- 56. Song H, Cerny J. Functional heterogeneity of marginal zone B cells revealed by their ability to generate both early antibody‐forming cells and germinal centers with hypermutation and memory in response to a T‐dependent antigen. J Exp Med 2003; 198:1923–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Cekic C, Sag D, Day Y‐J, Linden J. Extracellular adenosine regulates naive T cell development and peripheral maintenance. J Exp Med 2013; 210:2693–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Flores‐Santibáñez F, Fernández D, Meza D, Tejón G, Vargas L, Varela‐Nallar L et al CD73‐mediated adenosine production promotes stem cell‐like properties in mouse Tc17 cells. Immunology 2015; 146:582–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Deenick EK, Chan A, Ma CS, Gatto D, Schwartzberg PL, Brink R et al Follicular helper T cell differentiation requires continuous antigen presentation that is independent of unique B cell signaling. Immunity 2010; 33:241–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Weinstein JS, Herman EI, Lainez B, Licona‐Limón P, Esplugues E, Flavell R et al TFH cells progressively differentiate to regulate the germinal center response. Nat Immunol 2016; 17:1197–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Schena F, Volpi S, Faliti CE, Penco F, Santi S, Proietti M et al Dependence of immunoglobulin class switch recombination in B cells on vesicular release of ATP and CD73 ectonucleotidase activity. Cell Rep 2013; 3:1824–31. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figures S1 and S2. Gating strategy for cells, as labelled.


