Summary
Stem cell antigen‐1 (Sca‐1/Ly6A/E) is a cell surface glycoprotein that is often used as a biomarker for stem cells and cell stemness. However, it is not clear what factors can directly induce the expression of Sca‐1/Ly6A/E in T lymphocytes in vivo, and if induction of Sca‐1 is associated with T cell stemness. In this study, we show that interleukin‐27 (IL‐27), a member of the IL‐12 family of cytokines, directly induces Sca‐1 expression in T cells in vivo. We found that mice‐deficient for IL‐27 (either P28 or EBI3) or its signalling (IL‐27Rα) had profound reduction of Sca‐1 expression in naive (CD62L+ CD44−), memory (CD62L+ CD44+) and effector (CD62L− CD44+) T cells. In contrast, in vivo delivery of IL‐27 using adeno‐associated viral vectors strongly induced the expression of Sca‐1 in naive and memory/effector T‐cell populations in an IL‐27 receptor‐ or signal transducer and activator of transcription 1‐dependent manner. Interestingly, IL‐27‐induced Sca‐1+ T cells do not express or up‐regulate classic stem cell‐associated genes such as Nanog, Oct4, Sox2 and Ctnnb1. However, IL‐27‐induced Sca‐1+ T cells had increased expression of effector/memory‐associated transcription factor T‐bet, Eomes and Blimp1. Hence, IL‐27 signalling directly induces the expression of Sca‐1/Ly6A/E expression in T cells. Direct expansion of Sca‐1+ CD62L+ CD44− T memory stem cells may explain why IL‐27 enhances T‐cell memory.
Keywords: interleukin‐27, stem cell antigen‐1, T‐cell memory
Introduction
Interleukin‐27 (IL‐27) is a member of the IL‐12 cytokine family that consists of two subunits, i.e. Epstein–Barr virus‐induced gene 3 (EBI3) and P28 (also known as IL‐30).1 IL‐27 is produced by activated antigen‐presenting cells such as dendritic cells and macrophages,2, 3, 4 and signals through a heterodimeric receptor (IL‐27R) consisting of the WSX‐1 and the gp130 subunits in a variety of cell types including T cells.5 IL‐27 activates both the signal transducer and activator of transcription 1 (Stat1) and Stat3 signalling cascade,6, 7 with the activation of Stat1 being dominant.8, 9, 10 Hence, IL‐27 has potent activity in regulating T helper types 1, 2 and 17, and FoxP3+ regulatory T cell responses.11, 12, 13 IL‐27 is also known to induce T‐cell expression of IL‐1014, 15, 16 and programmed death ligand 1 (PD‐L1),17 two inhibitory pathways that are associated with T‐cell tolerance.
In addition to the well‐appreciated anti‐inflammatory effects of IL‐27, we and others have shown that IL‐27 can also enhance T‐cell survival18, 19, 20 and promote T‐cell memory.18, 21, 22 Our in vitro analysis of IL‐27‐stimulated cytotoxic T lymphocytes revealed that IL‐27 induces T‐cell expression of stemness‐associated molecule Sca‐1/Ly6A.18 Sca‐1 is a cell surface glycoprotein that is expressed in all adult mouse haematopoietic stem cells23 and also in activated T cells.24 Haematopoietic stems cells deficient for Sca‐1 are defective25 and T lymphocytes deficient for Sca‐1 have altered proliferative responses.24 Recently, we also showed that Sca‐1 could be induced in T cells by in vivo delivery of IL‐27 using adeno‐associated viral (AAV) vectors.26 However, it remains unclear if IL‐27 directly induces Sca‐1 expression in T cells, and if its induction is associated with T‐cell stemness.
Previous studies have revealed a group of CD62L+ CD44− Sca‐1+ T cells that are termed as T memory stem cells (TSCM). TSCM cells are an early‐stage T memory subset that has robust proliferative potential, long‐term survival capacity and the ability to mediate superior tumour regression upon adoptive transfer into tumour‐bearing mice.27, 28 TSCM cells can be generated by programming naive T cells in the presence of glycogen synthase‐3β inhibitors27, 28 or cytokines such as IL‐1529 and IL‐21.30 It would therefore be interesting to determine if IL‐27 can induce the expansion of TSCM cells.
In this study, we have examined whether IL‐27 signalling directly induces Sca‐1 expression in T cells in vivo and if induction of Sca‐1 is associated with T‐cell stemness. We found that mice deficient for IL‐27 (either P28 or EBI3) or its receptor (IL‐27Rα) had profound reduction of Sca‐1 expression in both naive and memory T‐cell populations. In contrast, in vivo delivery of IL‐27 by AAV significantly induced the expression of Sca‐1 in naive and memory T‐cell populations in IL‐27 receptor‐ and Stat1‐dependent manners. Interestingly, IL‐27‐induced Sca‐1 expression is not associated with T‐cell stemness, as IL‐27‐stimulated T cells failed to up‐regulate traditionally stemness‐associated genes such as Nanog, Oct4, Sox2 and Ctnnb1. In vivo delivery of IL‐27 by AAV induced an effector/memory phenotype in T cells characterized by the expression of T‐bet, Eomes and Blimp1. Hence, IL‐27 signalling directly induces the expression of Sca‐1/Ly6A expression in T cells. Direct expansion of Sca‐1+ CD62L+ CD44− TSCM cells may explain why IL‐27 enhances T‐cell memory.
Materials and methods
Mice
C57BL/6 mice and IL‐27Rα −/− mice were purchased from the Jackson Laboratory (Bar Harbor, ME). EBI3‐deficient mice in the C57BL/631 and BALB/c background22 have been described. C57BL/6 mice with a targeted mutation of the P28 gene (IL‐27P28−/−)13 were obtained from Dr Daniel J. Cua via a material transfer agreement. Stat1‐deficient BALB/c mice32 have been described previously. All mice were maintained in OSU laboratory animal facilities that were fully accredited by Institutional Animal Care and Use Committee.
Antibodies and flow cytometry
Fluorescein isothiocyanate‐, phycoerythrin‐, allophycocyanin‐ or Peridinin chlorophyll protein‐labelled antibodies to CD4, CD8a, CD44, CD62L, PD‐L1, Sca‐1 and isotype control antibodies were purchased from BD Biosciences (San Diego, CA). For staining of cell surface markers, cells (single‐cell suspensions of spleen) were stained with various antibodies in staining buffer (PBS with 1% fetal calf serum) on ice for 30 min, after washing with staining buffer, cells were fixed in 1% paraformaldehyde in PBS and were analysed on a FACSCalibur flow cytometer. Data were analysed using flowjo software (Tree Star, Inc., Ashland, OR).
Production of adeno‐associated viral vectors and mice treatment
We used rAAV vector to express IL‐27 and IL‐30 in vivo. The IL‐30 expression plasmids33 were obtained from Dr Shulin Li (MD Anderson Cancer Center). Briefly, IL‐27 or IL‐30 cDNA was inserted into an AAV carrier vector under the control of the cytomegalovirus‐chicken β‐actin hybrid (CAG) promoter.34, 35 The IL‐27 or IL‐30 carrier AAV vector was compacted with a helper vector in 293K cells into the AAV serotype 8 (AAV8). AAV8 is known to be particularly suitable for expression in muscle cells.36, 37 Intramuscular injection of 2 × 1011 DNAse resistant particle (DRP)/mouse of AAV‐IL‐27 or AAV‐IL‐30 achieved high concentrations of IL‐27 or IL‐30 production in the peripheral blood of mice. Hence, we evaluated the in vivo effects of AAV‐IL‐27 and AAV‐IL‐30 on T‐cell activation in the context of a concanavalin A‐induced liver injury model.
ELISA
Blood was drawn from mice treated with AAV‐IL‐27, AAV‐IL‐30 and AAV‐ctrl vectors at 2 weeks after viral injection. Serum was investigated for the presence of IL‐27 and IL‐30 using ELISA kits purchased from eBioscience (San Diego, CA) for IL‐27 and R&D Systems, Inc. (Minneapolis, MN) for IL‐30.
Real‐time PCR
Quantitative real‐time PCR was performed using an ABI 7900‐HT sequence system (PE Applied Biosystems, Foster City, CA) with the QuantiTect SYBR Green PCR kit (Qiagen, Hilden, Germany) in accordance with the manufacturer's instructions. PCR was performed using previously determined conditions.38 The following primers were used for amplifying specific genes: Actin: 5′‐GAGACCTTCAACACCCCAGC‐3′ (forward) and 5′‐ATGTCACGCACGATTTCCC‐3′ (reverse); Bcl2: 5′‐TGCGGAGGAAGTAGACTGATA‐3′ (forward) and 5′‐TGGCATGAGATGCAGGAAA‐3′ (reverse); Bcl6: 5′‐CATACAGAGATGTGCCTCCATAC‐3′ (forward) and 5′‐CCCATTCTCACAGCTAGAATCC‐3′ (reverse); Blimp1: 5′‐TCTACCCTCGGGTGGTTTAT‐3′ (forward) and 5′‐TGAGTTATGTAGGTGGGTCTCT‐3′ (reverse); Ctnnb1: 5′‐ GCTGCTCATCCCACTAATGT‐3′ (forward) and 5′‐CCGCGTCATCCTGATAGTTAAT‐3′ (reverse); Eomes: 5′‐CGTTCACCCAGAATCTCCTAAC‐3′ (forward) and 5′‐GCAGAGACTGCAACACTATCA‐3′ (reverse); Foxo1: 5′‐CGTGCCCTACTTCAAGGATAAG‐3′ (forward) and 5′‐GCACTCGAATAAACTTGCTGTG‐3′ (reverse); ID2: 5′‐CTACTCCAAGCTCAAGGAACTG‐3′ (forward) and 5′‐GATCTGCAGGTCCAAGATGTAA‐3′ (reverse); ID3: 5′‐AGACTACATCCTCGACCTTCA‐3′ (forward) and 5′‐GATCACAAGTTCCGGAGTGAG‐3′ (reverse); Klf4: 5′‐CCCTTCGGTCATCAGTGTTAG‐3′ (forward) and 5′‐GGACCGCCTCTTGCTTAAT‐3′ (reverse); Lef1: 5′‐AGAACACCCTGATGAAGGAAAG‐3′ (forward) and 5′‐GTACGGGTCGCTGTTCATATT‐3′ (reverse); Nanog: 5′‐GGCAGCCCTGATTCTTCTAC‐3′ (forward) and 5′‐GAGAACACAGTCCGCATCTT‐3′ (reverse); NFATc1: 5′‐CCGTCCAAGTCAGTTTCTATGT‐3′ (forward) and 5′‐GTCCGTGGGTTCTGTCTTTAT‐3′ (reverse); Oct4: 5′‐CCTACAGCAGATCACTCACATC‐3′ (forward) and 5′‐GCCGGTTACAGAACCATACTC‐3′ (reverse); Stat4: 5′‐GAAGTGCAGTACTGGGAGTAAA‐3′ (forward) and 5′‐GGTTAATGGTGAGGCCATAGAG‐3′ (reverse); Sox2: 5′‐TGAACGCCTTCATGGTATGG‐3′ (forward) and 5′‐GATCTCCGAGTTGTGCATCTT‐3′ (reverse); TCF1: 5′‐CCTTGGTGGAGGAGTGTAATAG‐3′ (forward) and 5′‐GTTGGCAAACCAGTTGTAGAC‐3′ (reverse); T‐bet: 5′‐CCAGGGAACCGCTTATATGT‐3′ (forward) and 5′‐CCTTGTTGTTGGTGAGCTTTAG‐3′ (reverse). Each sample was assayed in triplicate and the experiments were repeated twice. The relative gene expression was calculated by plotting the Ct (cycle number) and the average relative expression for each group was determined using the comparative method (2−ΔΔCt).
Statistics
Data are expressed as means of individual determinations ± SE. Statistical analysis was performed using the unpaired Student's t‐test.
Results
Reduced Sca‐1/Ly6A expression in T cells in IL‐27 and IL‐27 receptor‐deficient mice
Previous studies18, 21, 22 have revealed that IL‐27 contributes to T‐cell memory, and expression of Sca‐1/Ly6A, a cell surface glycoprotein, is considered to be a biomarker for TSCM.28 We therefore examined if the lack of IL‐27 or IL‐27 receptor signalling affected T‐cell expression of Sca‐1 in naive and memory T‐cell populations. In the peripheral lymphoid organs of naive mice, T cells can be sub‐divided into three populations based on their expression of CD62L and CD44, i.e. CD62L+ CD44− (naive), CD62L+ CD44+ (central memory) and CD62L− CD44+ (effector memory) T cells. As shown in Fig. 1(a), CD4+ and CD8+ T cells from IL‐27P28‐deficient and wild‐type B6 mice were subdivided into three populations based on their expression of CD62L and CD44. We found that CD4+ and CD8+ T cells and their subpopulations from IL‐27P28−/− mice had significantly reduced expression of Sca‐1, and the reduction of Sca‐1 expression was particularly significant among memory T‐cell populations in both CD4+ and CD8+ T cells (Fig. 1b,c). Similarly, CD4+ and CD8+ T cells from IL‐27EBI3−/− and wild‐type mice were also subdivided into three populations based on their expression of CD62L and CD44 (Fig. 2a). We observed reduced Sca‐1 expression in all subpopulations of CD8+ T cells in IL‐27EBI3−/− mice, whereas a trend of Sca‐1 reduction in CD4+ T cells from IL‐27EBI3−/− mice was also observed (Fig. 2b,c). Finally, the T‐cell subpopulations in IL‐27Rα −/− mice (Fig. 3a) were also analysed in a similar manner. Significant reduction of Sca‐1/Ly6A expression was observed in all subpopulations of CD4+ and CD8+ T cells in IL‐27Rα −/− mice (Fig. 3b,c). Hence, IL‐27‐IL‐27R signalling appears to be required for the expression of Sca‐1/Ly6A in both naive and memory/effector T cells under steady state.
Figure 1.
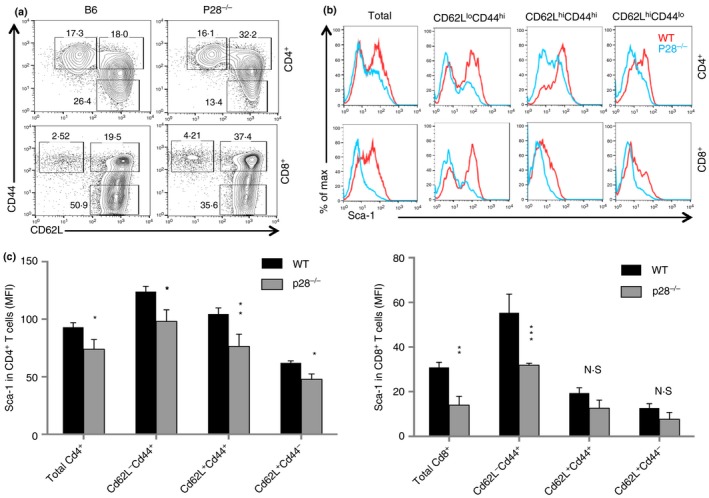
IL‐27P28‐deficient mice had a reduced Sca‐1+ memory pool of T cells. Splenocytes from naive wild‐type (WT) B6 and P28−/− mice were analysed by flow cytometry. Spleen CD4+ and CD8+ T cells and their subsets (a), based on the expression of CD62L and CD44, were analysed for the expression of Sca‐1 (b). Sca‐1 expression in CD4+ and CD8+ T cells and their subsets were quantified (c). Three to five mice were used in each group for this experiment. Data are expressed as means of individual determinations ± SE and represent three experiments using both male and female mice. Statistical analysis was performed using the unpaired Student's t‐test. *P < 0·05; **P < 0·01; ***P < 0·001. N.S., not significant.
Figure 2.
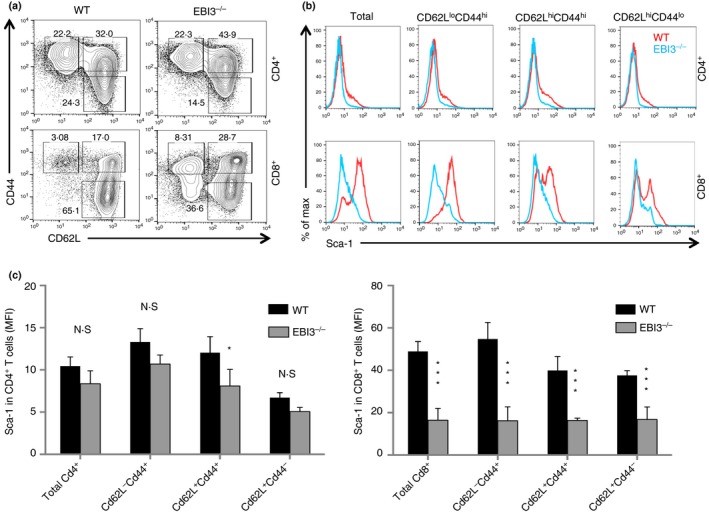
IL‐27EBI3‐deficient mice had a reduced Sca‐1+ memory pool of T cells. Splenocytes from naive wild‐type (WT) and EBI3−/− mice were analysed by flow cytometry. Spleen CD4+ and CD8+ T cells and their subsets (a) were analysed for the expression of Sca‐1 (b). Sca‐1+ T cells in CD4+ and CD8+ T cells and their subsets were quantified (c). At least three mice were used in each group for this experiment. Data are expressed as means of individual determinations ± SE and represent three experiments using both male and female mice. Statistical analysis was performed using the unpaired Student's t‐test. *P < 0·05; ***P < 0·001. N.S., not significant.
Figure 3.
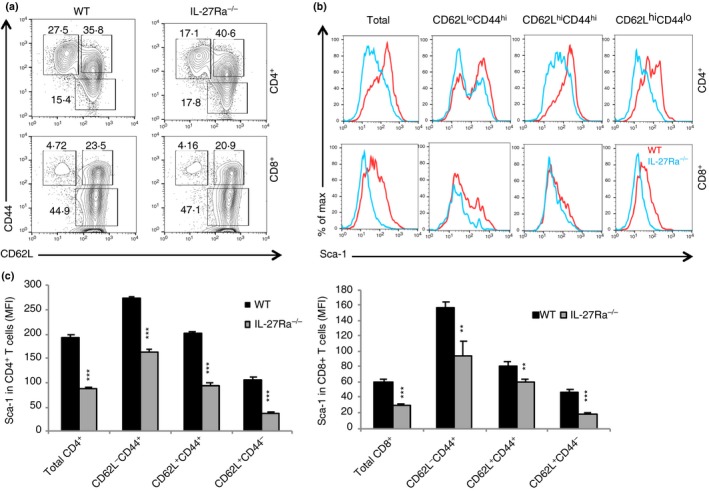
Interleukin‐27 receptor α (IL‐27Rα) ‐deficient mice had a reduced Sca‐1+ memory pool of T cells. Splenocytes from naive wild‐type (WT) B6 and IL‐27Rα −/− mice were analysed by flow cytometry. Spleen CD4+ and CD8+ T cells and their subsets (a) were analysed for the expression of Sca‐1 (b). Sca‐1 expression in CD4+ and CD8+ T cells and their subsets was quantified (c). Four to five mice were used in each group for this experiment. Data are expressed as means of individual determinations ± SE and represent two experiments. Statistical analysis was performed using the unpaired Student's t‐test. **P < 0·01; ***P < 0·001.
IL‐27 directly induce Sca‐1/Ly6A in T cells in an IL‐27R‐ or Stat1‐dependent manner
We previously showed that cultured T cells stimulated with IL‐27 up‐regulated Sca‐1/Ly6A.18 We also observed that T cells in AAV‐IL‐27‐treated mice had increased expression of Sca‐1/Ly6A.26 To determine if IL‐27 directly induces Sca‐1 expression in T cells in vivo, we generated AAV‐IL‐27, AAV‐IL‐30 (IL‐27P28) or AAV‐Ctrl viral vectors and injected the vectors intramuscularly into wild‐type mice. As shown in Fig. 4(a), 2 weeks after the injection of viral vectors, significant levels of IL‐27 or IL‐30 were detected only in the serum from mice receiving respective AAV vectors. Multi‐coloured flow cytometry analysis was used to determine the expression of Sca‐1. As shown in Fig. 4(b) and summarized in Fig. 4(c,d), T cells from AAV‐IL‐27, but not AAV‐IL‐30 or AAV‐Ctrl viral vector‐treated mice had significantly up‐regulated expression of Sca‐1, essentially in all subpopulations. These results confirm that IL‐27, but not its subunit P28 (IL‐30), induces Sca‐1 expression in all T‐cell subpopulations in vivo. Moreover, we found that AAV‐IL‐27‐induced Sca‐1 expression was IL‐27R and Stat1‐dependent, as AAV‐IL‐27‐induced Sca‐1 expression was only detected in T cells from wild‐type, but not IL‐27Rα (Fig. 5a) and Stat1‐deficient mice (Fig. 5b).
Figure 4.
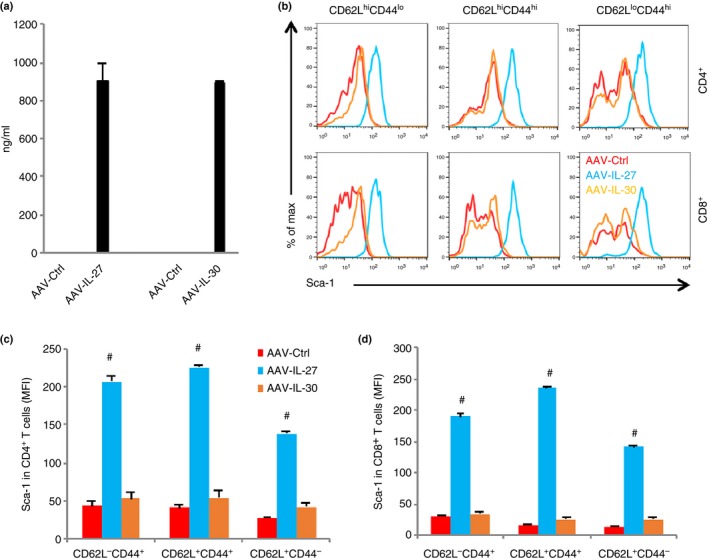
Adeno‐associated virus (AAV) vector‐delivered interleukin‐27 (IL‐27) induces Sca‐1 expression in T cells. (a) Wild‐type (WT) mice were injected with AAV‐ctrl, AAV‐IL‐27 or AAV‐IL‐30 viral vectors intramuscularly at a dose of 2 × 1011 DNAse resistant particle (DRP)/mouse, and serum IL‐27 or IL‐30 levels were measured by ELISA 2 weeks later. Spleen CD4+ and CD8+ T cells and their subsets were analysed for the expression of Sca‐1 (b). Sca‐1 expression in CD4+ (c) and CD8+ (d) subsets was quantified. Three mice were used in each group for this experiment. Data are expressed as means of individual determinations ± SE and represent three independent experiments using both male and female mice. Statistical analysis was performed using the unpaired Student's t‐test. #P < 1 × 10−5.
Figure 5.
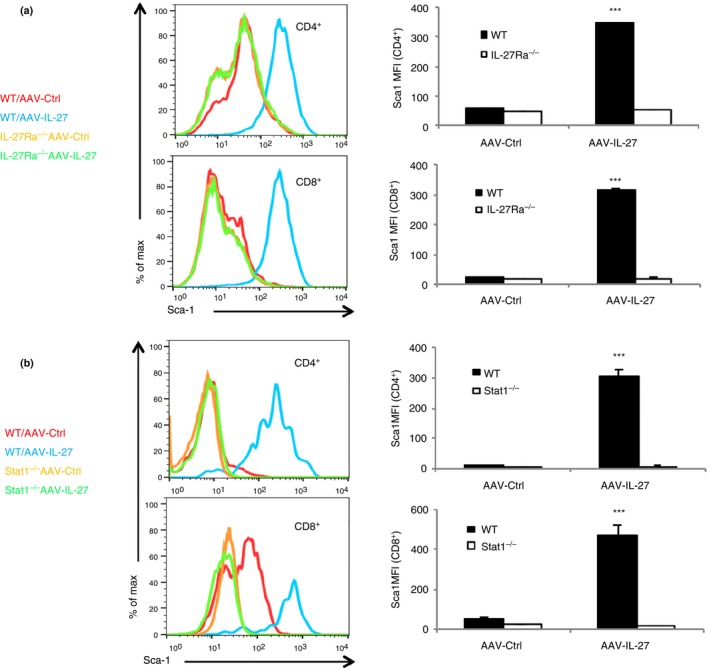
Adeno‐associated virus–interleukin‐27 (AAV‐IL‐27) ‐induced Sca‐1 expression in T cells is interleukin‐27 receptor (IL‐27R) and signal transducer and activator of transcription 1 (Stat1) ‐dependent. (a) IL‐27Rα −/− and wild‐type (WT) mice were treated with AAV‐IL‐27 or AAV‐Ctrl vectors. Two weeks later, the expression of Sca‐1 in spleen was examined by flow cytometry and mean fluorescence intensity (MFI) of Sca‐1 was quantified. (b) Stat1−/− and WT mice were treated with AAV‐IL‐27 or AAV‐Ctrl vectors. Two weeks later, the expression of Sca‐1 in spleen was examined by flow cytometry and MFI of Sca‐1 was quantified. Three to five mice were used in each group for the experiments. Data are expressed as means of individual determinations ± SE and represent two independent experiments using both male and female mice. Statistical analysis was performed using the unpaired Student's t‐test. ***P < 1 × 10−3.
Transcription factors in IL‐27‐stimulated Sca‐1+ T cells
To understand the phenotypes of AAV‐IL‐27‐induced Sca‐1+ T cells, we sorted CD4+ and CD8+ T cells from AAV‐IL‐27 and AAV‐ctrl virus‐treated mice by FACS, and used quantitative PCR to measure T‐cell differentiations and stemness‐associated transcription factors. As shown in Fig. 6, AAV‐IL‐27 treatment significantly up‐regulated the expression of T‐bet, Eomes and Blimp1, transcription factors typically expressed by effector memory T cells.39 We failed to detect the expression of stem cell‐associated transcription factors40 such as Nanog, Oct4 and Sox2, in T cells from either AAV‐IL‐27 or AAV‐ctrl virus‐treated mice. Induction of Ctnnb1 (β‐catenin) has been shown to be associated with T‐cell stemness.28 However, we found that Ctnnb1 was slightly reduced in T cells from AAV‐IL‐27‐treated mice. Finally, Klf4 up‐regulation was found in CD4+, but not CD8+, T cells from AAV‐IL‐27‐treated mice. Hence, IL‐27‐induced Sca‐1+ T cells express transcription factors of effector memory T cells but do not show many features of typical stem cells.
Figure 6.
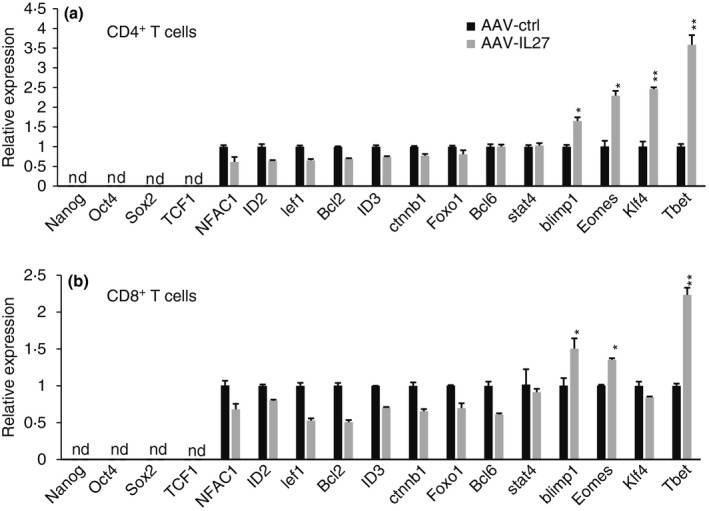
Expression of transcription factors and stemness genes in T cells from Adeno‐associated virus–interleukin‐27 (AAV‐IL‐27) and AAV‐ctrl‐treated mice. C57BL/6 mice were treated with AAV‐ctrl or AAV‐IL‐27 viral vectors intramuscularly at a dose of 2 × 1011 DNAse resistant particle (DRP)/mouse. Two weeks later CD4+ and CD8+ T cells were sorted from the spleens by FACS. Total RNA was prepared from purified T cells and quantitative PCR was used to quantify transcription factors associated with T‐cell differentiation and stemness in CD4+ (a) and CD8+ T cells (b). Samples were pooled from three mice in each group. Data are expressed as means of individual determinations ± SE of three replicates, and represent two independent experiments. Statistical analysis was performed using the unpaired Student's t‐test. *P < 0·05; **P < 0·01. nd, not detectable.
Discussion
In this study, we have found that IL‐27 directly induces Sca‐1 expression in naive and memory T cells in vivo. In IL‐27 and IL‐27 receptor‐deficient mice, Sca‐1 expression is greatly reduced in all three populations of T cells, i.e. CD62L+ CD44− naive, CD62L+ CD44+ central memory and CD62L− CD44+ effector memory T cells. In contrast, in mice treated with AAV‐IL‐27, Sca‐1 expression is greatly increased in naive, central memory and effector memory T cells, and AAV‐IL‐27‐induced Sca‐1 expression is IL‐27R‐ and Stat1‐dependent. Hence, our results suggest that IL‐27 signalling directly induces the expression of Sca‐1/Ly6A/E in T cells in vivo.
Demoulin et al.41 showed that IL‐6 and IL‐9 induced the expression of Sca‐1 in T lymphoma cells and mature T lymphocytes in vitro. They found that both IL‐6 and IL‐9 mediated the transcriptional activation of Sca‐1 through a GAS element in the Sca‐1 promotor, which was able to bind Stat1 and Stat3. In this study, we found that IL‐27 signalling induced Sca‐1 expression in vivo exclusively through Stat1 (Fig. 5b). Hence, it is likely that IL‐27‐induced activation of Stat1 directly binds to GAS element in the Sca‐1 promoter, leading to the expression of Sca‐1 in T cells.
Sca‐1/Ly6A/E is a cell surface glycoprotein that is expressed in all adult mouse haematopoietic stem cells23 and has been shown to be necessary for haematopoietic stem cell self‐renewal and the development of committed progenitor cells.25 Sca‐1 was also shown to play a role in c‐kit expression in haematopoietic stem cells42 and in haematopoietic commitment to granulocyte development.43 However, our current results suggest that IL‐27‐induced Sca‐1 expression does not appear to be associated with stemness of mature T cells, as the classic transcription factors associated with cell stemness, including Nanog, Oct4 and Sox2, were undetectable in Sca‐1+ T cells from AAV‐IL‐27‐treated mice (Fig. 6), whereas Klf4 expression was only found to be elevated in CD4+ but not CD8+ T cells. Induction of Ctnnb1 (β‐catenin) has also been shown to be associated with T‐cell stemness.28 However, we found that Ctnnb1 was slightly reduced in T cells from AAV‐IL‐27‐treated mice (Fig. 6). Hence, IL‐27‐induced Sca‐1+ T cells do not show many features of conventional stem cells. These results suggest that Sca‐1 expression in haematopoietic stem cells and mature T lymphocytes play different roles.
Although IL‐27‐induced Sca‐1+ T cells do not express many stem cell‐associated genes, they have elevated expression of memory T‐cell‐associated molecules such as Eomes, which have been shown to promote T‐cell memory.44, 45 In this study, we have found that IL‐27 induces Sca‐1 expression in all three populations of T cells, i.e. CD62L+ CD44− naive, CD62L+ CD44+ central memory and CD62L− CD44+ effector memory T cells. Although the significance for induction of Sca‐1 in central memory and effector memory T cells remains to be studied, Gattinoni et al. 27, 28 have identified a new subset of CD62L+ CD44− Sca‐1+ TSCM. They have shown that TSCM cells can be generated in vitro by programming naive T cells in the presence of small molecules such as glycogen synthase‐3β inhibitors27, 28 and cytokines such as IL‐1529 and IL‐21.30 Our results provide the first evidence that IL‐27 signalling induces the expansion of CD62L+ CD44− Sca‐1+ TSCM cells in vivo. These findings, taken together, explain why IL‐27 promotes T‐cell memory.18, 21, 22 However, these results do not suggest that Sca‐1 itself is important for T‐cell memory, as Sca‐1‐deficient mice have normal T‐cell memory responses.46
Taken together, we have found that IL‐27 signalling can directly induce Sca‐1/Ly6A/E expression in naive and memory populations of T cells. However, IL‐27‐induced Sca‐1+ T cells do not express the classic transcription factors for stemness. IL‐27 induces the expansion of a memory pool of T cells including TSCM cells. Given that IL‐27 may potentially be used as a therapeutic for cancer47 and autoimmune diseases,48 identification of Sca‐1/Ly6A/E as an IL‐27‐responsive biomarker in T cells in vivo may potentially be useful for determining therapeutic response in pre‐clinical settings.
Author contributions
ZL, LW, JZ, XZ and JZ performed experiments; JQL, JZ and JPD produced AAV viruses; SV and ARS provided Stat1 knockout mice and helped in designing the experiments; JZ, MSL and XFB designed experiments and generated funding support for this study. XFB wrote the manuscript.
Disclosures
The authors have no conflicts of interest to disclose.
Acknowledgements
This study was supported in part by a grant from the National Cancer Institute (R21CA198037) and a grant from China Natural Science Foundation (81572805). The authors declare no financial or commercial conflict of interest.
Contributor Information
Ming‐Song Li, Email: lims@smu.edu.cn.
Xue‐Feng Bai, Email: Xue-Feng.Bai@osumc.edu.
References
- 1. Pflanz S, Timans JC, Cheung J, Rosales R, Kanzler H, Gilbert J et al IL‐27, a heterodimeric cytokine composed of EBI3 and p28 protein, induces proliferation of naive CD4+ T cells. Immunity 2002; 16:779–90. [DOI] [PubMed] [Google Scholar]
- 2. Murugaiyan G, Mittal A, Weiner HL. Identification of an IL‐27/osteopontin axis in dendritic cells and its modulation by IFN‐γ limits IL‐17‐mediated autoimmune inflammation. Proc Natl Acad Sci USA 2010; 107:11495–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Pirhonen J, Siren J, Julkunen I, Matikainen S. IFN‐α regulates Toll‐like receptor‐mediated IL‐27 gene expression in human macrophages. J Leukoc Biol 2007; 82:1185–92. [DOI] [PubMed] [Google Scholar]
- 4. Remoli ME, Gafa V, Giacomini E, Severa M, Lande R, Coccia EM. IFN‐β modulates the response to TLR stimulation in human DC: involvement of IFN regulatory factor‐1 (IRF‐1) in IL‐27 gene expression. Eur J Immunol 2007; 37:3499–508. [DOI] [PubMed] [Google Scholar]
- 5. Pflanz S, Hibbert L, Mattson J, Rosales R, Vaisberg E, Bazan JF et al WSX‐1 and glycoprotein 130 constitute a signal‐transducing receptor for IL‐27. J Immunol 2004; 172:2225–31. [DOI] [PubMed] [Google Scholar]
- 6. Hibbert L, Pflanz S, De Waal Malefyt R, Kastelein RA. IL‐27 and IFN‐α signal via Stat1 and Stat3 and induce T‐Bet and IL‐12Rβ2 in naive T cells. J Interferon Cytokine Res 2003; 23:513–22. [DOI] [PubMed] [Google Scholar]
- 7. Hunter CA. New IL‐12‐family members: IL‐23 and IL‐27, cytokines with divergent functions. Nat Rev Immunol 2005; 5:521–31. [DOI] [PubMed] [Google Scholar]
- 8. Neufert C, Becker C, Wirtz S, Fantini MC, Weigmann B, Galle PR et al IL‐27 controls the development of inducible regulatory T cells and Th17 cells via differential effects on STAT1. Eur J Immunol 2007; 37:1809–16. [DOI] [PubMed] [Google Scholar]
- 9. Takeda A, Hamano S, Yamanaka A, Hanada T, Ishibashi T, Mak TW et al Cutting edge: role of IL‐27/WSX‐1 signaling for induction of T‐bet through activation of STAT1 during initial Th1 commitment. J Immunol 2003; 170:4886–90. [DOI] [PubMed] [Google Scholar]
- 10. Kamiya S, Owaki T, Morishima N, Fukai F, Mizuguchi J, Yoshimoto T. An indispensable role for STAT1 in IL‐27‐induced T‐bet expression but not proliferation of naive CD4+ T cells. J Immunol 2004; 173:3871–7. [DOI] [PubMed] [Google Scholar]
- 11. Hall AO, Silver JS, Hunter CA. The immunobiology of IL‐27. Adv Immunol 2012; 115:1–44. [DOI] [PubMed] [Google Scholar]
- 12. Lucas S, Ghilardi N, Li J, de Sauvage FJ. IL‐27 regulates IL‐12 responsiveness of naive CD4+ T cells through Stat1‐dependent and ‐independent mechanisms. Proc Natl Acad Sci USA 2003; 100:15047–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Diveu C, McGeachy MJ, Boniface K, Stumhofer JS, Sathe M, Joyce‐Shaikh B et al IL‐27 blocks RORc expression to inhibit lineage commitment of Th17 cells. J Immunol 2009; 182:5748–56. [DOI] [PubMed] [Google Scholar]
- 14. Stumhofer JS, Silver JS, Laurence A, Porrett PM, Harris TH, Turka LA et al Interleukins 27 and 6 induce STAT3‐mediated T cell production of interleukin 10. Nat Immunol 2007; 8:1363–71. [DOI] [PubMed] [Google Scholar]
- 15. Awasthi A, Carrier Y, Peron JP, Bettelli E, Kamanaka M, Flavell RA et al A dominant function for interleukin 27 in generating interleukin 10‐producing anti‐inflammatory T cells. Nat Immunol 2007; 8:1380–9. [DOI] [PubMed] [Google Scholar]
- 16. Fitzgerald DC, Ciric B, Touil T, Harle H, Grammatikopolou J, Das Sarma J et al Suppressive effect of IL‐27 on encephalitogenic Th17 cells and the effector phase of experimental autoimmune encephalomyelitis. J Immunol 2007; 179:3268–75. [DOI] [PubMed] [Google Scholar]
- 17. Hirahara K, Ghoreschi K, Yang XP, Takahashi H, Laurence A, Vahedi G et al Interleukin‐27 priming of T cells controls IL‐17 production in trans via induction of the ligand PD‐L1. Immunity 2012; 36:1017–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Liu Z, Liu JQ, Talebian F, Wu LC, Li S, Bai XF. IL‐27 enhances the survival of tumor antigen‐specific CD8+ T cells and programs them into IL‐10‐producing, memory precursor‐like effector cells. Eur J Immunol 2013; 43:468–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kim G, Shinnakasu R, Saris CJ, Cheroutre H, Kronenberg M. A novel role for IL‐27 in mediating the survival of activated mouse CD4 T lymphocytes. J Immunol 2013; 190:1510–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Harker JA, Dolgoter A, Zuniga EI. Cell‐intrinsic IL‐27 and gp130 cytokine receptor signaling regulates virus‐specific CD4+ T cell responses and viral control during chronic infection. Immunity 2013; 39:548–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Pennock ND, Gapin L, Kedl RM. IL‐27 is required for shaping the magnitude, affinity distribution, and memory of T cells responding to subunit immunization. Proc Natl Acad Sci USA 2014; 111:16472–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Liu Z, Liu JQ, Shi Y, Zhu X, Liu Z, Li MS et al Epstein–Barr virus‐induced gene 3‐deficiency leads to impaired antitumor T‐cell responses and accelerated tumor growth. Oncoimmunology 2015; 4:e989137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ma X, Robin C, Ottersbach K, Dzierzak E. The Ly‐6A (Sca‐1) GFP transgene is expressed in all adult mouse hematopoietic stem cells. Stem Cells 2002; 20:514–21. [DOI] [PubMed] [Google Scholar]
- 24. Stanford WL, Haque S, Alexander R, Liu X, Latour AM, Snodgrass HR et al Altered proliferative response by T lymphocytes of Ly‐6A (Sca‐1) null mice. J Exp Med 1997; 186:705–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ito CY, Li CY, Bernstein A, Dick JE, Stanford WL. Hematopoietic stem cell and progenitor defects in Sca‐1/Ly‐6A‐null mice. Blood 2003; 101:517–23. [DOI] [PubMed] [Google Scholar]
- 26. Zhu X, Liu Z, Liu JQ, Zhu J, Zhang J, Davis JP et al Systemic delivery of IL‐27 by an adeno‐associated viral vector inhibits T cell‐mediated colitis and induces multiple inhibitory pathways in T cells. J Leukoc Biol 2016; 100:403–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Gattinoni L, Lugli E, Ji Y, Pos Z, Paulos CM, Quigley MF et al A human memory T cell subset with stem cell‐like properties. Nat Med 2011; 17:1290–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Gattinoni L, Zhong XS, Palmer DC, Ji Y, Hinrichs CS, Yu Z et al Wnt signaling arrests effector T cell differentiation and generates CD8+ memory stem cells. Nat Med 2009; 15:808–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Cieri N, Camisa B, Cocchiarella F, Forcato M, Oliveira G, Provasi E et al IL‐7 and IL‐15 instruct the generation of human memory stem T cells from naive precursors. Blood 2013; 121:573–84. [DOI] [PubMed] [Google Scholar]
- 30. Hinrichs CS, Spolski R, Paulos CM, Gattinoni L, Kerstann KW, Palmer DC et al IL‐2 and IL‐21 confer opposing differentiation programs to CD8+ T cells for adoptive immunotherapy. Blood 2008; 111:5326–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Nieuwenhuis EE, Neurath MF, Corazza N, Iijima H, Trgovcich J, Wirtz S et al Disruption of T helper 2‐immune responses in Epstein–Barr virus‐induced gene 3‐deficient mice. Proc Natl Acad Sci USA 2002; 99:16951–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Roth KM, Gunn JS, Lafuse W, Satoskar AR. Francisella inhibits STAT1‐mediated signaling in macrophages and prevents activation of antigen‐specific T cells. Int Immunol 2009; 21:19–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Dibra D, Cutrera J, Xia X, Kallakury B, Mishra L, Li S. Interleukin‐30: a novel antiinflammatory cytokine candidate for prevention and treatment of inflammatory cytokine‐induced liver injury. Hepatology 2012; 55:1204–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Liu Y, Okada T, Nomoto T, Ke X, Kume A, Ozawa K et al Promoter effects of adeno‐associated viral vector for transgene expression in the cochlea in vivo . Exp Mol Med 2007; 39:170–5. [DOI] [PubMed] [Google Scholar]
- 35. Song S, Laipis PJ, Berns KI, Flotte TR. Effect of DNA‐dependent protein kinase on the molecular fate of the rAAV2 genome in skeletal muscle. Proc Natl Acad Sci USA 2001; 98:4084–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Wang Z, Zhu T, Qiao C, Zhou L, Wang B, Zhang J et al Adeno‐associated virus serotype 8 efficiently delivers genes to muscle and heart. Nat Biotechnol 2005; 23:321–8. [DOI] [PubMed] [Google Scholar]
- 37. Qiao C, Li J, Jiang J, Zhu X, Wang B, Xiao X. Myostatin propeptide gene delivery by adeno‐associated virus serotype 8 vectors enhances muscle growth and ameliorates dystrophic phenotypes in mdx mice. Hum Gene Ther 2008; 19:241–54. [DOI] [PubMed] [Google Scholar]
- 38. Bai XF, Liu J, Li O, Zheng P, Liu Y. Antigenic drift as a mechanism for tumor evasion of destruction by cytolytic T lymphocytes. J Clin Invest 2003; 111:1487–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Kaech SM, Cui W. Transcriptional control of effector and memory CD8+ T cell differentiation. Nat Rev Immunol 2012; 12:749–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 2006; 126:663–76. [DOI] [PubMed] [Google Scholar]
- 41. Demoulin JB, Maisin D, Renauld JC. Ly‐6A/E induction by interleukin‐6 and interleukin‐9 in T cells. Eur Cytokine Netw 1999; 10:49–56. [PubMed] [Google Scholar]
- 42. Bradfute SB, Graubert TA, Goodell MA. Roles of Sca‐1 in hematopoietic stem/progenitor cell function. Exp Hematol 2005; 33:836–43. [DOI] [PubMed] [Google Scholar]
- 43. Shi X, Siggins RW, Stanford WL, Melvan JN, Basson MD, Zhang P. Toll‐like receptor 4/stem cell antigen 1 signaling promotes hematopoietic precursor cell commitment to granulocyte development during the granulopoietic response to Escherichia coli bacteremia. Infect Immun 2013; 81:2197–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Intlekofer AM, Takemoto N, Wherry EJ, Longworth SA, Northrup JT, Palanivel VR et al Effector and memory CD8+ T cell fate coupled by T‐bet and eomesodermin. Nat Immunol 2005; 6:1236–44. [DOI] [PubMed] [Google Scholar]
- 45. Laidlaw BJ, Cui W, Amezquita RA, Gray SM, Guan T, Lu Y et al Production of IL‐10 by CD4+ regulatory T cells during the resolution of infection promotes the maturation of memory CD8+ T cells. Nat Immunol 2015; 16:871–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Whitmire JK, Eam B, Whitton JL. Mice deficient in stem cell antigen‐1 (Sca1, Ly‐6A/E) develop normal primary and memory CD4+ and CD8+ T‐cell responses to virus infection. Eur J Immunol 2009; 39:1494–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Li MS, Liu Z, Liu JQ, Zhu X, Liu Z, Bai XF. The Yin and Yang aspects of IL‐27 in induction of cancer‐specific T‐cell responses and immunotherapy. Immunotherapy 2015; 7:191–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Meka RR, Venkatesha SH, Dudics S, Acharya B, Moudgil KD. IL‐27‐induced modulation of autoimmunity and its therapeutic potential. Autoimmun Rev 2015; 14:1131–41. [DOI] [PMC free article] [PubMed] [Google Scholar]


