Summary
Immunotherapies have been traditionally applied in malignant melanoma, which represent one of the most immunogenic tumours. Recently, immune checkpoint modulation has shown high therapeutic efficacy and may provide long‐term survival in a significant proportion of affected patients. T cells are the major players in tumour rejection and recognize tumour cells predominantly in an MHC‐dependent way. The immunopeptidome comprises the peptide repertoire presented by MHC class I and II molecules on the surface of the body's cells including tumour cells. To understand characteristics of suitable rejection antigens as well as respective effective T‐cell responses, determination of the immunopeptidome is of utmost importance. Suitable rejection antigens need to be further characterized and validated not only to systematically improve current therapeutic approaches, but also to develop individualized treatment options. In this review, we report on current tools to explore the immunopeptidome in human melanoma and discuss current understanding and future developments to specifically detect and select those antigens that may be most relevant and promising for effective tumour rejection.
Keywords: immunogenicity, immunopeptidome, melanoma, T cells, tumor antigens, tumor rejection
Recognition of melanoma by the immune system
Malignant melanoma represents one of the most aggressive but also highly immunogenic tumours, which has traditionally been targeted by immunotherapies. High‐risk resectable melanoma has long been treated by the immune‐modulating cytokine interferon‐α in an adjuvant setting following surgery.1 Moreover, over a long period, a high number of clinical immunotherapeutic approaches have been tested in clinical studies aiming to specifically stimulate T cells by vaccination or to target a defined antigens by directed adoptive T‐cell transfer.2, 3 However, the success of these approaches was either limited or associated with major side effects.4, 5 In contrast, high efficacy of adoptive T‐cell transfer of tumour‐infiltrating lymphocytes (TIL) with mainly unknown specificity was reported repeatedly over a long period of time although larger, phase three clinical trials were missing.6 The discovery of immune checkpoints potentially exerting a major inhibitory effect on tumour‐reactive T cells was an outstanding break‐through in the emerging clinical application of novel immunotherapies.7, 8 Over the last decade, immune checkpoint modulators became available preventing exhaustion of endogenous T‐cell populations with unknown specificity and improving tumour‐cell recognition by supporting T‐cell priming and depletion of regulatory T cells in vivo.9 Immune‐checkpoint‐modulating antibodies targeting the programmed death‐1(PD‐1)/ programmed death ligand‐1(PD‐L1) axis meanwhile represent standard therapies in the treatment of diverse malignant diseases including melanoma, lung cancer, renal cell carcinoma, and head and neck squamous cell carcinoma, but also other malignant disease entities.10 These therapeutic agents have proven exceptionally effective in patients with melanoma,11 indicating that highly suitable target structures for tumour rejection are presented on melanoma cells. The identification and understanding of the nature of such target structures and the characterization of respective T‐cell responses is of fundamental importance to improve current therapies not only in melanoma but also in other malignant diseases.
The immunopeptidome represents the sum of peptide ligands presented by the MHC class I and II complexes on the body's cells including tumour cells and therefore in principle recognizable by T cells (Box 1). Recognition and elimination of target cells by T cells has been intensively studied for decades accompanied by immunological milestone discoveries such as MHC presenting antigenic peptides potentially representing epitopes to be recognized by T cells.12, 13 Until now, numerous approaches aimed at the mostly focused identification of immunogenic peptides suitable for further clinical application. In this regard, a prime example for MHC‐restricted antigen recognition by T cells was the detection of foreign antigens.13, 14 Antigenic peptides derive from intracellularly degraded exogenous or endogenous proteins processed by the antigen‐presenting machinery and presented by MHC class II and I molecules as peptide–MHC complex, respectively.15 T cells recognize such presented peptide ligands by their T‐cell receptor (TCR) and may subsequently eliminate target cells by a professional lytic machinery.16, 17, 18 The precondition for this highly fine‐tuned recognition is thought to base on the co‐evolution of MHC genes and TCR genes.19, 20 Education of the immune cells in the thymus is an essential prerequisite for the critical distinction between foreign and self and therefore maintenance of self tolerance as recently reviewed.21
Box 1. Definition of the immunopeptidome.
The immunopeptidome comprises the whole set of peptides represented on the cell surface in the context of MHC and recognizable by T cells. Parts of the immunopeptidome are presented on both, healthy and tumour cells. Tumour‐specific peptide ligands representing neoepitopes seem to significantly contribute to the detectability of the tumour by the autologous immune system. However, other target structures may serve as rejection antigens depending on the source of T cells (autologous versus allogeneic/xenogeneic) recognizing respective MHC–peptide ligands presented by tumour cells.
Although recognition of tumour cells by the patient's immune system has been long‐term propagated,22 routine recognition of tumour cells was questioned over the years and tolerance or ignorance of such transformed cells seemed to be dominant in the discourse of the immune system and cancer. Our understanding of the coherence of cancer and the immune system has improved and immune evasion has been recognized as one of the hallmarks of cancer.23 Evolution of tumours under immune pressure and immune editing has been determined as a key element of immune evasion and subsequent tumour progression.24 The tumour‐related immunopeptidome is also affected by such immune editing25, 26 and characteristics of peptide ligands suitable for effective tumour rejection may depend on a number of factors, such as relevance of the source protein for cell survival, dependence of peptide presentation on the antigen presentation machinery and affinity of the peptide towards MHC.27 Otherwise, target recognition is highly dependent on the relevant T‐cell population and higher‐affinity TCR towards MHC–peptide may be more effective in tumour cell rejection. Such TCR are generally observed against non‐self antigens or in the context of a non‐educated T‐cell repertoire or mismatched MHC environment.28, 29, 30 Hence, definition of the quality and antigenicity of peptide ligands presented by MHC is at least in part inevitably associated with a defined T‐cell population displaying respective reactivity.
Target structures presented on melanoma cells
Melanoma has been identified as a tumour entity that is potentially recognized by the immune system.31 Extensive efforts of several groups have been aimed at the identification of exactly those peptides of the immunopeptidome rendering tumour cells immunogenic. The basis of these efforts was primarily the presence of T cells within peripheral blood mononuclear cells (PBMC) or TIL of melanoma patients correlating with anti‐tumour activity in vitro or in vivo.32, 33 Potential antigen candidates can be assigned to two major classes, tumour‐associated antigens (TAA) and tumour‐specific antigens (TSA) (Table 1). TAA are antigens that might be dominantly presented by malignant cells but may also exist in normal cells. Differentiation antigens, as a subgroup of TAA, are representative for a defined cell type or tissue and eventually expressed in tumours originating from these cells. Peptide ligands derived from differentiation antigens in melanoma, such as gp100, MART1/Melan‐A and tyrosinase,34, 35 may represent promising target structures inducing also spontaneous immune responses in patients with disease.36 Responses against these antigens have been shown to be associated with vitiligo correlating with a good prognosis in patients with melanoma.37 Another class of TAA are represented by cancer‐germline antigens defined by exclusive expression in tumour and germline tissue and thereby representing an attractive means for targeting different tumour entities.38 In melanoma, a number of cancer‐germline antigens have been described, such as NY‐ESO‐1 or members of the Melanoma antigen encoding genes.39 However, vaccination strategies targeting both classes of TAA have shown only limited activity so far.4, 40, 41 Limited efficacy of vaccination studies targeting this class of antigens may be associated with the lack of high‐avidity T‐cell responses due to thymic negative depletion. However, both classes of TAA may still represent attractive target antigens in a non‐self immune environment. Peptides derived from proteins that quantitatively have higher expression in tumour cells compared with normal cells represent another class of TAA used for targeting in melanoma and other tumour entities,42 given Survivin as an example.43 Problems may arise from proper quantification of gene or protein expression in different cell types, especially in proteins with highly dynamic expression as well as critical functions in normal cells rendering them difficult targets, especially in a non‐self T‐cell environment potentially selected to improve efficacy.44 Another source of antigens is represented by proteasome‐generated spliced peptides obviously representing a substantial part of the immunopeptidome.45, 46, 47, 48 However, cancer specificity and a potential role for targeting in malignant diseases has so far not been elucidated. The most attractive source of antigens is currently estimated to be represented by TSA or neoantigens not presented on normal cells (Table 1). Mutated peptide antigens resulting from non‐synonymous point mutations are proposed to present an important source of such target antigens.49 Mutations may affect binding of peptides to respective MHC by altered anchor residues or may lead to recognition of affected amino acids by mutation‐specific T cells.50 These neoantigens represent highly attractive targets when these peptides derive from public mutations,26 although they may be also highly suitable for the design of personalized therapies. Moreover, mutations may result in non‐canonical reading frames additionally representing an important source for neoantigens, as shown for autoimmune diseases and cancer.51, 52, 53, 54, 55, 56 In addition, post‐translational modifications may produce aberrantly processed peptides potentially representing neoantigens.57 So far, it is not clear to what extent these diverse alterations and modifications resulting in neoantigens may contribute to the immunopeptidome. However, the predominant role of neoantigens resulting from mutations became obvious in the context of data indicating a better response rate to checkpoint modulation for those patients with tumours with high mutational load, as observed in diverse disease settings.58, 59, 60 In general, the high number of somatic mutations, as well as the limited tumour heterogeneity, in comparison to other malignant entities are most likely key issues in the high and prolonged response rates observed in patients with melanoma treated with immune checkpoint modulators.61, 62, 63
Table 1.
Classes of tumour antigens rendering melanoma cells immunogeneic
| Classes of tumour antigens | Description | Examples | Literature |
|---|---|---|---|
| Tumour‐associated antigens (TAA) | |||
| Differentiation antigens | Proteins mainly expressed in melanocytes and melanoma cells | MART1/MELAN‐A, gp100, tyrosinase | 34, 121, 122, 123 |
| Cancer germline antigens (CGA) | Expression restricted to germline tissues and various tumours | MAGE family, NY‐ESO‐1 | 67, 124 |
| Over‐expressed antigens | Antigens more highly expressed in malignant cells in relation to healthy tissue | Survivin | 43 |
| Tumour‐specific antigens (TSA) | |||
| Peptides containing mutations | Peptide fragments exclusively expressed in tumour cells due to genomic alterations |
SNV → aa exchange; In/Del → frame shifts; Chromosomal rearrangement → fused peptides |
49, 66, 125, 126, 127 |
| Peptides resulting from non‐canonical translation | Changes on the transcriptional level | Alternative ORF; retained introns | 54, 55 |
| Aberrantly spliced peptides | Tumour‐specific distinct proteasomal processing | Fused peptides resulting from different protein regions | 45, 46, 47, 48 |
| Peptides derived from TAP‐ and proteasome‐independent pathways | Unconventional sources for peptides assembled in the pMHC complex | TAP‐independent pathway of tyrosinase‐derived signal sequence | 128 |
| Peptides containing tumour‐specific post‐translational modifications | Aa modification after ribosomal translation | Phosphorylation and deamidation | 107, 129 |
aa, amino acid; In/Del, Insertions/Deletions of one or two bases; MAGE, melanoma antigen encoding genes; ORF, open reading frame; SNV, single nucleotide variant.
Strategies for tumour antigen identification on melanoma
Although all the diverse classes of antigens have been described, it is currently unclear how widely they are presented. Moreover, their defined role as suitable rejection antigens needs to be further clarified. Depending on the origin of the antigen of interest, the focus is on the selection of the most potent rejection antigens in a personalized approach or to be applied in a defined patient collective. As described above, definition of suitable target antigens also needs to be regarded in the context of the T‐cell repertoire that is therapeutically addressed. Early approaches mainly focused on highly selective target identification based on tumour‐reactive T cells and a number of diverse techniques have been applied to identify such antigens (Table 2). With respect to technical improvements and implementation of high‐throughput technologies, new methods aim to detect the most relevant target antigens in a more personalized way and hence define more general determinants for tumour rejection.
Table 2.
Techniques used for identification of relevant tumour rejection antigens
| Technique | Examples | Approach | Antigen class | Presentation of candidate epitopes for testing | Interrogated TCR repertoire | Antigen validation |
|---|---|---|---|---|---|---|
| cDNA and peptide library of preselected target antigen | 64, 65 | Direct immunology | TAA + TSA |
|
|
|
| In vitro proteasomal digest of preselected target antigen | 130 | Direct immunology | TAA | |||
| RNA/protein expression + in silico epitope prediction | 43 | Reverse immunology | TAA | |||
| Exome sequencing + in silico epitope prediction | 92 | Reverse immunology | TSA | |||
| Mass spectrometry | 98, 99 | MS‐based Immunopeptidomics | TAA | |||
| Exome/RNA sequencing + Mass spectrometry | 104, 105, 106, 107 | MS‐based Immunopeptidomics | TAA + TSA |
Direct immunology approach
For definition of target structures, TIL and PBMC from patients with a favourable course are a highly attractive source for subsequent identification of tumour antigens. For direct identification of tumour rejection antigens, isolated TIL or PBMC were co‐cultured with tumour cells without any knowledge of the defined target structure. Lack of reactivity against autologous healthy control cells hinted at tumour‐specific determinants. Great progress was made regarding the identification of specific target structures by the development of tum– variants in combination with the preparation of cDNA libraries and subsequent identification of immunogenic epitopes.64, 65 Subsequently, first TSA and TAA were detected in melanoma and characterized revealing marked tumour‐directed T‐cell responses.66, 67, 68
In concordance with the increasing number of identified targets, databases were established comprising identified relevant TAA.69, 70 In addition databases such as the immune epitope database (IEDB) were built, gathering published information about the origin of the identified epitopes and the results of functional analyses.71
Reverse immunology approach
The so‐called reverse immunology approach comprises all those methods identifying immunogenic tumour‐derived epitopes by the implementation of three sequential steps: (i) the selection of antigen based on expression or tumour relevance, (ii) prediction of putative antigens, and (iii) experimental validation.72 For step (i), putative antigen candidates are either selected by literature search of previously described over‐expressed antigens or based on disease‐specific expression profiles obtained from single patients or a compilation of different patient samples. As another possible starting point, proteins eliciting autologous humoral immune responses in cancer patients were identified by a recombinant expression cloning (SEREX) approach73 and were then investigated with regard to their potential as a T‐cell‐mediated target.29 As a prerequisite for (ii), public in silico binding prediction tools pave the way to broader application by the research community. In the early 1990s, a first structural approach for the provision of motifs was pursued for MHC class I,74 followed by the identification of motifs for MHC class II binding peptides.75 After proposals of several motifs in the following years, these efforts resulted in the development of a first public database for peptide‐binding motifs.76 Currently, a constantly growing number of binding prediction algorithms is available for both human HLA and mouse MHC class I and II.76, 77, 78, 79 The importance of peptide ligand quality is strengthened by the fact that peptides with high affinity towards MHC have a higher probability of representing immunodominant peptides and being suitable for tumour rejection.80, 81 The prediction of the stability of the peptide–MHC complex is another important aspect for selecting the most attractive candidate epitopes.82 In addition, the prediction of peptide‐processing steps, such as proteasomal cleavage and transporter associated with antigen processing (TAP)‐dependent transport to the endoplasmic reticulum, have found integration into more recent versions of prediction algorithms as recently reviewed.83 Comparison and validation of large experimental data sets using different tools or combinations of them will foster an even more precise definition of rules for actual presentation.84 Implementation of structural in silico analyses may provide deeper insights into conformational binding properties of potential HLA ligands.85 One of the major drawbacks of prediction‐based antigen selection is that not all epitope candidates with high predicted immunogenicity are actually presented as expected. For instances, MART127–35‐specific T cells only recognized about half of the MART1‐expressing HLA‐matched melanoma cell lines.86 Moreover, a distorted relationship between gene or protein expression and peptide presentation has also been reported.87 Validation by a defined T‐cell repertoire is essential for this approach and may be facilitated by the invention of novel technologies and further optimization of existing high‐throughput technologies.88
Owing to the previous observation of immunogenic tumour‐specific mutations and the generally high mutational load of melanomas,61, 66 systematic assessment of immunogenic tumour‐restricted mutations was pursued by exome sequencing in combination with in silico epitope prediction.25, 89, 90, 91, 92 This combinatorial approach was applied for instances on the analysis of tumour tissue from three melanoma patients followed by screening assays using 19‐mer peptides encompassing the respective mutation.92 In all patients, mutation‐specific T cells were detectable and all three individuals responded to the transfer of autologous TIL products. The same approach was used for the identification of immunogenic MHC‐II‐restricted neoepitopes in a murine model showing tumour rejection upon vaccination with pre‐screened candidates.89 Recently, two early‐phase clinical vaccination trials focusing on tumour‐specific mutations reported promising efficacy in patients with melanoma. Ott and colleagues used mixtures of long peptides comprising patient‐tailored single nucleotide variants for the vaccination of six patients and observed in four patients with stage III melanoma a stable clinical course with no reccurrence of disease up to 25 months.93 Within the second study, 13 patients with advanced melanoma have been vaccinated with an RNA‐based poly‐neoepitope vaccine encoding multiple single nucleotide variants per construct and experienced a significant decrease of relapses compared with their pre‐vaccination disease course.94 The described observations are highly encouraging and demand larger clinical trials. In particular, the value of vaccination at defined disease stages compared with immune checkpoint modulation alone or in combination needs to be elucidated. Moreover, the characteristics of neoantigens representing most effective tumour rejection antigens as well as respective T‐cell responses need to be investigated in detail to improve neoantigen‐specific therapeutic strategies.
Mass spectrometry‐based immunopeptidomics
HLA‐bound ligands that are presented on the cell surface can be directly identified as such. Since the identification of naturally presented ligands using the combination of high‐performance liquid chromatography and tandem mass spectrometry,95, 96 this approach profited largely from recent developments and inventions for the optimization of mass spectrometers.
The ability to directly measure HLA‐presented peptides by mass spectrometry rapidly broadened possibilities for epitope‐specific (onco)immunological research and currently several strategies for the detection of HLA‐bound peptides by MS are available, as has been reviewed recently.97 Cell lines have been mostly used for antigen identification by MS due to the unlimited material that is available.98 However, direct identification of ligands derived from primary tumour cells of patients reflects heterogeneity within one given sample and therefore may support the identification of those peptides that are well presented. In fact, primary patient tumour samples led to the designation of several novel and known immunogenic target structures with interesting potential for clinical applications.99, 100, 101 In addition, the comparison of ligands identified in tumour material and patient sera may be attractive for biomarker development.102, 103
The identification of neoantigens in solid tumours by MS immunopeptidomics among previously unassigned mass spectra was first shown by the analysis of murine tissues104, 105 and human cell lines.106 Further development led to identification of naturally presented neoepitopes on cryopreserved human solid tumour tissue derived from melanoma by mass spectrometry.107 Key issues of this analysis were the large number of peptides that were eluted, corresponding to a comparably deeper level of sensitivity in comparison with other studies. In addition, an optimized workflow for bioinformatics was developed using maxquant.107
Mass spectrometry‐based immunopeptidomic neoantigen identification has been shown to be successful also in lymphomas.108 Several epitopes derived from the immunoglobulin constant region were presented on MHC I, but no epitope was detected mapping to the mutated variable region. In contrast, 14 epitopes presented on MHC II were detected that derived from the hypermutated variable region, therefore presenting true neoepitopes. Due to the authors' hypothesis, this rather unexpected distribution of naturally presented tumour‐derived HLA ligands may derive from immunoediting of respective tumour cells. Hence, comparison of different modes of processing and epitope editing will help us to learn from analysed data sets and draw conclusions on similarities and differences of neoepitope presentation between different tumour entities.
There are a number of advantages of using mass spectrometry for the analysis of the immunopeptidome. The mass spectrometry‐based immunopeptidomic approach represents an HLA‐independent strategy for the identification of HLA ligands with direct proof of actual presentation. This is especially important as prediction analyses are highly limited to frequent HLA types for which a large data set is already available. Using a computational approach for the assignment of a data set of mass spectrometry‐identified epitopes to its respective HLA restriction element, existing in silico prediction tools can be further improved in their accuracy if they are trained with data sets derived from mass spectrometry analyses.109 In addition, measurement of an immunopeptidome that can be clearly assigned to one single HLA molecule leads to more input and a high‐quality data set for adjustment of prediction algorithms, such as the detection of novel anchor residues.84 Immunopeptidomes identified by mass spectrometry may therefore contribute to a further improvement of epitope prediction. Moreover, the unbiased search by mass spectrometry analysis enables the detection of ligands derived from post‐translational modification, which might be missed by conventional epitope prediction.107 Other examples are spliced peptide variants46 and the so far less well described MHC II immunopeptidome,110 although these peptides are currently also often difficult to detect by MS. In addition, so‐called ‘hot spots’ of preferential proteasomal processing and presentation within highly expressed proteins may be identified by comparison of ligandomes derived from different samples and mapping of frequently detected regions in a protein sequence.107, 111 Processing of retrieved mass spectra represents one key component for the valid interpretation of the analysed data and requires integration of state‐of‐the‐art bioinformatics and computational analyses.112 Nonetheless, mass spectrometry‐based immunopeptidomics currently inherits several limitations, such as the predominant dependence on those databases that are used for the assignment of analysed mass spectra. This bias may be overcome by the implementation of de novo sequencing.113 Despite the application of stringent filters, the verification of true binders remains another objective that is currently faced throughout laborious validation procedures. Moreover, the reported sensitivity for mass spectrometry is still rather low, ranging between 0·5% and 3% yield of peptides captured by immunoprecipitation.97 Further challenges are based on the robust identification of the actually presented HLA peptides, including the correct assignment of isobaric amino acids such as leucine versus isoleucine.114 As another technical limitation, very hydrophobic or hydrophilic peptides are less well detected by current mass spectrometry technologies, leading to biased acquisition of the individual immunopeptidome.115 In addition, as mentioned above, the integration of algorithms for the systematic detection of spliced peptides may contribute to a more comprehensive characterization of the melanoma immunopeptidome. With the high velocity of novel developments in the field, sensitivity in peptide ligand identification is expected to be substantially improved in the near future.
Clinical relevance and future challenges
As a result of the high efficacy of immune checkpoint modulation and T‐cell‐based therapies in melanoma we are currently able to learn much about suitable target antigens and respective T‐cell responses in a broader patient population. This will be instrumental in obtaining better understanding of the efficacy and rejection capabilities of T‐cell responses directed against differentiation antigens and/or mutated epitopes,116, 117 stressing the importance of thorough functional characterization of tumour‐specific immune responses. Currently, we have no clear picture of which antigens are the most relevant for tumour rejection or what aspects may contribute to immunodominance, mainly characterized by the reactive T‐cell population. Large‐scale analyses may move the field forward, including a comprehensive description of immunogenic epitopes presented on an (individual) melanoma tissue and a combination of permanently improving techniques (Figure 1). However, detailed sequential functional T‐cell analyses are essential.107 There are central questions including a better understanding of the role of the whole antigen‐presenting machinery, the role of CD8 versus CD4 responses and the contribution of each population to efficient tumour rejection. One of the biggest challenges is represented by tumour heterogeneity and immune evasion.25, 62, 63 Analysis of the immunopeptidome of different metastatic lesions may therefore help to understand inter‐ and intraindividual heterogeneity and its impact on plasticity, immunogenicity, responsiveness to immunotherapy and immune escape. It will probably become more complex as soon as a better understanding exists of the impact of other systems, for example the respective microenvironment118 and the microbiome,119, 120 on respective tumour‐related immunopeptidomes and the outcome of immunotherapies encouraging an integrative view of systemic immunotherapies.
Figure 1.
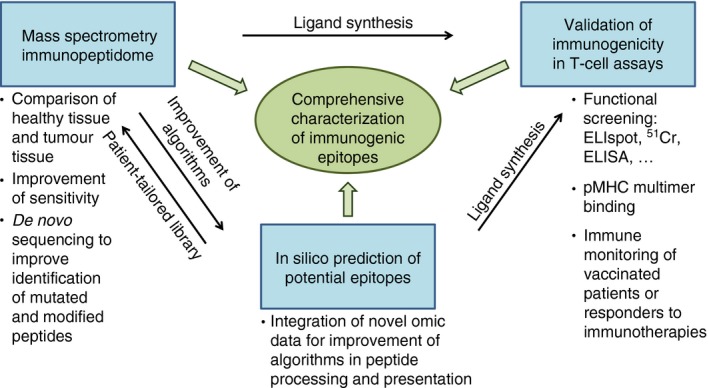
Schematic overview of relevant determinants for the comprehensive characterization of immunogenic epitopes.
Disclosures
The authors have no competing interests to declare.
Acknowledgements
We are grateful to the Wilhelm‐Sander‐Stiftung (2015·030·01) for the funding.
References
- 1. Eggermont AM, Suciu S, Testori A, Santinami M, Kruit WH, Marsden J et al Long‐term results of the randomized phase III trial EORTC 18991 of adjuvant therapy with pegylated interferon α‐2b versus observation in resected stage III melanoma. J Clin Oncol 2012; 30:3810–8. [DOI] [PubMed] [Google Scholar]
- 2. Morgan RA, Dudley ME, Wunderlich JR, Hughes MS, Yang JC, Sherry RM et al Cancer regression in patients after transfer of genetically engineered lymphocytes. Science 2006; 314:126–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Terando AM, Faries MB, Morton DL. Vaccine therapy for melanoma: current status and future directions. Vaccine 2007; 25(Suppl. 2):B4–16. [DOI] [PubMed] [Google Scholar]
- 4. Schwartzentruber DJ, Lawson DH, Richards JM, Conry RM, Miller DM, Treisman J et al gp100 peptide vaccine and interleukin‐2 in patients with advanced melanoma. N Engl J Med 2011; 364:2119–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Johnson LA, Morgan RA, Dudley ME, Cassard L, Yang JC, Hughes MS et al Gene therapy with human and mouse T‐cell receptors mediates cancer regression and targets normal tissues expressing cognate antigen. Blood 2009; 114:535–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Rosenberg SA, Restifo NP. Adoptive cell transfer as personalized immunotherapy for human cancer. Science 2015; 348:62–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Freeman GJ, Long AJ, Iwai Y, Bourque K, Chernova T, Nishimura H et al Engagement of the PD‐1 immunoinhibitory receptor by a novel B7 family member leads to negative regulation of lymphocyte activation. J Exp Med 2000; 192:1027–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Leach DR, Krummel MF, Allison JP. Enhancement of antitumor immunity by CTLA‐4 blockade. Science 1996; 271:1734–6. [DOI] [PubMed] [Google Scholar]
- 9. Topalian SL, Drake CG, Pardoll DM. Immune checkpoint blockade: a common denominator approach to cancer therapy. Cancer Cell 2015; 27:450–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Balar AV, Weber JS. PD‐1 and PD‐L1 antibodies in cancer: current status and future directions. Cancer Immunol Immunother 2017; 66:551–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Larkin J, Chiarion‐Sileni V, Gonzalez R, Grob JJ, Cowey CL, Lao CD et al Combined nivolumab and ipilimumab or monotherapy in untreated melanoma. N Engl J Med 2015; 373:23–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Zinkernagel RM, Doherty PC. MHC‐restricted cytotoxic T cells: studies on the biological role of polymorphic major transplantation antigens determining T‐cell restriction‐specificity, function, and responsiveness. Adv Immunol 1979; 27:51–177. [DOI] [PubMed] [Google Scholar]
- 13. Zinkernagel RM, Doherty PC. Restriction of in vitro T cell‐mediated cytotoxicity in lymphocytic choriomeningitis within a syngeneic or semiallogeneic system. Nature 1974; 248:701–2. [DOI] [PubMed] [Google Scholar]
- 14. Townsend AR, Rothbard J, Gotch FM, Bahadur G, Wraith D, McMichael AJ. The epitopes of influenza nucleoprotein recognized by cytotoxic T lymphocytes can be defined with short synthetic peptides. Cell 1986; 44:959–68. [DOI] [PubMed] [Google Scholar]
- 15. Vyas JM, Van der Veen AG, Ploegh HL. The known unknowns of antigen processing and presentation. Nat Rev Immunol 2008; 8:607–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hedrick SM, Cohen DI, Nielsen EA, Davis MM. Isolation of cDNA clones encoding T cell‐specific membrane‐associated proteins. Nature 1984; 308:149–53. [DOI] [PubMed] [Google Scholar]
- 17. Yanagi Y, Yoshikai Y, Leggett K, Clark SP, Aleksander I, Mak TW. A human T cell‐specific cDNA clone encodes a protein having extensive homology to immunoglobulin chains. Nature 1984; 308:145–9. [DOI] [PubMed] [Google Scholar]
- 18. Lanzavecchia A. Is the T‐cell receptor involved in T‐cell killing? Nature 1986; 319:778–80. [DOI] [PubMed] [Google Scholar]
- 19. Garcia KC, Adams JJ, Feng D, Ely LK. The molecular basis of TCR germline bias for MHC is surprisingly simple. Nat Immunol 2009; 10:143–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Sharon E, Sibener LV, Battle A, Fraser HB, Garcia KC, Pritchard JK. Genetic variation in MHC proteins is associated with T cell receptor expression biases. Nat Genet 2016; 48:995–1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Klein L, Kyewski B, Allen PM, Hogquist KA. Positive and negative selection of the T cell repertoire: what thymocytes see (and don't see). Nat Rev Immunol 2014; 14:377–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ehrlich P. Über den jetzigen Stand der Karzinomforschung. Beiträge zur experimentellen Pathologie und Chemotherapie. Leipzig, Akademische Verlagsgesellschaft 1909; 5:117–64. [Google Scholar]
- 23. Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell 2011; 144:646–74. [DOI] [PubMed] [Google Scholar]
- 24. Schreiber RD, Old LJ, Smyth MJ. Cancer immunoediting: integrating immunity's roles in cancer suppression and promotion. Science 2011; 331:1565–70. [DOI] [PubMed] [Google Scholar]
- 25. Verdegaal EM, de Miranda NF, Visser M, Harryvan T, van Buuren MM, Andersen RS et al Neoantigen landscape dynamics during human melanoma‐T cell interactions. Nature 2016; 536:91–5. [DOI] [PubMed] [Google Scholar]
- 26. Tran E, Robbins PF, Lu YC, Prickett TD, Gartner JJ, Jia L et al T‐cell transfer therapy targeting mutant KRAS in cancer. N Engl J Med 2016; 375:2255–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Reeves E, James E. Antigen processing and immune regulation in the response to tumours. Immunology 2017; 150:16–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Sadovnikova E, Stauss HJ. Peptide‐specific cytotoxic T lymphocytes restricted by nonself major histocompatibility complex class I molecules: reagents for tumor immunotherapy. Proc Natl Acad Sci USA 1996; 93:13114–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Schuster IG, Busch DH, Eppinger E, Kremmer E, Milosevic S, Hennard C et al Allorestricted T cells with specificity for the FMNL1‐derived peptide PP2 have potent antitumor activity against hematologic and other malignancies. Blood 2007; 110:2931–9. [DOI] [PubMed] [Google Scholar]
- 30. Stanislawski T, Voss RH, Lotz C, Sadovnikova E, Willemsen RA, Kuball J et al Circumventing tolerance to a human MDM2‐derived tumor antigen by TCR gene transfer. Nat Immunol 2001; 2:962–70. [DOI] [PubMed] [Google Scholar]
- 31. Sumner WC, Foraker AG. Spontaneous regression of human melanoma: clinical and experimental studies. Cancer 1960; 13:79–81. [DOI] [PubMed] [Google Scholar]
- 32. Knuth A, Danowski B, Oettgen HF, Old LJ. T‐cell‐mediated cytotoxicity against autologous malignant melanoma: analysis with interleukin 2‐dependent T‐cell cultures. Proc Natl Acad Sci USA 1984; 81:3511–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Rosenberg SA, Packard BS, Aebersold PM, Solomon D, Topalian SL, Toy ST et al Use of tumor‐infiltrating lymphocytes and interleukin‐2 in the immunotherapy of patients with metastatic melanoma. A preliminary report. N Engl J Med 1988; 319:1676–80. [DOI] [PubMed] [Google Scholar]
- 34. Coulie PG, Brichard V, Van Pel A, Wolfel T, Schneider J, Traversari C et al A new gene coding for a differentiation antigen recognized by autologous cytolytic T lymphocytes on HLA‐A2 melanomas. J Exp Med 1994; 180:35–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Storkus WJ, Zeh HJ III, Maeurer MJ, Salter RD, Lotze MT. Identification of human melanoma peptides recognized by class I restricted tumor infiltrating T lymphocytes. J Immunol 1993; 151:3719–27. [PubMed] [Google Scholar]
- 36. Maczek C, Berger TG, Schuler‐Thurner B, Schultz ES, Hamann A, Dunbar PR et al Differences in phenotype and function between spontaneously occurring melan‐A‐, tyrosinase‐ and influenza matrix peptide‐specific CTL in HLA‐A*0201 melanoma patients. Int J Cancer 2005; 115:450–5. [DOI] [PubMed] [Google Scholar]
- 37. Jacobs JF, Aarntzen EH, Sibelt LA, Blokx WA, Boullart AC, Gerritsen MJ et al Vaccine‐specific local T cell reactivity in immunotherapy‐associated vitiligo in melanoma patients. Cancer Immunol Immunother 2009; 58:145–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Boon T, Old LJ. Cancer tumor antigens. Curr Opin Immunol 1997; 9:681–3. [DOI] [PubMed] [Google Scholar]
- 39. Caballero OL, Chen YT. Cancer/testis (CT) antigens: potential targets for immunotherapy. Cancer Sci 2009; 100:2014–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Lawson DH, Lee S, Zhao F, Tarhini AA, Margolin KA, Ernstoff MS et al Randomized, placebo‐controlled, phase III trial of yeast‐derived granulocyte‐macrophage colony‐stimulating factor (GM‐CSF) versus peptide vaccination versus GM‐CSF plus peptide vaccination versus placebo in patients with no evidence of disease after complete surgical resection of locally advanced and/or stage IV melanoma: a trial of the eastern cooperative oncology Group‐American College of Radiology Imaging Network Cancer Research Group (E4697). J Clin Oncol 2015; 33:4066–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. van Baren N, Bonnet MC, Dreno B, Khammari A, Dorval T, Piperno‐Neumann S et al Tumoral and immunologic response after vaccination of melanoma patients with an ALVAC virus encoding MAGE antigens recognized by T cells. J Clin Oncol 2005; 23:9008–21. [DOI] [PubMed] [Google Scholar]
- 42. Melero I, Gaudernack G, Gerritsen W, Huber C, Parmiani G, Scholl S et al Therapeutic vaccines for cancer: an overview of clinical trials. Nat Rev Clin Oncol 2014; 11:509–24. [DOI] [PubMed] [Google Scholar]
- 43. Andersen MH, Pedersen LO, Becker JC, Straten PT. Identification of a cytotoxic T lymphocyte response to the apoptosis inhibitor protein survivin in cancer patients. Can Res 2001; 61:869–72. [PubMed] [Google Scholar]
- 44. Leisegang M, Wilde S, Spranger S, Milosevic S, Frankenberger B, Uckert W et al MHC‐restricted fratricide of human lymphocytes expressing survivin‐specific transgenic T cell receptors. J Clin Investig 2010; 120:3869–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Warren EH, Vigneron NJ, Gavin MA, Coulie PG, Stroobant V, Dalet A et al An antigen produced by splicing of noncontiguous peptides in the reverse order. Science 2006; 313:1444–7. [DOI] [PubMed] [Google Scholar]
- 46. Liepe J, Marino F, Sidney J, Jeko A, Bunting DE, Sette A et al A large fraction of HLA class I ligands are proteasome‐generated spliced peptides. Science 2016; 354:354–8. [DOI] [PubMed] [Google Scholar]
- 47. Ebstein F, Textoris‐Taube K, Keller C, Golnik R, Vigneron N, Van den Eynde BJ et al Proteasomes generate spliced epitopes by two different mechanisms and as efficiently as non‐spliced epitopes. Sci Rep 2016; 6:24032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Vigneron N, Stroobant V, Chapiro J, Ooms A, Degiovanni G, Morel S et al An antigenic peptide produced by peptide splicing in the proteasome. Science 2004; 304:587–90. [DOI] [PubMed] [Google Scholar]
- 49. Lennerz V, Fatho M, Gentilini C, Frye RA, Lifke A, Ferel D et al The response of autologous T cells to a human melanoma is dominated by mutated neoantigens. Proc Natl Acad Sci USA 2005; 102:16013–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Fritsch EF, Rajasagi M, Ott PA, Brusic V, Hacohen N, Wu CJ. HLA‐binding properties of tumor neoepitopes in humans. Cancer Immunol Res 2014; 2:522–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Kracht MJ, van Lummel M, Nikolic T, Joosten AM, Laban S, van der Slik AR et al Autoimmunity against a defective ribosomal insulin gene product in type 1 diabetes. Nat Med 2017; 23:501–7. [DOI] [PubMed] [Google Scholar]
- 52. Ronsin C, Chung‐Scott V, Poullion I, Aknouche N, Gaudin C, Triebel F. A non‐AUG‐defined alternative open reading frame of the intestinal carboxyl esterase mRNA generates an epitope recognized by renal cell carcinoma‐reactive tumor‐infiltrating lymphocytes in situ . J Immunol 1999; 163:483–90. [PubMed] [Google Scholar]
- 53. Laumont CM, Daouda T, Laverdure JP, Bonneil E, Caron‐Lizotte O, Hardy MP et al Global proteogenomic analysis of human MHC class I‐associated peptides derived from non‐canonical reading frames. Nat Commun 2016; 7:10238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Aarnoudse CA, van den Doel PB, Heemskerk B, Schrier PI. Interleukin‐2‐induced, melanoma‐specific T cells recognize CAMEL, an unexpected translation product of LAGE‐1. Int J Cancer 1999; 82:442–8. [DOI] [PubMed] [Google Scholar]
- 55. Lupetti R, Pisarra P, Verrecchia A, Farina C, Nicolini G, Anichini A et al Translation of a retained intron in tyrosinase‐related protein (TRP) 2 mRNA generates a new cytotoxic T lymphocyte (CTL)‐defined and shared human melanoma antigen not expressed in normal cells of the melanocytic lineage. J Exp Med 1998; 188:1005–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Turajlic S, Litchfield K, Xu H, Rosenthal R, McGranahan N, Reading JL et al Insertion‐and‐deletion‐derived tumour‐specific neoantigens and the immunogenic phenotype: a pan‐cancer analysis. Lancet Oncol 2017; 18:1009–21. [DOI] [PubMed] [Google Scholar]
- 57. Skipper JC, Hendrickson RC, Gulden PH, Brichard V, Van Pel A, Chen Y et al An HLA‐A2‐restricted tyrosinase antigen on melanoma cells results from posttranslational modification and suggests a novel pathway for processing of membrane proteins. J Exp Med 1996; 183:527–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Snyder A, Makarov V, Merghoub T, Yuan J, Zaretsky JM, Desrichard A et al Genetic basis for clinical response to CTLA‐4 blockade in melanoma. N Engl J Med 2014; 371:2189–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Rizvi NA, Hellmann MD, Snyder A, Kvistborg P, Makarov V, Havel JJ et al Cancer immunology. Mutational landscape determines sensitivity to PD‐1 blockade in non‐small cell lung cancer. Science 2015; 348:124–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Le DT, Uram JN, Wang H, Bartlett BR, Kemberling H, Eyring AD et al PD‐1 blockade in tumors with mismatch‐repair deficiency. N Engl J Med 2015; 372:2509–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Alexandrov LB, Nik‐Zainal S, Wedge DC, Aparicio SA, Behjati S, Biankin AV et al Signatures of mutational processes in human cancer. Nature 2013; 500:415–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. McGranahan N, Furness AJ, Rosenthal R, Ramskov S, Lyngaa R, Saini SK et al Clonal neoantigens elicit T cell immunoreactivity and sensitivity to immune checkpoint blockade. Science 2016; 351:1463–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. McGranahan N, Swanton C. Clonal heterogeneity and tumor evolution: past, present, and the future. Cell 2017; 168:613–28. [DOI] [PubMed] [Google Scholar]
- 64. Boon T, Kellermann O. Rejection by syngeneic mice of cell variants obtained by mutagenesis of a malignant teratocarcinoma cell line. Proc Natl Acad Sci USA 1977; 74:272–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Wolfel T, Van Pel A, De Plaen E, Lurquin C, Maryanski JL, Boon T. Immunogenic (tum‐) variants obtained by mutagenesis of mouse mastocytoma P815. VIII. Detection of stable transfectants expressing a tum‐ antigen with a cytolytic T cell stimulation assay. Immunogenetics 1987; 26:178–87. [DOI] [PubMed] [Google Scholar]
- 66. Wolfel T, Hauer M, Schneider J, Serrano M, Wolfel C, Klehmann‐Hieb E et al A p16INK4a‐insensitive CDK4 mutant targeted by cytolytic T lymphocytes in a human melanoma. Science 1995; 269:1281–4. [DOI] [PubMed] [Google Scholar]
- 67. van der Bruggen P, Traversari C, Chomez P, Lurquin C, De Plaen E, Van den Eynde B et al A gene encoding an antigen recognized by cytolytic T lymphocytes on a human melanoma. Science 1991; 254:1643–7. [DOI] [PubMed] [Google Scholar]
- 68. Wang RF, Wang X, Atwood AC, Topalian SL, Rosenberg SA. Cloning genes encoding MHC class II‐restricted antigens: mutated CDC27 as a tumor antigen. Science 1999; 284:1351–4. [DOI] [PubMed] [Google Scholar]
- 69. Cheever MA, Allison JP, Ferris AS, Finn OJ, Hastings BM, Hecht TT et al The prioritization of cancer antigens: a national cancer institute pilot project for the acceleration of translational research. Clin Cancer Res 2009; 15:5323–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Almeida LG, Sakabe NJ, deOliveira AR, Silva MC, Mundstein AS, Cohen T et al CTdatabase: a knowledge‐base of high‐throughput and curated data on cancer‐testis antigens. Nucleic Acids Res 2009; 37:D816–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Vita R, Overton JA, Greenbaum JA, Ponomarenko J, Clark JD, Cantrell JR et al The immune epitope database (IEDB) 3.0. Nucleic Acids Res 2015; 43:D405–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Viatte S, Alves PM, Romero P. Reverse immunology approach for the identification of CD8 T‐cell‐defined antigens: advantages and hurdles. Immunol Cell Biol 2006; 84:318–30. [DOI] [PubMed] [Google Scholar]
- 73. Sahin U, Tureci O, Schmitt H, Cochlovius B, Johannes T, Schmits R et al Human neoplasms elicit multiple specific immune‐responses in the autologous host. Proc Natl Acad Sci USA 1995; 92:11810–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Falk K, Rotzschke O, Stevanovic S, Jung G, Rammensee HG. Allele‐specific motifs revealed by sequencing of self‐peptides eluted from MHC molecules. Nature 1991; 351:290–6. [DOI] [PubMed] [Google Scholar]
- 75. Rudensky A, Preston‐Hurlburt P, Al‐Ramadi BK, Rothbard J, Janeway CA Jr. Truncation variants of peptides isolated from MHC class II molecules suggest sequence motifs. Nature 1992; 359:429–31. [DOI] [PubMed] [Google Scholar]
- 76. Rammensee H, Bachmann J, Emmerich NP, Bachor OA, Stevanovic S. SYFPEITHI: database for MHC ligands and peptide motifs. Immunogenetics 1999; 50:213–9. [DOI] [PubMed] [Google Scholar]
- 77. Andreatta M, Nielsen M. Gapped sequence alignment using artificial neural networks: application to the MHC class I system. Bioinformatics 2016; 32:511–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Parker KC, Bednarek MA, Coligan JE. Scheme for ranking potential HLA‐A2 binding peptides based on independent binding of individual peptide side‐chains. J Immunol 1994; 152:163–75. [PubMed] [Google Scholar]
- 79. Hoof I, Peters B, Sidney J, Pedersen LE, Sette A, Lund O et al NetMHCpan, a method for MHC class I binding prediction beyond humans. Immunogenetics 2009; 61:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Engels B, Engelhard VH, Sidney J, Sette A, Binder DC, Liu RB et al Relapse or eradication of cancer is predicted by peptide‐major histocompatibility complex affinity. Cancer Cell 2013; 23:516–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Yewdell JW, Bennink JR. Immunodominance in major histocompatibility complex class I‐restricted T lymphocyte responses. Annu Rev Immunol 1999; 17:51–88. [DOI] [PubMed] [Google Scholar]
- 82. Jorgensen KW, Rasmussen M, Buus S, Nielsen M. NetMHCstab ‐ predicting stability of peptide‐MHC‐I complexes; impacts for cytotoxic T lymphocyte epitope discovery. Immunology 2014; 141:18–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Soria‐Guerra RE, Nieto‐Gomez R, Govea‐Alonso DO, Rosales‐Mendoza S. An overview of bioinformatics tools for epitope prediction: implications on vaccine development. J Biomed Inform 2015; 53:405–14. [DOI] [PubMed] [Google Scholar]
- 84. Abelin JG, Keskin DB, Sarkizova S, Hartigan CR, Zhang W, Sidney J et al Mass spectrometry profiling of HLA‐associated peptidomes in mono‐allelic cells enables more accurate epitope prediction. Immunity 2017; 46:315–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Antes I, Siu SW, Lengauer T. DynaPred: a structure and sequence based method for the prediction of MHC class I binding peptide sequences and conformations. Bioinformatics 2006; 22:e16–24. [DOI] [PubMed] [Google Scholar]
- 86. Sorensen RB, Junker N, Kirkin A, Voigt H, Svane IM, Becker JC et al The immunodominant HLA‐A2‐restricted MART‐1 epitope is not presented on the surface of many melanoma cell lines. Cancer Immunol Immunother 2009; 58:665–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Weinzierl AO, Lemmel C, Schoor O, Muller M, Kruger T, Wernet D et al Distorted relation between mRNA copy number and corresponding major histocompatibility complex ligand density on the cell surface. Mol Cell Proteomics 2007; 6:102–13. [DOI] [PubMed] [Google Scholar]
- 88. Bentzen AK, Hadrup SR. Evolution of MHC‐based technologies used for detection of antigen‐responsive T cells. Cancer Immunol Immunother 2017; 66:657–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Kreiter S, Vormehr M, van de Roemer N, Diken M, Lower M, Diekmann J et al Mutant MHC class II epitopes drive therapeutic immune responses to cancer. Nature 2015; 520:692–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Gros A, Parkhurst MR, Tran E, Pasetto A, Robbins PF, Ilyas S et al Prospective identification of neoantigen‐specific lymphocytes in the peripheral blood of melanoma patients. Nat Med 2016; 22:433–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Linnemann C, van Buuren MM, Bies L, Verdegaal EM, Schotte R, Calis JJ et al High‐throughput epitope discovery reveals frequent recognition of neo‐antigens by CD4+ T cells in human melanoma. Nat Med 2015; 21:81–5. [DOI] [PubMed] [Google Scholar]
- 92. Robbins PF, Lu YC, El‐Gamil M, Li YF, Gross C, Gartner J et al Mining exomic sequencing data to identify mutated antigens recognized by adoptively transferred tumor‐reactive T cells. Nat Med 2013; 19:747–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Ott PA, Hu Z, Keskin DB, Shukla SA, Sun J, Bozym DJ et al An immunogenic personal neoantigen vaccine for patients with melanoma. Nature 2017; 547:217–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Sahin U, Derhovanessian E, Miller M, Kloke BP, Simon P, Lower M et al Personalized RNA mutanome vaccines mobilize poly‐specific therapeutic immunity against cancer. Nature 2017; 547:222–6. [DOI] [PubMed] [Google Scholar]
- 95. Hunt DF, Henderson RA, Shabanowitz J, Sakaguchi K, Michel H, Sevilir N et al Characterization of peptides bound to the class I MHC molecule HLA‐A2.1 by mass spectrometry. Science 1992; 255:1261–3. [DOI] [PubMed] [Google Scholar]
- 96. Rotzschke O, Falk K, Deres K, Schild H, Norda M, Metzger J et al Isolation and analysis of naturally processed viral peptides as recognized by cytotoxic T cells. Nature 1990; 348:252–4. [DOI] [PubMed] [Google Scholar]
- 97. Caron E, Kowalewski DJ, Chiek Koh C, Sturm T, Schuster H, Aebersold R. Analysis of major histocompatibility complex (MHC) immunopeptidomes using mass spectrometry. Mol Cell Proteomics 2015; 14:3105–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Gloger A, Ritz D, Fugmann T, Neri D. Mass spectrometric analysis of the HLA class I peptidome of melanoma cell lines as a promising tool for the identification of putative tumor‐associated HLA epitopes. Cancer Immunol Immunother 2016; 65:1377–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Klar R, Schober S, Rami M, Mall S, Merl J, Hauck SM et al Therapeutic targeting of naturally presented myeloperoxidase‐derived HLA peptide ligands on myeloid leukemia cells by TCR‐transgenic T cells. Leukemia 2014; 28:2355–66. [DOI] [PubMed] [Google Scholar]
- 100. Berlin C, Kowalewski DJ, Schuster H, Mirza N, Walz S, Handel M et al Mapping the HLA ligandome landscape of acute myeloid leukemia: a targeted approach toward peptide‐based immunotherapy. Leukemia 2015; 29:647–59. [DOI] [PubMed] [Google Scholar]
- 101. Walz S, Stickel JS, Kowalewski DJ, Schuster H, Weisel K, Backert L et al The antigenic landscape of multiple myeloma: mass spectrometry (re)defines targets for T‐cell‐based immunotherapy. Blood 2015; 126:1203–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Bassani‐Sternberg M, Barnea E, Beer I, Avivi I, Katz T, Admon A. Soluble plasma HLA peptidome as a potential source for cancer biomarkers. Proc Natl Acad Sci USA 2010; 107:18769–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Ritz D, Gloger A, Neri D, Fugmann T. Purification of soluble HLA class I complexes from human serum or plasma deliver high quality immuno peptidomes required for biomarker discovery. Proteomics 2017; doi:10.1002/pmic.201600364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Yadav M, Jhunjhunwala S, Phung QT, Lupardus P, Tanguay J, Bumbaca S et al Predicting immunogenic tumour mutations by combining mass spectrometry and exome sequencing. Nature 2014; 515:572–6. [DOI] [PubMed] [Google Scholar]
- 105. Gubin MM, Zhang X, Schuster H, Caron E, Ward JP, Noguchi T et al Checkpoint blockade cancer immunotherapy targets tumour‐specific mutant antigens. Nature 2014; 515:577–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Kalaora S, Barnea E, Merhavi‐Shoham E, Qutob N, Teer JK, Shimony N et al Use of HLA peptidomics and whole exome sequencing to identify human immunogenic neo‐antigens. Oncotarget 2016; 7:5110–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Bassani‐Sternberg M, Braunlein E, Klar R, Engleitner T, Sinitcyn P, Audehm S et al Direct identification of clinically relevant neoepitopes presented on native human melanoma tissue by mass spectrometry. Nat Commun 2016; 7:13404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Khodadoust MS, Olsson N, Wagar LE, Haabeth OA, Chen B, Swaminathan K et al Antigen presentation profiling reveals recognition of lymphoma immunoglobulin neoantigens. Nature 2017; 543:723–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Bassani‐Sternberg M, Gfeller D. Unsupervised HLA peptidome deconvolution improves ligand prediction accuracy and predicts cooperative effects in peptide–HLA interactions. J Immunol 2016; 197:2492–9. [DOI] [PubMed] [Google Scholar]
- 110. Sofron A, Ritz D, Neri D, Fugmann T. High‐resolution analysis of the murine MHC class II immunopeptidome. Eur J Immunol 2016; 46:319–28. [DOI] [PubMed] [Google Scholar]
- 111. Pearson H, Daouda T, Granados DP, Durette C, Bonneil E, Courcelles M et al MHC class I‐associated peptides derive from selective regions of the human genome. J Clin Investig 2016; 126:4690–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Aebersold R, Mann M. Mass‐spectrometric exploration of proteome structure and function. Nature 2016; 537:347–55. [DOI] [PubMed] [Google Scholar]
- 113. Menschaert G, Vandekerckhove TT, Baggerman G, Schoofs L, Luyten W, Van Criekinge W. Peptidomics coming of age: a review of contributions from a bioinformatics angle. J Proteome Res 2010; 9:2051–61. [DOI] [PubMed] [Google Scholar]
- 114. Deutsch EW, Overall CM, Van Eyk JE, Baker MS, Paik YK, Weintraub ST et al Human proteome project mass spectrometry data interpretation guidelines 2.1. J Proteome Res 2016; 15:3961–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Olsen JV, Mann M. Status of large‐scale analysis of post‐translational modifications by mass spectrometry. Mol Cell Proteomics 2013; 12:3444–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Andersen R, Donia M, Ellebaek E, Borch TH, Kongsted P, Iversen TZ et al Long‐lasting complete responses in patients with metastatic melanoma after adoptive cell therapy with tumor‐infiltrating lymphocytes and an attenuated IL2 regimen. Clin Cancer Res 2016; 22:3734–45. [DOI] [PubMed] [Google Scholar]
- 117. Prickett TD, Crystal JS, Cohen CJ, Pasetto A, Parkhurst MR, Gartner JJ et al Durable complete response from metastatic melanoma after transfer of autologous T cells recognizing 10 mutated tumor antigens. Cancer Immunol Res 2016; 4:669–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Anderson KG, Stromnes IM, Greenberg PD. Obstacles posed by the tumor microenvironment to T cell activity: a case for synergistic therapies. Cancer Cell 2017; 31:311–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Vetizou M, Pitt JM, Daillere R, Lepage P, Waldschmitt N, Flament C et al Anticancer immunotherapy by CTLA‐4 blockade relies on the gut microbiota. Science 2015; 350:1079–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Sivan A, Corrales L, Hubert N, Williams JB, Aquino‐Michaels K, Earley ZM et al Commensal Bifidobacterium promotes antitumor immunity and facilitates anti‐PD‐L1 efficacy. Science 2015; 350:1084–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Kawakami Y, Eliyahu S, Delgado CH, Robbins PF, Rivoltini L, Topalian SL et al Cloning of the gene coding for a shared human melanoma antigen recognized by autologous T cells infiltrating into tumor. Proc Natl Acad Sci USA 1994; 91:3515–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Kawakami Y, Eliyahu S, Delgado CH, Robbins PF, Sakaguchi K, Appella E et al Identification of a human melanoma antigen recognized by tumor‐infiltrating lymphocytes associated with in vivo tumor rejection. Proc Natl Acad Sci USA 1994; 91:6458–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Wolfel T, Van Pel A, Brichard V, Schneider J, Seliger B, Meyer zum Buschenfelde KH et al Two tyrosinase nonapeptides recognized on HLA‐A2 melanomas by autologous cytolytic T lymphocytes. Eur J Immunol 1994; 24:759–64. [DOI] [PubMed] [Google Scholar]
- 124. Chen YT, Scanlan MJ, Sahin U, Tureci O, Gure AO, Tsang S et al A testicular antigen aberrantly expressed in human cancers detected by autologous antibody screening. Proc Natl Acad Sci USA 1997; 94:1914–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Robbins PF, El‐Gamil M, Li YF, Kawakami Y, Loftus D, Appella E et al A mutated β‐catenin gene encodes a melanoma‐specific antigen recognized by tumor infiltrating lymphocytes. J Exp Med 1996; 183:1185–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Huang J, El‐Gamil M, Dudley ME, Li YF, Rosenberg SA, Robbins PF. T cells associated with tumor regression recognize frameshifted products of the CDKN2A tumor suppressor gene locus and a mutated HLA class I gene product. J Immunol 2004; 172:6057–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Wang RF, Wang X, Rosenberg SA. Identification of a novel major histocompatibility complex class II‐restricted tumor antigen resulting from a chromosomal rearrangement recognized by CD4+ T cells. J Exp Med 1999; 189:1659–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Wolfel C, Drexler I, Van Pel A, Thres T, Leister N, Herr W et al Transporter (TAP)‐ and proteasome‐independent presentation of a melanoma‐associated tyrosinase epitope. Int J Cancer 2000; 88:432–8. [PubMed] [Google Scholar]
- 129. Dalet A, Robbins PF, Stroobant V, Vigneron N, Li YF, El‐Gamil M et al An antigenic peptide produced by reverse splicing and double asparagine deamidation. Proc Natl Acad Sci USA 2011; 108:E323–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Ayyoub M, Stevanovic S, Sahin U, Guillaume P, Servis C, Rimoldi D et al Proteasome‐assisted identification of a SSX‐2‐derived epitope recognized by tumor‐reactive CTL infiltrating metastatic melanoma. J Immunol 2002; 168:1717–22. [DOI] [PubMed] [Google Scholar]


