Summary
Diffuse large B‐cell lymphoma (DLBCL), the most common type of malignant lymphoma, accounts for 30% of adult non‐Hodgkin lymphomas. Epstein–Barr virus (EBV) ‐positive DLBCL of the elderly is a newly recognized subtype that accounts for 8–10% of DLBCLs in Asian countries, but is less common in Western populations. Five DLBCL‐derived cell lines were employed to characterize patterns of EBV latent gene expression, as well as response to cytokines and chemotaxis. Interleukin‐4 and interleukin‐21 modified LMP1, EBNA1 and EBNA2 expression depending on cell phenotype and type of EBV latent programme (type I, II or III). These cytokines also affected CXCR4‐ or CCR7‐mediated chemotaxis in two of the cell lines, Farage (type III) and Val (type II). Further, we investigated the effect of EBV by using dominant‐negative EBV nuclear antigen 1(dnEBNA1) to eliminate EBV genomes. This resulted in decreased chemotaxis. By employing an alternative way to eliminate EBV genomes, Roscovitine, we show an increase of apoptosis in the EBV‐positive lines. These results show that EBV plays an important role in EBV‐positive DLBCL lines with regard to survival and chemotactic response. Our findings provide evidence for the impact of microenvironment on EBV‐carrying DLBCL cells and might have therapeutic implications.
Keywords: chemotaxis, diffuse large B‐cell lymphoma, Epstein–Barr virus, Epstein–Barr virus‐positive diffuse large B‐cell lymphoma, microenvironment
Abbreviations
- ABC
activated B cell
- BL
Burkitt Lymphoma
- Blimp1
B‐lymphocyte‐induced maturation protein 1
- DLBCL
diffuse large B‐cell lymphoma
- dnEBNA1
dominant negative EBNA1
- EBNA1
Epstein–Barr virus nuclear antigen‐1
- EBV
Epstein–Barr virus
- GC
germinal centre
- IL‐4
interleukin‐4
- IRF4
interferon regulatory factor 4
- LMP1
latent membrane protein 1
- STAT3
signal transducer and activator of transcription 3
Introduction
Diffuse large B‐cell lymphoma (DLBCL), the most common type of malignant lymphoma, accounts for 30% of adult non‐Hodgkin lymphomas representing a heterogeneous group of tumours with regard to morphology, phenotype, molecular profile, clinical course and therapeutic response.1
Based on morphology, immunophenotype, viral association [e.g. Epstein–Barr virus (EBV) or human herpesvirus 8] and genetic abnormalities, DLBCLs can be divided into subgroups. According to immunophenotyping and gene expression there are three subgroups: germinal centre (GC) derived, activated B cell (ABC) derived and others. GC DLBCLs express Bcl6 and CD10,2 whereas ABC‐derived DLBCLs express a set of genes that are up‐regulated in peripheral B cells in response to mitogens.
Several non‐random chromosomal translocations have been reported in DLBCL, involving the Bcl6 (35–40%), Bcl2 (13%) or c‐MYC (15%) gene.3 About 45% of the cases carry somatic hypermutations affecting Bcl6, c‐MYC or PAX5.3
EBV‐positive DLBCL is a newly recognized subgroup of DLBCLs according to the current World Health Organization classification.4 It is defined as a clonal EBV carrying B‐cell proliferation. EBV‐positive DLBCL most commonly occurs in patients older than 50 years, in the absence of any known immunodeficiency or previous lymphoma. EBV‐positive DLBCL accounts for 8–10% of the DLBCLs in Asian countries, but is less common in Western populations.5 The clinical features of age‐related EBV‐positive DLBCL are significantly different from those of EBV‐negative DLBCL with a less favourable prognosis.5, 6, 7 Unlike EBV‐positive Burkitt lymphomas (BLs) that only express EBV nuclear antigen 1 (EBNA1), EBV‐positive DLBCLs show different patterns of EBV latent gene expression.5, 8
EBV can be detected in both lymphoid and epithelial cell‐derived malignancies. The latent gene expression observed in the EBV‐carrying tumour cells varies according to the tissue of origin and the activation/differentiation stage of the malignant cells.9 EBV‐carrying BLs express only the EBNA1 protein and non‐coding RNAs including EBV‐encoded small RNAs (EBERs). This is designated type I latency.10 Type II latency, characterized by the expression of EBNA1, latent membrane protein 1 (LMP1) and LMP2, is observed in classical Hodgkin lymphomas11 and some DLBCLs.12 EBV immortalized lymphoblastoid cell lines express six nuclear (EBNA1–6) and three membrane localized (latent membrane proteins LMP1, 2A and 2B) proteins, designated type III latency.9 This pattern is seen in infectious mononucleosis, post‐transplant lymphoproliferative disease and immunoblastic/central nervous system lymphomas in individuals with AIDS.9
By using dominant negative EBNA1 (dnEBNA1) to eliminate EBV genomes, it was shown that the virus directly provides survival factors in BL cell lines and other EBV‐carrying cell lines.13, 14 In addition, small molecules targeting EBNA1 to eliminate EBV have been explored during the past few years. Recently, a selective inhibitor of cyclin‐dependent kinases, Roscovitine, was reported to be able to eliminate EBV genomes by inhibition of EBNA1 phosphorylation.15
In recent years it has been established that the microenvironment plays a role in the development and progression of B‐cell lymphomas, for review see Scott and Gascoyne.16 We have shown that the cytokines interleukin‐4 (IL‐4), IL‐10 and IL‐21 modulate EBV's latent gene expression.17, 18, 19 IL‐4 induces LMP1 expression in the EBV‐infected sub‐line of Hodgkin lymphoma‐derived cell line, KMH2‐EBV.17 IL‐21 was shown to impose a type II EBV gene expression profile on type III and type I BL cells by the repression of the C promoter and activation of the LMP‐1 promoter.18 Furthermore, IL‐21 can induce B‐lymphocyte‐induced maturation protein 1 (Blimp1) expression through the activation of signal transducer and activator of transcription 3 (STAT3).20
Recently the cytokine IL‐21 was reported to induce apoptosis through up‐regulation of c‐MYC in DLBCLs.21 We have shown that IL‐21 stimulation of a type III DLBCL line (Farage) results in enhanced proliferation. When EBV genomes were eliminated, proliferation decreased and apoptosis increased.22
Chemokines play an important role in cell migration, inflammation, haematopoiesis and tumour growth.23 The CXCR4–CXCL12 signalling pathway is involved in both tumour vasculature development and in the growth and metastatic spread of CXCR4‐expressing tumours.24 CCR7 expression is associated with cancer metastases.25 We have previously shown that EBV modulates the CXCR4 and CCR7 receptor expression and the corresponding chemokine‐induced migration in primary tonsillar B cells.26, 27
The expression pattern of EBV‐encoded genes is associated with the cellular phenotype, as well as chemotactic response and apoptotic proneness. In this paper we have characterized four EBV‐carrying and one EBV‐negative DLBCL cell lines with regard to the expression of genes associated with GC‐related DLBCL phenotypes and EBV gene expression profile. We report the effect of IL‐4 or IL‐21 treatment on apoptosis, chemotaxis and EBV gene expression. Further, in one EBV‐positive DLBCL cell line we showed that EBV modulates the CXCR4‐ and CCR7‐mediated migration, by eliminating EBV genomes by dnEBNA1, which interrupts the maintenance function of EBNA1 in viral genomes.14 The role of EBV in EBV‐positive DLBCL lines was further investigated using Roscovitine, another tool to inhibit EBNA1 function.
Materials and methods
Cell lines
Farage was kindly provided by Dr Hannah Ben‐Bassat (Laboratory of Experimental Surgery, Hadassah University Hospital, Jerusalem).Val was provided by Dr Christian Bastard (INSERM, France). OPL2 was provided by Dr Katsuyuki Aozasa (Department of Pathology, School of Medicine, Osaka University Graduate, Japan). DOHH2 was provided by Dr Jude Fitzgibbon (Centre for Medical Oncology Laboratory, London, UK). OCI‐Ly19 was provided by Dr Ari Melnick (Division of Hematology/Oncology, Department of Medicine, Weill Cornell Medical College, New York, NY). The EBV‐negative lines Jurkat (T‐cell acute lymphoblastic leukaemia), BJAB (B‐cell lymphoma cell line), DEV (Hodgkin's lymphoma cell line) and the EBV‐positive type III BL line Raji, the EBV‐positive CBM1 lymphoblastoid cell line and Granta (mantle cell lymphoma line) were used as controls.
Plasmid
pBSN‐tetOff‐dnEBNA1, which encodes amino acids (aa) 379 to 386 fused in frame to aa 451 to 641 of EBNA1 was a kind gift from Dr Seiji Maruo (Department of Tumour Virology, Institute for Genetic Medicine, Hokkaido University, Sapporo, Japan).
Establishment of stable dnEBNA1 cell line
Transfection of Farage with pBSN‐tetOff‐dnEBNA1 (dominant negative EBNA1 expression vector): Farage cells were transfected with the dominant negative EBNA1 expression vector,pBSN‐tetOff‐dnEBNA1 with the Nucleofector I device and Cell Line Nucleofector Kit V (Lonza, Basel, Switzerland). Briefly, after the PBS wash 100 μl of solution V was added to 2 × 106 cells. Thereafter, 2 μg of plasmid DNA was mixed with cells and solution V. The mixture was transferred to the specific cuvette and electroporated with the programme M13. After electroporation the mixture was transferred to pre‐warmed RPMI‐1640 medium with FCS but without antibiotics. Two days later the cells were cloned at 10 000 cells per well in 96‐well plates in complete RPMI‐1640 medium supplemented with 700 μg/ml G418 and 1 μg/ml doxycycline. The emerging clones were expanded and studied.
Cytokines
The recombinant human IL‐4 and IL‐21 were purchased from Peprotech (Rocky Hill, NJ, United States). Cells were cultured at 0.5 × 106 cells per 3 ml of complete RPMI‐1640 medium and treated with 100 ng/ml IL‐21 or 50 ng/ml IL‐4. For long‐term treatments cells were replated every third day with 0.5 × 106 cells per 3 ml with fresh IL‐21 (100 ng/ml) or IL‐4 (50 ng/ml).
Flow cytometry
Incubation of 1 ×106 cells with saturating amounts of the following monoclonal antibodies was performed for 30 min, at + 4°: phycoerythrin‐conjugated anti‐CXCR4 (BD Pharmingen, San Diego, CA), anti‐CCR7 (FAB197F), isotype‐matched controls, mouse IgG2A (IC003F) and mouse 2B (IC0041P) (R&D Systems, Minneapolis, MN). After washing, the cells were fixed in 1% formaldehyde in PBS and analysed in a FACScan using cellquest software (Becton Dickinson, Stockholm, Sweden).
SDS–PAGE and immunoblotting
Total cell lysates were prepared in loading buffer [60% glycerol, 50 mm Tris–HCl (pH 6.5), 2% SDS, 0.1% bromophenol blue, 0.02% 2‐mercaptoethanol] and then denatured by boiling for 10 min. Aliquots of total cell lysates corresponding to 1 × 105 to 2.5 × 105 cells (depending on the protein in question) were loaded in each well, electrophoretically separated on 8% SDS–PAGE gel, and transferred to PVDF membrane at 100 V for 1 hr. After blocking the membranes for 1 hr at room temperature with 7.5% non‐fat dried milk in PBS/0.1% Tween 20 (PBS‐T), they were incubated with the primary antibodies overnight at 4°. After extensive washes with PBS‐T the blots were incubated with horseradish peroxidase‐conjugated donkey anti‐rabbit IgG (GE Healthcare Biosciences, Little Chalfont, Buckinghamshire, UK; dilution 1 : 4000), goat anti‐mouse immunoglobulin, or rabbit anti‐goat‐immunoglobulin antibodies (both from Dako Cytomation, Fort Collins, CO, United States) for 1 hr at room temperature. After washing the membranes with balanced salt solution/0.5% Tween‐20, they were developed with Amersham ECL Plus detection reagent (GE Healthcare Biosciences). For visualization, Amersham Hyperfilm ECL (GE Healthcare Biosciences) film was exposed to the developed membranes. The following antibodies were used as primary antibodies in immunoblotting: S12 supernatant (anti‐LMP1), PE‐2 (anti‐EBNA2; Dako), NCL‐EBV‐CS1‐4 (mouse anti‐LMP1; Novocastra Laboratories, Newcastle upon Tyne, UK), OT1x (mouse anti‐EBNA‐1; gift from J.M. Middeldorp, Department of Pathology, Vrije Universiteit Medical Centre, Amsterdam, the Netherlands), AC‐15 (mouse anti‐human β‐actin; Sigma‐Aldrich, St Louis, MO, United States), D‐8 (mouse anti‐Bcl6), N‐262 (rabbit anti‐c‐myc), C‐20 (rabbit anti‐human Pax‐5), 9E10 (mouse anti‐c‐myc), MUM1p [mouse anti‐human‐interferon regulatory factor 4 (IRF4); Dako Cytomation], mouse anti‐Bcl2 (Dako Cytomation) and 3H2‐E8 (mouse anti‐human Blimp‐1; Novus Biologicals, Littleton, CO, United States).
Transmigration
Transwell culture was performed in duplicates on DLBCL cells using 5‐μm‐diameter pore filters (Transwell, 24‐well plate; Costar, Cambridge, MA): 2.5 × 105 cells were resuspended in 100 μl RPMI‐1640 medium supplemented with 1% fetal calf serum for Farage and Val and 10% for dnEBNA1‐Farage, 2 mm glutamine, penicillin and streptomycin and thereafter loaded into the upper chamber of the Transwell filter. To examine the migration of the cells towards CXCL12 and CCL21, 600 μl medium containing 1 μg/ml of CXCL12 or 250 ng/ml of CCL21 (Peprotech) was added to the lower well and the plate was incubated at 37° for 4 hr. Thereafter, the migrated cells in the lower wells were collected, fixed with 200 μl 1% formaldehyde in PBS and counted in a FACScan flow cytometer for 60 seconds with high speed (Becton Dickinson).
Growth curve and cell viability measurement of EBV knockout experiment in dnEBNA1‐Farage
Cells were washed with PBS four times and cultured in 5 ml of medium (1 × 105/ml) with or without doxycycline. Every 5 days, the viable cell number was estimated by the Erythrosin B (Sigma‐Aldrich) exclusion assay and the cultures were diluted 10 times by fresh medium. Cells were re‐plated every 60 hr with fresh medium to maintain optimum growth. Viable cell numbers were calculated based on the expansion from the initial 1× 105/ml cells.
Apoptosis detection
FITC‐conjugated Annexin V reagent (BD Pharmingen) was used to detect apoptosis according to the manufacturer's instructions.
Roscovitine treatment
Roscovitine (Calbiochem, San Diego, CA, United States) was dissolved in DMSO. Cells were cultured in 3 ml medium (2 × 105/ml) with 3 μm Roscovitine or DMSO. Every 3 days, the viable cell number was estimated by the Erythrosin B (Sigma‐Aldrich) exclusion assay and the cultures were diluted 10 times with fresh medium. Cells were re‐plated every 3 days with fresh medium containing 3 μm Roscovitine or DMSO to maintain optimum growth. Viable cell numbers were calculated based on the expansion from the initial 2× 105/ml cells.
Results
Characterization of EBV‐positive DLBCL lines
EBV gene expression in DLBCL cell lines
Five DLBCL lines were analysed for expression of three EBV‐encoded, growth transformation‐associated proteins (EBNA1, EBNA2 and LMP1) by Western blot. These proteins have a decisive role in the phenotype of EBV‐infected cells. Farage (characterized in our previous paper22) and DOHH2 expressed EBNA1, EBNA2 and LMP1, so demonstrating a latency III pattern. Val showed latency type II, expressing EBNA1 and LMP1 but not EBNA2. OPL2 cells expressed EBNA2 and LMP1 but not EBNA1 in line with published data that the virus is integrated into the genome.28 OCI‐Ly19 did not express any of the EBV‐encoded proteins (Fig. 1a and Table 1).
Figure 1.
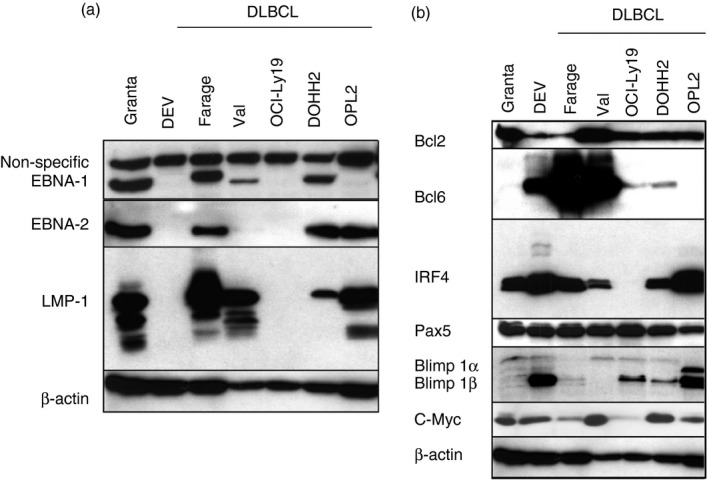
Characterization of Epstein–Barr virus‐positive (EBV +) diffuse large B‐cell lymphoma (DLBCL) lines. (a) EBV latent gene expression in DLBCL cell lines. Immunoblot analysis of total cell extracts of Granta, DEV, Farage, Val, OCI‐Ly19, DOHH2, OPL2 lines with EBV nuclear antigen 1 (EBNA1), EBNA2, latent membrane protein 1 (LMP1) (S12) and β‐actin antibodies. (b) Cellular gene expression in DLBCL cell lines. Immunoblot analysis of total cell extracts of Granta, DEV, Farage, Val, OCI‐Ly19, DOHH2, OPL2 cells with Bcl2, Bcl6, IRF4, β‐actin, PAX5 and Blimp1 antibodies.
Table 1.
Epstein–Barr virus and cellular protein characteristics of the diffuse large B‐cell lymphoma cell lines
| Farage | Val | OCI‐Ly19 | DOHH2 | OPL2 | |
|---|---|---|---|---|---|
| EBNA1 | + | + | − | + | − |
| EBNA2 | + | − | − | + | + |
| LMP1 | + | + | − | + | + |
| Bcl2 | + | +++ | ++ | ++ | ++ |
| Bcl6 | ++++++ | ++++ | + | + | − |
| Pax5 | ++ | ++ | ++ | ++ | + |
| IRF4 | ++ | + | − | + | ++++ |
| Blimp1α | − | − | − | − | + |
| Myc | ++ | ++++ | + | +++ | ++ |
Cellular gene expression in DLBCL cell lines
To characterize the cellular phenotype, we mapped the expression of cellular genes involved in B‐cell differentiation and/or B lymphoma development such as Bcl6, Bcl2, IRF4, Blimp1 and PAX5 (Fig. 1b). Farage and Val both expressed Bcl2 and Bcl6, but were Blimp1 negative. DOHH2 and OPL2 expressed Bcl2, IRF4 and Blimp1α. DOHH2 expressed Bcl6 at a low level whereas OPL2 was negative. OCI‐Ly19 expressed Blimp1β and a low level of Bcl6 but not IRF4. All lines expressed PAX5 (Fig. 1b). The expression of Bcl6 shows that Farage, Val, DOHH2 and OCI‐Ly19 adhere to the GC phenotype while Bcl6‐negative OPL2 represent the ABC subtype.
The effect of IL‐4 and IL‐21 on LMP1 expression in EBV‐positive DLBCL lines
Expression of LMP1 was mapped in the four EBV‐positive DLBCL cell lines after IL‐4 or IL‐21 treatment for 3 days. LMP1 was up‐regulated in EBV‐positive DLBCL lines, but with less induction after IL‐4 stimulation compared with that of IL‐21 (Fig. 2a,b).
Figure 2.
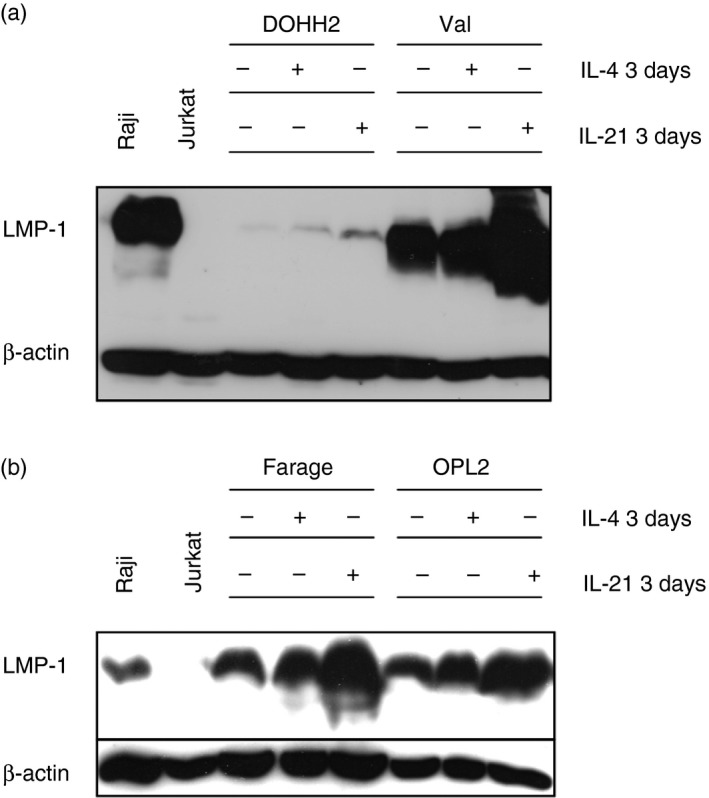
Latent membrane protein 1 (LMP1) expression in Epstein–Barr virus (EBV) ‐positive diffuse large B‐cell lymphoma (DLBCL) cell lines after 3 days of interleukin‐4 (IL‐4) or IL‐21 treatment. (a,b) Immunoblot analysis of total cell extracts of Farage, Val, DOHH2 and OPL2 lines treated with 50 ng/ml IL‐4 or 100 ng/ml IL‐21 for 3 days with β‐actin and LMP1 (S12) antibodies.
The effect of IL‐4 and IL‐21 on the modulation of EBV latent genes and cellular genes in Farage
As LMP1 and/or EBNA2 can be modulated by IL‐4 or IL‐21 treatment in EBV‐positive DLBCL lines, we performed kinetic experiments to examine whether the effect of these cytokines in Farage is transient. LMP1 was up‐regulated by day 3 and rose further until day 6 after both IL‐4 and IL‐21 treatment. EBNA1 and EBNA2 expression decreased significantly by day 3 and EBNA2 was further decreased on day 6 after IL‐21 treatment (Fig. 3a).
Figure 3.
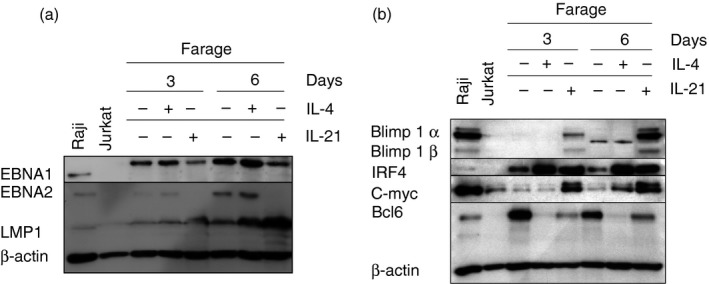
Effects of interleukin‐4 (IL‐4) and IL‐21 on the modulation of genes in Farage. (a) Epstein–Barr virus (EBV) latent gene expression after 3 days and 6 days IL‐4‐ or IL‐21‐treated Farage. Immunoblot analysis of total cell extracts of Farage cells treated with 100 ng/ml IL‐21 or 50 ng/ml IL‐4 for 3 days and 6 days with EBV nuclear antigen 1 (EBNA1), EBNA2, latent membrane protein 1 (LMP1) and β‐actin antibodies. Raji was used as a positive control and Jurkat was used as a negative control. (b) Cellular gene expression after 3 days and 6 days IL‐4 or IL‐21 treated Farage. Immunoblot analysis of total cell extracts of Farage cells treated with 100 ng/ml IL‐21 or 50 ng/ml IL‐4 for 3 days and 6 days with Bcl6, IRF4, Blimp1, c‐MYC and β‐actin antibodies. Raji was used as a positive control and Jurkat was used as a negative control.
Interleukin‐21 has been shown to modulate IRF4, Bcl6 and Blimp1 in a type I BL cell line, Jijoye M13.18 Therefore we studied the effect of IL‐4 and IL‐21 on the expression of these genes together with c‐MYC in Farage. Both cytokines induced IRF4 with concomitant down‐regulation of Bcl6. IL‐4 showed a stronger effect than IL‐21. Blimp1 protein was only detected in the IL‐21‐treated cells (Fig. 3b). c‐MYC was up‐regulated 3 days after IL‐21 treatment whereas IL‐4 up‐regulated c‐MYC only after 6 days, but to a lower level compared with IL‐21. The induction of Blimp1 and down‐regulation of Bcl6 is in line with a switch from the GC to the ABC phenotype (Fig. 3b). Kinetic experiments showed that the IL‐4 and IL‐21 effects on the EBV gene profile and on cellular genes are extended.
IL‐4 and IL‐21 treatment induced chemokine receptor modulation and receptor‐mediated migration
The effect of IL‐4 and IL‐21 on chemokine receptor expression
Two chemokine receptors that play a role in B‐cell migration and homing, CXCR4, and CCR7, were assayed in Val (type II) and Farage (type III). IL‐4 and IL‐21 induced different patterns of chemokine receptor expression. IL‐4 did not induce CXCR4 expression in Farage but significantly up‐regulated CCR7. In contrast, a significant up‐regulation of CXCR4 and down‐regulation of CCR7 was observed in IL‐4‐treated Val. IL‐21 up‐regulated both CXCR4 and CCR7 in Farage whereas it reduced CXCR4 and had no effect on CCR7 expression in Val (Fig. 4a). As expected, LMP1 was up‐regulated in both cell lines after IL‐4 and IL‐21 treatment. IL‐21 treatment resulted in EBNA2 down‐regulation in Farage whereas IL‐4 did not induce any significant change of EBNA2 expression (Fig. 4c). As a control, the modulation of LMP1 and EBNA2 were examined after IL‐4 and IL‐21 treatment (Fig. 4b).
Figure 4.
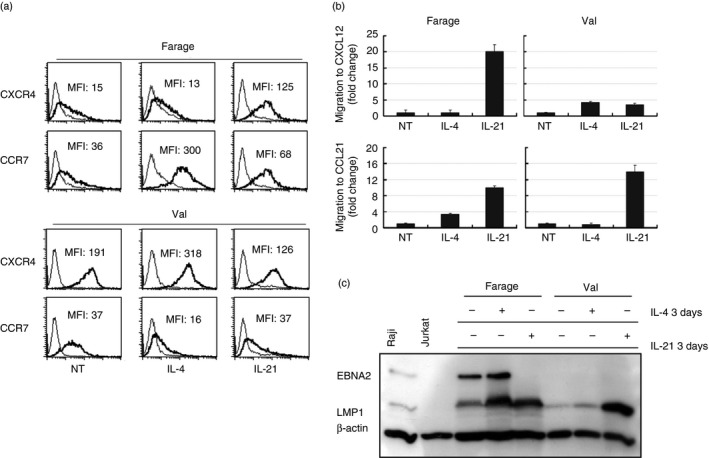
Interleukin‐4 (IL‐4) and IL‐21 treatment induced chemokine receptor modulation and cell migration in Val and Farage. (a) FACS analysis of CXCR4 and CCR7 expression on Farage and Val cells treated with either 50 ng/ml IL‐4 or 100 ng/ml IL‐21 after 3 days. MFI stands for mean fluorescence intensity (b) Chemotactic response of Farage and Val cells treated with either 50 ng/ml IL‐4 or 100 ng/ml IL‐21 for 3 days to CXCL12 and CCL21. Migrated cells were quantified by FACS for 60 seconds from the wells that contained the ligand and were compared with the input population after subtraction of background migration. The result is presented as relative fold change as the values of migration in non‐treated (NT) cells were arbitrarily defined as 1. The mean values are shown, calculated from three experiments. The error bars indicate ± SD (n = 3). (c) Immunoblot analysis of total cell extracts of Farage and Val treated with 50 ng/ml IL‐4 or 100 ng/ml IL‐21 for 3 days with β‐actin, LMP1 (S12) and EBV nuclear antigen 2 (EBNA2) antibodies.
Modulation of cell migration by IL‐4 and IL‐21 in Farage and Val
Next we studied the effect of IL‐4 and IL‐21 on the chemotactic response. In Farage, IL‐4 increased migration towards CCL21 (the ligand of CCR7) but not towards CXCL12 (the ligand of CXCR4); whereas in Val, IL‐4 only induced increased migration to CXCL12.
Interleukin‐21 induced a significant increase in chemotactic response to both CCL21 and CXCL12 in Farage. In Val the CCL21‐induced chemotaxis was increased whereas the response to CXCL12 was weaker (Fig. 4b).
EBV provides survival factors to EBV‐positive DLBCL lines
Expression of EBNA1 was gradually down‐regulated in dnEBNA1‐Farage transfectant cultured without doxycycline during 20 days compared with the control cultured with doxycycline (Fig. 5a). Following down‐regulation of EBV encoded genes by dnEBNA1, elevated apoptosis could be detected at 10 days after doxycycline removal in dnEBNA1‐Farage cells. The level of apoptosis further increased over time compared with the cells cultured with doxycycline (Fig. 5b,c). The effect was correlated with a decrease of viable cells over time (Fig. 5d).
Figure 5.
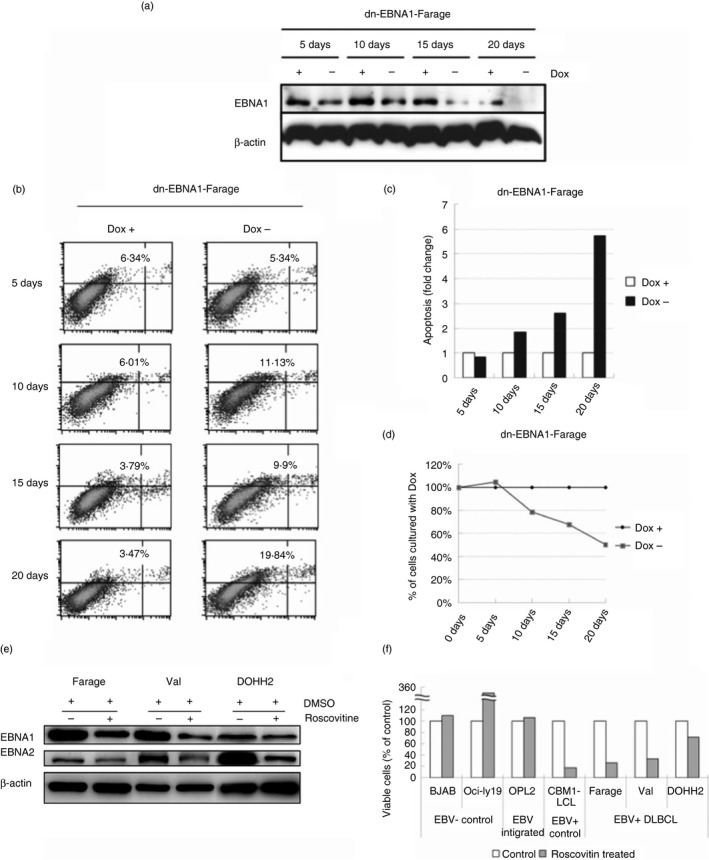
Epstein–Barr virus (EBV) provides survival factors to EBV‐positive diffuse large B‐cell lymphoma (DLBCL) lines. (a) Immunoblot analysis of total cell extracts of dominant negative EBV nuclear antigen 1 (dnEBNA1) ‐Farage cultured with or without 1 μg/ml doxycycline (Dox) for 5, 10, 15 and 20 days with β‐actin and EBNA1 antibodies. (b) dn‐EBNA1‐Farage cells were cultured with or without 1 μg/ml Dox for 5, 10, 15 and 20 days and apoptosis was assayed using Annexin V‐FITC and propidium iodide (PI) according to the manufacturer's instructions. The x‐axis of dual parametric dot plots represent PI fluorescence and y‐axis represent Annexin V‐FITC fluorescence. Cells were considered ‘apoptotic’ if positive for Annexin V. (c) Bar charts showing the relative percentage of apoptotic dnEBNA1‐Farage cells (Annexin V+) cultured with Dox for 5, 10, 15 and 20 days in comparison with the cells cultured without Dox. The result is presented as relative fold change of the percentage of apoptotic cells where the control cultured with Dox was arbitrarily defined as 1. (d) Relative viable dn‐EBNA1‐Farage cells (Annexin V‐) cultured without Dox for 5, 10, 15 and 20 days in comparison with the control cells cultured without Dox. The result is presented as a percentage of the control cells where control cells cultured with Dox, arbitrarily defined as 100%. (e) Immunoblot analysis of total cell extracts of Farage, Val and DOHH2 treated with 3 μg/ml Roscovitine for 12 days with β‐actin EBNA2 and EBNA1 antibodies. (f) Relative viable cells of the Roscovitine (3 μg/ml, 12 days)‐treated Farage (EBV + DLBCL), Val (EBV + DLBCL), DOHH2 (EBV + DLBCL), OCI‐Ly19 (EBV – DLBCL), BJAB (EBV – lymphoma), OPL2 (EBV‐integrated DLBCL) and CBM1 lymphoblastoid cell line (EBV + LCL) as measured by dye‐exclusion assay. The result is presented as percentage of the control cells treated with DMSO as the values of control cells were arbitrarily defined as 100%.
To further study the role of EBV in EBV‐positive DLBCLs, we treated EBV‐positive DLBCL lines with Roscovitine to eliminate EBV. After 12 days of culture with 3 μm Roscovitine, EBNA1 and EBNA2 expression was down‐regulated in Farage, Val and DOHH2 (Fig. 5e). Cell proliferation was inhibited after Roscovitine treatment in the three EBV‐positive DLBCL lines tested (Fig. 5f). In contrast the proliferation did not decrease in the EBV‐negative B‐cell lymphoma line BJAB, the EBV‐negative DLBCL line OCI‐Ly19, and in the DLBCL line OPL2 with integrated EBV. In fact, a significant induction of cell growth was even seen in the Roscovitine‐treated OCI‐Ly19 cell line.
Down‐regulation of EBNA1 in Farage resulted in decreased specific migration to CXCL12 and CCL21 induced by IL‐4 or IL‐21 treatment
As it was shown that EBV‐encoded genes can modulate migration,26, 27 we investigated the effect of down‐regulation of EBNA1 on migration (Fig. 6a). Treatment with IL‐4 or IL‐21 increased CXCR4 and CCR7 expression after the switch on of dnEBNA1 expression for 20 days (Fig. 6b). However, this elevated chemokine receptor expression did not increase the specific migration. On the contrary, a decreased induction of migration was observed after IL‐4 or IL‐21 treatment in dnEBNA1‐expressing cells compared with cells where dnEBNA1 was switched off (Fig. 6c).
Figure 6.
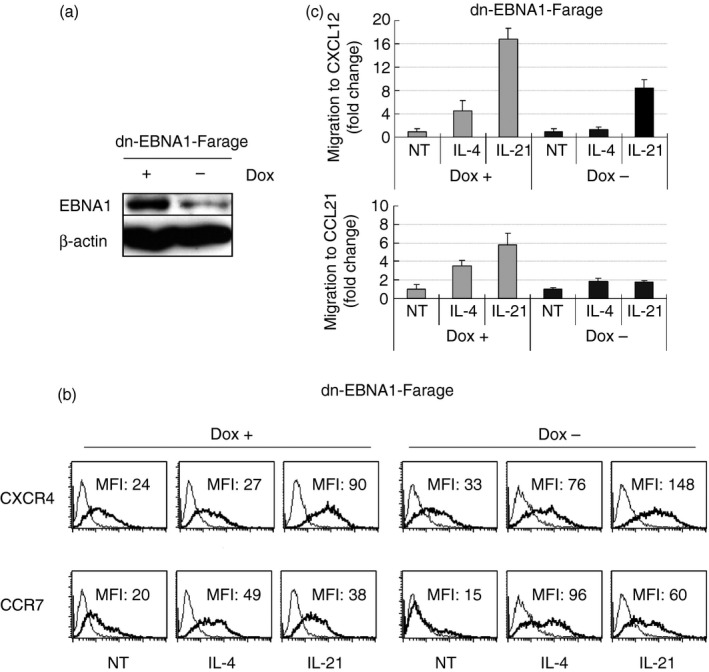
Down‐regulation of Epstein–Barr virus (EBV) encoded genes in Farage decreased specific chemotaxis induced by cytokines. (a) Immunoblot analysis of total cell extracts of dominant negative EBV nuclear antigen 1 (dnEBNA1) ‐Farage cultured with or without 1 μg/ml doxycycline (Dox) for 20 days with β‐actin and EBNA1 antibodies. (b) The expression of CXCR4, and CCR7 on dnEBNA1‐Farage cultured with or without 1 μg/ml Dox for 20 days and then treated with IL‐4 or IL‐21 for 24 hr was assessed by FACS analysis. (c) dnEBNA1‐Farage cultured with or without 1 μg/ml Dox for 20 days and then treated with IL‐4 or IL‐21 for 24 hr and the chemotactic response induced by CXCL12 and CCL21 in cytokine‐treated and control cells were compared in a 4‐hr Transwell migration assay. The migrated cells were counted by FACS for 60 seconds from the wells that contained cells that migrated to the ligand compared with the input population after subtraction of background migration. The result is presented as relative fold change where migration in non‐treated (NT) cells was arbitrarily defined as 1. The mean values are shown, calculated from three experiments. The error bars indicate ± SD (n = 3).
Discussion
In this study, we have characterized EBV status in five DLBCL lines for expression of EBV latency‐associated genes and of some relevant cellular genes, mapping the phenotype and DLBCL subtyping. Unlike EBV‐positive BL tumours, which only express EBNA1, EBV‐positive DLBCLs can also express EBNA2 and LMP1,5 consistent with latency type II or III patterns. We found both patterns in our cell lines, Farage being type III and Val being type II. In OPL2, the EBV genome is integrated in the host genome,28 which might explain how it can maintain EBV genomes without EBNA1 expression, and with EBNA2 and LMP1 expression. DOHH2 was reported to be EBV negative29 but we now show that it is EBV positive with a type III profile. Our results on EBV gene expression patterns conform with published data on the EBV status in DLBCL tumours.5, 6, 7
Blimp1α orchestrates plasma cell differentiation by repressing GC‐stage‐related genes, while at the same time activating those programmes associated with plasma cell functions. In contrast, Blimp1β may counteract the ability of Blimp1α to drive plasma cell differentiation. Therefore, Farage, Val, DOHH2 and OCI‐Ly19 showed a GC B‐cell phenotype whereas OPL2 represents an atypical ABC phenotype, as PAX5 and the plasma cell differentiation marker, Blimp1α, were both expressed. In DLBCLs, Bcl6 expression and EBNA2 expression are usually inversely correlated.30 However, Farage expressed both EBNA2 and Bcl6. This might relate to the low expression of EBV microRNAs in this cell as reported by Martin‐Perez et al.31 IRF4 and Bcl2 were also expressed, which are not normally expressed in GC B cells. The co‐expression might be a result of Bcl2 chromosomal translocation or due to the transcription factor Miz1 that disrupts the Bcl6 suppression of Bcl2.32 Expression of both Bcl2 and Bcl6 can be seen in Val and DOHH2 as well. OCI‐Ly19 was Bcl6‐positive, indicating a GC origin also of this cell.
LMP1 expression was up‐regulated in the EBV‐positive DLBCL lines by either IL‐4 or IL‐21. Due to the high level of LMP1 induced by IL‐4 and IL‐21, we pursued our experiments with the type III Farage cell line. LMP1 expression was demonstrated to be the most important marker for poor prognosis with short survival time compared with other tested molecular markers such as vascular endothelial growth factors A or C in DLBCLs.33 Importantly, this IL‐4‐ and IL‐21‐induced LMP1 expression might worsen the prognosis of EBV‐positive DLBCLs. This is particularly worrisome as IL‐21 has been suggested as a treatment option.21, 34, 35
Interleukin‐21 can induce human B‐cell activation, differentiation and proliferation.36 In both IL‐4‐treated and IL‐21‐treated Farage cells, LMP1 up‐regulation was followed by an elevated level of IRF4 and down‐regulation of Bcl6. This induction of IRF4 can be the result of the activation of nuclear factor‐κB by LMP1, a CD40 mimic.36, 37 IRF4 was shown to bind to the Bcl6 promoter and inhibit its expression.37 It may be conjectured that IL‐4‐ and IL‐21‐induced LMP1 could be responsible for the up‐regulation of IRF4 and the down‐regulation of Bcl6 expression. Additionally, in GC B‐cell‐like DLBCL, IL‐4 was reported to down‐regulate Bcl6 through induction of STAT6 whereas IL‐21‐mediated down‐regulation of Bcl6 and up‐regulation of IRF4 could be to the result of induction of STAT1 and STAT3.38, 39 Given the recent finding that IRF4 plays an essential oncogenic role in ABC DLBCL,40 the IL‐4‐ and IL‐21‐induced IRF4 expression in EBV‐positive DLBCL might contribute to adverse growth and survival of tumour cells in the patients. Blimp1α is the master regulator of plasma cell differentiation.41 The induction of Blimp1α by IL‐21 in the Farage cells indicated differentiation towards a plasma cell phenotype that is frequently associated with poor prognosis.
Recently, IL‐21 was reported to induce apoptosis in DLBCL cell lines with unknown EBV carrier status through up‐regulation of c‐MYC.21 In a recent study, we found that EBV counteracts IL‐21‐induced apoptosis in Farage, indicating an important role of EBV in DLBCL.22 With the help of dnEBNA1,13, 14, 15, 42, 43 it was shown that EBV blocks apoptosis and induces proliferation in EBV‐positive BLs.13, 14 Although the available data so far implicate EBV positivity as a potential predictor of worse prognosis in patients with DLBCL,44 the role of EBV in DLBCL is far from understood. Using dnEBNA1 and Roscovitine, we eliminated EBV from EBV‐positive DLBCL lines to dissect the role of the virus. This resulted in increased apoptosis. Furthermore, cell proliferation was inhibited, indicating that EBV contributes to sustain the growth of EBV‐positive DLBCLs. This is also supported by decreased cell proliferation after 12 days of treatment with Roscovitine in Farage, Val and DOHH2. Unexpectedly, the same dose of Roscovitine treatment promoted the growth of the EBV‐negative DLBCL line, OCI‐Ly19, pointing to some off‐target effects of the drug. Our findings speak for the use of small molecules targeting EBV genes as a future possibility in DLBCL treatment. Given the fact that EBV‐positive individuals with DLBCL showed a poorer treatment response and worse prognosis compared with EBV‐negative patients,5, 6, 7 alternative therapies need to be developed for use in EBV‐positive DLBCL.
Epstein–Barr virus modulated chemokine receptor expression in the DLBCL cell lines, in line with other observations on B cells. In the B lymphoma line BJAB, CXCR4 was down‐regulated by LMP1 or EBNA245 whereas CCR7 was up‐regulated by EBNA2 in the EBV‐negative Burkitt lymphoma line BL41.46 However, some established LMP+ BL lines express CXCR4 that may depend on other EBV proteins in contrast to EBNA2 or LMP1 transfectant, which express only one of the EBV proteins.42, 45 Primary EBV infection of tonsillar B cells with expression of EBNA2 and LMP1 led to reduction in the expression of CXCR4 and CCR7.26, 27 Immunohistochemical studies on chemokine receptor expression in DLBCL reveal that 15/16 expressed CXCR4 and 14/16 were positive for CXCR5. Only 3/15 had a low percentage of CCR7‐positive cells and the rest were negative.47 The EBV status of the DLBCLs in this study was not reported.
Our results show that EBNA2 down‐regulation by IL‐21 treatment of Farage may play a role in the increased CXCR4 expression and the ligand‐induced migration. This is in line with a previous report on EBNA2‐induced down‐regulation of CXCR4.45 After EBV suppression by dnEBNA1 in Farage, CXCL12‐ and CCL21‐induced migration decreased, indicating that EBV may modulate chemotaxis upon cytokine treatment. Our results together with published data suggest that the chemokine‐induced migration is due to the induction of LM 1 by IL‐21, as seen in Farage and Val. IL‐21 stimulation also induced CXCR4 and CCR7 expression in Farage, but there was no induction of these receptors in Val. However, the CCR7 receptor was functional as CCL21‐induced migration. The level of migration may not depend directly on receptor density. For example, we previously found that CD40 + IL‐4 treatment of primary tonsillar B cells increased the CXCL12‐induced chemotactic response although the expression level of its receptor CXCR4 decreased.26
CCR7 has been implicated to be involved in nodal dissemination and CXCL12, the ligand of CXCR4, promotes both tumour vasculature development and supports growth and metastasis in CXCR4‐positive tumours. CXCR4 is also associated with bone marrow involvement of nodal lymphomas.48 Recently, CXCR4 was suggested to be an independent prognostic marker in patients with DLBCL.49 Furthermore, plasma cells home to bone marrow through the CXCR4–CXCL12 axis.50 The IL‐21‐induced increase of CXCL12‐mediated migration and Blimp1 expression in Farage may therefore promote plasma cell differentiation, homing to bone marrow and, as a consequence tentatively even lead to activation of EBV lytic cycle and virus production.51
Interleukin‐21 treatment of patients with DLBCL with EBV‐carrying tumours may induce migration of tumour cells, so supporting metastatic spread. The mechanism would be increased CXCR4 expression with migration to vascularized areas where CXCL12 level is high. In a NOD/SCID mouse model CXCR4 neutralization before intravenous injection of the BL line Namalva lowered the tumour cell extravasation. Between 32 and 47% of the CXCR4‐neutralized cells circulated 24 hr after intravenous injection whereas none was found in the control.52 Recently, expression of CXCR4 has been shown to be an independent factor predicting poorer progression‐free survival in GC B‐cell‐like DLBCLs due to CXCR4‐associated chemotaxis.53 Furthermore, induced CCR7‐mediated migration may increase the nodal dissemination.
Our findings provide evidence for an impact of microenvironmental factors in EBV‐carrying DLBCLs. Together with the role of EBV this should be considered in future therapeutic approaches.
Disclosures
The authors have no conflicting financial interests.
Acknowledgements
We thank Dr Seiji Maruo for providing dnEBNA1 plasmid. We thank the authors mentioned in the text for kindly providing the cell lines. This work was supported by the Swedish Cancer Society, Cancer Research Institute (New York), Concern Foundation (Los Angeles), The Fundamental Research Funds for the Central Universities (ZD2014YW0029), National Natural Science Foundation of China (81402964), Natural Science Foundation of Jiangsu Province (BK20140665) and Jiangshu ‘Shuang Chuang’ team.
We report that Epstein–Barr virus (EBV) plays an important role in EBV‐positive diffuse large B‐cell lymphoma (DLBCL) lines with regard to survival and microenvironment‐induced chemotaxis. We believe that these findings not only provide evidence for the impact of microenvironmental factors on EBV‐carrying DLBCL cells but might also have future therapeutic implications.
References
- 1. A clinical evaluation of the international lymphoma study group classification of non‐Hodgkin's lymphoma. The non‐Hodgkin's lymphoma classification project. Blood 1997; 89:3909–18. [PubMed] [Google Scholar]
- 2. Alizadeh AA, Eisen MB, Davis RE, Ma C, Lossos IS, Rosenwald A et al Distinct types of diffuse large B‐cell lymphoma identified by gene expression profiling. Nature 2000; 403:503–11. [DOI] [PubMed] [Google Scholar]
- 3. Gurbuxani S, Anastasi J, Hyjek E. Diffuse large B‐cell lymphoma – more than a diffuse collection of large B cells: an entity in search of a meaningful classification. Arch Pathol Lab Med 2009; 133:1121–34. [DOI] [PubMed] [Google Scholar]
- 4. Swerdlow SH, Campo E, Pileri SA, Harris NL, Stein H, Siebert R et al The 2016 revision of the World Health Organization classification of lymphoid neoplasms. Blood 2016; 127:2375–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Adam P, Bonzheim I, Fend F, Quintanilla‐Martinez L. Epstein–Barr virus‐positive diffuse large B‐cell lymphomas of the elderly. Adv Anat Pathol 2011; 18:349–55. [DOI] [PubMed] [Google Scholar]
- 6. Oyama T, Ichimura K, Suzuki R, Suzumiya J, Ohshima K, Yatabe Y et al Senile EBV+ B‐cell lymphoproliferative disorders: a clinicopathologic study of 22 patients. Am J Surg Pathol 2003; 27:16–26. [DOI] [PubMed] [Google Scholar]
- 7. Park S, Lee J, Ko YH, Han A, Jun HJ, Lee SC et al The impact of Epstein–Barr virus status on clinical outcome in diffuse large B‐cell lymphoma. Blood 2007; 110:972–8. [DOI] [PubMed] [Google Scholar]
- 8. Wong HH, Wang J. Epstein–Barr virus positive diffuse large B‐cell lymphoma of the elderly. Leuk Lymphoma 2009; 50:335–40. [DOI] [PubMed] [Google Scholar]
- 9. Klein E, Kis LL, Klein G. Epstein–Barr virus infection in humans: from harmless to life endangering virus‐lymphocyte interactions. Oncogene 2007; 26:1297–305. [DOI] [PubMed] [Google Scholar]
- 10. Fahraeus R, Fu HL, Ernberg I, Finke J, Rowe M, Klein G et al Expression of Epstein–Barr virus‐encoded proteins in nasopharyngeal carcinoma. Int J Cancer 1988; 42:329–38. [DOI] [PubMed] [Google Scholar]
- 11. Pallesen G, Hamilton‐Dutoit SJ, Rowe M, Young LS. Expression of Epstein–Barr virus latent gene products in tumour cells of Hodgkin's disease. Lancet 1991; 337:320–2. [DOI] [PubMed] [Google Scholar]
- 12. Gloghini A, Gaidano G, Larocca LM, Pierconti F, Cingolani A, Dal Maso L et al Expression of cyclin‐dependent kinase inhibitor p27(Kip1) in AIDS‐related diffuse large‐cell lymphomas is associated with Epstein–Barr virus‐encoded latent membrane protein 1. Am J Pathol 2002; 161:163–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kennedy G, Komano J, Sugden B. Epstein–Barr virus provides a survival factor to Burkitt's lymphomas. Proc Natl Acad Sci USA 2003; 100:14269–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Watanabe A, Maruo S, Ito T, Ito M, Katsumura KR, Takada K. Epstein–Barr virus‐encoded Bcl‐2 homologue functions as a survival factor in Wp‐restricted Burkitt lymphoma cell line P3HR‐1. J Virol 2010; 84:2893–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kang MS, Lee EK, Soni V, Lewis TA, Koehler AN, Srinivasan V et al Roscovitine inhibits EBNA1 serine 393 phosphorylation, nuclear localization, transcription, and episome maintenance. J Virol 2011; 85:2859–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Scott DW, Gascoyne RD. The tumour microenvironment in B cell lymphomas. Nat Rev Cancer 2014; 14:517–34. [DOI] [PubMed] [Google Scholar]
- 17. Kis LL, Nishikawa J, Takahara M, Nagy N, Matskova L, Takada K et al In vitro EBV‐infected subline of KMH2, derived from Hodgkin lymphoma, expresses only EBNA‐1, while CD40 ligand and IL‐4 induce LMP‐1 but not EBNA‐2. Int J Cancer 2005; 113:937–45. [DOI] [PubMed] [Google Scholar]
- 18. Kis LL, Salamon D, Persson EK, Nagy N, Scheeren FA, Spits H et al IL‐21 imposes a type II EBV gene expression on type III and type I B cells by the repression of C‐ and activation of LMP‐1‐promoter. Proc Natl Acad Sci USA 2010; 107:872–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kis LL, Takahara M, Nagy N, Klein G, Klein E. IL‐10 can induce the expression of EBV‐encoded latent membrane protein‐1 (LMP‐1) in the absence of EBNA‐2 in B lymphocytes and in Burkitt lymphoma‐ and NK lymphoma‐derived cell lines. Blood 2006; 107:2928–35. [DOI] [PubMed] [Google Scholar]
- 20. Calame K. Activation‐dependent induction of Blimp‐1. Curr Opin Immunol 2008; 20:259–64. [DOI] [PubMed] [Google Scholar]
- 21. Sarosiek KA, Malumbres R, Nechushtan H, Gentles AJ, Avisar E, Lossos IS. Novel IL‐21 signaling pathway up‐regulates c‐Myc and induces apoptosis of diffuse large B‐cell lymphomas. Blood 2010; 115:570–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Wu L, Ehlin‐Henriksson B, Zhu H, Ernberg I, Klein G. EBV counteracts IL‐21‐induced apoptosis in an EBV‐positive diffuse large B‐cell lymphoma cell line. Int J Cancer 2013; 133:766–70. [DOI] [PubMed] [Google Scholar]
- 23. Lazennec G, Richmond A. Chemokines and chemokine receptors: new insights into cancer‐related inflammation. Trends Mol Med 2010; 16:133–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Salcedo R, Oppenheim JJ. Role of chemokines in angiogenesis: CXCL12/SDF‐1 and CXCR4 interaction, a key regulator of endothelial cell responses. Microcirculation 2003; 10:359–70. [DOI] [PubMed] [Google Scholar]
- 25. Moore MA. The role of chemoattraction in cancer metastases. BioEssays 2001; 23:674–6. [DOI] [PubMed] [Google Scholar]
- 26. Ehlin‐Henriksson B, Mowafi F, Klein G, Nilsson A. Epstein–Barr virus infection negatively impacts the CXCR4‐dependent migration of tonsillar B cells. Immunology 2006; 117:379–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ehlin‐Henriksson B, Liang W, Cagigi A, Mowafi F, Klein G, Nilsson A. Changes in chemokines and chemokine receptor expression on tonsillar B cells upon Epstein–Barr virus infection. Immunology 2009; 127:549–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Takakuwa T, Ham MF, Luo WJ, Nakatsuka S, Daibata M, Aozasa K. Loss of expression of Epstein‐Barr virus nuclear antigen‐2 correlates with a poor prognosis in cases of pyothorax‐associated lymphoma. Int J Cancer 2006; 118:2782–9. [DOI] [PubMed] [Google Scholar]
- 29. Kluin‐Nelemans HC, Limpens J, Meerabux J, Beverstock GC, Jansen JH, de Jong D et al A new non‐Hodgkin's B‐cell line (DoHH2) with a chromosomal translocation t(14;18)(q32;q21). Leukemia 1991; 5:221–4. [PubMed] [Google Scholar]
- 30. Boccellato F, Anastasiadou E, Rosato P, Kempkes B, Frati L, Faggioni A et al EBNA2 interferes with the germinal center phenotype by downregulating BCL6 and TCL1 in non‐Hodgkin's lymphoma cells. J Virol 2007; 81:2274–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Martin‐Perez D, Vargiu P, Montes‐Moreno S, Leon EA, Rodriguez‐Pinilla SM, Lisio LD et al Epstein–Barr virus microRNAs repress BCL6 expression in diffuse large B‐cell lymphoma. Leukemia 2012; 26:180–3. [DOI] [PubMed] [Google Scholar]
- 32. Saito M, Novak U, Piovan E, Basso K, Sumazin P, Schneider C et al BCL6 suppression of BCL2 via Miz1 and its disruption in diffuse large B cell lymphoma. Proc Natl Acad Sci USA 2009; 106:11294–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Paydas S, Ergin M, Seydaoglu G, Erdogan S, Yavuz S. Prognostic [corrected] significance of angiogenic/lymphangiogenic, anti‐apoptotic, inflammatory and viral factors in 88 cases with diffuse large B cell lymphoma and review of the literature. Leuk Res 2009; 33:1627–35. [DOI] [PubMed] [Google Scholar]
- 34. Wang H, Wang M, Feng Z, Chen L, Gao L, Li Q et al Functional interleukin‐21 polymorphism is a protective factor of diffuse large B‐cell lymphoma. DNA Cell Biol 2014; 33:775–80. [DOI] [PubMed] [Google Scholar]
- 35. Cha Z, Gu H, Guo H, Tu X, Zang Y, Zhao C et al Effect of interleukin 21 and its receptor on CD8+ T cells in the pathogenesis of diffuse large B‐cell lymphoma. Oncol Lett 2014; 8:421–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Ettinger R, Kuchen S, Lipsky PE. The role of IL‐21 in regulating B‐cell function in health and disease. Immunol Rev 2008; 223:60–86. [DOI] [PubMed] [Google Scholar]
- 37. Teng Y, Takahashi Y, Yamada M, Kurosu T, Koyama T, Miura O et al IRF4 negatively regulates proliferation of germinal center B cell‐derived Burkitt's lymphoma cell lines and induces differentiation toward plasma cells. Eur J Cell Biol 2007; 86:581–9. [DOI] [PubMed] [Google Scholar]
- 38. Basso K, Dalla‐Favera R. BCL6: master regulator of the germinal center reaction and key oncogene in B cell lymphomagenesis. Adv Immunol 2010; 105:193–210. [DOI] [PubMed] [Google Scholar]
- 39. Schrader A, Meyer K, von Bonin F, Vockerodt M, Walther N, Hand E et al Global gene expression changes of in vitro stimulated human transformed germinal centre B cells as surrogate for oncogenic pathway activation in individual aggressive B cell lymphomas. Cell Commun Signal 2012; 10:43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Yang Y, Shaffer AL 3rd, Emre NC, Ceribelli M, Zhang M, Wright G et al Exploiting synthetic lethality for the therapy of ABC diffuse large B cell lymphoma. Cancer Cell 2012; 21:723–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Vrzalikova K, Vockerodt M, Leonard S, Bell A, Wei W, Schrader A et al Down‐regulation of BLIMP1α by the EBV oncogene, LMP‐1, disrupts the plasma cell differentiation program and prevents viral replication in B cells: implications for the pathogenesis of EBV‐associated B‐cell lymphomas. Blood 2011; 117:5907–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Nasimuzzaman M, Kuroda M, Dohno S, Yamamoto T, Iwatsuki K, Matsuzaki S et al Eradication of Epstein–Barr virus episome and associated inhibition of infected tumor cell growth by adenovirus vector‐mediated transduction of dominant‐negative EBNA1. Mol Ther 2005; 11:578–90. [DOI] [PubMed] [Google Scholar]
- 43. Imai S, Kuroda M, Yamashita R, Ishiura Y. [Therapeutic inhibition of Epstein–Barr virus‐associated tumor cell growth by dominant‐negative EBNA1]. Uirusu 2005; 55:239–49. [DOI] [PubMed] [Google Scholar]
- 44. Castillo JJ, Beltran BE, Miranda RN, Paydas S, Winer ES, Butera JN. Epstein–Barr virus‐positive diffuse large B‐cell lymphoma of the elderly: what we know so far. Oncologist 2011; 16:87–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Nakayama T, Fujisawa R, Izawa D, Hieshima K, Takada K, Yoshie O. Human B cells immortalized with Epstein‐Barr virus upregulate CCR6 and CCR10 and downregulate CXCR4 and CXCR5. J Virol 2002; 76:3072–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Burgstahler R, Kempkes B, Steube K, Lipp M. Expression of the chemokine receptor BLR2/EBI1 is specifically transactivated by Epstein–Barr virus nuclear antigen 2. Biochem Biophys Res Commun 1995; 215:737–43. [DOI] [PubMed] [Google Scholar]
- 47. Rehm A, Anagnostopoulos I, Gerlach K, Broemer M, Scheidereit C, Johrens K et al Identification of a chemokine receptor profile characteristic for mediastinal large B‐cell lymphoma. Int J Cancer 2009; 125:2367–74. [DOI] [PubMed] [Google Scholar]
- 48. Deutsch AJ, Steinbauer E, Hofmann NA, Strunk D, Gerlza T, Beham‐Schmid C et al Chemokine receptors in gastric MALT lymphoma: loss of CXCR4 and upregulation of CXCR7 is associated with progression to diffuse large B‐cell lymphoma. Mod Pathol 2013; 26:182–94. [DOI] [PubMed] [Google Scholar]
- 49. Moreno MJ, Bosch R, Dieguez‐Gonzalez R, Novelli S, Mozos A, Gallardo A et al CXCR4 expression enhances diffuse large B cell lymphoma dissemination and decreases patient survival. J Pathol 2015; 235:445–55. [DOI] [PubMed] [Google Scholar]
- 50. Nagasawa T, Hirota S, Tachibana K, Takakura N, Nishikawa S, Kitamura Y et al Defects of B‐cell lymphopoiesis and bone‐marrow myelopoiesis in mice lacking the CXC chemokine PBSF/SDF‐1. Nature 1996; 382:635–8. [DOI] [PubMed] [Google Scholar]
- 51. Laichalk LL, Thorley‐Lawson DA. Terminal differentiation into plasma cells initiates the replicative cycle of Epstein–Barr virus in vivo . J Virol 2005; 79:1296–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Bertolini F, Dell'Agnola C, Mancuso P, Rabascio C, Burlini A, Monestiroli S et al CXCR4 neutralization, a novel therapeutic approach for non‐Hodgkin's lymphoma. Cancer Res 2002; 62:3106–12. [PubMed] [Google Scholar]
- 53. Chen J, Xu‐Monette ZY, Deng L, Shen Q, Manyam GC, Martinez‐Lopez A et al Dysregulated CXCR4 expression promotes lymphoma cell survival and independently predicts disease progression in germinal center B‐cell‐like diffuse large B‐cell lymphoma. Oncotarget 2015; 6:5597–614. [DOI] [PMC free article] [PubMed] [Google Scholar]


