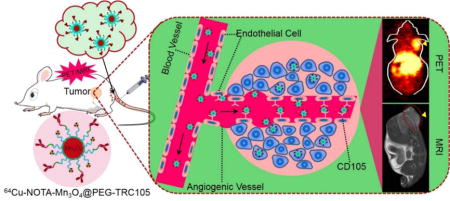
Abstract
Manganese oxide nanoparticles (Mn3O4 NPs) have attracted a great deal of attention in the field of biomedical imaging because of their ability to create an enhanced imaging signal in MRI as novel potent T1 contrast agents. In this study, we present tumor vasculature-targeted imaging in mice using Mn3O4 NPs through conjugation to the anti-CD105 antibody TRC105 and radionuclide copper-64 (64Cu, t1/2: 12.7 h). The Mn3O4 conjugated NPs, 64Cu-NOTA-Mn3O4@PEG-TRC105, exhibited sufficient stability in vitro and in vivo. Serial positron emission tomography (PET) and magnetic resonance imaging (MRI) studies evaluated the pharmacokinetics and demonstrated targeting of 64Cu-NOTA-Mn3O4@PEG-TRC105 to 4T1 murine breast tumors in vivo, compared to 64Cu-NOTA-Mn3O4@PEG. The specificity of 64Cu-NOTA-Mn3O4@PEG-TRC105 for the vascular marker CD105 was confirmed through in vivo, in vitro, and ex vivo experiments. Since Mn3O4 conjugated NPs exhibited desirable properties for T1 enhanced imaging and low toxicity, the tumor-specific Mn3O4 conjugated NPs reported in this study may serve as promising multifunctional nanoplatforms for precise cancer imaging and diagnosis.
Keywords: Manganese oxide nanoparticles, Cancer, Positron emission tomography (PET), Magnetic resonance imaging (MRI), CD105 (endoglin)
Graphical abstract
INTRODUCTION
Medical imaging techniques have come to play an important role in the early detection and diagnosis of disease due to their precise diagnostic abilities at the whole-body, molecular, and cellular levels.1,2 Various imaging technologies in widespread use today include computed tomography (CT), positron emission tomography (PET), magnetic resonance imaging (MRI), ultrasound (US), single-photon emission computed tomography (SPECT) and photoacoustic imaging (PA).3 Because each of these modalities has its own unique strengths and limitations regarding spatial and temporal resolution, tissue penetration depth, detection sensitivity, and cost, combinations of different imaging modalities are being developed and have shown promise to overcome clinical diagnostic challenges. Among these combinations, the use of PET and MRI together has been under vigorous development and is currently in clinical trials for applications in cancer detection thanks to the high sensitivity of PET and ultra-high spatial resolution of MRI.4, 5
To further make full use of PET/MR imaging, creation of dual-modal PET/MR imaging probes has been actively pursued since their emergence in the last decade.6–8 T1-positive paramagnetic and T2-negative super paramagnetic nanoparticles are among commonly-used MRI contrast components. In particular, T2 contrast agents based on iron oxide have been widely used as the MRI contrast moeity in PET/MRI probes over the last two decades and a few have advanced into clinical trials or received the approval of the United States’ Food and Drug Administration.9–12 However, these nanoparticles have been somewhat limited in clinical applications due to their drawbacks of negative contrast and high susceptibility; therefore, it is desirable to develop new PET/MRI probes with higher T1 or T2 relaxivity components.
T1 contrast agents based on gadolinium and manganese in the form of ionic complexes or colloidal nanoparticles have gained increased interest because of their signal-enhancing positive contrast characteristics.2, 13 However, the complex-based agents demonstrate downfalls such a relatively short vascular residence time and notable toxicity in vivo, which limit their extensive clinical application in disease diagnosis.13 In view of this, recent manganese (II)-based nanoparticles have been considered promising T1-weighted contrast agents, attributed to their relatively high magnetization spins and fast water proton exchange rates.14 Meanwhile, the manganese (II)-based agents also exhibit low side effects and good biocompatibility as manganese itself is an essential component of cells and a cofactor for many enzymes.15 Most importantly, monodisperse manganese-based nanoparticles of good crystallinity and uniformity have been demonstrated to be easily synthesized on a large scale under mild and ambient reaction conditions.16 This class of MRI contrast agents, particularly manganese oxides, indicates a promising new direction in biomedical imaging and tumor-targeted diagnosis.17,18 Multimodality imaging probes and their application in biomedical imaging have been reported in recent years. For instance, solid and hollow MnO nanoparticle-based T1 contrast agents have been reported for selectively imaging breast cancer cells and for drug delivery by several groups.19–24 Yang and co-workers further developed silica-coated Mn3O4 core-shell nanoparticles for tumor folate-receptor-targeted MRI and fluorescent imaging in vitro and tumor aptamer-receptor-targeted MRI imaging in vivo.25–27 Although the reported manganese-based NPs have exhibited good imaging capabilities as contrast agents, it is still urgent to develop novel multifunctional manganese-based imaging probes for future biomedical imaging, especially PET/MRI probes. As we all know, driven by the impending clinical requirement, the perfect combination of PET and MRI has been under rapid development in clinical cancer detection and diagnosis due to the high sensitivity of PET and ultra-high spatial resolution of MRI. Inspired by this, we were encouraged to develop a new PET/MRI imaging probe with a manganese oxide-based T1 contrast component and Cu-64 positron emission for in vivo tumor imaging.
Effective delivery of agents to specific sites in the body is a major barrier to tumor imaging and therapy.28–30 Compared to tumor cell-based targeting, targeting of nanoparticles to tumor vasculature is more reasonable due to the relative ease of access for nanoparticles to vasculature after intravenous injection. Additionally, extravasation is not required (as this is a known issue with many nanoplatforms). Furthermore, angiogenesis, or the formation of new blood vessels, is a critical process in tumor development and progression.31–34 CD105 (also called endoglin) is nearly exclusively expressed on proliferating endothelial cells, which are a strong marker for tumor angiogenesis.32–34 More importantly, various studies have confirmed that, in more than ten solid tumor types, high expression of CD105 is correlated with poor patient outcomes, which makes it a widely-applicable target in cancer.35–38 Using TRC105 (a chimeric IgG1 monoclonal antibody which binds to both human and murine CD10539,40) as the targeting ligand, our group has monitored CD105 expression using both antibody and nanoplatforms, demonstrating the great potential of CD105-targeted agents for future extensive applications in cancer diagnosis and therapy.41–47 Inspired by this previous success, we set out to develop our first PET/MRI probe targeted to CD105.
In this work, we investigated in vivo tumor vasculature targeting with surface functionalized Mn3O4 nanoparticles. We utilized a mild and ambient reaction method to synthesize uniform Mn3O4 nanocrystals, and subsequently conjugated them with TRC105 and S-2-(4-isothiocyanatobenzyl)-1,4,7-triazacyclononane-1,4,7-triacetic acid (p-SCN-Bn-NOTA) through polyethylene glycol (PEG) linkers, for radiolabeling with 64Cu (half-life: 12.7 h) to form the 64Cu-NOTA-Mn3O4@PEG-TRC105 conjugate (Scheme 1). To demonstrate the CD105 specificity of 64Cu-NOTA-Mn3O4@PEG-TRC105, in vivo PET/MRI imaging, biodistribution, and blocking studies were carried out in 4T1 tumor-bearing mice, the results of which were further validated by additional in vitro and ex vivo experiments. Moreover, serum biochemistry assays and histological assessments were also conducted to determine the potential toxicity of these nanoparticles.
Scheme 1.
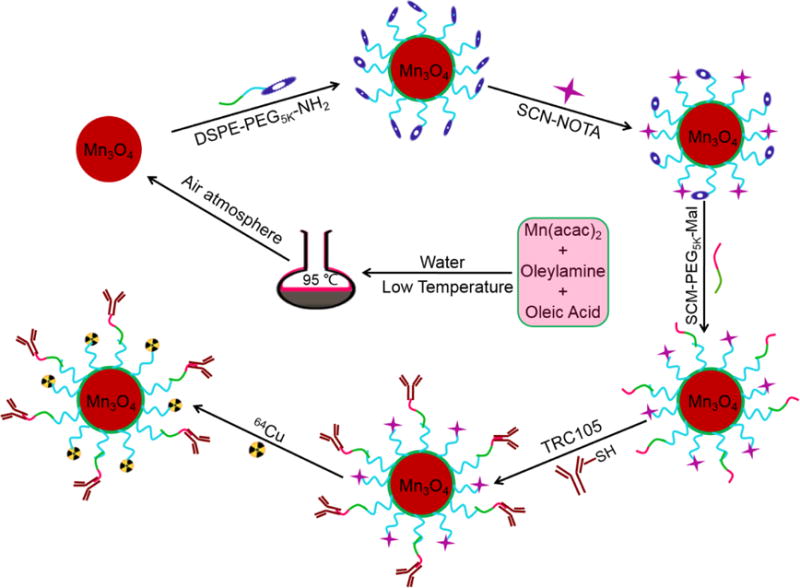
Synthesis of the Mn3O4 conjugated NPs (64Cu-NOTA-Mn3O4@PEG-TRC105).
2 MATERIALS AND METHODS
2.1. Reagents
Oleylamine (approximate C18-content 80–90%), oleic acid (technical grade 90%), xylene (98%), manganese (II) acetate (98%), CCK-8 and fluorescein isothiocyanate (FITC) were obtained from Sigma-Aldrich. S-2-(4-isothiocyanatobenzyl)-1, 4, 7-triazacyclononane-1, 4, 7-triacetic acid (p-SCN-Bn-NOTA) was purchased from Macrocyclics, Inc. (Dallas, TX). TRC105 was supplied by TRACON Pharmaceuticals Inc. (San Diego, CA). DSPE-PEG5000-NH2 and SCM-PEG5000-Mal were purchased from Creative PEGworks (Winston Salem, NC). AlexaFluor488- or Cy3-labeled secondary antibodies (Jackson Immunoresearch Laboratories, Inc., West Grove, CA), rat anti-mouse CD31 primary antibody (BD Biosciences, San Diego, CA), and PD-10 desalting columns (GE Healthcare, Piscataway, NJ) were all acquired from commercial sources. All buffers and water were of Millipore grade. All other reaction buffers and chemicals were obtained from Thermo Fisher Scientific (Fair Lawn, NJ).
2.2. Synthesis of the Mn3O4 NPs
Mn3O4 NPs were prepared according to a previously reported method with slight modifications.16 The nanoparticles were modified with DSPE-PEG5000-NH2 to obtain Mn3O4@PEG-NH2 NPs. Mn3O4@PEG-NH2 was then reacted with p-SCN-Bn-NOTA or FITC as described previously,45 at a molar ratio of 1:10 at pH 8.5 for 3 h. The nanoparticles were then further modified through the addition of SCM-PEG-Mal using similar procedures. In preparation for further reactions, TRC105 was incubated with Traut’s reagent at a molar ratio of 1:25 at pH 8.0 for 2 h. The final products (NOTA-Mn3O4@PEG-TRC105 or FITC-Mn3O4@PEG-TRC105) were generated as described previously.48 Final purification to remove excess TRC105 was achieved by passing the solution through a PD-10 size-exclusion column.
2.3. Characterization
The size and morphology of Mn3O4 NPs were determined using a JEOL JEM-2100 transmission electron microscope (TEM). X-ray diffraction (XRD) measurements were conducted on a Bruker D4 diffractometer. The surface zeta potential and hydrodynamic size were measured using a Malvern Zetasizer Nano ZS. The T1-relaxivities and T1 images were obtained with a conventional spin echo acquisition (repetition time, TR, 1000 ms) with echo time, TE, of 50 ms, and a section thickness of 1 mm in a 4.7 T small animal scanner (Agilent Technologies, Santa Clara, CA). Relaxivity values of r1 were calculated through curve fitting of 1/T1 (s−1) versus the manganese concentration (mM). The concentration of Mn was determined by ICP-AES (VISTAMPXICP Varian, USA).
2.4. Radiolabeling and serum stability studies
Cu-64 was produced using a GE PETrace cyclotron. 50 μg of NOTA-Mn3O4@PEG-TRC105 or NOTA-Mn3O4@PEG was mixed with 64CuCl2 (74 MBq), and the reaction proceeded at 37°C for 30 min, with the reaction and purification proceeding as outlined previously.38 To ensure that 64Cu-NOTA-Mn3O4@PEG-TRC105 and 64Cu-NOTA-Mn3O4@PEG were stable for in vivo applications, serum stability studies were performed. 64Cu-NOTA-Mn3O4@PEG-TRC105 or 64Cu-NOTA-Mn3O4@PEG were incubated in complete mouse serum at 37 °C for 48 h, and analysis was performed as previously described.46
2.5. Cell lines and animal model
4T1 murine breast cancer, human embryonic kidney 293 cells (HEK-293), human hepatoma cells (HUH-7), MCF-7 human breast cancer cells, and human umbilical vein endothelial cells (HUVECs) were obtained from the American Type Culture Collection (ATCC, Manassas, VA). Cells were cultured and 4T1 animal models were developed as previously described.46
2.6. In vitro cytotoxicity assay
The cytotoxicity of Mn3O4 conjugated NPs was assessed with a CCK-8 assay using HUH-7 cells and HEK-293 cells.47 Briefly, cells were seeded in 96-well plates at 20,000 cells per well in 200 μL culture medium. The cells were maintained in RPMI-1640 containing 10% fetal bovine serum (FBS) and incubated at 37 °C in a humidified cell culture incubator with 5% CO2 atmosphere for 24 h. Mn3O4 conjugated NPs solutions with different concentrations from 200 to 1000 μg/mL were added to each well, and the cells were subjected to a CCK-8 assay after being incubated for another 24 h. The cell viability was determined through measuring the absorption at 450 nm using a microplate reader. Cell viability was calculated using: cell viability (%) = (mean absorption value of treatment group/mean absorption value of control) ×100.
2.7. Flow cytometry
HUVECs (CD105 positive) and MCF-7 cells (CD105 negative) were harvested and incubated with FITC-Mn3O4@PEG-TRC105 or FITC-Mn3O4@PEG for flow cytometry analysis as described elsewhere.41,46 A blocking study was also performed in which cells were pre-incubated with 500 μg/mL of TRC105 prior to incubation with the nanoparticles. All cells were then analyzed using a BD FACSCalibur cytometer (Bectone-Dickinson, San Jose, CA) and FlowJo analysis software (Tree Star, Inc., Ashland, OR).
2.8. PET imaging and ex vivo biodistribution studies
PET scans of 4T1 tumor-bearing mice (n = 3 per group) at 0.5, 3, 6, and 24 h post-injection (p.i.) of 64Cu-NOTA-Mn3O4@PEG-TRC105 or 64Cu-NOTA-Mn3O4@PEG (5–10 MBq) were performed using an Inveon rodent model microPET/microCT scanner (Siemens Medical Solutions USA, Inc.) following tail vein injection. Detailed procedures for data acquisition and analysis have been reported previously.46 Blocking studies, in which 4T1-bearing mice were injected with 1 mg of TRC105 2 h before the injection of 64Cu-NOTA-Mn3O4@PEG-TRC105, were also carried out to confirm the in vivo specificity of the nanoparticles. Quantitative data from imaging and biodistribution studies are presented as percent of the injected dose per gram of tissue (%ID/g). To further validate the PET uptake values, ex vivo biodistribution studies were performed both at 6 and 24 h, at the time of peak tumor uptake and the terminal imaging timepoint, respectively, as previously reported.47
2.9. In vivo T1 MRI imaging
To detect Mn3O4 accumulation in tumors, in vivo T1-weighted MR imaging was performed at 6 h post-injection after intravenous injection of 400 μL NOTA-Mn3O4@PEG-TRC105 with a Mn concentration of 1.04 mM. Meanwhile, a blocking study was also conducted through injection of 1 mg of TRC105 before NOTA-Mn3O4@PEG-TRC105 administration and subsequent MRI imaging at 6 h p.i. using a 4.7 T small animal scanner (Agilent Technologies, Santa Clara, CA) with the following parameters: TR = 500 ms; TE = 12 ms; flip angle = 120°; FOV = 40 mm × 40 mm; matrix = 256 × 256; NEX = 8; slice thickness = 1 mm for axial images.
2.10. Histology
Three 4T1-bearing mice were each injected with NOTA-Mn3O4@PEG-TRC105 (5 mg/kg dose) and euthanized 6 h p.i. of the nanoparticles. The tumor, spleen, liver, and muscle were harvested, frozen, and sectioned for histological analysis as previously described.41, 45 Rat anti-mouse CD31 and Cy3-labeled donkey anti-rat antibodies served to visualize vasculature, while an AlexaFluor488-labeled goat anti-human antibody allowed staining of TRC105 in the nanoparticles. All images were acquired using a Nikon Eclipse Ti microscope.
2.11. In Vivo Biocompatibility Studies of Mn3O4 NPs
The toxicity of Mn3O4@PEG NPs to healthy male BALB/c mice was evaluated through injection of Mn3O4@PEG NPs (dose: 20 mg/kg) via the tail vein. Mice injected with PBS alone served as a control group (n = 3). Mice were sacrificed on Day 7 and Day 14 for blood serum biochemistry assays. At the same time, major organs from each mouse (heart, liver, spleen, lung, and kidney) were harvested and fixed in 4% paraformaldehyde solution for 1 day. These tissues were then embedded in paraffin, sliced, and stained with hematoxylin and eosin (H&E) and examined using a digital microscope (Leica DM5000). The serum chemistry data, including hepatic and kidney functions, was measured by the University of Wisconsin-Madison Veterinary Hospital.
3. RESULTS AND DISCUSSION
3.1. Synthesis and characterization of Mn3O4 conjugated NPs
As shown in Scheme 1, Mn3O4 conjugated NPs (64Cu-NOTA-Mn3O4@PEG-TRC105) were prepared according to our previous report.16,42,47 When it comes to the preparation of monodispersed Mn3O4 NPs, various methods have been reported such as solvothermal,48 oxidation-precipitation,49 thermal decomposition,27 surfactant-assisted synthesis,50 micro-wave irradiation51 and vapor phase growth.52 However, it is still difficult to synthesize uniform Mn3O4 NPs to meet the desired biomedical requirements. Fortunately, Hyeon and co-workers developed a new simple method to prepare Mn3O4 NPs with various shapes at low temperature in air atmosphere.16 Thus, in this present work, an improved method was used to prepare Mn3O4 NPs according to the method by Hyeon. Based on TEM measurements, Mn3O4 NPs were monodispersed in nonpolar organic solvent and had a small spherical shape of approximately 7 nm (Figure 1a), which was consistent with the previous reports.16,26,27 Furthermore, the hydrophobic Mn3O4 NPs were successfully transferred to the aqueous phase by coating with amine-functionalized PEG lipids (DSPE-PEG5000-NH2) and showed good stability in aqueous solution (Figure 1b). In addition, DLS measurements showed that Mn3O4@PEG-NH2 has a hydrodynamic diameter of 11 ± 3.5 nm, whereas the diameter of Mn3O4 NPs is 8 ± 4.6 nm (Figure 1d), which was similar to the observation from TEM. The zeta-potential value of Mn3O4@PEG-NH2 was −21.7 ± 2.3 mV. The crystallography of the Mn3O4 NPs was further verified by powder XRD (Figure 1c), which showed that all the diffraction peaks of the Mn3O4 NPs can be indexed as a tetragonal Mn3O4 phase (Joint Committee for Powder Diffraction Standards (JCPDS) card no: 24-0734).
Figure 1.
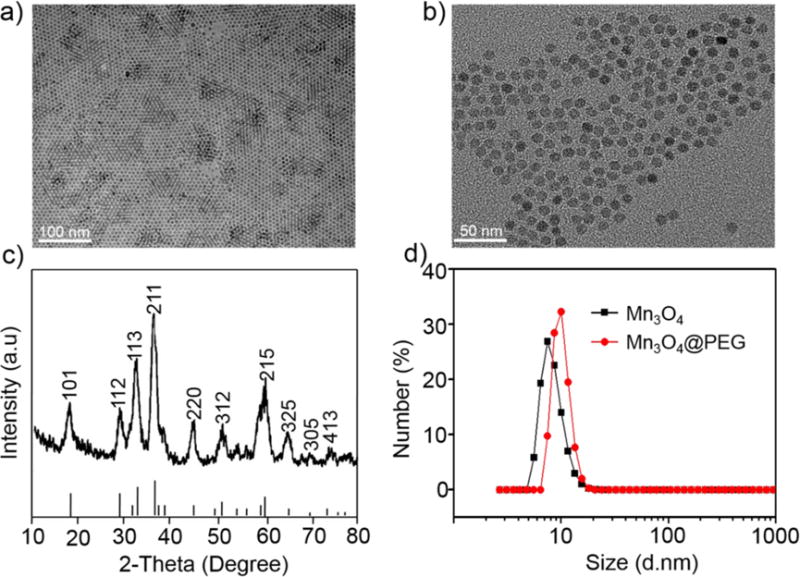
Characterization of Mn3O4@PEG. (a) TEM images of Mn3O4 NPs; (b) HRTEM images of Mn3O4@PEG NPs; (c) X-ray diffraction pattern of Mn3O4 NPs; (d) Size distribution of Mn3O4 NPs and Mn3O4@PEG as determined through DLS.
After further conjugation as previously reported,46 the zeta-potential values changed significantly to −14.8 ± 3.7 mV (NOTA-Mn3O4@PEG) and −3.2 ± 4.8 mV (NOTA-Mn3O4@PEG-TRC105), suggesting successful conjugation of NOTA and TRC105 to the surface of Mn3O4@PEG-NH2. Moreover, in order to further confirm the conjugation, the absorption spectra of the TRC105, Mn3O4@PEG and NOTA-Mn3O4@PEG-TRC105 were measured by UV spectrophotometer. It can be seen that the TRC105 and NOTA-Mn3O4@PEG-TRC105 NPs have obvious absorption peaks at 280 nm (Figure S1), which were ascribed to the protein itself. Furthermore, the size of NOTA-Mn3O4@PEG was also increased from 11 ± 3.5 nm to 32.6 ± 4.5 nm, which was attributed to the diameter of TRC105 itself (Figure S2). An estimated average of 1.67 TRC105 antibodies were conjugated to each nanoparticle.
To examine the MRI contrast capabilities of the Mn3O4 conjugated NPs, the relaxation properties of Mn3O4@PEG-NH2 and NOTA-Mn3O4@PEG-TRC105 in aqueous solution were measured by a 4.7 T MRI scanner. It was clear that both NOTA-Mn3O4@PEG-TRC105 and Mn3O4@PEG displayed signal enhancement in T1-weighted MR images with increasing manganese concentration (Figure 2a). The r1 values of the NOTA-Mn3O4@PEG-TRC105 and Mn3O4@PEG-NH2 were calculated as 0.54 mM−1s−1 and 0.57 mM−1s−1 respectively, from linear fitting of 1/T1 versus Mn2+ concentration (Figure 2b). These values are also similar to those of previously-reported Mn3O4 NPs, verifying their potential use as positive MRI contrast agents.22, 25–27 Other T1 contrast agents (such as those based on gadolinium) certainly possess higher r1 values; however, the promise of Mn-based agents lies in their relatively lower toxicity concerns. This study demonstrates that Mn-based nanoparticles do indeed provide MRI contrast; however, there certainly is room for improvement in their r1 values.
Figure 2.
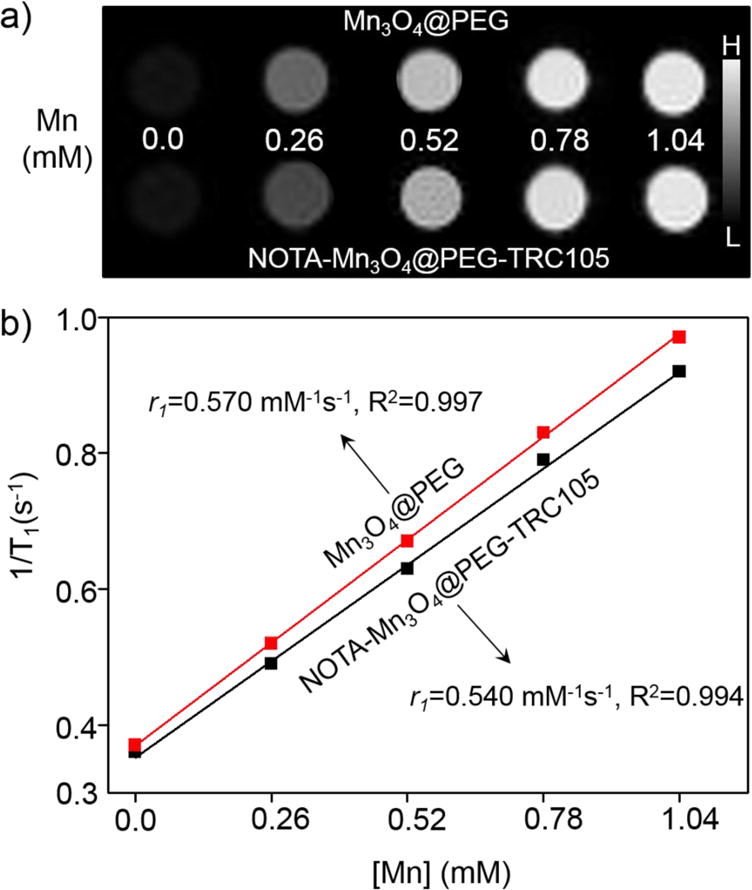
The relaxation properties of NOTA-Mn3O4@PEG-TRC105. (a) T1-weighted MRI of Mn3O4@PEG and NOTA-Mn3O4@PEG-TRC105 NPs; (b) T1 relaxivity plot of aqueous suspensions of Mn3O4@PEG and NOTA-Mn3O4@PEG-TRC105 NPs on a 4.7 T MRI system.
3.2. Flow cytometry and serum stability studies
HUVECs (CD105 positive) and MCF-7 cells (CD105 negative) were incubated in PBS containing NOTA-Mn3O4@PEG-TRC105 NPs at different concentrations and analyzed using flow cytometry. As shown in Figure 3a, the fluorescence signal of CD105 positive HUVECs was approximately 18 fold higher than that of the untreated cells upon incubation with FITC-Mn3O4@PEG-TRC105, whereas no fluorescence signal enhancement was observed in the blocking and FITC-Mn3O4@PEG treatment groups. In addition, MCF-7 cells, which are CD105 negative, demonstrated minimal fluorescence across all groups. Taken together, the results of flow cytometry indicate high CD105 specificity and minimal non-specific binding of TRC105-conjugated Mn3O4 NPs.
Figure 3.
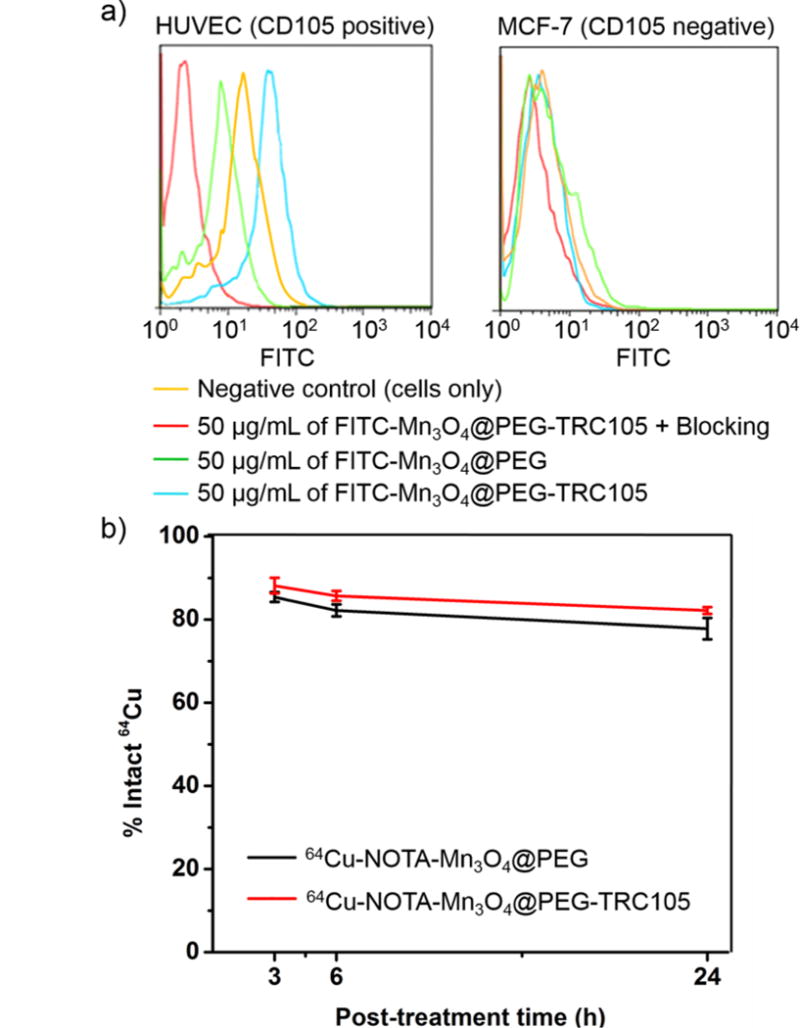
Flow cytometry analysis and serum stability of Mn3O4 conjugated NPs. (a) Flow cytometry analysis of the Mn3O4 conjugated NPs in HUVECs (CD105 positive) and MCF-7 breast cancer cells (CD105 negative); (b) Serum stability studies of 64Cu-NOTA-Mn3O4@PEG-TRC105 at 37 °C.
The stability of 64Cu labeling was evaluated through a serum stability study of 64Cu-NOTA-Mn3O4@PEG-TRC105. As shown in Figure 3b, after incubation in complete mouse serum at 37 °C for 24 h, nearly 80% of 64Cu remained on the Mn3O4 conjugated NPs, suggesting good stability of the radiolabel on the Mn3O4 conjugated NPs. Good radio-stability in serum indicated that 64Cu-NOTA-Mn3O4@PEG-TRC105 would have desirable properties in vivo.
3.3. Cytotoxicity assay
The cytotoxicity of Mn3O4 conjugated NPs was evaluated by CCK-8 assay with normal cells (HEK-293) and tumor cells (HUH-7). No obvious cytotoxicity of Mn3O4 conjugated NPs to HEK-293 and HUH-7 cells was observed at any studied concentration (from 200 to 1000 μg mL−1, Figure S3) or any timepoint (24 or 48 h). Even at the concentration of 1000 μg mL−1, the viability for both HEK-293 and HUH-7 cells remained above 80%, indicating that the Mn3O4 conjugated NPs should have little cytotoxicity at the given concentration range.
3.4. PET imaging and biodistribution studies
Since many nanomaterials extravasate poorly, targeting to the tumor vasculature is a promising strategy for tumor targeting. CD105 is an ideal marker primarily expressed on tumor neovasculature, and thus can serve as an attractive and universal vascular target for multiple solid tumor types.37, 38 Additionally, the 4T1 model is a fast-growing tumor that exhibits very high levels of angiogenesis, making targeting to CD105 quite simple. In view of this, the specific targeting capability of 64Cu-NOTA-Mn3O4@PEG-TRC105 was evaluated through PET imaging. As Cu-64 has a 12.7 h half-life, serial PET scans were performed at time points of 0.5, 3, 6 and 24 h p.i., as seen in Figure 4. The quantitative data obtained from the PET scans are shown in Figure 5a, b and c. Since the hydrodynamic diameter of the Mn3O4 conjugated NPs is above the cutoff for renal filtration,53 these nanoparticles were mainly cleared through the hepatobiliary pathway. The liver uptake of 64Cu-NOTA-Mn3O4@PEG-TRC105 was 25.3 ± 2.5, 24.1 ± 1.3, 23.2 ± 0.5 and 21.6 ± 0.7 %ID/g at 0.5, 3, 6, and 24 h p.i. respectively, while the blood pool radioactivity was 4.8 ± 0.8, 3.5 ± 0.7, 3.7 ± 0.6, and 3.2 ± 0.4 %ID/g at the same timepoints (n = 3; Figure 5a), suggesting a short circulation half-life (< 30 min). More importantly, the 4T1 tumor was clearly visible after 64Cu-NOTA-Mn3O4@PEG-TRC105 injection at 0.5 h p.i. (Figure 5a) and remained visible over time (5.8 ± 0.6, 7.8 ± 0.4, and 6.1 ± 0.2 %ID/g at 3, 6 and 24 h p.i. respectively; n = 3; Figure 5a). Pre-injection of a blocking dose of TRC105 was found to significantly reduce the tumor uptake of 64Cu-NOTA-Mn3O4@PEG-TRC105 to 1.5 ± 0.3, 2.1 ± 0.7, 2.8 ± 0.5, and 2.0 ± 0.8 %ID/g at 0.5, 3, 6, and 24 h p.i. respectively (n = 3; Figure 5c and 5d; p < 0.05 at all time points), which demonstrated in vivo specificity of 64Cu-NOTA-Mn3O4@PEG-TRC105 for CD105 on tumor vasculature.
Figure 4.
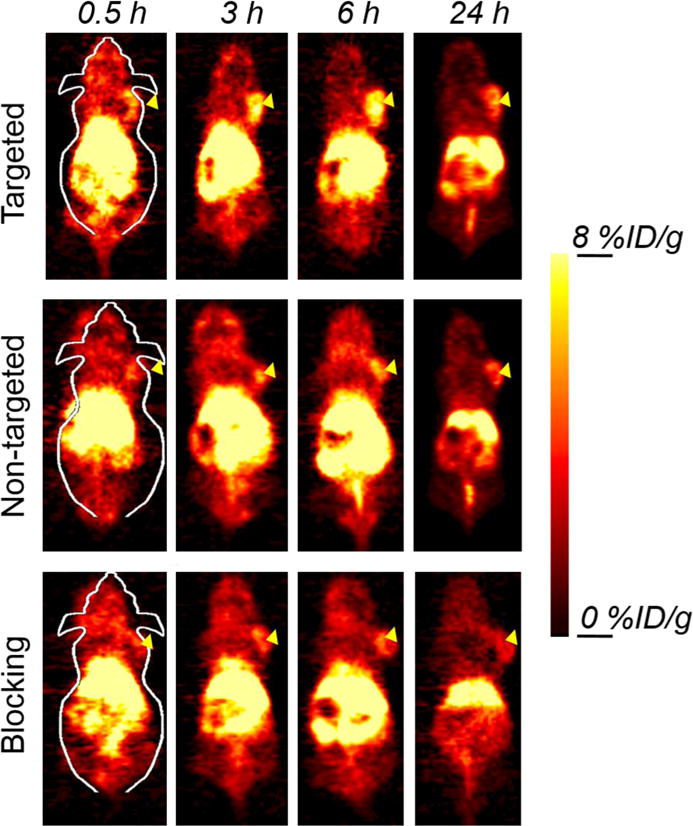
Serial coronal PET images of 4T1 tumor-bearing mice after injection of 64Cu-NOTA-Mn3O4@PEG-TRC105, 64Cu-NOTA-Mn3O4@PEG, or 64Cu-NOTA-Mn3O4@PEG-TRC105 after a pre-injected blocking dose of TRC105. Arrowheads indicate tumor locations.
Figure 5.
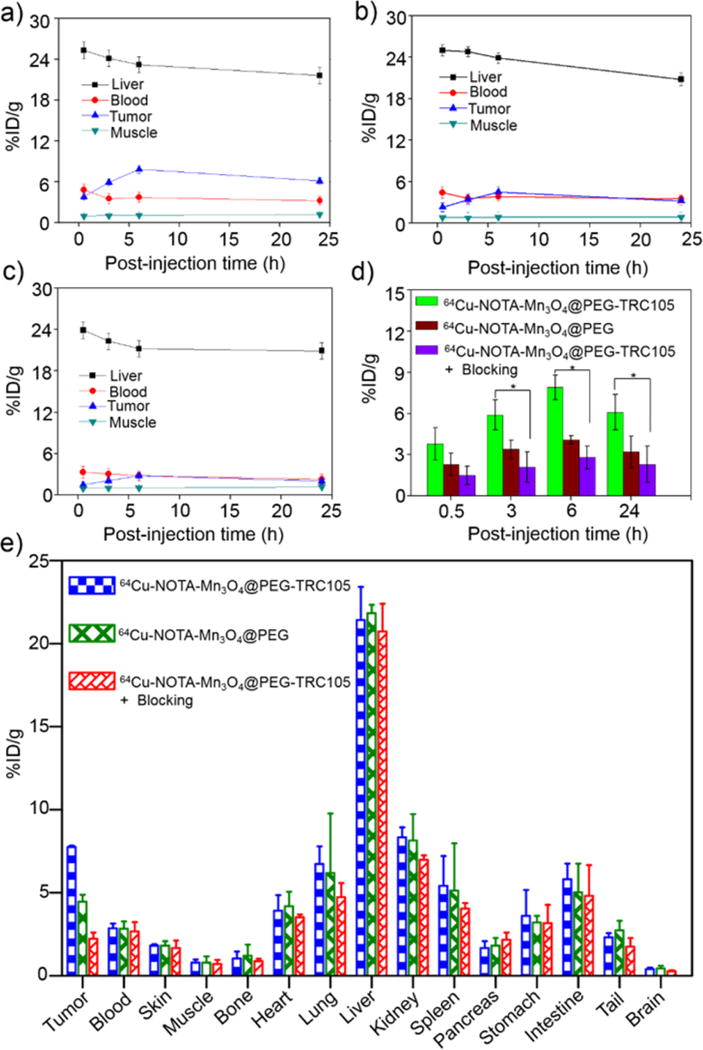
Quantitative analysis of the PET data. Time-activity curves of the liver, 4T1 tumor, blood, and muscle following intravenous injection of (a) 64Cu-NOTA-Mn3O4@PEG-TRC105, (b) non-targeted 64Cu-NOTA-Mn3O4@PEG, and (c) 64Cu-NOTA-Mn3O4@PEG-TRC105, after a blocking dose of TRC105; (d) 4T1 tumor uptake in the three groups. The differences between uptake in 4T1 tumors of 64Cu-NOTA-Mn3O4@PEG-TRC105 and the two control groups were statistically significant (p < 0.05) at all time points, except at 0.5 h post-injection between 64Cu-NOTA-Mn3O4@PEG-TRC105 and 64Cu-NOTA-Mn3O4@PEG (n=3 per group); (e) Biodistribution studies in 4T1 tumor-bearing mice at 24 h post-injection of 64Cu-NOTA-Mn3O4@PEG-TRC105, 64Cu-NOTA-Mn3O4@PEG and 64Cu-NOTA-Mn3O4@PEG-TRC105, after a blocking dose of TRC105 (n=3 per group).
Uptake of the radiolabeled nanoparticle in the liver of the blocking group was similar to mice injected with 64Cu-NOTA-Mn3O4@PEG-TRC105 alone, at 23.9 ± 1.2, 22.3 ± 1.3, 21.2 ± 1.3 and 20.9 ± 0.3 %ID/g at 0.5, 3, 6, and 24 h p.i. (n = 3; Figure 5c). Blood pool activity (3.3 ± 0.3, 3.1 ± 0.6, 2.8 ± 0.3 and 2.3 ± 0.2 %ID/g at 0.5, 3, 6, and 24 h p.i. respectively; n = 3; Figure 5c), was slightly impacted by the excess TRC105. The 4T1 tumor uptake of 64Cu-NOTA-Mn3O4@PEG (2.3 ± 0.3, 3.5± 0.2, 4.5 ± 0.4, and 3.1 ± 0.3 %ID/g at 0.5, 3, 6, and 24 h p.i. respectively; n = 3; Figure 5b, d) was ∼ 1.5 fold lower than that of the targeted tracer, suggesting that the conjugation of TRC105 to Mn3O4 NPs clearly enhanced tumor uptake through binding to CD105. In addition, liver uptake (25.2 ± 1.9, 24.7 ± 1.3, 23.7 ± 1.2 and 21.3 ± 0.5 %ID/g at 0.5, 3, 6, and 24 h p.i. respectively; n = 3; Figure 5b) and radioactivity in the blood (4.4 ± 0.4, 3.6 ± 0.6, 3.8± 0.3, and 3.5 ± 0.3 %ID/g at 0.5, 3, 6, and 24 h p.i. respectively; n = 3; Figure 5b) for the nonspecific tracer were similar to those of mice injected with 64Cu-NOTA-Mn3O4@PEG-TRC105.
Biodistribution studies of 64Cu-NOTA-Mn3O4@PEG-TRC105 were carried out at 24 h p.i. (Figure 5e). The analysis based on both PET data and biodistribution studies were in agreement, confirming that in vivo serial PET imaging reflected the distribution of 64Cu-NOTA-Mn3O4@PEG-TRC105 and 64Cu-NOTA-Mn3O4@PEG in tumor-bearing mice. As these intravenously-injected nanomaterials are cleared through the hepatobiliary pathway, substantial radioactive content was commonly detected in the liver, intestine and spleen. To determine the fate of the nanoparticles themselves (rather than the radiolabel) in mice, quantitative biodistribution of NOTA-Mn3O4@PEG-TRC105 was investigated through the detection of manganese by ICP-AES at 24 h (Figure S4). After collection of the tumor, heart, liver, spleen, lung, kidney, blood, feces and urine and ICP-AES analysis, the reasonably efficient active targeting property of NOTA-Mn3O4@PEG-TRC105 NPs was confirmed, in accordance with the biodistribution of 64Cu by gamma counter at the same time. Importantly, the tumor uptake of 64Cu-NOTA-Mn3O4@PEG-TRC105 was significantly higher than that of 64Cu-NOTA-Mn3O4@PEG and the blocking group, indicating that using TRC105 as a targeting ligand to vascular CD105 could effectively improve the tumor uptake.
3.5. MR imaging in vivo
While PET imaging provides high sensitivity and quantitative capabilities, essential anatomical information is also indispensable for accurate tumor imaging.4 To further supplement the PET results, MRI was used to investigate the Mn3O4 conjugated NPs in 4T1 tumor-bearing mice. As the NPs exhibited significant T1 signal enhancement in vitro, in vivo T1 MR imaging of 4T1 tumor-bearing mice was conducted before and after intravenous injection of 64Cu-NOTA-Mn3O4@PEG-TRC105 (Targeted group) and 64Cu-NOTA-Mn3O4@PEG (Non-Targeted group (N-Targeted)) solution at a dose of 20 mg/kg nanoparticles. A positive T1 signal enhancement in the tumor was observed at 6 h post-injection of 64Cu-NOTA-Mn3O4@PEG-TRC105, compared with the same mice prior to the injection of the nanoparticles (Figure 6a). On the contrary, the non-targeted group exhibited slight T1 signal enhancement in the tumor. Furthermore, in the blocking group, mice were intravenously injected with TRC105 first and then 64Cu-NOTA-Mn3O4@PEG-TRC105 (20 mg/kg body weight). At 6 h post injection, the T1-weighted MR signal in the blocking group tumors also exhibited slight enhancement and the signal to noise ratio of the T1-MR in the tumor was about 3 times lower than that only injected with 64Cu-NOTA-Mn3O4@PEG-TRC105 (Figure 6b and b, p < 0.05), indicating that excess TRC105 blocked the CD105 binding sites, thereby limiting the binding of 64Cu-NOTA-Mn3O4@PEG-TRC105, consistent with the PET result at the same time point. Of note, the metabolism pathway for a large nanoparticle such as that evaluated herein is different from many commercially-available MRI contrast agents such as gadolinium chelates. Thus, the timescales of contrast enhancement are expected to be different, with large nanoparticles taking a longer time to accumulate in the tumor than small molecules.
Figure 6.
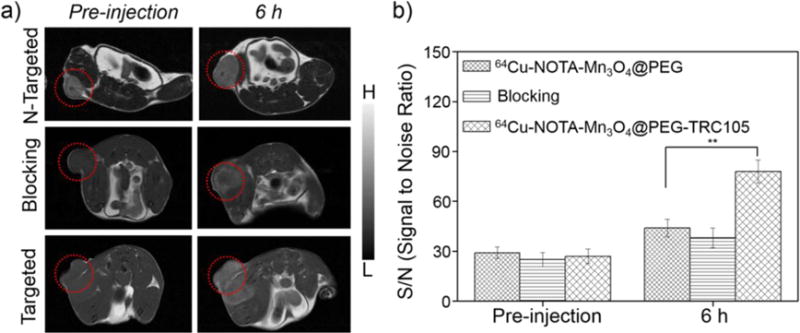
Serial T1-weighted MR images of 4T1 tumor-bearing mice. (a) In vivo T1-weighted MRIs acquired prior to and post-injection of 64Cu-NOTA-Mn3O4@PEG, 64Cu-NOTA-Mn3O4@PEG-TRC105 and 64Cu-NOTA-Mn3O4@PEG-TRC105 after a blocking dose of TRC105 (n = 3). Tumors are indicated by circles. (b) The corresponding signal to noise ratio of the tumor before and after intravenous injection of 64Cu-NOTA-Mn3O4@PEG, 64Cu-NOTA-Mn3O4@PEG-TRC105 and 64Cu-NOTA-Mn3O4@PEG-TRC105 after a blocking dose of TRC105.
3.6. Histology
Histological studies were conducted to certify that 64Cu-NOTA-Mn3O4@PEG-TRC105 was targeted to the tumor vasculature via CD105, as PET and MRI only reflect the distribution of 64Cu and Mn ions but not the Mn3O4 conjugated NPs per se. As indicated in Figure 7, the distribution of 64Cu-NOTA-Mn3O4@PEG-TRC105 in the 4T1 tumor was primarily on the vasculature, as indicated by the co-localization of the signals for the nanoparticle and CD31. Due to the relatively large size of 64Cu-NOTA-Mn3O4@PEG-TRC105, little extravasation was observed in the tumors. In addition, the nanoparticle-related signal in the liver was mostly outside the vasculature, indicating that 64Cu-NOTA-Mn3O4@PEG-TRC105 was intercepted by the liver through non-specific RES uptake rather than CD105 targeting. Meanwhile, little nanoparticle accumulation was observed in muscle tissues, in accordance with the results of PET imaging and biodistribution studies.
Figure 7.
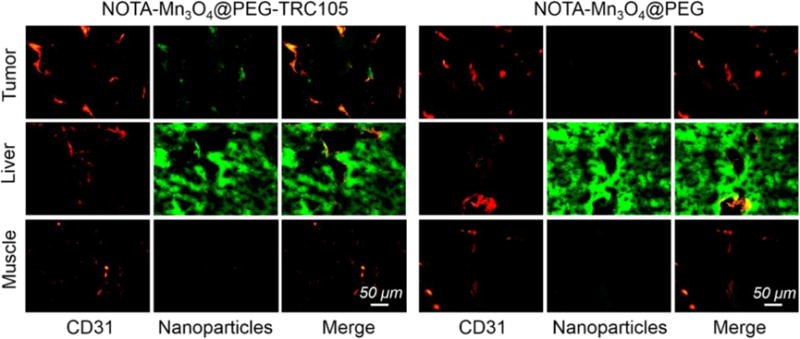
Immunofluorescent staining for CD31 (red, with anti-mouse CD31 primary antibody) and CD105 (green, using TRC105 within NOTA-Mn3O4@PEG-TRC105 as the primary antibody). Magnification: 200×. Scale bar: 50 μm.
3.7. Toxicity of Mn3O4 conjugated NPs in healthy mice
To investigate the potential in vivo toxicity of Mn3O4@PEG NPs, histological assessment was carried out by injecting Mn3O4@PEG NPs (20 mg/kg) to healthy BALB/c mice via the tail vein. PBS injections served as the control. As shown in Figure 8a, no clear tissue or cellular damage was observed in all major organs of mice, as compared to that obtained from the control group. Serum biochemistry assays were then also conducted to investigate the influence of Mn3O4@PEG NPs, especially on potential hepatic injury and kidney functions (Figure 8b). Analysis of four primary hepatic function indicators including aspartate aminotransferase (AST), alanine aminotransferase (ALT), alkaline phosphatase (ALP), and total bilirubin (TBIL), as well as two kidney function indicators including serum creatinine (CREA) and serum urea (UREA), demonstrated no obvious hepatic or kidney disorders in both the mice treated with Mn3O4@PEG NPs and the control injected with PBS on both Day 7 and Day 14 post-injection. These results suggested that Mn3O4@PEG NPs demonstrated no obvious toxicity in mice and may be a safe agent for tumor imaging.
Figure 8.
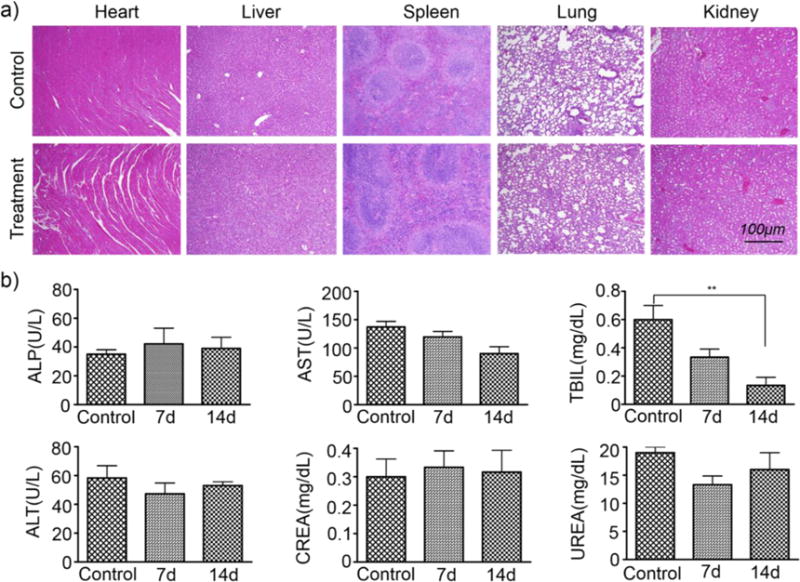
Biocompatibility studies of Mn3O4@PEG in healthy mice.(a) H&E staining of major organs (including heart, liver, spleen, lung, and kidney) from mice after injecting Mn3O4@PEG (dose: 20 mg/kg) at 7 d and14 d post-injection. Healthy mice treated with PBS were used as the control. (b) Analysis of liver and kidney function markers. Healthy BALB/c mice were intravenously injected with Mn3O4@PEG (dose: 20 mg/kg), and sacrificed on Day 7 and Day 14 post-injection (n = 3).
4. CONCLUSION
We herein report a biocompatible T1-MRI and PET contrast agent for in vivo tumor vasculature targeting based on Mn3O4 conjugated NPs, with Cu-64 as the radiolabel and TRC105 as the targeting ligand. CD105, as the specific receptor of TRC105, has been proven to be overexpressed in many proliferating tumor endothelial cells, making it suitable for tumor diagnosis and potential treatment through the use of nanomaterials. The Mn3O4 conjugated NPs (64Cu-NOTA-Mn3O4@PEG-TRC105) exhibited good radiostability and high specificity for tumor targeting. Bimodal PET/MRI imaging demonstrated that 64Cu-NOTA-Mn3O4@PEG-TRC105 accumulated in tumor sites rapidly, peaking at 6 h p.i. and remaining stable over time. Importantly, in vivo toxicity investigations revealed that the Mn3O4 NPs could be used as a safe nanoplatform for long-term targeted tumor imaging, diagnosis, and therapy.
Supplementary Material
Acknowledgments
Funding Sources
This work was supported, in part, by the University of Wisconsin-Madison, the National Institutes of Health (NIBIB/NCI 1R01CA169365, 1R01CA205101, 1R01EB021336, T32GM008505, T32CA009206, and P30CA014520), the American Cancer Society (125246-RSG-13-099-01-CCE), the National Natural Science Foundation of China under Grant Nos. 81227901, 11727813, 81571725, 81230033, 31371006, 61405149, 81660505 and 81627807, the Natural Science Basic Research Plan in Shaanxi Province of China under Grant No. 2017JM8057, and the Fundamental Research Funds for the Central Universities (JB171204).
Footnotes
Supporting Information
The Supporting Information is available free of charge on the ACS Publications website, which contains UV-Vis absorbance spectra, DLS measurements, cell viability assays, and ICP-AES analysis of tissue biodistribution.
Author Contributions
The manuscript was written with contributions from all authors. All authors have given approval of the final version of the manuscript.
References
- 1.McDonald DM, Choyke PL. Imaging of Angiogenesis: from Microscope to Clinic. Nat Med. 2003;9(6):713–725. doi: 10.1038/Nm0603-713. [DOI] [PubMed] [Google Scholar]
- 2.Na HB, Song IC, Hyeon T. Inorganic Nanoparticles for MRI Contrast Agents. Adv Mater. 2009;21(21):2133–2148. doi: 10.1002/adma.200802366. [DOI] [Google Scholar]
- 3.Weissleder R, Pittet MJ. Imaging in The Era of Molecular Oncology. Nature. 2008;452(7187):580–589. doi: 10.1038/nature06917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gambhir SS. Molecular Imaging of Cancer with Positron Emission Tomography. Nat Rev Cancer. 2002;2(9):683–693. doi: 10.1038/nrc882. [DOI] [PubMed] [Google Scholar]
- 5.Judenhofer MS, Wehrl HF, Newport DF, Catana C, Siegel SB, Becker M, Thielscher A, Kneilling M, Lichy MP, Eichner M, Klingel K, Reischl G, Widmaier S, Rocken M, Nutt RE, Machulla HJ, Uludag K, Cherry SR, Claussen CD, Pichler BJ. Simultaneous PET-MRI: A New Approach for Functional and Morphological Imaging. Nat Med. 2008;14(4):459–465. doi: 10.1038/nm1700. [DOI] [PubMed] [Google Scholar]
- 6.Glaus C, Rossin R, Welch MJ, Bao G. In Vivo Evaluation of Cu-64-Labeled Magnetic Nanoparticles as a Dual-Modality PET/MR Imaging Agent. Bioconjugate Chem. 2010;21(4):715–722. doi: 10.1021/bc900511j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pichler BJ, Kolb A, Nagele T, Schlemmer HP. PET/MRI: Paving the Way for the Next Generation of Clinical Multimodality Imaging Applications. J Nucl Med. 2010;51(3):333–336. doi: 10.2967/jnumed.109.061853. [DOI] [PubMed] [Google Scholar]
- 8.Kim J, Piao Y, Hyeon T. Multifunctional Nanostructured Materials for Multimodal Imaging, and Simultaneous Imaging and Therapy. Chem Soc Rev. 2009;38(2):372–390. doi: 10.1039/b709883a. [DOI] [PubMed] [Google Scholar]
- 9.Lee H, Lee E, Kim DK, Jang NK, Jeong YY, Jon S. Antibiofouling Polymer-Coated Superparamagnetic Iron Oxide Nanoparticles as Potential Magnetic Resonance Contrast Agents for in Vivo Cancer Imaging. J Am Chem Soc. 2006;128(22):7383–7389. doi: 10.1021/ja061529k. [DOI] [PubMed] [Google Scholar]
- 10.Ma LL, Feldman MD, Tam JM, Paranjape AS, Cheruku KK, Larson TA, Tam JO, Ingram DR, Paramita V, Villard JW, Jenkins JT, Wang T, Clarke GD, Asmis R, Sokolov K, Chandrasekar B, Milner TE, Johnston KP. Small Multifunctional Nanoclusters (Nanoroses) for Targeted Cellular Imaging and Therapy. Acs Nano. 2009;3(9):2686–2696. doi: 10.1021/nn900440e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wong RM, Gilbert DA, Liu K, Louie AY. Rapid Size-Controlled Synthesis of Dextran-Coated, Cu-64-Doped Iron Oxide Nanoparticles. Acs Nano. 2012;6(4):3461–3467. doi: 10.1021/nn300494k. [DOI] [PubMed] [Google Scholar]
- 12.Yang XQ, Hong H, Grailer JJ, Rowland IJ, Javadi A, Hurley SA, Xiao YL, Yang YA, Zhang Y, Nickles R, Cai WB, Steeber DA, Gong SQ. cRGD-Functionalized, DOX-Conjugated, and Cu-64-Labeled Superparamagnetic Iron Oxide Nanoparticles for Targeted Anticancer Drug Delivery and PET/MR Imaging. Biomaterials. 2011;32(17):4151–4160. doi: 10.1016/j.biomaterials.2011.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Na HB, Hyeon T. Nanostructured T1 MRI Contrast Agents. J Mater Chem. 2009;19(35):6267–6273. doi: 10.1039/b902685a. [DOI] [Google Scholar]
- 14.Xiao J, Tian XM, Yang C, Liu P, Luo NQ, Liang Y, Li HB, Chen DH, Wang CX, Li L, Yang GW. Ultrahigh Relaxivity and Safe Probes of Manganese Oxide Nanoparticles for in Vivo Imaging. Sci Rep. 2013;3:3424. doi: 10.1038/srep03424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Crossgrove J, Zheng W. Manganese Toxicity upon Overexposure. Nmr Biomed. 2004;17(8):544–553. doi: 10.1002/nbm.931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yu T, Moon J, Park J, Park YI, Na HB, Kim BH, Song IC, Moon WK, Hyeon T. Various-Shaped Uniform Mn3O4 Nanocrystals Synthesized at Low Temperature in Air Atmosphere. Chem Mater. 2009;21(11):2272–2279. doi: 10.1021/cm900431b. [DOI] [Google Scholar]
- 17.Na HB, Lee JH, An KJ, Park YI, Park M, Lee IS, Nam DH, Kim ST, Kim SH, Kim SW, Lim KH, Kim KS, Kim SO, Hyeon T. Development of a T-1 Contrast Agent for Magnetic Resonance Imaging Using MnO Nanoparticles. Angew Chem Int Edit. 2007;46(28):5397–5401. doi: 10.1002/anie.200604775. [DOI] [PubMed] [Google Scholar]
- 18.Jun YW, Lee JH, Cheon J. Chemical Design of Nanoparticle Probes for High-Performance Magnetic Resonance Imaging. Angew Chem Int Edit. 2008;47(28):5122–5135. doi: 10.1002/anie.200701674. [DOI] [PubMed] [Google Scholar]
- 19.Bennewitz MF, Lobo TL, Nkansah MK, Ulas G, Brudvig GW, Shapiro EM. Biocompatible and pH-Sensitive PLGA Encapsulated MnO Nanocrystals for Molecular and Cellular MRI. Acs Nano. 2011;5(5):3438–3446. doi: 10.1021/nn1019779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ha TL, Kim HJ, Shin J, Im GH, Lee JW, Heo H, Yang J, Kang CM, Choe YS, Lee JH, Lee IS. Development of Target-Specific Multimodality Imaging Agent by Using Hollow Manganese Oxide Nanoparticles as A Platform. Chem Commun. 2011;47(32):9176–9178. doi: 10.1039/c1cc12961a. [DOI] [PubMed] [Google Scholar]
- 21.Hao R, Yu J, Hou YL, Sun SH. One-Pot Synthesis of Hollow/Porous Mn-Based Nanoparticles via a Controlled Ion Transfer Process. Chem Commun. 2011;47(32):9095–9097. doi: 10.1039/c1cc12759d. [DOI] [PubMed] [Google Scholar]
- 22.Shin JM, Anisur RM, Ko MK, Im GH, Lee JH, Lee IS. Hollow Manganese Oxide Nanoparticles as Multifunctional Agents for Magnetic Resonance Imaging and Drug Delivery. Angew Chem Int Edit. 2009;48(2):321–324. doi: 10.1002/anie.200802323. [DOI] [PubMed] [Google Scholar]
- 23.Huang CC, Khu NH, Yeh CS. The Characteristics of Sub 10 nm Manganese Oxide T-1 Contrast Agents of Different Nanostructured Morphologies. Biomaterials. 2010;31(14):4073–4078. doi: 10.1016/j.biomaterials.2010.01.087. [DOI] [PubMed] [Google Scholar]
- 24.Huang J, Xie J, Chen K, Bu LH, Lee S, Cheng Z, Li XG, Chen XY. HSA Coated MnO Nanoparticles with Prominent MRI Contrast for Tumor Imaging. Chem Commun. 2010;46(36):6684–6686. doi: 10.1039/c0cc01041c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yang XY, Zhou ZG, Wang L, Tang CZ, Yang H, Yang SP. Folate Conjugated Mn3O4@SiO2 Nanoparticles for Targeted Magnetic Resonance Imaging in Vivo. Mater Res Bull. 2014;57:97–102. doi: 10.1016/j.materresbull.2014.05.023. [DOI] [Google Scholar]
- 26.Hu H, Dai AT, Sun J, Li XY, Gao FH, Wu LZ, Fang Y, Yang H, An L, Wu HX, Yang SP. Aptamer-Conjugated Mn3O4@SiO2 Core-Shell Nanoprobes for Targeted Magnetic Resonance Imaging. Nanoscale. 2013;5(21):10447–10454. doi: 10.1039/c3nr03490a. [DOI] [PubMed] [Google Scholar]
- 27.Yang H, Zhuang YM, Hu H, Du XX, Zhang CX, Shi XY, Wu HX, Yang SP. Silica-Coated Manganese Oxide Nanoparticles as a Platform for Targeted Magnetic Resonance and Fluorescence Imaging of Cancer Cells. Adv Funct Mater. 2010;20(11):1733–1741. doi: 10.1002/adfm.200902445. [DOI] [Google Scholar]
- 28.Ruoslahti E, Bhatia SN, Sailor MJ. Targeting of Drugs and Nanoparticles to Tumors. J Cell Biol. 2010;188(6):759–768. doi: 10.1083/jcb.200910104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hong H, Zhang Y, Sun JT, Cai WB. Molecular Imaging and Therapy of Cancer with Radiolabeled Nanoparticles. Nano Today. 2009;4(5):399–413. doi: 10.1016/j.nantod.2009.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cai WB, Chen XY. Nanoplatforms for Targeted Molecular Imaging in Living Subjects. Small. 2007;3(11):1840–1854. doi: 10.1002/smll.200700351. [DOI] [PubMed] [Google Scholar]
- 31.Hanahan D, Weinberg RA. The Hallmarks of Cancer. Cell. 2000;100(1)(00):57–70. 81683–9. doi: 10.1016/S0092-8674. [DOI] [PubMed] [Google Scholar]
- 32.Fonsatti E, Nicolay HJM, Altomonte M, Covre A, Maio M. Targeting Cancer Vasculature via Endoglin/CD105: A Novel Antibody-Based Diagnostic and Therapeutic Strategy in Solid Tumours. Cardiovasc Res. 2010;86(1):12–19. doi: 10.1093/cvr/cvp332. [DOI] [PubMed] [Google Scholar]
- 33.Seon BK, Haba A, Matsuno F, Takahashi N, Tsujie M, She XW, Harada N, Uneda S, Tsujie T, Toi H, Tsai H, Haruta Y. Endoglin-Targeted Cancer Therapy. Curr Drug Deliv. 2011;8(1):135–143. doi: 10.2174/156720111793663570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang Y, Yang YA, Cai WB. Multimodality Imaging of Integrin alpha(v)beta(3) Expression. Theranostics. 2011;1:135–148. doi: 10.7150/thno/v01p0135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Quon A, Gambhir SS. FDG-PET and Beyond: Molecular Breast Cancer Imaging. J Clin Oncol. 2005;23(8):1664–1673. doi: 10.1200/Jco.2005.11.024. [DOI] [PubMed] [Google Scholar]
- 36.Engle JW, Hong H, Zhang Y, Valdovinos HF, Myklejord DV, Barnhart TE, Theuer CP, Nickles RJ, Cai WB. Positron Emission Tomography Imaging of Tumor Angiogenesis with a Ga-66-Labeled Monoclonal Antibody. Mol Pharmaceut. 2012;9(5):1441–1448. doi: 10.1021/mp300019c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dallas NA, Samuel S, Xia L, Fan F, Gray MJ, Lim SJ, Ellis LM. Endoglin (CD105): A Marker of Tumor Vasculature and Potential Target for Therapy. Clin Cancer Res. 2008;14(7):1931–1937. doi: 10.1158/1078-0432.CCR-07-4478. [DOI] [PubMed] [Google Scholar]
- 38.Zhang Y, Yang YN, Hong H, Cai WB. Multimodality Molecular Imaging of CD105 (Endoglin) Expression. Int J Clin Exp Med. 2011;4(1):32–42. [PMC free article] [PubMed] [Google Scholar]
- 39.Apolo AB, Karzai FH, Trepel JB, Alarcon S, Lee S, Lee MJ, Tomita Y, Cao L, Yu YK, Merino MJ, Madan RA, Parnes HL, Steinberg SM, Rodriguez BW, Seon BK, Gulley JL, Arlen PM, Dawson NA, Figg WD, Dahut WL. A Phase II Clinical Trial of TRC105 (Anti-Endoglin Antibody) in Adults with Advanced/Metastatic Urothelial Carcinoma. Clin Genitourin Canc. 2017;15(1):77–85. doi: 10.1016/j.clgc.2016.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rosen LS, Hurwitz HI, Wong MK, Goldman J, Mendelson DS, Figg WD, Spencer S, Adams BJ, Alvarez D, Seon BK, Theuer CP, Leigh BR, Gordon MS. A Phase I First-in-Human Study of TRC105 (Anti-Endoglin Antibody) in Patients with Advanced Cancer. Clin Cancer Res. 2012;18(17):4820–4829. doi: 10.1158/1078-0432.CCR-12-0098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhang Y, Hong H, Severin GW, Engle JW, Yang YA, Goel S, Nathanson AJ, Liu G, Nickles RJ, Leigh BR, Barnhart TE, Cai WB. ImmunoPET and Near-Infrared Fluorescence Imaging of CD105 Expression Using a Monoclonal Antibody Dual-Labeled with Zr-89 and IRDye 800CW. Am J Transl Res. 2012;4(3):333–346. [PMC free article] [PubMed] [Google Scholar]
- 42.Hong H, Yang K, Zhang Y, Engle JW, Feng LZ, Yang YA, Nayak TR, Goel S, Bean J, Theuer CP, Barnhart TE, Liu Z, Cai WB. In Vivo Targeting Imaging of Tumor Vasculature with Radiolabeled, Antibody-Conjugated Nanographene. Acs Nano. 2012;6(3):2361–2370. doi: 10.1021/nn204625e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hong H, Zhang Y, Engle JW, Nayak TR, Theuer CP, Nickles RJ, Barnhart TE, Cai WB. In Vivo Targeting Positron Emission Tomography Imaging of Tumor Vasculature with Ga-66-Labeled Nano-Graphene. Biomaterials. 2012;33(16):4147–4156. doi: 10.1016/j.biomaterials.2012.02.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hong H, Zhang Y, Severin GW, Yang YN, Engle JW, Niu G, Nickles RJ, Chen XY, Leigh BR, Barnhart TE, Cai WB. Multimodality Imaging of Breast Cancer Experimental Lung Metastasis with Bioluminescence and a Monoclonal Antibody Dual-Labeled with Zr-89 and IRDye 800CW. Mol Pharmaceut. 2012;9(8):2339–2349. doi: 10.1021/mp300277f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Guo JT, Hong H, Chen GJ, Shi SX, Zheng QF, Zhang Y, Theuer CP, Barnhart TE, Cai WB, Gong SQ. Image-Guided and Tumor-Targeted Drug Delivery with Radiolabeled Unimolecular Micelles. Biomaterials. 2013;34(33):8323–8332. doi: 10.1016/j.biomaterials.2013.07.085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shi SX, Yang K, Hong H, Valdovinos HF, Nayak TR, Zhang Y, Theuer CP, Barnhart TE, Liu Z, Cai WB. Tumor Vasculature Targeting and Imaging in Living Mice with Reduced Graphene Oxide. Biomaterials. 2013;34(12):3002–3009. doi: 10.1016/j.biomaterials.2013.01.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chen F, Hong H, Zhang Y, Valdovinos HF, Shi SX, Kwon GS, Theuer CP, Barnhart TE, Cai WB. In Vivo Tumor Targeting and Image-Guided Drug Delivery with Antibody-Conjugated, Radio labeled Mesoporous Silica Nanoparticles. Acs Nano. 2013;7(10):9027–9039. doi: 10.1021/nn403617J. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhang YC, Qiao T, Hu XY. Preparation of Mn3O4 Nanocrystallites by Low-Temperature Solvothermal Treatment of Gamma-MnOOH Nanowires. J Solid State Chem. 2004;177(11):4093–4097. doi: 10.1016/j.jssc.2004.05.034. [DOI] [Google Scholar]
- 49.Ozkaya T, Baykal A, Kavas H, Koseoglu Y, Toprak MS. A Novel Synthetic Route to Mn(3)O(4) Nanoparticles and Their Magnetic Evaluation. Physica B. 2008;403(19–20):3760–3764. doi: 10.1016/j.physb.2008.07.002. [DOI] [Google Scholar]
- 50.Chen ZW, Lai JKL, Shek CH. Shape-Controlled Synthesis and Nanostructure Evolution of Single-Crystal Mn3O4 Nanocrystals. Scripta Mater. 2006;55(8):735–738. doi: 10.1016/j.scriptamat.2006.05.041. [DOI] [Google Scholar]
- 51.Yang LX, Zhu YJ, Tong H, Wang WW, Cheng GF. Low Temperature Synthesis of Mn3O4 Polyhedral Nanocrystals and Magnetic Study. J Solid State Chem. 2006;179(4):1225–1229. doi: 10.1016/j.jssc.2006.01.033. [DOI] [Google Scholar]
- 52.Chang YQ, Xu XY, Luo XH, Chen CP, Yu DP. Synthesis and Characterization of Mn3O4 Nanoparticles. J Cryst Growth. 2004;264(1–3):232–236. doi: 10.1016/j.crysgro.2003.11.117. [DOI] [Google Scholar]
- 53.Choi HS, Liu W, Misra P, Tanaka E, Zimmer JP, Ipe BI, Bawendi MG, Frangioni JV. Renal Clearance of Quantum Dots. Nat Biotechnol. 2007;25(10):1165–1170. doi: 10.1038/nbt1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


