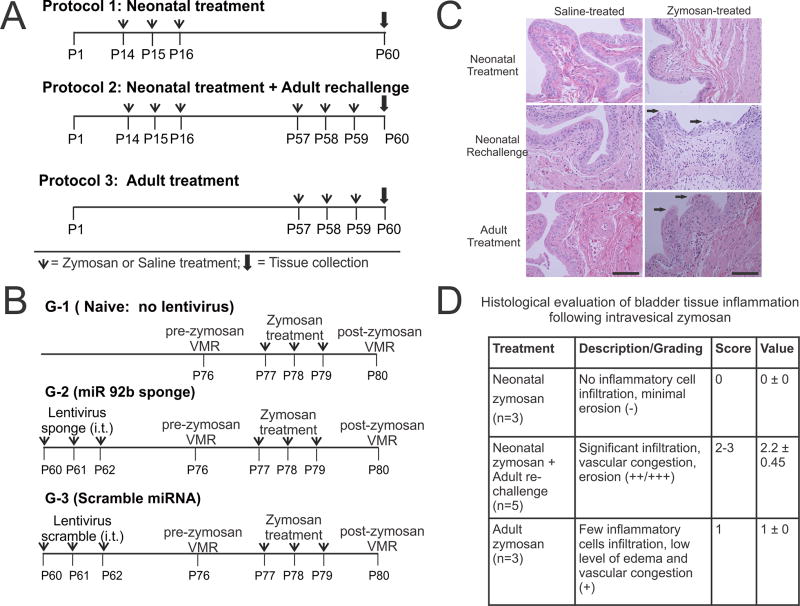
Figure 1.
Schematic representation of three zymosan treatment protocols used in this study. (A) In protocol 1, neonatal zymosan. In protocol 2, similar neonatal treatment as in protocol 1 followed by re-challenge with zymosan or saline at adult stage and in protocol 3, adult zymosan or saline treatment from P57 thro and tissue collection on P60. (B) Schematic representation of intrathecal Lentiviral sponge and scramble miRNA administration preemptively before inducing cystitis in adult rats. (C) Histological examination of the bladder tissues at P60 for rats in all 3 protocols. Hematoxylin and eosin staining reveals no epithelial damage and inflammation in neonatally zymosan-treated rats in protocol 1. Zymosan-treated rats in protocol 2 demonstrate the most prominent epithelial damage and inflammatory cell infiltration among the three treatment protocols. Arrows indicate urothelial damage. Scale bar indicates 100µm. (D). Histopathological changes of the bladder tissue in protocol 2 is significantly higher than in bladder tissues from rats in protocol 3 (fig. 1D, p<0.05).