Abstract
HPV-related multiphenotypic sinonasal carcinoma (HMSC), originally known as HPV-related carcinoma with adenoid cystic carcinoma-like features, is a peculiar neoplasm that is restricted to the sinonasal tract, exhibits features of both a surface-derived and salivary gland carcinoma (particularly adenoid cystic carcinoma), and is associated with high-risk human papillomavirus (HPV). Given the limited number of published cases, the full clinicopathologic spectrum of this neoplasm is unclear. Here, we present an updated experience of 49 cases.
All cases of HMSC were obtained from the authors' files. Immunohistochemistry for p16, c-kit, and myoepithelial cell markers (S100, actin, calponin, p63, and/or p40) was performed along with RNA in situ hybridization for HPV (type 33-specific as well as a high-risk cocktail). Fluorescence in situ hybridization studies for fusions of MYB, NFIB, and MYBL1 was performed on a subset of cases. Clinical follow-up was obtained from medical records.
A total of 49 cases of HMSC were collected. Twenty-eight (57%) were from women and 18 (43%) from men, ranging in age from 28 to 90 years (mean, 54). Of 40 cases with detailed staging information, 43% HMSCs presented with a high T-stage (T3 or T4). Histologically, most grew predominantly as solid nests of basaloid cells exhibiting high mitotic rates and frequent necrosis, with histologic and immunohistochemical evidence of myoepithelial differentiation. Most cases also demonstrated foci of cribriform and/or tubular growth, along with an inconspicuous population of ducts. Thirty-four (69%) cases demonstrated an unusual pattern of surface involvement where markedly atypical squamous cells colonized tracts of the sinonasal mucosa. Less consistent histologic features included squamous differentiation within the invasive tumor (n=6), sarcomatoid transformation (n=5) including overt chondroid differentiation (n=3), and prominent epithelial-myoepithelial carcinoma-like growth (n=3). All cases were positive for p16 by immunostaining and HPV by RNA in situ hybridization. Thirty-three (67%) were positive for HPV 33. No cases tested for MYB, MYBL1, or NFIB gene fusions were positive. In the 38 cases with follow-up data, (mean follow-up, 42 months) 14 recurred locally and 2 metastasized (lung, finger). There were no regional lymph node metastases, and no tumor-related deaths.
HMSC is a distinct sinonasal neoplasm characterized by myoepithelial differentiation, frequent surface epithelial involvement, and the presence of high-risk HPV (especially type 33). Although it classically exhibits a cribriforming pattern that closely resembles adenoid cystic carcinoma, our expanded series highlights a histologic spectrum that is much broader than previously recognized, warranting a change in terminology. HMSC usually presents as a large and destructive sinonasal mass with high-grade histologic features, but it paradoxically behaves in a relatively indolent manner, underscoring the importance of distinguishing HMSC from true adenoid cystic carcinoma, squamous cell carcinoma, and other histologic mimickers.
Keywords: Human papillomavirus, multiphenotypic sinonasal carcinoma, adenoid cystic carcinoma, squamous cell carcinoma, carcinoma with adenoid cystic-like features, MYB, MYBL1
Introduction
Human papillomavirus (HPV) is now well established as a causative agent in approximately 20-25% of head and neck carcinomas overall.(1) Although most HPV-related head and neck carcinomas arise from the oropharynx,(2) the sinonasal tract represents another anatomic “hot spot.” Twenty to 25% of sinonasal carcinomas harbor high risk HPV.(3-7) In the sinonasal tract, HPV positivity is largely associated with the non-keratinizing squamous cell carcinoma phenotype, but Bishop, et al. described a novel form of HPV-positive carcinoma characterized by areas that were highly reminiscent of solid adenoid cystic carcinoma and thus were originally designated as “HPV-related carcinoma with adenoid cystic-like features.”(8) In that original series of 8 cases, all cases: 1) arose from the sinonasal tract, 2) exhibited morphologic features of a salivary gland tumor including admixed ductal and myoepithelial elements, 3) displayed an unusual pattern of surface involvement where markedly atypical squamous cells colonized tracts of the sinonasal mucosa, and 4) failed to demonstrate the MYB gene fusions that characterize many true adenoid cystic carcinomas.(8) Since this sentinel publication, only one additional series (n=6) and a single-case report have been published.(9, 10) Given the small number of reported cases and limited clinical follow-up, the full pathologic spectrum of this tumor remains unclear and its clinical behavior remains undefined. Indeed, while “HPV-related carcinoma with adenoid cystic-like features” was included provisionally in the new WHO classification of head and neck tumors, more cases were needed to justify its inclusion as a full-fledged tumor entity.(11)
Our current documentation of 35 additional cases helps: 1) confirm this carcinoma as a genuine tumor entity that is distinct from true adenoid cystic carcinoma and squamous cell carcinoma, 2) define its clinical behavior, and 3) provide a more complete description of its full morphologic spectrum. Indeed, based on its more expansive histologic features that includes lines of myoepithelial, ductal and squamous differentiation, we propose the term HPV-related multiphenotypic sinonasal carcinoma (HMSC).
Methods
Cases
All cases of HMSC were pulled from the authors' files. Fourteen of the cases had been previously published.(8, 10) Study approval was obtained from the Johns Hopkins University Internal Review Board (IRB00096402). The cases were all reviewed centrally (JAB) and various histologic features were characterized.
Immunohistochemistry
Immunohistochemical studies were performed on five-micrometer thick sections prepared from formalin-fixed and paraffin embedded tissue. Most of the immunohistochemistry was done at The Johns Hopkins Hospital, where the primary antibodies were: p16 (clone INK4a; MTM Laboratories, Heidelberg, Germany); p63 (clone 4A4; Cell Marque Corp., Rocklin, CA); p40 (clone Ab-1; Oncogene Research Products, Cambridge, MA), muscle specific actin (clone HHF35; Ventana; Phoenix, AZ); calponin (clone CALP; Dako, Carpinteria, CA); S100 (clone 4C4.9; Ventana); c-kit (clone YR145; Cell Marque); and AE1/AE3 (clone PCK26; Ventana). For a minority of cases, immunohistochemistry had been done at an outside institution and the reported results were recorded. As a surrogate marker for the presence of HPV, p16 immunostaining was regarded as positive only if staining was both nuclear and cytoplasmic, and present in ≥70% of the tumor cells.
HPV RNA in situ hybridization
HPV testing was performed by RNA in situ hybridization via the RNAscope method (Advanced Cell Diagnostics, Hayward, CA), as previously detailed.(12) Five micron sections from formalin-fixed and paraffin-embedded tissue blocks were evaluated for the presence of HPV RNA using the RNAscope HPV-HR18 Probe (Advanced Cell Diagnostics, Hayward, CA) which recognizes 18 high-risk HPV genotypes (16, 18, 26, 31, 33, 35, 39, 45, 51, 52, 53, 56, 58, 59, 66, 68, 73, and 82). In addition, all cases were tested with an HPV type 33-specific probe, and a subset were tested with an HPV type 16-specific probe. HPV positive controls included an HPV33-positive head and neck squamous cell carcinoma, while an HPV-negative head and neck squamous cell carcinoma served as a negative control.
Quantitative polymerase chain reaction
As previously published, fourteen HMSCs underwent HPV genotyping via quantitative HPV-specific PCR.(8, 10) DNA was extracted from five micron-thick slides containing formalin-fixed paraffin-embedded tumor samples. Briefly, tissues were deparaffinized using xylene, macrodissected from the slides, and underwent digestion with 50 μg/mL proteinase K (Boehringer Mannheim) in the presence of 1% sodium dodecylsuflate at 48°C for 2 days. Then DNA was extracted using UltraPure Phenol:Chloroform:Isoamyl Alcohol reagents (Invitrogen, Carlsbad, CA) utilizing manufacturer's instructions.
For 8 cases, the procedure was as follows, as previously described.(8) The general consensus primers Gp5-Gp6 and Gp5+-Gp6+ were used to amplify the L1 region in the HPV genome as described previously (13)using the following 30μl PCR solution: 16.6mM Ammonium Sulfate, 67.0 mM Tris-Trizma pre-set crystals (pH 8.8), 6.7 mM Magnesium Chloride, 10.0 mM 2-MercaptoEthanol, 0.1% Dimethyl Sulfoxide, 3.3% DMSO, 20 pmol of each primer and 0.5 U Platinum Taq. The fast PCR program used was: 95°C for 30sec, 44°C for 60sec and 72°C for 90sec for 40 cycles in a Veriti thermal cycler (Applied Biosciences). In addition, type-specific primers for E6 and E7 regions for HPV types 11, 16, 18, 31, 33, and 35 were used as described previously.(14) The PCR conditions used were the same as described above except that the amplification cycle was shortened to 35 and the annealing temperature for the HPV 33 and 35 primers was 57°C for 30 seconds.
For another 6 cases, amplicon-based next-generation sequencing 50–100ng of DNA was ligated using the KAPA HTP Library Preparation Kit (Roche, Basel, Switzerland) as previously described.(10) Adaptor-ligated amplicons were sequenced on a MiSeq (Illumina, San Diego, CA) using MiSeq reagent kit version 2 in 500 cycles (Illumina). Raw reads were subjected to a standard Basic Local Alignment Search Tool (BLAST) nucleotide search (http://blast.ncbi.nlm.nih.gov) to identify the HPV type. All reads were mapped against the reference genome of the relevant HPV genotype.
Fluorescence in situ hybridization
A break apart fluorescence in situ hybridization (FISH) assay for MYB (Empire Genomics, Buffalo, NY, USA) was performed on 15 cases, while break apart FISH assays for MYBL1 (Empire Genomics) and NFIB (Empire Genomics) were performed on 6 cases according to the manufacturers' protocol using the HYBrite platform (Abbott Molecular, Des Plaines, IL, USA)., as previously described.(8, 10) Following hybridization, the tumor nuclei were counterstained with 4′,6-diamidino-2-phenylindole (DAPI) II (ZytoVision GmbH, Bremerhaven, Germany). One hundred nuclei were counted, with only nuclei where the entire nuclear membrane could be visualized scored. Break-apart signals in ≥ 10% of cells was considered to represent rearrangement.
Results
Forty-nine HPV-related multiphenotypic sinonasal carcinomas (HMSCs) were identified. The clinical features are summarized in Table 1. All cases arose in the sinonasal tract, with 28 (57%) confined to the nasal cavity (Figure 1), 5 (10%) confined to the paranasal sinuses, and 16 (33%) involving the nasal cavity and paranasal sinuses. The most frequently involved paranasal sinuses were the maxillary (n=10, 48%) and ethmoid (n=9, 43%) sinuses, followed by the sphenoid sinus (n=2, 10%) and frontal sinus (n=2, 10%)). Twenty-eight (57%) of the patients were women and 21 (43%) were men, ranging in age from 28 to 90 years (mean 54 years). The presenting symptoms included nasal obstruction/stenosis (n=26), epistaxis (n=20), nasal drainage/discharge (n=6), pain (n=3), and ocular symptoms (e.g., epiphora or exophthalmos) (n=3). The presenting symptom was unknown in 9 cases. The tumor sizes ranged in size from 0.7 to 8.5 cm (mean, 3.8 cm). Stage information was available for 39 patients. At the time of presentation, the patients were stage T1 (n=16, 41%), T2 (n=7, 17%), T3 (n=8, 21%) and T4 (n=9, 23%). All 39 were limited to the primary site with no evidence of local or distant metastasis at the time of initial diagnosis.
Table 1. Summary of clinical features HPV-related multiphenotypic sinoansal carcinomas.
| Case | Patient | Tumor | Treatment | Follow-up (months) | |||||
|---|---|---|---|---|---|---|---|---|---|
| Age | Sex | Presentation | Site | Size (cm) | Stage | 1° | Recurrence(s) or Metastasis | ||
| 1 | 46 | F | Epistaxis, obstruction | Nasal | 4 | T1N0M0 | Surgery, RT | NED (35) | |
| 2 | 63 | F | Epistaxis | Maxillary/Ethmoid | 3 | T3N0M0 | Surgery | Surgery, RT | LR (24) NED (27) |
| 3 | 48 | F | Obstruction | Ethmoid | 3 | T1N0M0 | Surgery | Surgery, RT | LR (24) NED (68) |
| 4 | 57 | M | Epistaxis | Nasal, Ethmoid/Sphenoid | 3 | T4aN0M0 | Surgery | NED (2) | |
| 5 | 61 | M | Epistaxis, obstruction | Nasal | 3 | Unknown | Unknown | Unknown | |
| 6 | 51 | F | Epistaxis | Nasal | 1 | T1N0M0 | Surgery, RT | NED (55) | |
| 7 | 73 | F | Obstruction, epiphora | Nasal, Maxillary, Orbit | 5 | T4aN0M0 | Surgery | NED (37) | |
| 8 | 40 | F | Obstruction | Nasal, Ethmoid/Sphenoid | 3 | T4bN0M0 | RT, CT | Surgery, RT, CT | LR (23) AWD (24) |
| 9 | 35 | M | Unknown | Nasal | Unknown | Unknown | Unknown | Unknown | |
| 10 | 38 | F | Obstruction | Nasal | 3.2 | T1N0M0 | Surgery, RT | Unknown | LR (38) |
| 11 | 64 | M | Unknown | Maxillary | Unknown | Unknown | Surgery, RT | 1stLR: Surgery 2nd LR: RT |
LR (48) LR (84) |
| 12 | 72 | M | Unknown | Nasal | 3.5 | T1N0M0 | Unknown | Unknown | |
| 13 | 45 | M | Obstruction, epistaxis | Nasal, Maxillary | 8.5 | T3N0M0 | Surgery, RT | NED (12) | |
| 14 | 35 | F | Obstruction, epistaxis, exophthalmo | Nasal, Frontal, Lacrimal duct | 4.3 | T2N0M0 | Surgery, RT | NED (23) | |
| 15 | 49 | F | Epistaxis | Nasal | 1 | T1N0M0 | Surgery | NED (19) | |
| 16 | 58 | F | Epistaxis, sinusitis | Nasal | 0.7 | T1N0M0 | Surgery, RT | LR: surgery DMs: surgery |
LR (72) DM (144, 192, 204) AWD (204) |
| 17 | 46 | F | Epistaxis, drainage | Nasal | 3.6 | T1N0M0 | Surgery, RT | Surgery, RT | LR (24) NED (36) |
| 18 | 36 | M | Unknown | Nasal | Unknown | Unknown | Unknown | Unknown | |
| 19 | 28 | F | Unknown | Nasal | Unknown | Unknown | Unknown | LR (72) | |
| 20 | 57 | F | Obstruction | Nasal, Ethmoid, Orbit | 3.9 | T4aN0M0 | Surgery, RT | NED (12) | |
| 21 | 76 | M | Nasal swelling | Nasal, Orbit | 2.5 | T4aN0M0 | Surgery, RT | NED (8) | |
| 22 | 84 | F | Unknown | Nasal, Orbit, Cranial Fossa | 6.1 | T4aN0M0 | Unknown | Unknown | |
| 23 | 90 | M | Epistaxis | Nasal | 4.5 | T3N0M0 | XRT | Unknown | |
| 24 | 65 | F | Obstruction | Nasal, Maxillary | 2.5 | T3N0M0 | Surgery, RT | NED (21) | |
| 25 | 46 | F | Obstruction | Nasal | Unknown | Unknown | Unknown | Unknown | LR (96) LR (120) |
| 26 | 50 | M | Epistaxis, Drainage | Nasal, Ethmoid | 7.2 | T2N0M0 | Surgery, RT, CT | NED (6) | |
| 27 | 52 | F | Epistaxis | Nasal | Unknown | T1N0M0 | Surgery, RT | Surgery | LR (36) NED (44) |
| 28 | 60 | F | Stenosis, Epistaxis | Nasal | 3.9 | T3N0M0 | Surgery, RT | Surgery | LR (130) NED (256) |
| 29 | 53 | F | Stenosis | Nasal | 3 | T1N0M0 | Surgery | NED (56) | |
| 30 | 48 | F | Stenosis | Nasal | 4 | T1N0M0 | Surgery | NED (39) | |
| 31 | 51 | F | Stenosis, epiphora | Nasal, Ethmoid/Sphenoid | 2 | T4N0M0 | Surgery, RT | NED (27) | |
| 32 | 29 | M | Stenosis, epistaxis | Nasal | 5 | T2N0M0 | Surgery | Surgery | LR (23) |
| 33 | 53 | M | Stenosis | Nasal | 3 | T3N0M0 | Surgery | NED (15) | |
| 34 | 61 | M | Stenosis, epistaxis | Nasal | 7 | T4aN0M0 | Surgery | NED (3) | |
| 35 | 53 | M | Unknown | Nasal, frontal | Unknown | T2N0M0 | Surgery | 1st LR: surgery, RT 2nd LR: surgery DM: CT |
LR (36) LR and DM (96) |
| 36 | 51 | M | Stenosis | Nasal | 3.6 | T2N0M0 | Surgery, RT | Surgery | LR (42) |
| 37 | 53 | M | Epistaxis, Obstruction | Nasal | 5 | T1N0M0 | Surgery | NED (3) | |
| 38 | 63 | F | Obstruction | Nasal, Maxillary | 6 | T3N0M0 | Unknown | Unknown | |
| 39 | 66 | M | Epistaxis | Maxillary | 3.7 | T1N0M0 | Surgery | NED (3) | |
| 40 | 50 | M | Obstruction, discharge | Nasal, Maxillary/Ethmoid | 6.5 | T4N0M0 | Surgery | NED (6) | |
| 41 | 53 | F | Obstruction, pain | Nasal | 3.8 | T1N0M0 | Surgery | NED (1) | |
| 42 | 71 | F | Unknown | Ethmoid | Unknown | Unknown | Unknown | Unknown | |
| 43 | 37 | F | Obstruction, Discharge | Nasal, Maxillary | 3.5 | T2N0M0 | Surgery, RT, CT | NED (42) | |
| 44 | 51 | F | Obstruction, Epistaxis | Nasal | Unknown | Unknown | Unknown | Unknown | |
| 45 | 48 | M | Obstruction, Epistaxis | Nasal | 2.2 | T1N0M0 | Surgery | NED (22) | |
| 46 | 58 | M | Epistaxis | Nasal | 3.4 | T3N0M0 | Surgery | NED (3) | |
| 47 | 46 | M | Epistaxis | Nasal | 1.3 | T1N0M0 | Surgery | NED (8) | |
| 48 | 46 | M | Unknown | Nasal | Unknown | Unknown | Unknown | Unknown | |
| 49 | 60 | F | Discharge, pain | Nasal, Maxillary | 4.2 | T2N0M0 | Surgery, RT, CT | NED (10) | |
Figure 1.
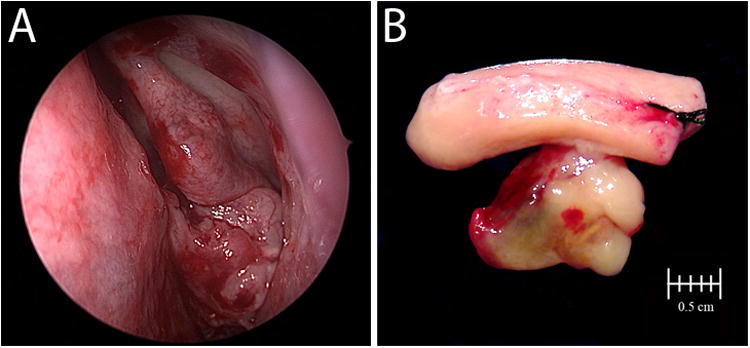
Clinical and gross appearance of HPV-related multiphenotypic sinonasal carcinoma.
Treatment and follow up information was available for 38 patients and 39 patients, respectively. For initial treatment, 18 patients underwent surgical resection alone, 15 patients received surgery with post-operative radiation therapy, 3 patients underwent surgery followed by chemoradiation, 1 patient received chemoradiation without surgery, and 1 patient received radiation therapy alone. The length of follow-up ranged from 1 to 256 months (mean, 42 months). Fourteen of 39 (36%) patients developed local recurrences. The recurrences occurred 23 to 130 months following original treatment. The treatment of the recurrences was known in 11 of 14 patients and included surgery only (n=7), surgery and radiation (n=4), radiation only (n=1), and surgery plus chemoradiation (n=1). Two of 39 (5%) patients developed distant metastases at 96, 144, 192, and 204 months post-treatment. The involved distant sites were the lung (in both patients) and finger (in one patient). The metastases were treated with surgical excision in one patient and chemotherapy in the other. At last known follow-up, 30 patients were alive with no evidence of disease, and 9 patients were alive with disease. None of the patients developed lymph node metastasis or died as a result of their disease.
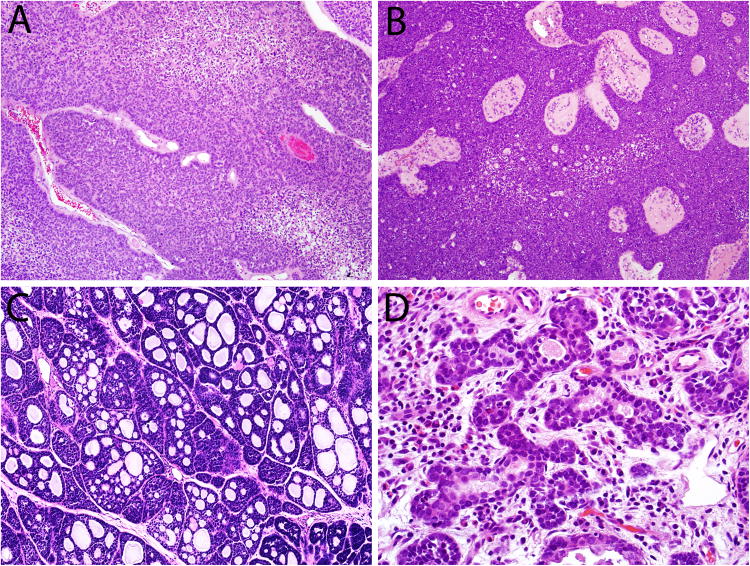
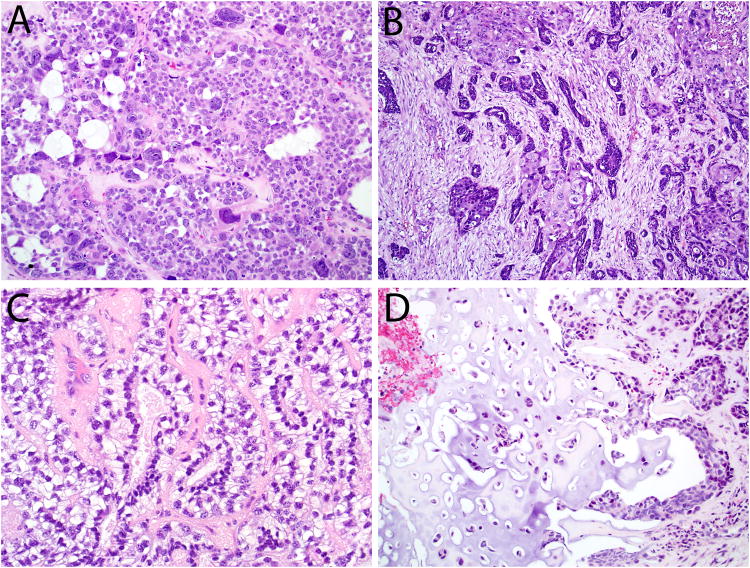
Microscopically, the carcinomas consisted of highly cellular proliferations of basophilic cells. The most common architectural pattern was solid, with large lobules and sheets separated by thin fibrous bands (Figure 2A). The solid pattern was present in 48 of 49 cases, ranging from 5 to 100% of the tumor volume (mean, 76%). Six cases demonstrated fibrovascular cores among the solid areas, with a pushing, ribbon-like growth pattern reminiscent of non-keratinizing squamous cell carcinoma or inverted sinonasal papilloma (Figure 2B). In addition to the predominant solid growth pattern, 34 of 49 cases exhibited cribriform architecture in 5 to 95% of the tumor (mean, 24%) where basaloid cells aligned around cylindromatous microcystic spaces filled with mucoid material (Figure 2C). The least common pattern was tubular which was seen in 24 of 49 cases, and ranged from 5 to 100% (mean, 17%) of the tumor volume (Figure 2D). Thirty of 49 (61%) tumors had areas of cellular tumor necrosis, and the mitotic rates ranged from 3 to 150 per 10 high power fields (mean, 38). Bone invasion was present in 11 of 49 cases, while perineural invasion was identified in just 4 of 49 cases. Lymphovascular invasion was not seen in any of the cases.
Figure 2.
Most HPV-related multiphenotypic sinonasal carcinomas demonstrated a predominantly solid growth pattern (A), sometimes with an inverted growth pattern and fibrovascular cores (B). A cribriform growth pattern was commonly seen, imparting a highly adenoid cystic-like appearance (C). A focal tubular growth pattern was also seen in some cases (D).
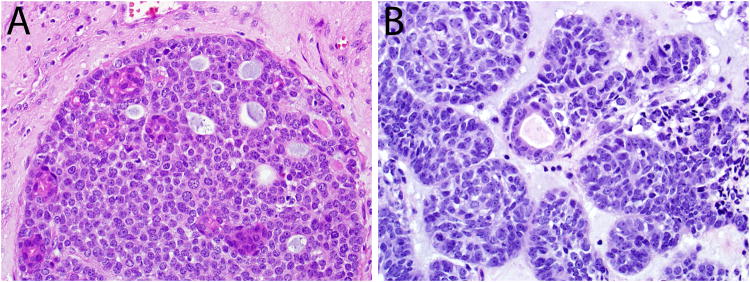
Forty-three (88%) cases were biphasic, consisting of two tumor cell populations: eosinophilic ductal cells scattered among the basaloid cells (Figure 3). The predominant basaloid cell type had hyperchromatic nuclei, a high nuclear to cytoplasmic ratio (Figure 3), and commonly exhibited histologic evidence of myoepithelial differentiation in the form of focal cell spindling (n=26), cytoplasmic clearing (n=44), plasmacytoid cytomorphology (n=5), and extracellular deposition of hyaline matrix-like material (n=13) (Figure 4). The second cell type was the true ductal cell that was cuboidal with eosinophilic cytoplasm and often situated at the center of the nests and surrounded by zones of basaloid cells (Figure 3).
Figure 3.
Most HPV-related multiphenotypic sinonasal carcinomas were biphasic, with subtle eosinophilic ducts scattered among more prominent basaloid cells (A, B).
Figure 4.
The basaloid cells of HPV-related multiphenotypic sinonasal carcinoma variably demonstrated histologic evidence of myoepithelial differentiation in the form of cell spindling (A), clear cell change (B), plasmacytoid morphology (C), and extracellular hyaline matrix deposition (D).
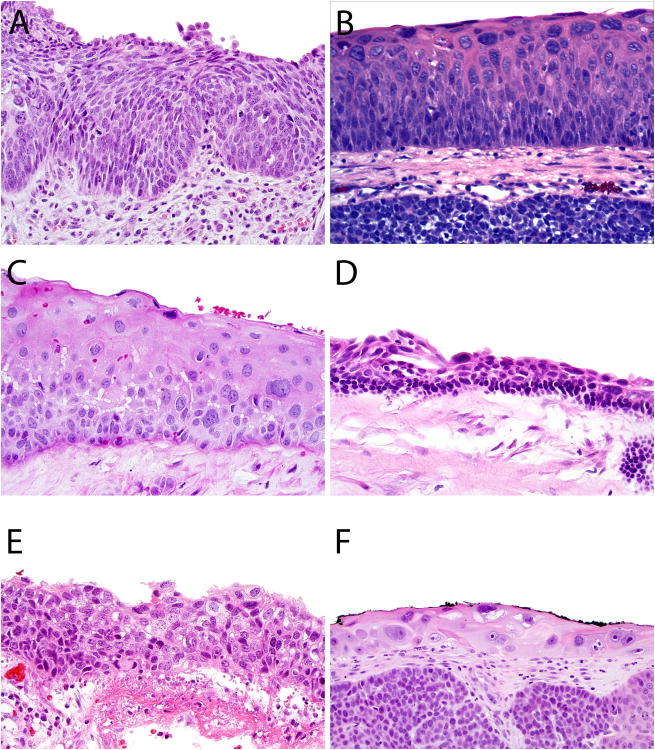
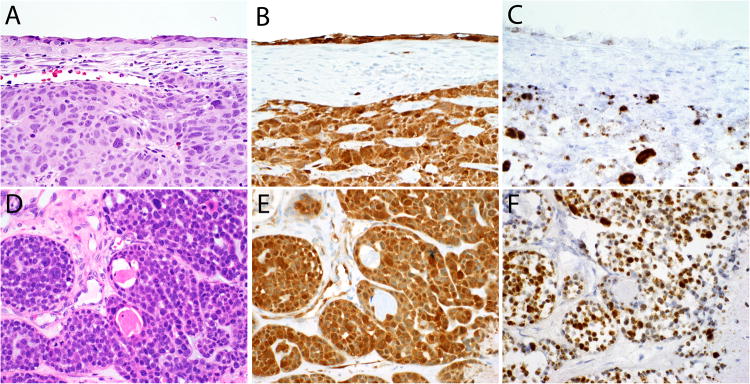
Surface tumor involvement in the form of severe squamous epithelial atypia was observed in the overlying surface epithelium in 34 of 49 (69%) cases. The atypical epithelium was variably thick, ranging from 2-3 cell layers to >20 cell layers thick, with a quality that also ranged from maturing and squamous to primitive and transitional in appearance. It is not clear whether this surface involvement represented a true premalignant lesion or secondary tumor extension. In a few of the cases the atypical epithelium resembled squamous dysplasia that could be encountered in other head and neck sites, but in most examples, the cellular atypia was very bizarre, with randomly distributed, hyperchromatic, giant nuclei with variably prominent nucleoli (Figures 5 and 8A).
Figure 5.
The variable histologic appearances of the tumor involvement of the surface epithelium seen in HPV-related multiphenotypic sinonasal carcinomas (A-F). It is not clear whether this finding is a truly premalignant (dysplastic) process.
Figure 8.
The HPV-related multiphenotypic sinonasal carcinomas (A and D) were diffusely positive for p16 by immunohistochemistry (B and E) and high-risk HPV by RNA in situ hybridization (C and F) in both the surface and invasive components (A-C) and in both the ductal and abluminal tumor cells (D-F). The pattern of in situ hybridization signals was both punctate and diffuse (C and F), with the diffuse signals localizing to the tumor giant cells (C).
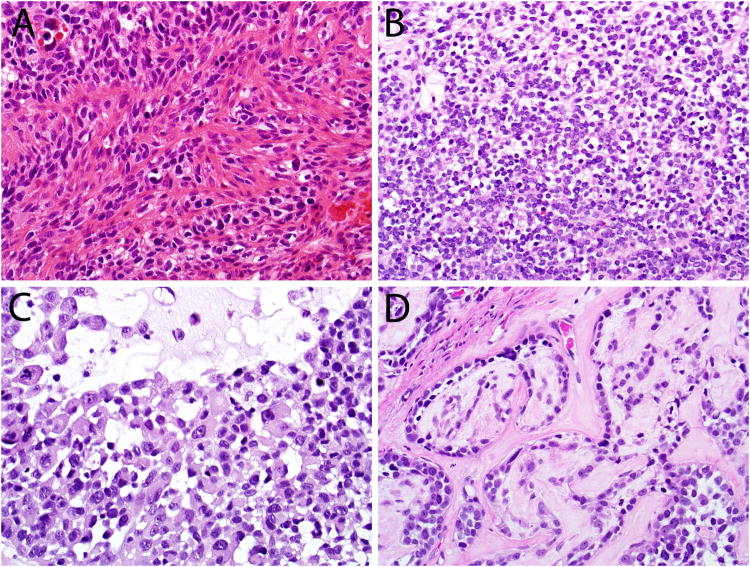
In this expanded cohort, some histologic features were seen that were not noted in our previous series. Twenty-two of 49 cases exhibited anaplastic giant cells randomly scattered in the invasive tumor (Figures 6A and 8A). Six of 49 cases demonstrated scattered foci of squamous differentiation within the invasive tumor component (Figure 6B). Three cases exhibited an epithelial-myoepithelial carcinoma-like appearance with a tightly coupled population of ductal and myoepithelial cells with a prominently clear cell appearance (Figure 6C). Two cases exhibited prominent dilated, slit-like or “hemangiopericytoma-like” vessels. Five cases demonstrated a minor component of sarcomatoid differentiation with undifferentiated malignant spindled cells. In 3 of these cases, heterologous mesenchymal tumor elements were seen in the form of chondroid (n=3) and osseous (n=1) differentiation (Figure 6D).
Figure 6.
The histologic spectrum of HPV-related multiphenotypic sinonasal carcinoma includes the presence of scattered anaplastic giant cells (A), squamous differentiation within the invasive tumor (B), an epithelial-myoepithelial carcinoma-like appearance (C) and sarcomatoid differentiation including heterologous cartilage formation (D).
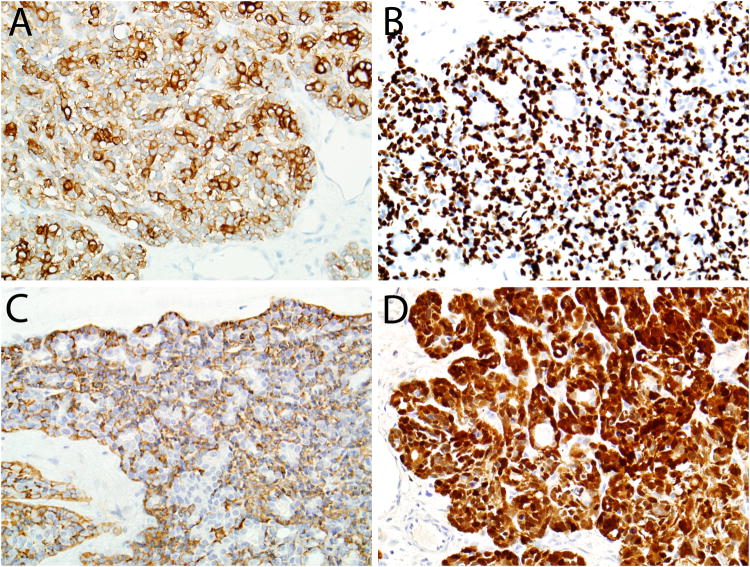
By immunohistochemistry, 39 of 39 (100%) cases were positive for the cytokeratin cocktail AE1/AE3. In 31 of 39 cases, cytokeratin staining was biphasic, with strong staining in the ductal cells and weaker staining in the abluminal basaloid cells (Figure 7A). The basal/myoepithelial cell marker p40 (or p63) was positive in 48 of 49 cases (98%). In 42 of these cases, the pattern was abluminal, sparing true ductal elements (Figure 7B). Calponin was positive in 29 of 30 cases and smooth muscle actin was positive in 30 of 37, with the majority of cases showing an abluminal staining pattern with these smooth muscle markers (Figure 7C). C-kit was positive in 41 of 43 HMSCs, with 29 showing a ductal staining pattern. S100 was positive in 44 of 46 cases (Figure 7D), with a variety of patterns: 19 abluminal, 11 focal, 7 diffuse, 4 ductal, and 3 mixed.
Figure 7.
HPV-related multiphenotypic sinonasal carcinomas were consistently positive for pancytokeratin, usually in a biphasic pattern with dark staining in ducts and lighter staining in the basaloid cells (A). Immunostaining for myoepithelial markers p40 (B) and calponin (C) tended to be in an abluminal pattern, staining ducts, while the staining distribution was more variable for S100, including a diffuse pattern (D).
P16 showed strong and diffuse immunoreactivity in 49 of 49 cases (Figure 8B), and RNA in situ hybridization signals for the high-risk HPV cocktail were present in all cases as well. RNA in situ hybridization studies specific for type 33 were positive in 33 of 49 cases (67%). PCR-based HPV typing, performed on 14 cases, revealed HPV type 33 (n=9), type 35 (n=3), type 56 (n=1), and HPV type unknown (n=1). Of the 24 cases also tested specifically for HPV type 16 by either RNA in situ hybridization or PCR-based detection, only one was positive. The p16 immunopositivity and in situ hybridization signals were detected in both cell types (basaloid and ductal) in the invasive tumor, as well as the involved surface epithelium (Figure 8).
Because of the morphologic resemblance that many of the HMSCs shared with adenoid cystic carcinoma, a subset of the tumors was evaluated for rearrangements of MYB (n=15), MYBL1 (n=6), and NFIB (n=6). All cases tested were negative, as previously described.(8, 10)
Discussion
The classification of sinonasal tumors has undergone major revisions in recent years, with the introduction of several new molecularly-defined entities including NUT carcinoma,(15, 16) biphenotypic sinonasal sarcoma,(17, 18) and SMARCB1-deficient sinonasal carcinoma.(19, 20) Furthermore, the sinonasal tract has been identified as another “hot spot” for HPV-related carcinomas, with 20-25% of sinonasal carcinomas harboring transcriptionally active high-risk HPV.(3-7) In the sinonasal tract, HPV positivity usually is associated with non-keratinizing squamous cell carcinoma, but we have previously drawn attention to a peculiar form of HPV-related sinonasal carcinoma characterized by a component of cribriforming growth and a strong tendency to be misclassified as high grade adenoid cystic carcinoma.(8) Since its initial description in 2012 under the term “HPV-related carcinoma of the sinonasal tract with adenoid cystic-like features,” just a handful of additional cases have been reported, and it was only provisionally included in the WHO classification of head and neck tumors (as a form of non-keratinizing squamous cell carcinoma).(9-11) We now report a more comprehensive experience that permits a more complete description of its histologic features, draws attention to its paradoxical clinical behavior, and helps to validate its nature as a bona fide tumor entity that is quite distinct from both squamous cell carcinoma and adenoid cystic carcinoma.
Many of the characteristics in the original description of “HPV-related carcinoma with adenoid cystic like features” were confirmed in the expanded cohort. 1) All of the tumors arose from the sinonasal tract. In our collective consultation practices, this distinct tumor type was not encountered outside of the sinonasal tract. 2) Architecturally, the tumors were predominantly solid but with zones of cribriform and/or tubular growth – an appearance reminiscent of the solid variant of adenoid cystic carcinoma. 3) The tumors were comprised of mixed phenotypes including basaloid cells showing myoepithelial differentiation, luminal cells showing true ductal differentiation, and surface lining cells showing squamous differentiation. 4) The tumors invariably harbored transcriptionally active high risk HPV, most commonly HPV type 33. 5) the tumors do not harbor any of the gene fusions that have been identified in true adenoid cystic carcinomas. At the same time, the increased numbers of cases allowed for the recognition of histologic features not seen in the initial publication. While all tumors demonstrated a “salivary-like” appearance, their resemblance to certain salivary gland tumor types varied tremendously. For example, some tumors demonstrated almost purely cribriform architectural growth reminiscent of classic adenoid cystic carcinoma, while others more closely resembled epithelial-myoepithelial carcinoma, basal cell adenocarcinoma, or simply adenocarcinoma, not otherwise specified. Indeed, the presence of high-grade “salivary like” features that are difficult to designate into a specific category is an important clue to the diagnosis. Other newly recognized features included cellular anaplasia (22 of 49), an inverted growth pattern with fibrovascular cores (6 of 49), squamous differentiation within the invasive tumor (5 of 49), hemangiopericytoma-like vasculature (2 of 49), and sarcomatoid features (5 of 49) including chondro-osseous differentiation (3 of 49). These findings further expand the differential diagnosis to include squamous cell carcinoma, carcinosarcoma, and carcinoma ex-pleomorphic adenoma, among others.
This larger case series prompts reconsideration of the most appropriate nomenclature for this distinct tumor type. We had previously introduced the term “HPV-related sinonasal carcinoma with adenoid cystic-like features” for the way it consistently showed morphologic overlap with the solid variant of adenoid cystic carcinoma. We now propose the designation “HPV-related multiphenotypic sinonasal carcinoma” (HMSC) for 3 primary reasons. First, the morphologic spectrum is broader than initially appreciated. The adenoid cystic-like component, while a consistent feature, is not a prevailing feature in all cases. Some cases, for example, may more easily be mistaken for epithelial-myoepithelial carcinoma or basal cell adenocarcinoma. Second, the tumor is partially defined by the invariable presence of multidirectional phenotypes including myoepithelial, ductal, and squamous lines of differentiation that characterizes it. The term “multiphenotypic” captures this histologic diversity. The cell of origin that accounts for these multiple lines of differentiation is unknown, though one possibility is the transitional epithelium between the excretory ducts and the overlying surface epithelium. While many cases demonstrate surface involvement in the form of bizarre squamous atypia, it is unclear whether these foci represent a truly premalignant process or secondary intraepithelial tumor extension. Finally, we have found that the term “adenoid cystic-like” is troublesome for the way it invites clinicians to regard this tumor as a true adenoid cystic carcinoma. Finally, the term HPV-related emphasizes yet another defining feature of this tumor, its strong association with high risk HPV.
The increased number of patients and extended follow-up time allowed for a greater appreciation of the clinical behavior of HMSC. The presenting tumor stage was variable, with 23 presenting at a low stage (T1 or T2) and 17 at a high stage (T3 or T4). The nasal cavity was involved in most cases (44 of 49). Treatments given were variable, with most patients receiving surgery with or without radiation therapy. While recurrences were relatively frequent (14 of 39 patients with follow up), only 2 patients developed distant metastases, none developed regional metastases and none died of their disease within the follow-up. Even compared to other sinonasal carcinomas (both HPV-positive and HPV-negative), this behavior appears to be uniquely indolent.(4, 5, 7) These observations would seem to justify a therapeutic approach that would include surgical resection of the primary tumor followed by radiation therapy for close or positive margins. In the absence of documented regional or distant disease, routine lymph node dissection and chemotherapy do not appear to be indicated for HMSC although our experience with these tumors is still too limited for any definitive treatment conclusions.
High-risk HPV may be a common etiologic factor for various carcinomas of the head and neck, but it does not necessarily follow that all HPV-related carcinomas of the head and neck are equivalent. This expanded series highlights striking differences between HPV-related carcinoma of the oropharynx and this distinct carcinoma of the sinonasal tract (Table 2). In addition to the differences in location and morphology, these tumors differ in patient demographics. For example, while HPV-related carcinoma of the oropharynx typically affects adults in their 5th to 7th decades, HMSC may be seen in broader age range, from 28 to 90 years old. In addition, while HPV-related carcinoma of the oropharynx affects predominantly men (3:1 male:female ratio), HMSC has a female predominance (1:1.5 male:female ratio). While both tumors seem to carry a good prognosis, oropharyngeal HPV-related carcinoma frequently metastasizes to cervical lymph nodes, while lymph node metastases have not been recorded in HMSC. Finally, while HPV type 16 is by far the most common HPV type in oropharyngeal squamous cell carcinoma, the uncommon HPV type 33 is most common in HMSC (seen in two-thirds of cases), with only one case harboring HPV16.
Table 2.
Comparison between HPV-related oropharyngeal squamous cell carcinoma and HPV-related multiphenotypic sinonasal carcinoma.
| HPV-related oropharyngeal squamous cell carcinoma | HPV-related multiphenotypic sinonasal carcinoma | |
|---|---|---|
| Age | 40s-60s | 20s-90s |
| Sex | Men > women (3:1) | Women > men (1.5:1) |
| Histologic origin | Tonsillar crypts | Unclear |
| Morphology | Non-keratinizing squamous cell carcinoma | Salivary-like |
| Most common HPV type | 16 | 33 |
| Clinical behavior | ||
| • Regional metastases | • Very common | • Not observed |
| •Outcome | • Favorable | • Favorable |
To summarize, HMSC is a distinct sinonasal neoplasm characterized by a salivary-like appearance with myoepithelial and ductal cells, often high-grade histologic features, frequent surface squamous atypia, and a consistent association with uncommon high-risk HPV types (especially type 33). Its histologic spectrum is broader than previously realized, however, with cases often exhibiting cellular anaplasia and occasionally showing squamous or sarcomatoid differentiation. Importantly, HMSC does not always closely resemble adenoid cystic carcinoma, and can resemble squamous cell carcinoma or other salivary tumor types. Although HMSC presents at high tumor stage in close to half of cases and has a high-grade histologic appearance, it paradoxically behaves in a relatively indolent manner with frequent local recurrences but only rare metastases and no reported tumor-related deaths. As a result, it is important to diagnose HMSC correctly and communicate the significance of this diagnosis to treating clinicians. HMSC should be separated from sinonasal adenoid cystic carcinoma and, indeed, the diagnosis should be considered for any unusual-appearing carcinoma, especially if it resembles a salivary gland tumor. P16 is a useful immunohistochemical screen because all HMSCs are diffusely positive, but a definitive diagnosis requires subsequent HPV-specific testing. The potential clinical management differences will need to be defined by future studies, as more cases of this unique entity are identified and studied.
Acknowledgments
This work has been partially funded by the National Institute of Dental and Craniofacial Research (R01 DE013152-11) and State of Maryland Department of Health and Mental Hygiene (Cigarette Restitution Fund Program) (PHPA-G2034)
References
- 1.Gillison ML, Koch WM, Capone RB, et al. Evidence for a causal association between human papillomavirus and a subset of head and neck cancers. Journal of the National Cancer Institute. 2000;92:709–720. doi: 10.1093/jnci/92.9.709. [DOI] [PubMed] [Google Scholar]
- 2.Singhi AD, Westra WH. Comparison of human papillomavirus in situ hybridization and p16 immunohistochemistry in the detection of human papillomavirus-associated head and neck cancer based on a prospective clinical experience. Cancer. 2010;116:2166–2173. doi: 10.1002/cncr.25033. [DOI] [PubMed] [Google Scholar]
- 3.El-Mofty SK, Lu DW. Prevalence of high-risk human papillomavirus DNA in nonkeratinizing (cylindrical cell) carcinoma of the sinonasal tract: a distinct clinicopathologic and molecular disease entity. Am J Surg Pathol. 2005;29:1367–1372. doi: 10.1097/01.pas.0000173240.63073.fe. [DOI] [PubMed] [Google Scholar]
- 4.Bishop JA, Guo TW, Smith DF, et al. Human papillomavirus-related carcinomas of the sinonasal tract. Am J Surg Pathol. 2013;37:185–192. doi: 10.1097/PAS.0b013e3182698673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Alos L, Moyano S, Nadal A, et al. Human papillomaviruses are identified in a subgroup of sinonasal squamous cell carcinomas with favorable outcome. Cancer. 2009;115:2701–2709. doi: 10.1002/cncr.24309. [DOI] [PubMed] [Google Scholar]
- 6.Larque AB, Hakim S, Ordi J, et al. High-risk human papillomavirus is transcriptionally active in a subset of sinonasal squamous cell carcinomas. Mod Pathol. 2014;27:343–351. doi: 10.1038/modpathol.2013.155. [DOI] [PubMed] [Google Scholar]
- 7.Laco J, Sieglova K, Vosmikova H, et al. The presence of high-risk human papillomavirus (HPV) E6/E7 mRNA transcripts in a subset of sinonasal carcinomas is evidence of involvement of HPV in its etiopathogenesis. Virchows Arch. 2015;467:405–415. doi: 10.1007/s00428-015-1812-x. [DOI] [PubMed] [Google Scholar]
- 8.Bishop JA, Ogawa T, Stelow EB, et al. Human papillomavirus-related carcinoma with adenoid cystic-like features: a peculiar variant of head and neck cancer restricted to the sinonasal tract. Am J Surg Pathol. 2013;37:836–844. doi: 10.1097/PAS.0b013e31827b1cd6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hwang SJ, Ok S, Lee HM, et al. Human papillomavirus-related carcinoma with adenoid cystic-like features of the inferior turbinate: a case report. Auris Nasus Larynx. 2015;42:53–55. doi: 10.1016/j.anl.2014.07.005. [DOI] [PubMed] [Google Scholar]
- 10.Andreasen S, Bishop JA, Hansen TV, et al. Human papillomavirus-related carcinoma with adenoid cystic-like features of the sinonasal tract: clinical and morphological characterization of six new cases. Histopathology. 2017;70:880–888. doi: 10.1111/his.13162. [DOI] [PubMed] [Google Scholar]
- 11.Bishop JA, Brandwein-Gensler M, Nicolai P, et al. Non-keratinizing squamous cell carcinoma. In: el-Naggar AK, Chan JKC, Grandis JR, et al., editors. WHO Classification of Head and Neck Tumours. Lyon, France: IARC Press; 2017. pp. 15–17. [Google Scholar]
- 12.Bishop JA, Ma XJ, Wang H, et al. Detection of transcriptionally active high-risk HPV in patients with head and neck squamous cell carcinoma as visualized by a novel E6/E7 mRNA in situ hybridization method. Am J Surg Pathol. 2012;36:1874–1882. doi: 10.1097/PAS.0b013e318265fb2b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jacobs MV, de Roda Husman AM, van den Brule AJ, et al. Group-specific differentiation between high- and low-risk human papillomavirus genotypes by general primer-mediated PCR and two cocktails of oligonucleotide probes. Journal of clinical microbiology. 1995;33:901–905. doi: 10.1128/jcm.33.4.901-905.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hagmar B, Johansson B, Kalantari M, et al. The incidence of HPV in a Swedish series of invasive cervical carcinoma. Medical oncology and tumor pharmacotherapy. 1992;9:113–117. doi: 10.1007/BF02987743. [DOI] [PubMed] [Google Scholar]
- 15.French CA, Bishop JA, Lewis JS, et al. NUT carcinoma. In: el-Naggar AK, Chan JKC, Grandis JR, et al., editors. WHO Classification of Head and Neck Tumours. Lyon, France: IARC Press; 2017. pp. 20–21. [Google Scholar]
- 16.French C. NUT midline carcinoma. Nat Rev Cancer. 2014;14:149–150. doi: 10.1038/nrc3659. [DOI] [PubMed] [Google Scholar]
- 17.Lewis JT, Oliveira AM, Nascimento AG, et al. Low-grade sinonasal sarcoma with neural and myogenic features: a clinicopathologic analysis of 28 cases. Am J Surg Pathol. 2012;36:517–525. doi: 10.1097/PAS.0b013e3182426886. [DOI] [PubMed] [Google Scholar]
- 18.Lewis JE, Oliveira AM. Biphenotypic sinonasal sarcoma. In: el-Naggar AK, Chan JKC, Grandis JR, et al., editors. WHO Classification of Head and Neck Tumours. Lyon, France: IARC Press; 2017. pp. 40–41. [Google Scholar]
- 19.Bishop JA, Antonescu CR, Westra WH. SMARCB1 (INI-1)-deficient carcinomas of the sinonasal tract. Am J Surg Pathol. 2014;38:1282–1289. doi: 10.1097/PAS.0000000000000285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Agaimy A, Hartmann A, Antonescu CR, et al. SMARCB1 (INI-1)-deficient Sinonasal Carcinoma: A Series of 39 Cases Expanding the Morphologic and Clinicopathologic Spectrum of a Recently Described Entity. Am J Surg Pathol. 2017;41:458–471. doi: 10.1097/PAS.0000000000000797. [DOI] [PMC free article] [PubMed] [Google Scholar]