Abstract
Hypertension is associated with increased activity of the kallikrein-kinin system. Kinin B1 receptor (B1R) activation leads to vasoconstriction and inflammation. Despite evidence supporting a role for the B1R in blood pressure (BP) regulation, the mechanisms by which B1R could alter autonomic function and participate in the pathogenesis of hypertension remain unidentified. We sought to explore whether B1R-mediated inflammation contribute to hypertension and investigate the molecular mechanisms involved. In this study, we tested the hypothesis that activation of B1R in the brain is involved in the pathogenesis of hypertension, using the DOCA-salt model of neurogenic hypertension in wild-type (WT) and B1R knockout mice (B1RKO). DOCA-salt treatment in WT mice led to significant increases in B1R mRNA and protein levels and bradykinin levels, enhanced gene expression of carboxypeptidase N supporting an increase in the B1R ligand, associated with enhanced BP, inflammation, sympatho-excitation, autonomic dysfunction and impaired baroreflex sensitivity, while these changes were blunted or prevented in B1RKO mice. B1R stimulation was further shown to involve activation of the ASK1-JNK-ERK1/2 and NF-kB pathways in the brain. To dismiss potential developmental alterations in KO mice, we further used B1R blockade selectively in the brain of WT mice. Supporting the central origin of this mechanism, intracerebroventricular infusion of a specific B1R antagonist, attenuated the DOCA-salt-induced increase in BP in WT mice. Our data provide the first evidence of a central role for B1R-mediated inflammatory pathways in the pathogenesis of DOCA-salt hypertension, and offer novel insights into possible B1R-targeteted therapies for the treatment of neurogenic hypertension.
Keywords: Autonomic function, central nervous system, hypertension, inflammation, kinin B1 receptor
Introduction
Hypertension is the single most important predisposing factor for cardiovascular and renal impairments, and remains an important public health issue. The mechanisms involved in the pathogenesis of human essential hypertension are multifactorial and remain unknown.1 The majority of current therapeutics are targeted against the peripheral overactive renin-angiotensin system.2 These therapies have clearly reduced the morbidity and mortality in hypertensive patients; however, the long-term prognosis remains poor and new therapeutic approaches are needed. Although several factors have been implicated in the pathogenesis of salt-induced hypertension, recent studies have highlighted the role played by inflammatory cytokines in the brain.3–6
Kinins are a family of vasoactive pro-inflammatory peptides of the kallikrein-kinin system that mediate a variety of physiological actions related to cardiovascular homeostasis, inflammatory responses and pain induction mechanisms.7 Kinins, particularly Bradykinin and lysyl-bradykinin (Lys-BK) or kallidin are important mediators of inflammatory responses.7 Physiological effects of kinins are mediated by two pharmacologically distinct G-protein coupled receptor subtypes, kinin B1 (B1R) and B2 (B2R) receptors.8, 9 Bradykinin and Lys-BK are relatively selective ligands for the B2R, whereas, bradykinin metabolites des-Arg9BK and Lys-des-Arg9BK are selective ligands for the B1R.8, 9 Carboxypeptidase N enzymatically removes the carboxy-terminal arginine from BK, forming des-Arg9-BK. Typically, the B2R is constitutively expressed whereas B1R expression is up-regulated in the presence of cytokines and endotoxins or following tissue injury.9, 10 Interestingly, unlike B2R, once B1R is activated by an agonist, it is not internalized or desensitized.10 Thus, B1R are better equipped to mediate the chronic actions of the bradykinin system and represent a novel therapeutic target for chronic inflammatory diseases.
Observations from clinical and experimental studies suggest an important role for kinins and its receptors in the maintenance of normotension and the development of hypertension.11–15 However, the role of B1R expression in the cardiovascular system is still poorly understood. Evidence has been presented that B1R blockade contributes to the protective effects of angiotensin converting enzyme inhibitors in mice after myocardial infarction16 and in diabetic nephropathy.17 On the other hand, significant up-regulation of B1R gene expression was observed in cardiac and renal tissues of salt dependent and renovascular hypertensive animals.13 Up-regulation of B1R and increased systolic pressure were observed in a rat model of insulin resistance induced by glucose feeding, which was prevented by treatment with B1R antagonist.18 Acute blockade of B1R using specific antagonist or antisense oligodeoxynucleotides infusion decreased blood pressure (BP) in spontaneously hypertensive (SH) but not in Wistar-Kyoto (WKY) rats.19 These studies suggest that B1R expression may play a role in the pathogenesis of hypertension. However, studies addressing the effect of chronic blockade of B1R on neurogenic hypertension have not been performed. Despite the body of evidence that supports a role for the kinin B1R in hypertension, the mechanisms through which the B1R participates in the pathogenesis of hypertension remains unknown. Therefore, in the present study we determined the effects of kinin B1R deletion and brain specific antagonism of the B1R on Deoxycorticosterone acetate (DOCA)-salt hypertension, a neurogenic hypertension model. Additionally, we examined the role of B1R in neuro-inflammation, sympathetic activation, baroreflex sensitivity, as well as the cellular and molecular mechanisms involved during neurogenic hypertension. The specific objectives of the present study were to investigate the following: 1) the involvement of B1R activation in the brain cardiovascular regulatory centers in hypertension; 2) the role of B1R blockade either globally or specifically in the brain on hypertension; 3) elucidate the molecular signaling mechanisms activated by B1R in the pathogenesis of neurogenic hypertension.
Methods
A detailed Methods section is available in the online data supplement.
Animals
Mice were housed in a temperature- and humidity-controlled facility under a 12-hour dark/light cycle, fed standard mouse chow and water ad libitum. The experiments were performed on adult male mice (12–16 weeks old). Kinin B1 receptor knockout (B1RKO) mice were a kind gift from Dr. Michael Bader (Charité Hospital, Berlin, Germany) and originated from 10 generations of backcrossing of an initially mixed genetic background (129/Sc and C57Bl/6) with C57Bl/6 mice.20 Wild-type (WT) C57Bl/6J mice (stock no. 000664) were purchased from the Jackson Laboratory. All animal studies were approved by the Louisiana State University Health Sciences Center-New Orleans Animal Care and Use Committee (IACUC #3271) and were performed in accordance with the National Institutes of Health Guidelines for the Care and Use of Laboratory Animals.
Data analysis
Data are presented as mean ±SEM. Statistical analyses were performed using GraphPad Prism 7 (GraphPad Software). Experiments involving two groups were compared using unpaired, 2-tailed t tests. Multiple comparisons were made using 1-way ANOVA, or two-way ANOVA, followed by Bonferroni’s post hoc analysis or Tukey’s multiple comparisons test, as appropriate. Mean arterial pressure data was analyzed by two-way repeated measures ANOVA with Tukey’s multiple comparisons test. Differences were considered statistically significant at P<0.05.
Results
Kinin B1R expression is up-regulated in the brain of hypertensive mice
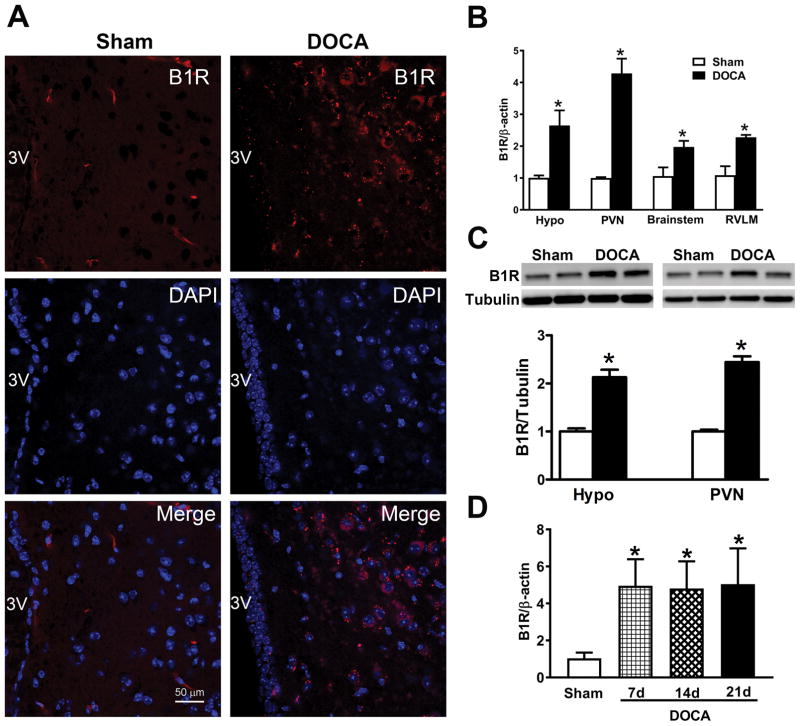
To determine whether B1R expression is involved in hypertension, we first performed immunohistochemistry for this receptor in the brain of mice submitted to the DOCA-salt paradigm. Immunostaining of coronal brain sections with a B1R specific antibody showed a dramatic up-regulation of the receptor expression in the hypothalamus of hypertensive mice compared to control mice (Figure 1A). In addition, we performed double immuno labelling of B1R and MAP2, a neuronal marker, which showed co-localization of B1R immunoreactivity within neurons, suggesting that B1R expression was upregulated in neurons during hypertension (Supplemental Figure S1). Gene expression assessed by real time RT-PCR revealed that B1R mRNA was significantly up-regulated in hypothalamus and brainstem, notably the PVN and RVLM of DOCA-salt-treated hypertensive mice when compared with sham-treated controls (Figure 1B, P<0.01). However, B1R gene expression was higher in the PVN than in the RVLM. Western blot analysis revealed that B1R protein levels in the hypothalamus and PVN were also significantly increased in DOCA-salt hypertensive mice compared with controls (Figure 1C, P<0.01). B1R mRNA expression was increased at days 7, 14 and 21 of DOCA-salt treatment compared with sham mice (Figure 1D, P<0.01) suggesting an early upregulation of B1R which remained elevated throughout the study. In addition, bradykinin levels in the plasma and hypothalamus (Supplemental Figure S2, P<0.01) were significantly increased in DOCA-salt-treated mice compared with sham mice. Gene and protein expression of carboxypeptidase N, which converts bradykinin into the B1R endogenous ligand des-Arg9-BK, were significantly enhanced in the hypothalamic PVN of DOCA-salt treated mice (Supplemental Figure S2, P<0.01), confirming the central activation of this pathway in hypertension.
Figure 1. B1R gene and protein expression increased in DOCA-salt hypertension.
Immunofluorescence staining showing increased B1R expression in the paraventricular nucleus (PVN) of DOCA-salt treated wild-type (WT) mice compared to sham treated mice (A, B1R red, DAPI blue, Scale bar: 50 μm). Real time PCR shows significantly increased mRNA expression in hypothalamus (hypo), PVN, brainstem and rostral ventrolateral medulla (RVLM) of DOCA-salt treated wild-type mice. (B, n=4, Unpaired, 2-tailed t test, *P<0.01 vs. Sham). Representative western blot and quantification showing significantly increased B1R protein expression in the hypothalamus and PVN of hypertensive mice (C, n=4–6, Unpaired, 2-tailed t test, *P<0.01 vs. Sham). Time course study showing that the PVN B1R gene expression was increased at 7, 14 and 21 days of DOCA-salt treated hypertensive mice compared with sham mice (D, n=4, One-way ANOVA, *P<0.01 vs. Sham).
Kinin B1R deletion attenuates neurogenic hypertension by preserving autonomic function and baroreflex sensitivity
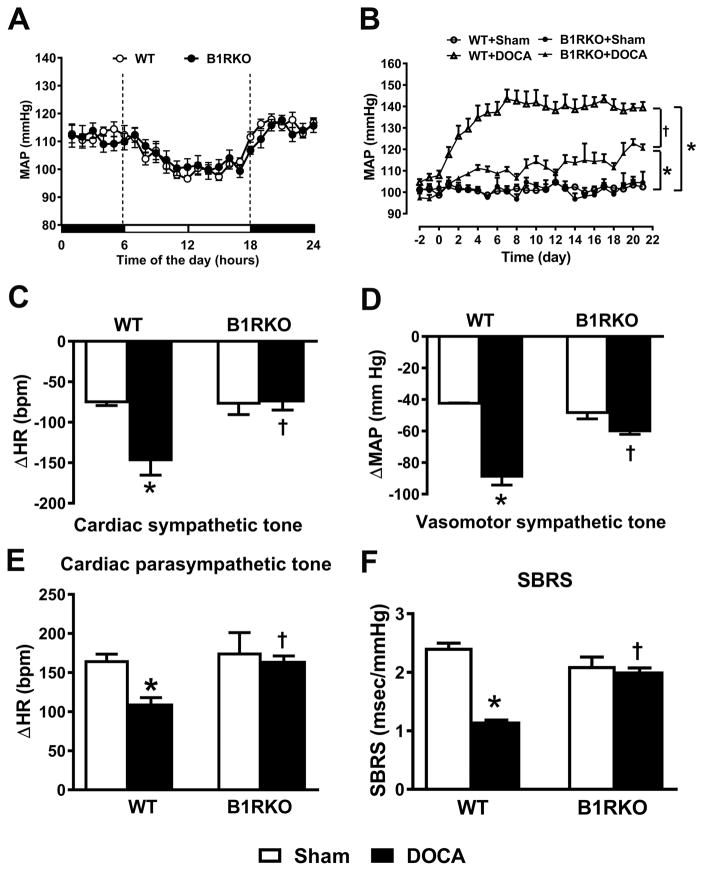
To further determine the role of B1R in DOCA-salt hypertension, we used B1R global knockout (KO) mice (Supplemental figure S3) and WT controls. Mean arterial pressure (MAP) was similar in both WT and B1RKO mice at baseline (WT: 102 ±2 vs. B1RKO: 104 ±5 mmHg, P>0.05) without significant change over the 24-hour period (Figure 2A). Three weeks of DOCA-salt treatment (Figure 2B) significantly increased MAP in WT mice (138 ±3 mmHg, P<0.01 vs. sham: 102 ±2). However, this increase was blunted in B1RKO mice (121 ±2 mmHg, P<0.05 vs. WT+DOCA), suggesting that B1R are required for the development of DOCA-salt-induced hypertension. Previous studies from our lab and others showed that DOCA-salt hypertension, a model of neurogenic hypertension, is associated with autonomic dysfunction and elevated sympathetic activation.4, 21 Autonomic function was identical at baseline between sham-treated WT and B1RKO mice (Figure 2C–E). DOCA-salt administration resulted in significant increases in both cardiac (increased bradycardia to propranolol, −145 ±21 beats/minutes, P<0.01 vs. sham) and vascular (decreased MAP to chlorisondamine, −88 ±7 mmHg, P<0.01 vs. sham) sympathetic drive, as well as reduction of vagal tone (reduced tachycardia to atropine, +111 ±8 beats/minute, P<0.01 vs. sham) in WT mice, which were normalized in B1RKO mice (−72 ±13 beats/minutes, −59 ±3 mmHg, and +165 ±6 beats/minute, respectively, P<0.01 vs. WT+DOCA).
Figure 2. DOCA-salt-induced hypertension and dysautonomia are significantly attenuated in mice with B1R deletion.
The baseline mean arterial pressure (MAP) measured continuously by telemetry over a period of 24 hours, was similar in both wild-type (WT) control and B1R knockout (B1RKO) mice showing a clear diurnal variation (A, n=6–12). Deoxycorticosterone acetate (DOCA) implanted subcutaneously and combined with 1% salt as drinking solution induced a progressive rise of MAP in sham-treated controls that was attenuated in B1RKO mice (B, n=8 mice/group, Repeated measures two-way ANOVA, *P<0.01 vs. Sham, †P<0.05 vs. WT+DOCA). After 21 days of DOCA-salt treatment, autonomic function was assessed pharmacologically by determining the changes in MAP (△MAP) and heart rate (△HR) following ip injections of a β-blocker (propranolol: 4 mg/kg, Cardiac sympathetic tone, C), ganglionic blocker (chlorisondamine: 5 mg/kg, vascular sympathetic tone, D) and muscarinic antagonist (atropine: 1 mg/kg, cardiac parasympathetic tone, E). Spontaneous Baroreceptor Reflex Sensitivity (SBRS) was calculated using the sequence method (F) was improved in B1RKO mice treated with DOCA compared to WT mice. n=6–8/group. Data are presented as mean ±SEM. Two-way ANOVA, *P<0.01 vs. Sham, †P<0.01 vs. WT+DOCA.
The baroreceptor reflex is the main mechanism involved in the beat-to-beat maintenance of BP within a normal range and we previously showed that its sensitivity is impaired in DOCA-salt hypertension.5 To assess the effects of B1R deletion on baroreflex function, we determined spontaneous baroreflex sensitivity (SBRS) using the sequence method. SBRS was significantly reduced in WT+DOCA mice compared to sham (Figure 2F). However, B1RKO mice showed no impairment of SBRS during DOCA-salt treatment (Figure 2F), further suggesting that B1R contribute to impaired autonomic and baroreflex functions in hypertension. In addition, DOCA-salt treatment resulted in sympatho-excitation in WT mice, as indicated by significantly increased plasma (13 ±1 ng/ml, P<0.05 vs. sham: 6 ±1 ng/ml) and urinary (299 ±23 ng/ml, P<0.05 vs. sham: 151 ±7 ng/ml) norepinephrine levels, but these increases were blunted in B1RKO mice with DOCA (4 ±1 and 172 ±30 ng/ml, respectively, P<0.05 vs. WT+DOCA) (Supplemental Figure S4). Furthermore, in WT mice DOCA-salt treatment led to a significant increase in urinary arginine vasopressin, measured by the C-terminal portion of the arginine vasopressin precursor, Copeptin. This increase was blunted in B1RKO with DOCA-salt treatment (Supplemental Figure S4).
Kinin B1R blockade reduces DOCA-salt-induced neuro-inflammation
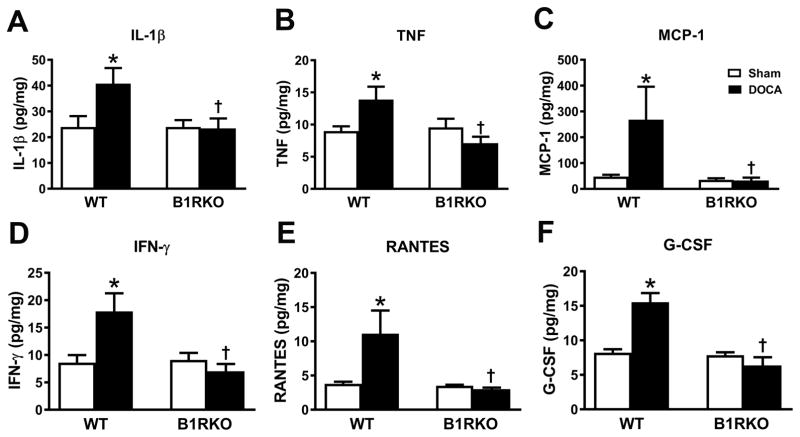
To understand the contribution of B1R activation to the production of inflammatory markers, we extended our study by measuring pro-inflammatory cytokines and chemokines in hypothalamic PVN homogenates after 3 weeks of sham or DOCA-salt hypertension. In WT DOCA-salt hypertensive mice, protein levels of IL-1β, TNF, MCP-1, IFN-γ, RANTES and G-CSF were significantly increased indicating elevated neuro-inflammation (Figure 3). However, these increases were completely abrogated in B1RKO+DOCA mice, suggesting that B1R knockdown reduces neuro-inflammation during hypertension.
Figure 3. B1R gene deletion blunts DOCA-salt induced inflammation.
Wild-type mice treated with DOCA-salt hypertension showed increase in concentrations of IL-1β, TNF, MCP-1, IFN-γ, RANTES and G-CSF levels in the brain hypothalamus. B1R knockout mice did not show this increase in inflammatory mediators. Brain tissue samples were extracted using lysis buffer, analyzed using a MILLIPLEX MAP Mouse Cytokine/Chemokine kit normalized to the amount of proteins in each sample. n=6/group. Data are presented as mean ±SEM. Two-way ANOVA, *P<0.05 vs. Sham, †P<0.05 vs. WT+DOCA.
Kinin B1R blockade blunts DOCA-salt-induced oxidative stress
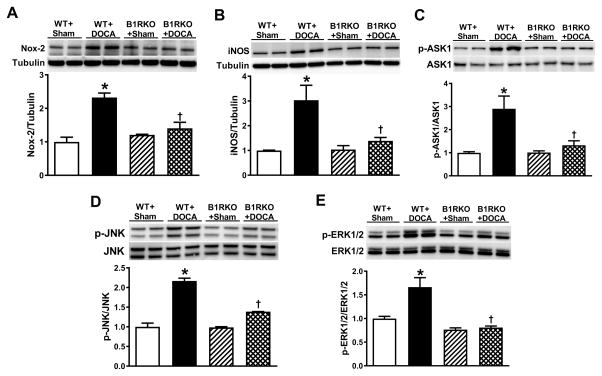
To elucidate the possible mechanisms involved in the attenuation of hypertension after B1R knockdown, we investigated oxidative stress markers that we previously reported as elevated in DOCA-salt hypertension.5 Notably, we investigated the gene and protein expression of Nox2, a catalytic subunit of NADPH Oxidase and iNOS in the PVN. Real time RT-PCR analysis showed significant increases in Nox2 and iNOS gene expression in the PVN of WT+DOCA mice but not in B1RKO+DOCA mice (Supplemental Figure S5). Western blot analysis revealed that protein levels of Nox2 and iNOS were significantly increased in the PVN after 3 weeks of DOCA-salt hypertension in WT mice. In contrast, these proteins showed blunted expression in B1RKO following DOCA treatment, indicating decreased oxidative stress in the brain (Figure 4A, B).
Figure 4. Deletion of B1R reduces oxidative stress mediated signaling.
Representative western blots and quantification data for protein expression in the hypothalamic PVN homogenates of wild-type (WT) and B1R gene deletion (B1RKO) mice treated with sham or DOCA-salt for three weeks. Protein expression of Nox2, a catalytic subunit of NADPH Oxidase (A), and inducible nitric oxide synthase (iNOS, B) were significantly increased in hypothalamic PVN indicating increased oxidative stress. However, this increase in oxidative stress was not observed in B1RKO mice. In addition, phosphorylated and total ASK1 (C), phosphorylated and total JNK (D), and phosphorylated and total ERK1/2 (E) protein expression in the PVN indicates activation of ASK1, JNK and ERK1/2 in DOCA-salt hypertension, which was prevented by B1R gene deletion. n=4/group, PVN tissues from 3 mice were pooled for each sample. Data are presented as mean ±SEM. Two-way ANOVA, *P<0.05 vs. WT+Sham, †P<0.05 vs. WT+DOCA.
Kinin B1R blockade reduces activation of ASK1-JNK-ERK1/2 pathway
Apoptosis signal regulating kinase (ASK) 1 is activated in response to various cytotoxic stresses, including inflammation and oxidative stress, and is required for sustained activation of JNK and p38 MAP kinases. In this study, we examined whether B1R activation can activate the ASK1-JNK-ERK1/2 signaling pathway in neurogenic hypertension. DOCA-salt treatment for 3 weeks significantly increased the phosphorylation of ASK1 in WT mice, but not in B1RKO mice (Figure 4C). Next, we examined whether B1R are involved in activating MAPK signaling. Phosphorylation at residues Thr183/Tyr185, which results in the activation of SAPK/JNK, was increased in the PVN of WT mice under DOCA-salt treatment, but was blunted in B1RKO mice treated with DOCA-salt (Figure 4D). Since phosphorylation at residues Thr202/Tyr204 results in activation of ERK1/2, it was used as an index of ERK1/2 activation. No differences in total ERK1/2 protein levels in the PVN were observed among the groups (Figure 4E). However, phosphorylation of ERK1/2 at Thr202/Tyr204 was increased in the PVN of WT mice treated with DOCA, but not in B1RKO mice treated with DOCA. These results suggest that ASK1-JNK-ERK1/2 signaling pathways are involved in B1R-mediated DOCA-salt hypertension.
Kinin B1R blockade prevents NF-κB activation
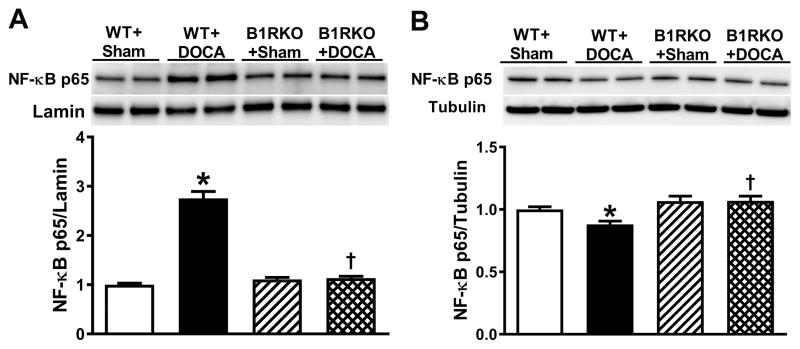
To determine the involvement of B1R-mediated ASK1-JNK-ERK1/2 signaling in NF-κB activation, we assessed NF-κB p65 expression in the PVN nuclear and cytoplasmic fractions using western blot. In WT mice, DOCA-salt hypertension significantly increased the expression of nuclear NF-κB p65 (Figure 5A) and decreased cytoplasmic NF-κB p65 (Figure 5B) suggesting a translocation of NF-κB p65 subunit from cytosol to nucleus. In contrast, in B1RKO mice with DOCA-salt, there was no significant difference in nuclear and cytoplasmic NF-κB p65 expression when compared to sham mice, suggesting a lack of NF-κB activation in these animals (Figure 5).
Figure 5. B1R mediated downstream signaling involves NF-κB activation.
NF-κB p65 protein expression was quantified using western blot in brain hypothalamic nuclear and cytosolic fractions from wild-type (WT) and B1R knockout (B1RKO) mice following a 3-week DOCA-salt, or sham treatment. Protein expression was normalized to housekeeping genes Lamin (nuclear proteins) and tubulin (cytosolic proteins). NF-κB p65 levels were significantly increased in nuclear fractions (A) and decreased in cytosolic fractions (B) in hypertensive DOCA-salt-treated mice, indicating activation and translocation of NF-κB into the nucleus. However, no change in NF-κB p65 expression was observed in B1RKO mice with DOCA-salt compared to sham, suggesting that B1R gene deletion prevented NF-κB activation. Data are presented as mean ±SEM. n=6/group. Two-way ANOVA, *P<0.05 vs. WT+Sham, †P<0.05 vs. WT+DOCA.
Blockade of B1R within the brain attenuates hypertension, improves autonomic function and reduces neuro-inflammation
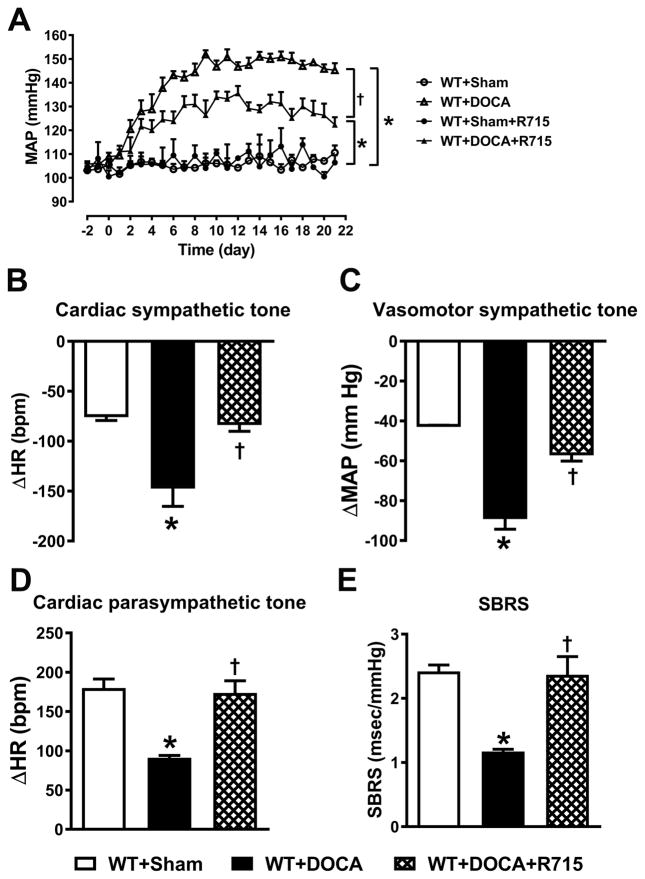
Though our studies using B1RKO mice provided evidence that B1R genetic deletion attenuates the development of neurogenic hypertension, deletion of a gene may result in compensatory changes in other vasoactive factors during ontogenesis, whose altered status may contribute to the phenotype of transgenic mice. It has been reported that deletion of kinin receptor gene can affect specific tissues differently, resulting in down- or up-regulation of related receptors.13 Real time PCR results show that genetic deletion of B1R results in compensatory increase in B2R gene expression (Supplemental Figure S6). In order to rule out contribution of B2R in the beneficial effects of B1R gene deletion, we used a pharmacological approach to examine the role of B1R in the brain during hypertension. We used R715, a specific antagonist to block B1R within the brain of WT mice with or without DOCA-salt hypertension. In line with our findings in B1RKO mice, chronic intracerebroventricular (icv) infusion of R715 led to a significant attenuation of DOCA-salt hypertension (Figure 6A). In addition, the DOCA-salt hypertension-induced cardiac dysautonomia was prevented, sympathetic drive to the vasculature was reduced, while vagal tone and baroreflex function were restored by R715 treatment (Figure 6B–E). Furthermore, ruling out a peripheral effect of this antagonist, subcutaneous infusion of same dose of R715 for 3 weeks with DOCA-salt did not prevent the development of hypertension (Supplemental Figure S7). The DOCA-salt hypertension-induced increases in urinary and plasma norepinephrine, and urinary copeptin levels were blunted by treatment with R715 suggesting decreased sympathetic drive in these mice (Supplemental Figure S8). We also observed that similar to our B1RKO mice, R715 treatment reduced the inflammatory response within the CNS, as evidenced by decreased expression of cytokine and chemokines in the PVN (Supplemental Figure S9 A). In addition, central R715 treatment also reduced oxidative stress in the brain as indicated by reductions in Nox2 and iNOS mRNA expression in the PVN (Supplemental Figure S9 B–C). These data provide strong evidence that B1R expression within the brain plays a critical role in maintaining the functionality of baroreflex and autonomic regulations, and reduction in neuro-inflammation and oxidative stress during the development of neurogenic hypertension.
Figure 6. Central treatment with B1R antagonist prevents DOCA-salt-induced hypertension and autonomic dysfunction.
Three weeks of DOCA-salt treatment resulted in significant increase in mean arterial pressure (MAP) in sham treated wild-type (WT) mice. Intracerebroventricular infusion of R715, a B1R specific antagonist (70 μg/kg/day) prevented the DOCA-salt mediated increase in blood pressure (A n=4–8 mice/group, Repeated measures two-way ANOVA, *P<0.01 vs. Sham, †P<0.05 vs. WT+DOCA). In addition, after 21 days of DOCA-salt treatment, autonomic function was assessed pharmacologically and spontaneous baroreceptor reflex sensitivity (SBRS) was calculated using the sequence method. Treatment with B1R antagonist prevented autonomic dysfunction, decreased cardiac (B) and vasomotor (C) sympathetic tone and improved cardiac parasympathetic tone (D) and SBRS (E). Data are presented as mean ±SEM. n=4 mice/group, One-way ANOVA *P<0.05 vs. Sham and †P<0.05 vs. WT+DOCA.
Discussion
The major findings of our present study are that 1) B1R expression is significantly up-regulated in brain cardiovascular regulatory nuclei during DOCA-salt hypertension; 2) global deletion of B1R protected mice from the development of DOCA-salt-induced hypertension; 3) Importantly, selective pharmacological blockade of B1R in the brain attenuated DOCA-salt-induced hypertension suggesting a central origin mechanism; 4) B1R blockade prevented autonomic dysfunction, reduced neuro-inflammation and decreased oxidative stress thereby blunting neurogenic hypertension; 5) the beneficial effects of B1R deletion are mediated by ASK1-JNK-ERK1/2 and NF-kB pathways inhibition in the PVN. Our study offers new insights into the possible signaling pathways of kinin B1R activation in the brain and provides the first evidence, to our knowledge, of a central role for B1R-mediated inflammatory pathway in the pathogenesis of DOCA-salt hypertension.
Despite the body of evidence supporting a role for B1R activation in hypertension, the beneficial effects of B1R blockade on hypertension remain inconclusive. It was previously reported that neither the B1R agonist des-Arg9-BK nor the B1R antagonist Leu8-des-Arg9-BK injected acutely into the fourth ventricle modified BP in SH or WKY rats.22 In contrast, another study reported that the same B1R antagonist, Leu8-des-Arg9-BK, injected into the lateral ventricle (icv) caused a long lasting reduction in BP and heart rate in SH but not in WKY rats.23 Emanueli et al. also reported that icv activation of B1R in SH and WKY rats evokes increases in BP, while icv injection of the B1R antagonist R715 causes a mild decrease of BP (−14 mmHg) within 15 min in SH rats.19 On the other hand, Cloutier et al. showed that icv injection of R715 failed to alter MAP and HR for a period up to 24 h post injection in SH and WKY rats.24 Therefore, to elucidate the mechanisms of chronic B1R blockade on hypertension (and to circumvent the lack of tissue-specific deletion mouse models), we used two complementary approaches, genetic deletion and pharmacological antagonism, to prevent B1R activation selectively and chronically. The B1R global knockout mouse is normotensive compared to WT controls, and shows a clear nycthemeral variation in BP recorded continuously with telemetry over a period of 24 hours. Our data clearly demonstrate that DOCA-salt-induced hypertension is attenuated in B1R-deficient mice, and suggest that B1R is indeed responsible for the development of neurogenic hypertension. In addition, to investigate the importance of B1R in central regulation of BP, we used a pharmacological approach. Infusion of a specific B1R antagonist R715 chronically through icv route for 3 weeks resulted in attenuation of DOCA-salt hypertension. In contrast, the same dose of B1R antagonist when administered systemically did not prevent the development of hypertension. Similar to B1RKO mice being normotensive, central treatment with R715 for 3 weeks did not alter the BP in control sham mice, implying that at baseline B1R are not involved in the physiological control of BP. Taken together, these data suggest that the activation of B1R in the brain is critical for the development of hypertension and its blockade attenuates the development of the disease. Although B1R knockout mice or the B1R antagonist resulted in an attenuated hypertension, the BP was not completely reduced to control level, confirming that other pathways may be involved, independently of B1R. Interaction between the kinin kallikrein system and the renin-angiotensin system is well known and future studies are warranted to evaluate the combination of kinin B1R blockade with renin-angiotensin system blockers.
To the best of our knowledge, this is the first demonstration that B1R gene and protein expression are significantly increased in brain nuclei critical for BP regulation, during low renin DOCA-salt hypertension model, which is clinically relevant to African-American and type 2 diabetes patients. Previous studies have reported elevated densities of B1R binding sites24 or increased B1R mRNA expression25 in the hypothalamus of 12–13 week old SH rats when BP was significantly higher compared to WKY rats. Indeed, our time course experiments show that B1R mRNA expression was significantly increased at 7 days of DOCA-salt treatment, which coincided with a maximum rise in mean arterial pressure and this B1R expression remained elevated over the course of the experiment. Moreover, increase in bradykinin levels coupled with increased expression of carboxypeptidase N might be responsible for the elevated des-Arg9-BK formation leading to B1R activation. In fact, it has been previously shown that once B1R is activated by an agonist, it is not internalized or desensitized7 and in the inflammatory context, the B1R agonist is able to up-regulate the expression of its own receptor via the activation of the transcription factor NF-κB.26 These findings suggest that once B1R is activated, it can create a vicious cycle of its own activation leading to lasting effects.
Recently, it has become apparent that inflammation plays a pivotal role in the development of hypertension. Pro-inflammatory cytokines such as tumor necrosis factor (TNF)-α27, interleukin (IL)-1β27, 28, and IL-628, 29 have been reported to be increased in hypertensive patients. Recent studies from our group and others demonstrate a link between inflammation and activated renin angiotensin system in neurogenic hypertension.3–5, 30 Inflammation in the brain involves primarily the activation of microglia. We have previously shown that neurogenic hypertension involves activation of microglia in the PVN and indeed inhibition of PVN microglia activation fully abolishes Ang-II-induced hypertension.30 Expression of kinin B1R in microglia, and increase in inflammatory cytokine production following activation of B1R was previously reported.31, 32 Furthermore, B1R expression is also induced by various pro-inflammatory cytokines and growth factors such as EGF.9 In the present study, we demonstrate that in DOCA-salt hypertension, elevated B1R expression is correlated with elevated inflammation, as indicated by increased protein levels of cytokines and chemokines in PVN. Importantly, B1R gene deletion was able to prevent inflammation in PVN, suggesting that B1R activation is important in initiating the inflammatory response observed in neurogenic hypertension. Previous findings have emphasized the role of inflammatory cytokines in the PVN in mediating sympatho-excitation and autonomic function during various cardiovascular diseases, including hypertension.4, 5, 30 In our study, we found that B1R gene deletion reversed the DOCA-salt hypertension-induced autonomic dysfunction and sympatho-excitation, and improved baroreflex sensitivity, suggesting a central role for B1R activation during neurogenic hypertension.
We previously showed that in DOCA-salt hypertension, elevated neuro-inflammation could induce oxidative stress and activate MAPK-mediated signaling mechanisms.4, 5 ASK1 was identified as a ROS-sensitive MAPK kinase kinase, which activates the c-jun-terminal kinase (JNK) and p44/42 MAPK (ERK1/2) cascade.33 Activation of kinin B1R with a selective agonist either in vitro or in vivo enhanced aortic superoxide anion production through activation of NADPH oxidase, which was abolished by apocynin, a selective inhibitor of NADPH oxidase.15 It has been previously reported that B1R activation involves NF-κB mediated signaling mechanisms in human34 and rat35 vascular smooth muscle cells. In this study, B1R blockade significantly inhibited oxidative stress markers including Nox2 and iNOS, phosphorylation of ASK1, JNK and ERK1/2 within the PVN. Furthermore, B1R blockade also prevented the activation of NF-κB, an early transcriptional factor that modulates gene expression initiating rapid inflammatory response. Here, we provide the first evidence that the activation of this ASK1-MAPK-NF-κB-mediated inflammatory signaling pathway is dependent on B1R activation. Thus, we might suggest that ASK1, JNK, ERK1/2 and NF-κB, are acting downstream of B1R activation, mediating the expression of pro-inflammatory molecules in a feed-forward fashion and are responsible for driving the neuro-inflammation and sympatho-excitation thereby contributing to neurogenic hypertension (Supplemental Figure S10). However, a limitation of our study is the inability to determine whether these signaling pathways are activated within one cell type or whether activation occurs simultaneously within multiple cell types (e.g. within neurons and microglia).
Since DOCA-salt hypertension is mainly driven by an alteration in central mechanisms regulating BP,21 we further investigated the importance of kinin B1R in BP control, using a brain targeted pharmacological approach. Chronic icv infusion of R715, a B1R specific antagonist, attenuated the development of DOCA-salt hypertension. On the other hand, subcutaneous infusion of R715 for 3 weeks with DOCA-salt did not prevent the development of hypertension, suggesting that this mechanism is not due to peripheral activation of compensatory mechanisms. In addition, hypertension-induced inflammation, oxidative stress, cardiac and vascular dysautonomia were prevented, and baroreflex function was restored by icv R715 treatment. These data clearly support a central role for B1R activation in neurogenic hypertension. Using these 2 different approaches, genetic KO animal model and pharmacological blockade of B1R model, allowed us 1) to confirm the importance of B1R in neurogenic hypertension, 2) to study the brain specificity of the effects of B1R blockade and 3) to study the long term effect of B1R deletion before the onset of hypertension in KO mice vs. blockade of B1R in parallel to the development of hypertension.
Perspectives
Dysregulation of the kallikrein-kinin system and its receptors is involved in many pathological conditions and cardiovascular diseases that are associated with or induced by inflammation.8, 13, 31 In the last decade, numerous seminal works showed evidence for immune cell infiltration and inflammation in the brain in the pathogenesis of hypertension.3, 30, 36–38 Patients with essential hypertension exhibit a significant up-regulation of B1R gene expression in peripheral monocytes, leading to functional changes and the development of target organ damage.39 Recent evidence suggest that novel orally active, selective non-peptide B1R antagonists are effective in the treatment of various in vivo models of inflammation and insulin resistance.40–42 It has been postulated that the kinin receptor signaling pathway may act as a molecular link between changes in hemodynamic forces and the activation of inflammatory pathways.39 Our data clearly show the beneficial effect of central B1R blockade in reducing inflammation and preventing the development of hypertension. Accordingly, Kinin B1R blockade may provide a novel strategy to reduce neuro-inflammation, oxidative stress, and sympatho-excitation in neurogenic hypertension.
Supplementary Material
Novelty and Significance.
What is new?
This study demonstrates that bradykinin levels, and kinin B1 receptor (B1R) expression in the brain cardiovascular regulatory nuclei, are elevated during DOCA-salt hypertension.
Global deletion of B1R or selective pharmacological blockade of B1R in the brain attenuated DOCA-salt hypertension.
What is relevant?
B1R activation mediated inflammatory pathways play a pivotal role in the pathophysiology of DOCA-salt hypertension, a model of neurogenic hypertension.
Summary
Kinin B1R blockade may provide a novel strategy to reduce neuro-inflammation, oxidative stress, and sympatho-excitation, thereby attenuating the development of neurogenic hypertension.
Acknowledgments
Sources of funding
This study was supported in part by research grants from The American Heart Association (15SDG25720021 and 13POST16500025 to SS) and from the National Institutes of Health (HL093178 to EL, and COBRE P30GM106392).
Footnotes
Disclosures
None
References
- 1.Writing Group M. Mozaffarian D, Benjamin EJ, et al. Heart disease and stroke statistics-2016 update: A report from the american heart association. Circulation. 2016;133:e38–360. doi: 10.1161/CIR.0000000000000350. [DOI] [PubMed] [Google Scholar]
- 2.Heidenreich PA, Trogdon JG, Khavjou OA, et al. Forecasting the future of cardiovascular disease in the united states: A policy statement from the american heart association. Circulation. 2011;123:933–944. doi: 10.1161/CIR.0b013e31820a55f5. [DOI] [PubMed] [Google Scholar]
- 3.Sriramula S, Cardinale JP, Francis J. Inhibition of TNF in the brain reverses alterations in RAS components and attenuates angiotensin II-induced hypertension. PLoS One. 2013;8:e63847. doi: 10.1371/journal.pone.0063847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Xia H, Sriramula S, Chhabra KH, Lazartigues E. Brain angiotensin-converting enzyme type 2 shedding contributes to the development of neurogenic hypertension. Circ Res. 2013;113:1087–1096. doi: 10.1161/CIRCRESAHA.113.301811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sriramula S, Xia H, Xu P, Lazartigues E. Brain-targeted angiotensin-converting enzyme 2 overexpression attenuates neurogenic hypertension by inhibiting cyclooxygenase-mediated inflammation. Hypertension. 2015;65:577–586. doi: 10.1161/HYPERTENSIONAHA.114.04691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Solak Y, Afsar B, Vaziri ND, Aslan G, Yalcin CE, Covic A, Kanbay M. Hypertension as an autoimmune and inflammatory disease. Hypertens Res. 2016;39:567–573. doi: 10.1038/hr.2016.35. [DOI] [PubMed] [Google Scholar]
- 7.Regoli D, Barabe J. Pharmacology of bradykinin and related kinins. Pharmacol Rev. 1980;32:1–46. [PubMed] [Google Scholar]
- 8.Marceau F, Hess JF, Bachvarov DR. The B1 receptors for kinins. Pharmacol Rev. 1998;50:357–386. [PubMed] [Google Scholar]
- 9.Leeb-Lundberg LM, Marceau F, Muller-Esterl W, Pettibone DJ, Zuraw BL International union of pharmacology. XlV. Classification of the kinin receptor family: From molecular mechanisms to pathophysiological consequences. Pharmacol Rev. 2005;57:27–77. doi: 10.1124/pr.57.1.2. [DOI] [PubMed] [Google Scholar]
- 10.Calixto JB, Medeiros R, Fernandes ES, Ferreira J, Cabrini DA, Campos MM. Kinin B1 receptors: Key G-protein-coupled receptors and their role in inflammatory and painful processes. Br J Pharmacol. 2004;143:803–818. doi: 10.1038/sj.bjp.0706012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Margolius HS, Horwitz D, Pisano JJ, Keiser HR. Urinary kallikrein excretion in hypertensive man. Relationships to sodium intake and sodium-retaining steroids. Circ Res. 1974;35:820–825. doi: 10.1161/01.res.35.6.820. [DOI] [PubMed] [Google Scholar]
- 12.Ferri C, Bellini C, Carlomagno A, Perrone A, Santucci A. Urinary kallikrein and salt sensitivity in essential hypertensive males. Kidney Int. 1994;46:780–788. doi: 10.1038/ki.1994.333. [DOI] [PubMed] [Google Scholar]
- 13.Duka I, Kintsurashvili E, Gavras I, Johns C, Bresnahan M, Gavras H. Vasoactive potential of the B(1) bradykinin receptor in normotension and hypertension. Circ Res. 2001;88:275–281. doi: 10.1161/01.res.88.3.275. [DOI] [PubMed] [Google Scholar]
- 14.De Brito Gariepy H, Carayon P, Ferrari B, Couture R. Contribution of the central dopaminergic system in the anti-hypertensive effect of kinin B1 receptor antagonists in two rat models of hypertension. Neuropeptides. 2010;44:191–198. doi: 10.1016/j.npep.2009.12.011. [DOI] [PubMed] [Google Scholar]
- 15.Dias JP, Talbot S, Senecal J, Carayon P, Couture R. Kinin B1 receptor enhances the oxidative stress in a rat model of insulin resistance: Outcome in hypertension, allodynia and metabolic complications. PLoS One. 2010;5:e12622. doi: 10.1371/journal.pone.0012622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xu J, Carretero OA, Shesely EG, Rhaleb NE, Yang JJ, Bader M, Yang XP. The kinin B1 receptor contributes to the cardioprotective effect of angiotensin-converting enzyme inhibitors and angiotensin receptor blockers in mice. Exp Physiol. 2009;94:322–329. doi: 10.1113/expphysiol.2008.045583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Couture R, Girolami JP. Putative roles of kinin receptors in the therapeutic effects of angiotensin 1-converting enzyme inhibitors in diabetes mellitus. Eur J Pharmacol. 2004;500:467–485. doi: 10.1016/j.ejphar.2004.07.045. [DOI] [PubMed] [Google Scholar]
- 18.Lungu C, Dias JP, Franca CE, Ongali B, Regoli D, Moldovan F, Couture R. Involvement of kinin B1 receptor and oxidative stress in sensory abnormalities and arterial hypertension in an experimental rat model of insulin resistance. Neuropeptides. 2007;41:375–387. doi: 10.1016/j.npep.2007.09.005. [DOI] [PubMed] [Google Scholar]
- 19.Emanueli C, Chao J, Regoli D, Chao L, Ni A, Madeddu P. The bradykinin B1 receptor and the central regulation of blood pressure in spontaneously hypertensive rats. Br J Pharmacol. 1999;126:1769–1776. doi: 10.1038/sj.bjp.0702527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pesquero JB, Araujo RC, Heppenstall PA, Stucky CL, Silva JA, Jr, Walther T, Oliveira SM, Pesquero JL, Paiva AC, Calixto JB, Lewin GR, Bader M. Hypoalgesia and altered inflammatory responses in mice lacking kinin B1 receptors. Proc Natl Acad Sci U S A. 2000;97:8140–8145. doi: 10.1073/pnas.120035997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Basting T, Lazartigues E. Doca-salt hypertension: An update. Curr Hypertens Rep. 2017;19:32. doi: 10.1007/s11906-017-0731-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Martins DT, Fior DR, Nakaie CR, Lindsey CJ. Kinin receptors of the central nervous system of spontaneously hypertensive rats related to the pressor response to bradykinin. Br J Pharmacol. 1991;103:1851–1856. doi: 10.1111/j.1476-5381.1991.tb12341.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Alvarez AL, Delorenzi A, Santajuliana D, Finkielman S, Nahmod VE, Pirola CJ. Central bradykininergic system in normotensive and hypertensive rats. Clin Sci (Lond) 1992;82:513–519. doi: 10.1042/cs0820513. [DOI] [PubMed] [Google Scholar]
- 24.Cloutier F, Ongali B, Campos MM, Thibault G, Neugebauer W, Couture R. Correlation between brain bradykinin receptor binding sites and cardiovascular function in young and adult spontaneously hypertensive rats. Br J Pharmacol. 2004;142:285–296. doi: 10.1038/sj.bjp.0705759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Qadri F, Hauser W, Johren O, Dominiak P. Kinin B1 and B2 receptor mRNA expression in the hypothalamus of spontaneously hypertensive rats. Can J Physiol Pharmacol. 2002;80:258–263. doi: 10.1139/y02-051. [DOI] [PubMed] [Google Scholar]
- 26.Schanstra JP, Bataille E, Marin Castano ME, Barascud Y, Hirtz C, Pesquero JB, Pecher C, Gauthier F, Girolami JP, Bascands JL. The B1-agonist [des-arg10]-kallidin activates transcription factor NF-kappaB and induces homologous upregulation of the bradykinin B1-receptor in cultured human lung fibroblasts. J Clin Invest. 1998;101:2080–2091. doi: 10.1172/JCI1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dorffel Y, Latsch C, Stuhlmuller B, Schreiber S, Scholze S, Burmester GR, Scholze J. Preactivated peripheral blood monocytes in patients with essential hypertension. Hypertension. 1999;34:113–117. doi: 10.1161/01.hyp.34.1.113. [DOI] [PubMed] [Google Scholar]
- 28.Peeters AC, Netea MG, Janssen MC, Kullberg BJ, Van der Meer JW, Thien T. Pro-inflammatory cytokines in patients with essential hypertension. Eur J Clin Invest. 2001;31:31–36. doi: 10.1046/j.1365-2362.2001.00743.x. [DOI] [PubMed] [Google Scholar]
- 29.Chae CU, Lee RT, Rifai N, Ridker PM. Blood pressure and inflammation in apparently healthy men. Hypertension. 2001;38:399–403. doi: 10.1161/01.hyp.38.3.399. [DOI] [PubMed] [Google Scholar]
- 30.Shi P, Diez-Freire C, Jun JY, Qi Y, Katovich MJ, Li Q, Sriramula S, Francis J, Sumners C, Raizada MK. Brain microglial cytokines in neurogenic hypertension. Hypertension. 2010;56:297–303. doi: 10.1161/HYPERTENSIONAHA.110.150409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Noda M, Kariura Y, Amano T, Manago Y, Nishikawa K, Aoki S, Wada K. Expression and function of bradykinin receptors in microglia. Life Sci. 2003;72:1573–1581. doi: 10.1016/s0024-3205(02)02449-9. [DOI] [PubMed] [Google Scholar]
- 32.Ifuku M, Farber K, Okuno Y, Yamakawa Y, Miyamoto T, Nolte C, Merrino VF, Kita S, Iwamoto T, Komuro I, Wang B, Cheung G, Ishikawa E, Ooboshi H, Bader M, Wada K, Kettenmann H, Noda M. Bradykinin-induced microglial migration mediated by B1-bradykinin receptors depends on Ca2+ influx via reverse-mode activity of the na+/ca2+ exchanger. J Neurosci. 2007;27:13065–13073. doi: 10.1523/JNEUROSCI.3467-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ichijo H, Nishida E, Irie K, ten Dijke P, Saitoh M, Moriguchi T, Takagi M, Matsumoto K, Miyazono K, Gotoh Y. Induction of apoptosis by ASK1, a mammalian MAPKKK that activates SAPK/JNK and p38 signaling pathways. Science. 1997;275:90–94. doi: 10.1126/science.275.5296.90. [DOI] [PubMed] [Google Scholar]
- 34.Moreau ME, Bawolak MT, Morissette G, Adam A, Marceau F. Role of nuclear factor-kappab and protein kinase c signaling in the expression of the kinin B1 receptor in human vascular smooth muscle cells. Mol Pharmacol. 2007;71:949–956. doi: 10.1124/mol.106.030684. [DOI] [PubMed] [Google Scholar]
- 35.Morand-Contant M, Anand-Srivastava MB, Couture R. Kinin B1 receptor upregulation by angiotensin ii and endothelin-1 in rat vascular smooth muscle cells: Receptors and mechanisms. Am J Physiol Heart Circ Physiol. 2010;299:H1625–1632. doi: 10.1152/ajpheart.00735.2009. [DOI] [PubMed] [Google Scholar]
- 36.Zubcevic J, Waki H, Raizada MK, Paton JF. Autonomic-immune-vascular interaction: An emerging concept for neurogenic hypertension. Hypertension. 2011;57:1026–1033. doi: 10.1161/HYPERTENSIONAHA.111.169748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Santisteban MM, Ahmari N, Carvajal JM, Zingler MB, Qi Y, Kim S, Joseph J, Garcia-Pereira F, Johnson RD, Shenoy V, Raizada MK, Zubcevic J. Involvement of bone marrow cells and neuroinflammation in hypertension. Circ Res. 2015;117:178–191. doi: 10.1161/CIRCRESAHA.117.305853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Marvar PJ, Thabet SR, Guzik TJ, Lob HE, McCann LA, Weyand C, Gordon FJ, Harrison DG. Central and peripheral mechanisms of t-lymphocyte activation and vascular inflammation produced by angiotensin ii-induced hypertension. Circ Res. 2010;107:263–270. doi: 10.1161/CIRCRESAHA.110.217299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Marketou ME, Kontaraki J, Zacharis E, Parthenakis F, Maragkoudakis S, Gavras I, Gavras H, Vardas PE. Differential gene expression of bradykinin receptors 1 and 2 in peripheral monocytes from patients with essential hypertension. J Hum Hypertens. 2014;28:450–455. doi: 10.1038/jhh.2013.133. [DOI] [PubMed] [Google Scholar]
- 40.Dias JP, Couture R. Blockade of kinin B(1) receptor reverses plasma fatty acids composition changes and body and tissue fat gain in a rat model of insulin resistance. Diabetes Obes Metab. 2012;14:244–253. doi: 10.1111/j.1463-1326.2011.01521.x. [DOI] [PubMed] [Google Scholar]
- 41.Dias JP, Couture R. Suppression of vascular inflammation by kinin B1 receptor antagonism in a rat model of insulin resistance. J Cardiovasc Pharmacol. 2012;60:61–69. doi: 10.1097/FJC.0b013e3182576277. [DOI] [PubMed] [Google Scholar]
- 42.Murugesan P, Hildebrandt T, Bernlohr C, Lee D, Khang G, Doods H, Wu D. Inhibition of kinin B1 receptors attenuates pulmonary hypertension and vascular remodeling. Hypertension. 2015;66:906–912. doi: 10.1161/HYPERTENSIONAHA.115.05338. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.