Abstract
The brain is highly susceptible to injury caused by hypertension because the increased blood pressure causes artery remodeling that can limit cerebral perfusion. Mineralocorticoid receptor antagonism prevents hypertensive cerebral artery remodeling, but the vascular cell types involved have not been defined. In the periphery, the endothelial mineralocorticoid receptor mediates hypertension-induced vascular injury, but cerebral and peripheral arteries are anatomically distinct; thus these findings cannot be extrapolated to the brain. The parenchymal arterioles determine cerebrovascular resistance. Determining the effects of hypertension and mineralocorticoid receptor signaling on these arterioles could lead to a better understanding of cerebral small vessel disease. We hypothesized that endothelial mineralocorticoid receptor signaling mediates inward cerebral artery remodeling and reduced cerebral perfusion during angiotensin-II hypertension. The biomechanics of the parenchymal arterioles and posterior cerebral arteries were studied in male C57Bl/6 and endothelial cell specific mineralocorticoid receptor knockout mice and their appropriate controls using pressure myography. Angiotensin-II increased plasma aldosterone and decreased cerebral perfusion in C57Bl/6 and mineralocorticoid receptor-intact littermates. Endothelial cell mineralocorticoid receptor deletion improved cerebral perfusion in angiotensin-II treated mice. Angiotensin-II hypertension resulted in inward hypotrophic remodeling; this was prevented by mineralocorticoid receptor antagonism and endothelial mineralocorticoid receptor deletion. Our studies suggest that endothelial cell mineralocorticoid receptor mediates hypertensive remodeling in the cerebral microcirculation and large pial arteries. Angiotensin II-induced inward remodeling of cerebral arteries and arterioles was associated with a reduction in cerebral perfusion that could worsen the outcome of stroke or contribute to vascular dementia.
Keywords: Hypertension, remodeling, endothelium, microcirculation, vasculature
Introduction
Hypertension is a modifiable risk factor for cerebral small vessel disease (CSVD)1-3. In animal models of hypertension, remodeling occurs in the middle cerebral artery (MCA)4 and parenchymal arterioles (PAs)5, producing arteries with smaller lumens and thicker walls and the same effect is observed in patients with hypertension1. However, our understanding of the mechanisms responsible for these changes in the cerebral circulation remains incomplete.
Our studies focus on two artery types: PAs and posterior cerebral arteries (PCAs). The PAs have a limited number of anastomoses and arise from pial arteries to perfuse the cerebral microcirculation. These arterioles play a critical role in the outcome of ischemia and the development of vascular dementia5-7. The PCA, a large pial artery, regulates perfusion of the hippocampus, the temporal cortex and parts of the parieto-occipital cortex8 which are associated with memory formation.
Aldosterone and mineralocorticoid receptor (MR) activation have been linked to vascular damage in hypertension5, 9. However, the specific cell type involved in MR-mediated artery remodeling in the brain has not been defined. Identifying the cell type that drives the MR-mediated remodeling will allow us to define the specific cellular pathways activated by the MR, and this may allow for the development of more specific therapies to prevent or reverse cerebral vascular remodeling in hypertensive patients. Cell-specific MR knockout mice have been developed to facilitate studies defining the role played by the endothelial MR (EC-MR). Mueller et al, utilized a specific EC-MR knockout driven by the VE-cadherin promoter to show that the EC-MR regulates mesenteric artery vasodilation but not coronary artery function in response to hypertension10. These studies suggest that EC-MR activation is an important mediator of hypertensive vascular injury. These studies also highlight the need to study the cerebral vasculature separately, not only because it is anatomically unique but also because it is clear that different vascular beds have varying sensitivities to EC-MR signaling.
In this study, we used a pharmacological and genetic approach to test the hypothesis that the EC-MR activation is required for Angiotensin II (AngII)-hypertensive cerebral artery remodeling. We used eplerenone (EPL; 100mg/kg/day)5 to pharmacologically inhibit the receptor to confirm that MR signaling mediates hypertensive remodeling of PAs and PCAs. EC-MR knockout (ECMRKO) mice generated using the VE-cadherin promoter were used to determine if EC-MR is involved in cerebral artery remodeling in AngII-hypertension. PAs were used as a model of the cerebral microcirculation and the PCA as a model of a large cerebral resistance artery.
Methods
Materials and detailed methods are available in the online supplement.
Animal Models
All experimental protocols were approved by the Michigan State University Animal Care and Use Committee and were performed in accordance with the American Physiological Society's Guiding Principles in the Care and Use of Animals. C57Bl/6 mice (n=6-8 per group) were purchased from Charles River Laboratories. ECMRKO mice (n=6-8 per group) were generated by Dr. Iris Z. Jaffe at Tufts Medical Center by crossing VE-cadherin Cad-Cre+ mice with MRf/f mice as described previously10, 11. MRf/f mice had exons 5 and 6 of the MR gene flanked by loxP sites10, 11. These EC-MR knockouts have been shown to be specific for the endothelium with MR recombination confirmed in endothelial cells from all vascular beds tested including lung, heart, and aorta without MR recombination in splenic leukocytes or lymph nodes and with intact MR mRNA expression in isolated leukocytes10, 12. MR-intact littermates were used as controls. All mice studied were males and housed on 12h: 12h light/dark cycle with food and water ad libitum.
Statistical Analyses
All data are presented as mean ± SEM. Blood pressure, plasma aldosterone, mRNA expression and cerebral perfusion data were analyzed by one-way analysis of variance. For analysis of artery structure, two-way analysis of variance with repeated measures in one factor (pressure) was utilized followed by Bonferroni- adjustments for post-hoc comparison. All statistical analyses were performed using GraphPad Prism 7.0 software (GraphPad, San Diego, CA). In all cases statistical significance was denoted by p<0.05.
Results
MR antagonism does not change PA and PCA structure under control conditions
To test the possibility that under normal conditions MR activation regulates artery structure, Sham-operated mice were treated with EPL. MR inhibition in normotensive mice did not cause PA or PCA remodeling as evidenced by the lack of difference in the outer diameter, lumen diameter, or wall cross-sectional area when compared to Sham mice (Fig. S1). Measurements are presented at the physiological intralumenal pressure of 40mmHg for the PAs and 60mmHg for the PCA. As previously demonstrated in the aorta10, we confirmed that the ECMRKO mice also had decreased MR mRNA expression in the PCA when compared to the MR-intact littermates (Fig. S2). There was still some MR mRNA expression in the PCAs from the ECMRKO mice because the vascular smooth muscle cells (VSMC) also express the receptor. While we expect MR gene recombination in all ECs, due to insufficient tissue from the PAs, we could not measure changes in gene expression of the MR. Specific EC-MR deletion did not change the structure of the PAs or PCAs under normotensive conditions. Outer diameter, lumen diameter and wall area were not changed when compared to MR-intact littermates (Fig. S3). MR inhibition and specific EC-MR deletion also did not alter cerebral perfusion as shown in the C57Bl/6 mice treated with EPL and the ECMRKO mice (Fig. 1).
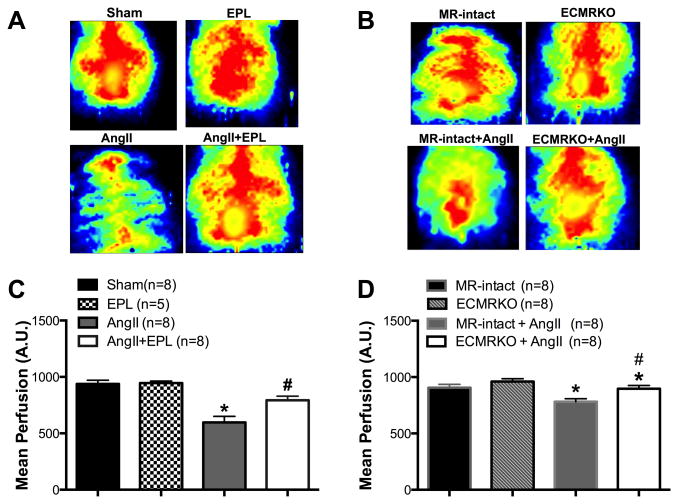
Figure 1. Endothelial MR deletion prevents a reduction in cerebral perfusion with AngII-hypertension.
Cerebral perfusion was measured prior to euthanasia in anesthetized mice using scanning laser Doppler. Representative images of the scanning laser Doppler are shown (A, B). In Figures C and D, data are presented as mean ± SEM. (C) Mean cerebral perfusion was reduced in AngII treated mice compared to Sham. Cerebral perfusion was improved in the AngII+EPL treated C57Bl/6 mice. (D) EC-MR deletion did not change baseline perfusion compared to MR-intact. AngII-hypertension decreased cerebral perfusion in MR-intact mice. EC-MR deletion in the ECMRKO+AngII significantly increased perfusion compared to the MR-intact+AngII mice. *p<0.05; vs. Sham, MR-intact or ECMRKO. #p<0.05 AngII vs. AngII+EPL or MR-intact+AngII vs. ECMRKO+AngII. Legend: Eplerenone (EPL); Angiotensin II (AngII); Mineralocorticoid receptor-intact mice (MR-intact); Endothelial cell MR knockout (ECMRKO).
AngII increases systolic blood pressure and plasma aldosterone
C57Bl/6 mice
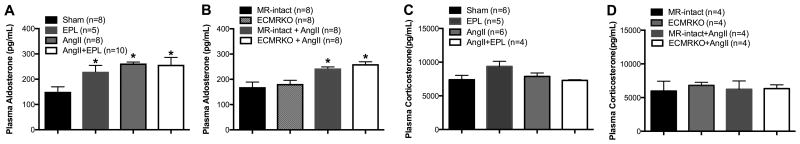
To assess the role of the MR in the genesis of AngII-hypertension, C57Bl/6 mice were treated with EPL during the 4 weeks of AngII infusion (800ng/kg/min). AngII treatment increased systolic blood pressure (Table S1) but EPL did not reduce blood pressure suggesting that MR activation is not the major driving force for the elevation in blood pressure caused by AngII administration. As expected due to its role in activating adrenal mineralocorticoid production, AngII infusion increased plasma aldosterone levels. This was not altered by EPL treatment (Fig. 2A). The mice treated with AngII and the vehicle for EPL had a similar increase in aldosterone (Table S2). Plasma corticosterone levels remained unchanged in all groups (Fig. 2C).
Figure 2. AngII-hypertension increases plasma aldosterone in C57bl/6 and EMCRKO mice.
Plasma aldosterone and corticosterone were measured after 4 weeks of AngII or vehicle treatment. (A) EPL treatment increased plasma aldosterone levels when compared to Sham C57bl/6 mice. AngII-hypertension also increased plasma aldosterone, AngII+EPL treatment did not further increase aldosterone levels in C57Bl/6 mice. (B) EC-MR deletion did not alter plasma aldosterone levels when compared to MR-intact littermates in the absence of hypertension. AngII infusion significantly increased aldosterone levels in MR-intact and ECMRKO mice. (C-D) AngII-induced hypertension did not change plasma corticosterone levels. Data are presented as mean ± SEM. *p<0.05 vs Sham or MR-intact and ECMRKO. Legend: Eplerenone (EPL); Angiotensin II (AngII); Mineralocorticoid receptor-intact mice (MR-intact); Endothelial cell MR knockout (ECMRKO).
ECMRKO mice
As previously reported10, EC-MR specific deletion is not associated with changes in baseline blood pressure (Table S1). ECMRKO mice were infused with AngII and compared to MR-intact littermates with AngII treatment or Sham surgery. AngII increased systolic blood pressure to the same extent in the MR-intact and ECMRKO mice when compared to untreated MR-intact and ECMRKO mice (Table S1). Plasma aldosterone levels were not different between control MR-intact and ECMRKO mice and AngII treatment increased plasma aldosterone to the same degree in both mouse strains (Fig 2B). Plasma corticosterone was not changed in any group (Fig 2 D).
Specific deletion of the endothelial MR attenuated the reduction in cerebral perfusion induced by AngII-hypertension
We next determined the effect of AngII-hypertension on cerebral perfusion. As expected, AngII-hypertension reduced cerebral perfusion. We cannot confirm that EPL treatment improved perfusion because the vehicle for EPL (Fig 1C) also had a protective effect (Table S2). However, the changes in perfusion were prevented by EC-MR deletion (Fig 1D), which suggests that MR signaling in EC is involved in the process independent of changes in blood pressure. As the improvement in cerebral perfusion could be in part due to alterations in artery remodeling, we next examined the effect of MR inhibition on cerebral vascular structure in response to AngII-hypertension.
MR antagonism prevents PA and PCA AngII-hypertensive remodeling in C57BL/6 mice
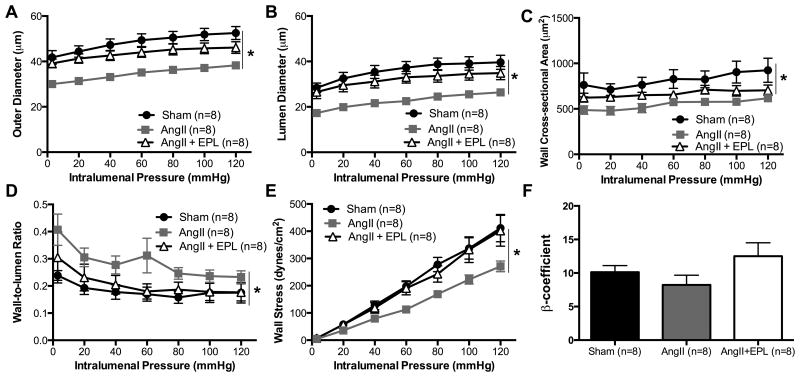
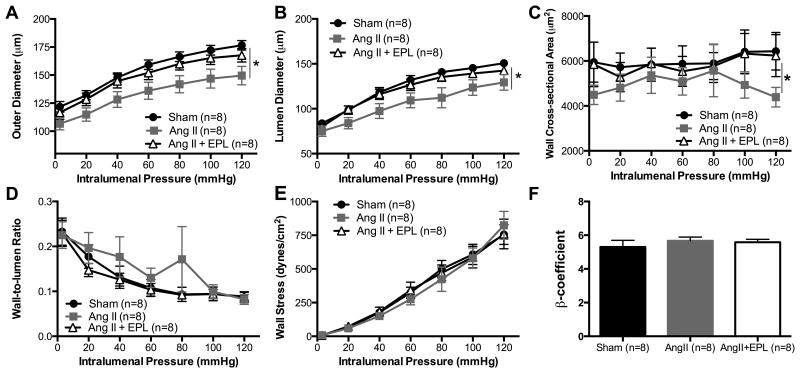
As expected, AngII-hypertension caused inward hypotrophic remodeling of the PAs and PCA as evidenced by smaller outer diameters (Fig. 3A, 4A), lumen diameters (Fig. 3B, 4B) and wall areas (Fig. 3C, 4C) in the AngII-treated mice when compared to Sham C57BL/6 mice. Wall-to-lumen ratio was increased by AngII-hypertension in the PAs but not in the PCAs (Fig. 4D, 4E). In both artery types, artery cross sectional and lumen cross sectional area were reduced by AngII treatment, and MR antagonism prevented this reduction (Fig. S5, S6). The changes in the mechanical properties of the PAs and PCAs in response to AngII-hypertension were distinct suggesting that the cerebral arteries and arterioles respond differently to the effects of AngII infusion and hypertension. The distensibility of the PAs and PCAs was not changed by AngII-hypertension nor by addition of EPL (Fig. S5, S6). Conversely, AngII-hypertension reduced wall stress in the PAs (Fig. 3E), but not in the PCA (Fig. 4E); EPL treatment prevented the changes in PA wall stress. Wall stiffness was not changed by AngII-hypertension in either vessel type (Fig. 3F). Our data shows that MR inhibition prevented inward remodeling (Fig. 3, 4) without changing blood pressure (Table S1) suggesting that the MR contributes directly to the process of vascular remodeling in response to AngII-hypertension independent of changes in blood pressure. The vehicle for EPL treatment did not improve AngII-induced artery remodeling (Fig. S4).
Figure 3. MR antagonism prevents PA inward hypotrophic remodeling with AngII-hypertension.
The biomechanical properties were assessed in isolated PAs using pressure myography. AngII infusion resulted in a reduced (A) outer diameter, (B) lumen diameter, and (C) wall-cross sectional area. (D) Wall-to-lumen ratio was increased in the AngII treated mice. EPL treatment prevented these changes. (E) Wall stress was significantly reduced. However, (F) artery wall stiffness was not changed. Data are presented as mean ± SEM. *p<0.05 vs Sham or AngII+EPL. Legend: Eplerenone (EPL); Angiotensin II (AngII).
Figure 4. MR antagonism prevents inward remodeling of the PCA during AngII-hypertension.
Structure was assessed in isolated PCAs using pressure myography. The (A) outer and (B) lumen diameter were reduced in AngII treated mice. (C)Wall cross sectional area was reduced at 120mmHg. (D) Wall-to-lumen ratio was not changed. EPL treatment prevented the inward remodeling of the posterior cerebral artery. (E) Wall stress and (F) wall stiffness remained unchanged. Data are presented as mean ± SEM. *p<0.05 vs Sham or Ang+EPL. Legend: Eplerenone (EPL); Angiotensin II (AngII).
Endothelial MR signaling mediates PA and PCA remodeling in AngII-hypertension
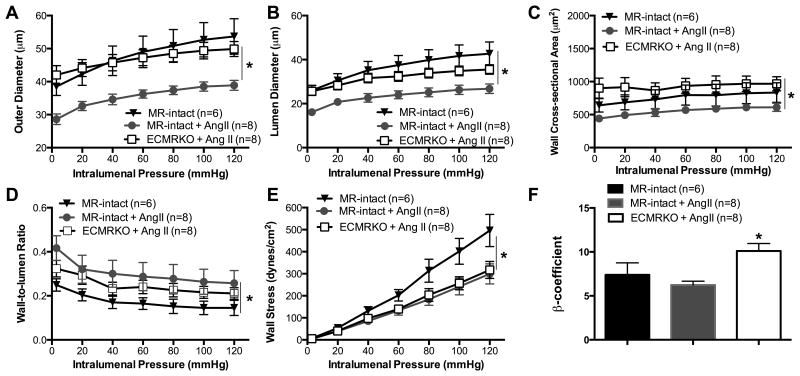
The benefits of MR antagonism were independent of blood pressure suggesting a potential direct effect on the vasculature. To explore this possibility, we determined the role of EC-MR signaling in the AngII-induced remodeling observed in the PAs and PCA. In the PAs, deletion of the MR, specifically from endothelial cells (ECs), prevented the inward remodeling as ECMRKO mice treated with AngII had increased outer diameter (Fig. 5A), lumen diameter (Fig. 5B), vessel area (Fig. S7), lumen area (Fig. S7), and wall area (Fig. 5C) when compared to MR-intact+AngII mice. As a result, there were no significant differences in cerebral vessel structure between MR-intact sham-treated mice and ECMRKO littermates treated with AngII. EC-MR specific deletion also prevented the change in wall-to-lumen ratio caused by AngII infusion (Fig. 5D). However, the decreased wall stress caused by AngII-hypertension in the MR-intact+AngII was not reversed by EC-MR deletion in the ECMRKO+AngII mice (Fig. 5E). This was contrary to the EPL study suggesting that changes in wall stress are mediated by MR outside the ECs, maybe in the VSMCs. As in the EPL study, wall stiffness (Fig. 5F) and artery distensibility were not significantly different in either group (Fig. S7 C).
Figure 5. Endothelial MR signaling mediates PA remodeling during AngII-hypertension.
AngII-hypertension significantly reduced the (A) outer diameter, (B) lumen diameter, and (C) wall area in MR-intact+AngII compared to MR-intact mice. (D) Wall-to-lumen ratio was increased in MR-intact mice. (E) Wall stress was also changed, but (F) wall stiffness was not significantly changed. Endothelial MR deletion prevented the inward hypotrophic remodeling but it did not increase the wall stress. Data are presented as mean ± SEM. *p<0.05 vs MR-intact or ECMRKO+AngII. Legend: Angiotensin II (AngII); Mineralocorticoid receptor-intact mice (MR-intact); Endothelial cell MR knockout (ECMRKO).
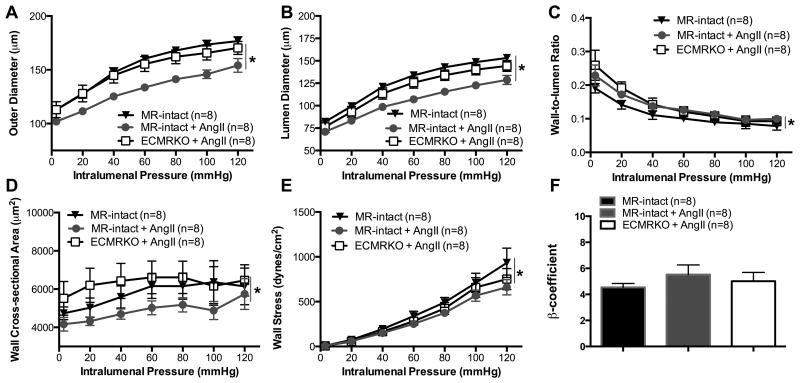
In the PCA, EC-MR deletion also prevented the AngII-induced remodeling as evidenced by the increase in the outer diameter (Fig. 6A), lumen diameter (Fig. 6B), vessel area (Fig. S8 A), lumen area (Fig. S8 B) and wall area (Fig. 6C) but the changes were not as marked as in the PAs. In the PCA, EC-MR deletion did not prevent the increase in wall-to-lumen ratio caused by AngII-hypertension (Fig. 6D) as it had in the PA. Distensibility (Fig. S8 C) and artery wall stiffness (Fig. 6F) were not significantly changed by EC-MR deletion. EC-MR deletion did not reverse the reduced wall stress that was observed in MR-intact+AngII mice (Fig. 6E).
Figure 6. Endothelial MR deletion prevents posterior cerebral artery remodeling during AngII-hypertension.
Structure was assessed in isolated arteries using pressure myography. AngII infusion resulted in inward remodeling evidenced by the reduced (A) outer, and (B) lumen diameter in the MR-intact+AngII compared to MR-intact mice. EC-MR deletion prevented the inward remodeling in the ECMRKO+AngII mice. (C) Wall area was not significantly changed in the MR-intact+AngII compared to MR-intact, but it was significantly increased in the ECMRKO+AngII compared to MR-intact+AngII mice. (D) Wall-to-lumen ratio did not change. (E) Wall stress was reduced in the MR-intact+AngII and ECMRKO+AngII compared to MR-intact. (F)Artery wall stiffness was not changed. Data are presented as mean ± SEM. *p<0.05 vs MR-intact or ECMRKO+AngII; #p<0.05 MR-intact+AngII vs ECMRKO+AngII. Legend: Angiotensin II (AngII); Mineralocorticoid receptor-intact mice (MR-intact); Endothelial cell MR knockout (ECMRKO).
AngII-hypertension and MR antagonism change the mRNA expression of MCP-1, IL-6 and MMP-2 in the PCA
To explore potential mechanisms by which the MR contributes to cerebral artery remodeling, we assessed the PCA mRNA expression for markers of inflammation, macrophage infiltration, oxidative stress, and matrix metallaproteases (MMP) which have been previously associated with AngII-induced vascular remodeling. Importantly, MR and angiotensin II receptor type 1 (agtr1a) mRNA expression were unchanged in the PCA in response to AngII-hypertension and EPL treatment (Fig. S9 A, M). Previous studies have shown that AngII-hypertension increases the expression of MMP-2 in the aorta and in VSMC and that this is important for the process of arterial remodeling13, 14. Consistent with these previous findings, AngII treatment significantly increased the mRNA expression of MMP-2 in the PCA and this appears to be mediated, at least in part, by MR signaling as EPL prevented the increase (Fig. S9 B). AngII was also shown to increase the expression of MMP-9 in the aorta15. However, in the PCA, MMP-9 mRNA level was not significantly changed by AngII or EPL treatment (Fig. S9 C). MMP-12, produced by macrophages, may also play an important role in flow-induced artery remodeling16. In this study, we show that AngII-hypertension increases the expression of MMP-12 mRNA in the PCA, but this does not appear to be mediated by MR signaling as MMP-12 expression was unchanged by EPL treatment (Fig. S9 D).
AngII has also been shown to increase the production of Nox2 17 as well as Nox4 which has been shown to be regulated by the MR in human coronary arteries ECs 18. However, in the PCA the mRNA expression of Nox2 and Nox4 genes were not changed in response to AngII-hypertension or MR inhibition (Fig. S9 E, F). Inflammatory markers including monocyte chemoattractant protein 1 (MCP1) and interlukin-6 (IL-6) were increased in the PCA in response to AngII (Fig. S9 G, H). Other inflammatory markers including tumor necrosis factor alpha (TNFa) and the alternative macrophage marker YM1 tended to increase, but these changes were not statistically significant (Fig. S9 I, J). The mRNA expression of two collagen genes, Col2a1 and Col3a1, were not significantly changed by AngII-hypertension or MR antagonism (Fig. S9 K, L). In summary, MR antagonism did not change the degree of AngII induction of inflammatory genes nor did AngII change the mRNA expression of Nox2. However, AngII significantly increased mRNA expression of MMP-2 and this was prevented by EPL.
Discussion
The novel findings in our study are that: 1) in the absence of hypertension, cerebral artery structure does not depend on MR activation; 2) the protective effect of MR inhibition in the cerebral circulation could be almost completely reproduced by EC-specific MR deletion suggesting that during AngII-hypertension, EC-MR is necessary for hypertensive vascular remodeling and that cerebrovascular protection by MR inhibition is mediated by EC-MR. These findings enhance our understanding of the role of vascular MR signaling in cerebral artery remodeling.
PA and PCA remodeling in response to AngII-hypertension
AngII promotes the secretion of aldosterone by activation of the agt1r in the adrenal gland and animal studies have shown that increases in circulating levels of AngII or aldosterone are associated with adverse vascular remodeling19, 20. As expected, AngII-hypertension resulted in PA and PCA inward remodeling. The PAs also exhibited an increased wall-to-lumen ratio which has been shown to predict end organ damage21. Despite the increase in wall-to-lumen ratio, the wall area was reduced in the AngII-treated mice; this hypotrophic remodeling was an unexpected finding that will be discussed in more detail later. The AngII-hypertension-associated changes in the mechanical properties were different in the two artery types supporting the need to study changes in the macro- and microcirculation separately. In the PAs, wall stress was decreased, but the same was not observed in the PCA. These differences could be associated with the location and innervation of these arteries and arterioles. To the best of our knowledge this is the first time that AngII-dependent artery remodeling has been studied in the PAs and PCAs from the same animal; this is a strength of our study because all of the effects of circulating factors are the same.
A role for MR in ECs in Hypertensive PA and PCA remodeling
Our data further support previous studies5, 22,23 indicating that MR activation contributes to inward hypotropic remodeling in the PAs and PCAs during AngII-hypertension. However, those previous studies did not identify the cell type involved in MR-mediated cerebral artery remodeling. In the current study, we show that EC-MR is necessary for the AngII-dependent hypertensive cerebral artery remodeling. The role of EC-MR was independent of changes in blood pressure or basal cerebral vessel structure prior to exposure to AngII-hypertension suggesting a direct role for EC-MR in the response of the cerebral vasculature to hypertension induced by AngII. Whether EC-MR contributes to cerebral remodeling in response to hypertension caused by a distinct mechanism remains to be explored. Since hypertension is predominantly a disorder of the elderly and AngII signaling increases with aging and contributes to the rise in blood pressure with aging24, this mechanism has important implications for the growing aging population in which dementia and stroke are important causes of morbidity and mortality.
Possible mechanisms for the EC-MR mediated remodeling
This study implicates an important role for EC-MR in cerebral artery remodeling however, the mechanisms downstream of MR activation have not been identified. Additional in vivo studies that are beyond the scope of this study will be required to identify the mechanism because the remodeling process likely requires exposure to hypertension and circulating factors and hence cannot be reproduced in vitro. Previous studies have implicated oxidative stress, inflammation, and extracellular matrix remodeling in AngII induced artery remodeling17, 19, 20, 25-27. In the PCAs we found that AngII-hypertension increases the mRNA expression of MCP-1 and IL-6 suggesting a possible role for these cytokines in the remodeling process. However, these changes in cytokine mRNA were not mediated by MR activation, because EPL did not reduce MCP-1 or IL-6 mRNA levels. We did not observe any changes in the gene expression of collagen, but AngII-hypertension increased the mRNA expression of MMP-2 that was prevented by EPL treatment. The increase in MMP-2 mRNA appears to be specific, because no changes in MMP-9 and -12 mRNA were observed. These observations suggest the possibility that changes in MMP-2 induced by AngII-hypertension could involve MR signaling and contribute to vascular remodeling during AngII-hypertension, however additional studies are needed to confirm this potential mechanism. Although previous studies link aldosterone production to MMP-2 activity28, other studies have also shown that AngII increased MMP-9 production to mediate pial arteriole remodeling29 confirming regional heterogeneity in remodeling mechanisms, that the regulation of the MMPs is complex, and that MMP-2 might not be the only MMP regulating artery wall structure30. Studies from our lab support a role for MMP-2 in the hypertensive cerebral artery remodeling process as doxycycline, an MMP-2 inhibitor, prevented cerebral artery remodeling in hypertensive rats 31. A limitation in our study is that we could only measure changes in mRNA expression in the PCA, but not in PA due to limited tissue availability.
Differences in the role of EC-MR in small cerebral arterioles PA versus PCA
The mechanical properties of the PAs and PCAs were differentially affected by AngII-hypertension. In the PAs, the decrease in diameter was accompanied by a reduction in wall stress that suggests that these arterioles remodeled to maintain wall stress within a normal range during hypertension. The changes in wall stress were prevented in the hypertensive mice by EPL treatment, but not by EC-MR deletion; this suggests that the effect of EPL on wall stress might be mediated by MR in VSMC, or some other cell type. However, this is speculative and additional studies with VSMC-specific MR deletion will need to be conducted to confirm this possibility.
EC-MR signaling contributes to changes in cerebral blood flow in response to AngII-hypertension
EC-specific MR deletion enhanced cerebral blood flow in mice with AngII-hypertension suggesting that MR inhibition in the endothelium is sufficient to protect from impaired cerebral blood flow in this hypertension model. Previous studies have shown that artery structure changes are associated with changes in perfusion3, 32. Although the changes in artery structure correlate well with the reduction in blood flow, it is important to note that AngII could also negatively impact endothelium-dependent vasodilation and that, and other factors could also contribute to decreased blood flow33-37. Previous studies demonstrated that EC-MR deletion protects the mesenteric vasculature from endothelial dysfunction caused by AngII-hypertension10. Future studies will examine whether this is also occurring in the cerebral vasculature in addition to the structural benefits of EC-MR deletion. Since EC-MR deletion preserved cerebral blood flow in the setting of AngII-hypertension and AngII is an important driver of hypertension in the elderly24, 38, future studies could explore whether this could have implications for improving cognitive function and stroke outcomes.
Some limitations of our study must be acknowledged. First, VE-Cadherin is also expressed in a small compartment of hematopoietic cells. All of the studies were done in male mice, so we did not assess possible sex differences. Although we implicate EC-MR signaling in cerebral artery remodeling in AngII-hypertension, we have not determined the detailed molecular mechanism. Our studies suggest IL-6, MMP-2, -9 are in involved in the AngII-induced hypertensive remodeling process and the data we have thus far suggest that MMP-2 could be a critical mediator of the MR dependent remodeling, but additional studies are required to verify this. Another limitation is that the vehicle for EPL improved cerebral blood flow; however, it did not alter artery structure. This disconnect between structure and flow could be a consequence of the technique used to assess blood flow. The laser in the Pim3 system we used has a penetration of approximately 1mm, with the skull intact it is likely that we are measuring only the blood flow on the surface of the brain, and not the flow in the PAs. We have not studied remodeling of the pial arteries so it is unclear if changes in their structure can explain the differences in cerebral blood flow. However, the fact that in the AngII-treated ECMRKO mice the perfusion was improved suggests that EC MR signaling is involved in the changes in perfusion. Cerebral blood flow was measured in mice anesthetized with isoflurane which is a vasodilator 32, 39, we also did not measure blood gasses while the animals were under anesthetic, which is a limitation. However, the comparisons were made with appropriate controls also exposed to isoflurane. Nonetheless, additional studies using alternative methods are warranted. We recognize differences in dilator responses to isoflurane are possible between the groups, but this is a limitation of all studies of this type.
We only assessed changes in gene expression of the PCA because the limited tissue from the PAs did not allow accurate measurement of changes in gene expression. We measured blood pressure using tail-cuff plethysmography; we recognize that telemetry blood pressure measurements are generally considered to be more accurate than tail-cuff. The increase in blood pressure after AngII infusion was consistent in every mouse and, recent studies show that tail-cuff blood pressure measurements in AngII treated mice are very similar to those obtained by telemetry in an undisturbed mouse 40. The role of the MR in VSMC in hypertensive artery remodeling should also be considered as VMSC MR contributes to myogenic tone and arterial remodeling in response to carotid injury and to vascular stiffness in response to aldosterone-salt induced hypertension 41, 42. Future studies will also consider whether there are EC-MR-mediated changes in endothelium-dependent vasodilation and myogenic tone in cerebral vessels in response to hypertension. Previous studies have shown that PAs and PCA from hypertensive rats have increased myogenic tone compared to normotensive Wistar Kyoto rats 5, 43.
Perspectives
Our study fills a gap in our knowledge of how MR signaling mediates hypertensive remodeling in arterioles in the microcirculation and arteries in the pial circulation in the brain. Hypertension has been linked to cerebrovascular diseases such as ischemic stroke and the development of vascular dementia 44, 45. We utilized EC-specific MR-knockout mice to show, for the first time, the role-played by the EC-MR in cerebral artery remodeling and that MR inhibition or EC-MR deletion improved cerebral blood flow without changing blood pressure. Identification of the cell specific actions of aldosterone and MR signaling could allow us to better define the downstream mechanisms of MR-mediated cerebral artery remodeling in hypertension. This could contribute to the development of better therapeutic approaches to improve cerebrovascular health in hypertensive patients, a rapidly growing concern in our aging population.
Supplementary Material
Novelty and Significance.
1. What is new?
This study shows, for the first time, the role of endothelial cell mineralocorticoid receptor signaling in cerebral artery remodeling and cerebral perfusion during Angiotensin II-hypertension.
The study of the parenchymal arterioles in mice is particularly novel and exciting. These arterioles play a critical role in the outcome of ischemic stroke and the development of vascular dementia, yet they have not been widely studied.
2. What is relevant?
Identification of cell specific effects of MR activation in artery remodeling and dysfunction could allow us to define the downstream mechanisms of action more rapidly.
Defining the mechanisms responsible for the detrimental effects of MR activation could lead to the development of better therapeutic approaches to improve the outcome of stroke and dementia.
3. Summary
Mineralocorticoid receptor activation at the level of the endothelium mediates cerebral artery inward remodeling during AngII-hypertension. This hypertensive remodeling was associated with a decrease in cerebral blood flow and was prevented by endothelial mineralocorticoid receptor deletion or treatment with a mineralocorticoid receptor antagonist. Impairments in cerebral blood flow could increase the risk for ischemic stroke and development of vascular dementia.
Acknowledgments
The authors would like to acknowledge Erika Sarno and Brendan Mullan for technical assistance with the experiments. The MSU Pharmacology and Toxicology writing group is thanked for their editorial comments.
Sources of Funding: This study was supported by the National Institutes of Health grant PO1-HL-070687 to WF Jackson and AM Dorrance, the American Heart Association grant 13GRNT17210000 to AM Dorrance, and National Institutes of Health grant R01-HL095590 to IZ Jaffe. JM Diaz-Otero was supported by the National Institutes of Health grant 5T32GM092715-04.
Footnotes
Disclosures: None.
References
- 1.Sierra C. Cerebral small vessel disease, cognitive impairment and vascular dementia. Panminerva Med. 2012;54(3):179–88. [PubMed] [Google Scholar]
- 2.Dichgans M, Zietemann V. Prevention of vascular cognitive impairment. Stroke. 2012;43(11):3137–46. doi: 10.1161/STROKEAHA.112.651778. [DOI] [PubMed] [Google Scholar]
- 3.Pires PW, Dams Ramos CM, Matin N, Dorrance AM. The effects of hypertension on the cerebral circulation. Am J Physiol Heart Circ Physiol. 2013;304(12):H1598–614. doi: 10.1152/ajpheart.00490.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rigsby CS, Pollock DM, Dorrance AM. Spironolactone improves structure and increases tone in the cerebral vasculature of male spontaneously hypertensive stroke-prone rats. Microvasc Res. 2007;73(3):198–205. doi: 10.1016/j.mvr.2006.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pires PW, Jackson WF, Dorrance AM. Regulation of myogenic tone and structure of parenchymal arterioles by hypertension and the mineralocorticoid receptor. Am J Physiol Heart Circ Physiol. 2015;309(1):H127–36. doi: 10.1152/ajpheart.00168.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cipolla MJ, Chan SL, Sweet J, Tavares MJ, Gokina N, Brayden JE. Postischemic reperfusion causes smooth muscle calcium sensitization and vasoconstriction of parenchymal arterioles. Stroke. 2014;45(8):2425–30. doi: 10.1161/STROKEAHA.114.005888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Iddings JA, Kim KJ, Zhou Y, Higashimori H, Filosa JA. Enhanced parenchymal arteriole tone and astrocyte signaling protect neurovascular coupling mediated parenchymal arteriole vasodilation in the spontaneously hypertensive rat. J Cereb Blood Flow Metab. 2015;35(7):1127–36. doi: 10.1038/jcbfm.2015.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hayman LA, Berman SA, Hinck VC. Correlation of CT cerebral vascular territories with function: II. Posterior cerebral artery. AJR Am J Roentgenol. 1981;137(1):13–9. doi: 10.2214/ajr.137.1.13. [DOI] [PubMed] [Google Scholar]
- 9.Dorrance AM, Rupp NC, Nogueira EF. Mineralocorticoid receptor activation causes cerebral vessel remodeling and exacerbates the damage caused by cerebral ischemia. Hypertension. 2006;47(3):590–5. doi: 10.1161/01.HYP.0000196945.73586.0d. [DOI] [PubMed] [Google Scholar]
- 10.Mueller KB, Bender SB, Hong K, Yang Y, Aronovitz M, Jaisser F, Hill MA, Jaffe IZ. Endothelial Mineralocorticoid Receptors Differentially Contribute to Coronary and Mesenteric Vascular Function Without Modulating Blood Pressure. Hypertension. 2015;66(5):988–97. doi: 10.1161/HYPERTENSIONAHA.115.06172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jia G, Habibi J, DeMarco VG, Martinuez-Lemus LA, Ma L, Whaley-Connell AT, Aroor AR, Domeier TL, Zhu Y, Meininger GA, Mueller KB, Jaffe IZ, Sowers JR. Endothelial Mineralocorticoid Receptor Deletion Prevents Diet-Induced Cardiac Diastolic Dysfunction in Females. Hypertension. 2015;66(6):1159–67. doi: 10.1161/HYPERTENSIONAHA.115.06015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Salvador AM, Moss ME, Aronovitz M, Mueller KB, Blanton RM, Jaffe IZ, Alcaide P. Endothelial mineralocorticoid receptor contributes to systolic dysfunction induced by pressure overload without modulating cardiac hypertrophy or inflammation. Physiol Rep. 2017;5(12):e13313. doi: 10.14814/phy2.13313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Patel VB, Zhong JC, Fan D, Basu R, Morton JS, Parajuli N, McMurtry MS, Davidge ST, Kassiri Z, Oudit GY. Angiotensin-converting enzyme 2 is a critical determinant of angiotensin II-induced loss of vascular smooth muscle cells and adverse vascular remodeling. Hypertension. 2014;64(1):157–64. doi: 10.1161/HYPERTENSIONAHA.114.03388. [DOI] [PubMed] [Google Scholar]
- 14.Wang C, Qian X, Sun X, Chang Q. Angiotensin II increases matrix metalloproteinase 2 expression in human aortic smooth muscle cells via AT1R and ERK1/2. Exp Biol Med (Maywood) 2015;240(12):1564–71. doi: 10.1177/1535370215576312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ruddy JM, Jones JA, Stroud RE, Mukherjee R, Spinale FG, Ikonomidis JS. Differential effects of mechanical and biological stimuli on matrix metalloproteinase promoter activation in the thoracic aorta. Circulation. 2009;120(11 Suppl):S262–8. doi: 10.1161/CIRCULATIONAHA.108.843581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ota R, Kurihara C, Tsou TL, Young WL, Yeghiazarians Y, Chang M, Mobashery S, Sakamoto A, Hashimoto T. Roles of matrix metalloproteinases in flow-induced outward vascular remodeling. J Cereb Blood Flow Metab. 2009;29(9):1547–58. doi: 10.1038/jcbfm.2009.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.De Silva TM, Faraci FM. Effects of angiotensin II on the cerebral circulation: role of oxidative stress. Front Physiol. 2012;3:484. doi: 10.3389/fphys.2012.00484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Caprio M, Newfell BG, la Sala A, Baur W, Fabbri A, Rosano G, Mendelsohn ME, Jaffe IZ. Functional mineralocorticoid receptors in human vascular endothelial cells regulate intercellular adhesion molecule-1 expression and promote leukocyte adhesion. Circ Res. 2008;102(11):1359–67. doi: 10.1161/CIRCRESAHA.108.174235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Briet M, Barhoumi T, Mian MO, Coelho SC, Ouerd S, Rautureau Y, Coffman TM, Paradis P, Schiffrin EL. Aldosterone-Induced Vascular Remodeling and Endothelial Dysfunction Require Functional Angiotensin Type 1a Receptors. Hypertension. 2016;67(5):897–905. doi: 10.1161/HYPERTENSIONAHA.115.07074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Johnson RJ, Alpers CE, Yoshimura A, Lombardi D, Pritzl P, Floege J, Schwartz SM. Renal injury from angiotensin II-mediated hypertension. Hypertension. 1992;19(5):464–74. doi: 10.1161/01.hyp.19.5.464. [DOI] [PubMed] [Google Scholar]
- 21.Izzard AS, Rizzoni D, Agabiti-Rosei E, Heagerty AM. Small artery structure and hypertension: adaptive changes and target organ damage. J Hypertens. 2005;23(2):247–50. doi: 10.1097/00004872-200502000-00002. [DOI] [PubMed] [Google Scholar]
- 22.Rigsby CS, Ergul A, Portik Dobos V, Pollock DM, Dorrance AM. Effects of spironolactone on cerebral vessel structure in rats with sustained hypertension. Am J Hypertens. 2011;24(6):708–15. doi: 10.1038/ajh.2011.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Heistad DD, Mayhan WG, Coyle P, Baumbach GL. Impaired dilatation of cerebral arterioles in chronic hypertension. Blood Vessels. 1990;27(2-5):258–62. doi: 10.1159/000158817. [DOI] [PubMed] [Google Scholar]
- 24.Wang M, Khazan B, Lakatta EG. Central Arterial Aging and Angiotensin II Signaling. Curr Hypertens Rev. 2010;6(4):266–81. doi: 10.2174/157340210793611668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Martinez FA. Aldosterone inhibition and cardiovascular protection: more important than it once appeared. Cardiovasc Drugs Ther. 2010;24(4):345–50. doi: 10.1007/s10557-010-6256-6. [DOI] [PubMed] [Google Scholar]
- 26.Cascella T, Radhakrishnan Y, Maile LA, Busby WH, Jr, Gollahon K, Colao A, Clemmons DR. Aldosterone enhances IGF-I-mediated signaling and biological function in vascular smooth muscle cells. Endocrinology. 2010;151(12):5851–64. doi: 10.1210/en.2010-0350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ma J, Weisberg A, Griffin JP, Vaughan DE, Fogo AB, Brown NJ. Plasminogen activator inhibitor-1 deficiency protects against aldosterone-induced glomerular injury. Kidney Int. 2006;69(6):1064–72. doi: 10.1038/sj.ki.5000201. [DOI] [PubMed] [Google Scholar]
- 28.Sakamuri SS, Valente AJ, Siddesha JM, Delafontaine P, Siebenlist U, Gardner JD, Bysani C. TRAF3IP2 mediates aldosterone/salt-induced cardiac hypertrophy and fibrosis. Mol Cell Endocrinol. 2016;429:84–92. doi: 10.1016/j.mce.2016.03.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Umesalma S, Houwen FK, Baumbach GL, Chan SL. Roles of Caveolin-1 in Angiotensin II-Induced Hypertrophy and Inward Remodeling of Cerebral Pial Arterioles. Hypertension. 2016;67(3):623–9. doi: 10.1161/HYPERTENSIONAHA.115.06565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Galis ZS, Khatri JJ. Matrix metalloproteinases in vascular remodeling and atherogenesis: the good, the bad, and the ugly. Circ Res. 2002;90(3):251–62. doi: 10.1161/hh0302.105345. [DOI] [PubMed] [Google Scholar]
- 31.Pires PW, Rogers CT, McClain JL, Garver HS, Fink GD, Dorrance AM. Doxycycline, a matrix metalloprotease inhibitor, reduces vascular remodeling and damage after cerebral ischemia in stroke-prone spontaneously hypertensive rats. Am J Physiol Heart Circ Physiol. 2011;301(1):H87–97. doi: 10.1152/ajpheart.01206.2010. doi 10.1152/ajpheart.01206.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wildling L, Hinterdorfer P, Kusche-Vihrog K, Treffner Y, Oberleithner H. Aldosterone receptor sites on plasma membrane of human vascular endothelium detected by a mechanical nanosensor. Pflugers Arch. 2009;458(2):223–30. doi: 10.1007/s00424-008-0615-1. [DOI] [PubMed] [Google Scholar]
- 33.Lipsitz LA, Gagnon M, Vyas M, Iloputaife I, Keily DK, Sorond F, Serrador J, Cheng DM, Babikian V, Cupples LA. Antihypertensive therapy increases cerebral blood flow and carotid distensibility in hypertensive elderly subjects. Hypertension. 2005;45(2):216–21. doi: 10.1161/01.HYP.0000153094.09615.11. [DOI] [PubMed] [Google Scholar]
- 34.Beason-Held LL, Moghekar A, Zonderman AB, Kraut MA, Resnick SM. Longitudinal changes in cerebral blood flow in the older hypertensive brain. Stroke. 2007;38(6):1766–73. doi: 10.1161/STROKEAHA.106.477109. [DOI] [PubMed] [Google Scholar]
- 35.Dai W, Lopez OL, Carmichael OT, Becker JT, Kuller LH, Gach HM. Abnormal regional cerebral blood flow in cognitively normal elderly subjects with hypertension. Stroke. 2008;39(2):349–54. doi: 10.1161/STROKEAHA.107.495457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Waldstein SR, Lefkowitz DM, Siegel EL, Rosenberg WF, Spencer RJ, Tankard CF, Manukyan Z, Gerber EJ, Katzel LI. Reduced cerebral blood flow in older men with higher levels of blood pressure. J Hypertens. 2010;28(5):993–8. doi: 10.1097/hjh.0b013e328335c34f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Muller M, van der Graaf Y, Visseren FL, Mali WP, Geerlings MI, Group SS. Hypertension and longitudinal changes in cerebral blood flow: the SMART-MR study. Ann Neurol. 2012;71(6):825–33. doi: 10.1002/ana.23554. [DOI] [PubMed] [Google Scholar]
- 38.Dinh QN, Drummond GR, Kemp-Harper BK, Diep H, De Silva MT, Kim HA, Vinh A, Robertson AB, Cooper MA, Mansel A, Chrissobolis S, Sobey CG. Pressor response to angiotensin II is enhanced in aged mice and associated with inflammation, vasoconstriction and oxidative stress. Aging. 2017;9(6):1595–606. doi: 10.18632/aging.101255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schwinn DA, McIntyre RW, Reves JG. Isoflurane-induced vasodilation: role of the alpha-adrenergic nervous system. Anesth Analg. 1990;71(5):451–9. doi: 10.1213/00000539-199011000-00001. [DOI] [PubMed] [Google Scholar]
- 40.Wilde E, Aubdool AA, Thakore P, Baldissera L, Jr, Alawi KM, Keeble J, Nandi M, Brain SD. Tail-Cuff Technique and Its Influence on Central Blood Pressure in the Mouse. J Am Heart Assoc. 2017;6(6) doi: 10.1161/JAHA.116.005204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pruthi D, McCurley A, Aronovitz M, Galayda C, Karumanchi SA, Jaffe IZ. Aldosterone promotes vascular remodeling by direct effects on smooth muscle cell mineralocorticoid receptors. Arterioscler Thromb Vasc Biol. 2014;34(2):355–64. doi: 10.1161/ATVBAHA.113.302854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Galmiche G, Pizard A, Gueret A, El Moghrabi S, Ouvard-Pascaud A, Berger S, Challande P, Jaffe IZ, Labat C, Lacolley P, Jaisser F. Smooth muscle cell mineralocorticoid receptors are mandatory for aldosterone-salt to induce vascular stiffness. Hypertension. 2014;63(3):520–6. doi: 10.1161/HYPERTENSIONAHA.113.01967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Osol G, Halpern W. Myogenic properties of cerebral blood vessels from normotensive and hypertensive rats. Am J Physiol. 1985;249(5 Pt 2):H914–21. doi: 10.1152/ajpheart.1985.249.5.H914. [DOI] [PubMed] [Google Scholar]
- 44.Ramos-Estebanez C, Moral-Arce I, Gonzalez-Mandly A, Dhagubatti V, Gonzalez-Macias J, Munoz R, Hernandez-Hernandez JL. Vascular cognitive impairment in small vessel disease: clinical and neuropsychological features of lacunar state and Binswanger's disease. Age Ageing. 2011;40(2):175–80. doi: 10.1093/ageing/afq169. [DOI] [PubMed] [Google Scholar]
- 45.Sakurai H, Hanyu H, Sato T, Kanetaka H, Shimizu S, Hirao K, Kikukawa M, Iwamoto T. Vascular risk factors and progression in Alzheimer's disease. Geriatr Gerontol Int. 2011;11(2):211–4. doi: 10.1111/j.1447-0594.2010.00669.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.