Abstract
It has been proposed that Complex Regional Pain Syndrome (CRPS) is a post-traumatic autoimmune disease and we previously observed that B cells are required for the full expression of CRPS-like changes a mouse tibia fracture CRPS model. The current study used the mouse model to evaluate the progression of post-fracture CRPS-like changes in wildtype (WT) and muMT fracture mice lacking B cells and antibodies. The pronociceptive effects of injecting WT fracture mouse serum antibodies into muMT fracture mice were also evaluated. Post-fracture pain behaviors transitioned from being initially dependent on both innate and autoimmune inflammatory mechanisms at 3 weeks post-fracture to being entirely mediated by antibody responses at 12 weeks post-fracture and spontaneously resolving by 21 weeks post-fracture. Furthermore, serum IgM antibodies from WT fracture mice had pronociceptive effects in the fracture limb when injected into muMT fracture mice. IgM antibody levels gradually increased in the fracture limb hindpaw skin, sciatic nerve, and corresponding lumbar cord, peaking at 12–18 weeks post-fracture and then declining. Immunohistochemistry localized post-fracture IgM antibody binding to antigens in the fracture limb hindpaw dermal cell nuclei. We postulate that fracture induces expression of neoantigens in fracture limb skin, sciatic nerve, and cord, which trigger B cells to secret IgM antibodies that bind those antigens and initiate a pronociceptive antibody response. Autoimmunity plays a key role in the progression of nociceptive and vascular changes in the mouse fracture model and potentially contributors to the CRPS disease process.
Keywords: antigen, autoimmunity, pain, fracture, immunoglobulin, complex regional pain syndrome
1. Introduction
Complex regional pain syndrome (CRPS) usually develops after a regional injury and presents with distal limb nociceptive, vascular, and bone changes that exceed the expected clinical course of the inciting injury in both magnitude and duration, frequently resulting in significant motor impairment and disability [46]. Improving our understanding of the mechanisms supporting CRPS may help us to understand the temporal progression of the condition’s manifestations and suggest new avenues to treatment. The study of both the innate and adaptive systems of immunity in patients and in animal models may move us closer to those goals.
Population based studies indicate that distal limb fracture is the most common cause of CRPS [9,40] and we have developed a tibia fracture rodent model closely resembling CRPS. Distal tibia fractured rats and mice casted for 3–4 weeks develop hindpaw allodynia, unweighting, warmth, edema, increased spontaneous protein extravasation, and regional periarticular bone loss [17]. The fracture CRPS model has been used successfully to study the effects of fracture on neuropeptide signaling [17,19,51,53], the sympathetic nervous system [28], mast cell infiltration [33], keratinocyte [44,51,54] and microglia [34,43] inflammatory mediator (IL-1, IL-6, TNF, CCL2, NGF) production [14,30,32,38,39,52], and other CRPS-related phenomena [18].
We recently used the fracture model to investigate the effects of B cell depletion using anti-CD20 antibodies or B cell deficient muMT mice [29]. We observed that; 1) wildtype (WT) mice treated with intravenous anti-CD20 antibody had virtually no mature B cells and exhibited attenuated hindpaw allodynia, unweighting, warmth, and edema, 2) muMT mice lacking B cells and IgM had attenuated nociceptive and inflammatory changes at 3 weeks post-fracture, 3) intravenous anti-CD20 treatment did not prevent the up-regulation of cutaneous inflammatory mediators at 3 weeks post-fracture in WT mice, and 4) IgM-containing immune complexes were deposited in skin and sciatic nerve at 3 weeks after fracture in WT mice but not in muMT fracture mice, and 5) complement system membrane attack complex deposition in skin and sciatic nerve after fracture was partially reversed by anti-CD20 treatment. Collectively these results suggest that autoimmunity partially contributes to development of nociceptive changes and is crucial for the development of vascular changes in the mouse fracture CRPS model.
Based on these experiments we postulated that fracture can induce the regional expression of novel antigens in the fracture limb hindpaw skin and sciatic nerve, subsequently triggering B cells to secret autoantibodies capable of binding to those antigens and initiating a regionally restricted antibody-antigen-complement response resulting in complement sensitization of nociceptive neurons [23]. The current study tested the hypothesis that serum antibodies from fracture mice can induce regionally restricted pain behaviors in B cell deficient fracture mice and proceeded to identify the immunoglobulin isotype responsible for these pronociceptive effects. Additional experiments tested the hypothesis that in the first 12 weeks after fracture the hindpaw pain behaviors are dependent on both innate and autoimmune inflammatory mechanisms, but after 3 months the hindpaw keratinocyte, mast cell, and microglia mediated innate inflammation resolves and the residual chronic pain behavior is primarily mediated by autoantibody responses.
2. Materials and methods
2.1 Animals
These experiments were approved by the Veterans Affairs Palo Alto Health Care System Institutional Animal Care and Use Committee (Palo Alto, CA, USA) and followed the animal subjects guidelines of the International Association for the Study of Pain. Three-month-old male C57BL/6J mice (#000664, Jackson Laboratory, Bar Harbor, ME) were designated the wildtype (WT) mice and muMT mice lacking mature B cells and immunoglobulin, on a C57BL/6J congenic background (#002288, Jackson Laboratory, Bar Harbor, ME) were used in these experiments. The animals were housed 4 per group under pathogen-free conditions with soft bedding and were given food and water ad libitum, with a 12:12 light:dark cycle. During the experimental period the animals were fed Teklad lab rodent diet 2018 (Harlan Laboratories, Indianapolis, IN), which contains 1.0% calcium, 0.7% phosphorus, and 1.5 IU/g vitamin D3, and were kept under standard conditions with a 12-h light-dark cycle. Data collection was conducted blind to group assignment.
2.2. Surgery
The fracture model was performed in 3 month-old male mice as previously described [19]. Under isoflurane anesthesia a hemostat was used to make a closed fracture of the right tibia just distal to the middle of the tibia. The hindlimb was then wrapped in casting tape (Delta-Lite, BSN Medical, Hamburg, Germany) so the hip, knee and ankle were all fixed. After fracture and casting, the mice were given subcutaneously 2 days of buprenorphine (0.05 mg/kg) and enrofloxacin (5 mg/kg) as well as 1.0 ml of normal saline. At 3 weeks after surgery the mice were anesthetized with isoflurane and the cast removed. All mice had union at the fracture site by manual inspection.
2.3. Hindpaw nociceptive testing
Mechanical allodynia was assayed using nylon von Frey filaments according to the “up-down” algorithm as previously described [7]. The mice were placed on wire mesh platforms in clear cylindrical plastic enclosures 10 cm in diameter and 40 cm in height, and after 15 minutes of acclimation von Frey fibers of sequentially increasing stiffness were applied against the hindpaw plantar skin at approximately midsole, taking care to avoid the tori pads, and pressed upward to cause a slight bend in the fiber and left in place for 5 sec. Withdrawal of or licking the hindpaw after fiber application was scored as a response. When no response was obtained the next stiffest fiber in the series was applied to the same paw; if a response was obtained a less stiff fiber was applied. Testing proceeded in this manner until 4 fibers had been applied after “negative+positive or positive+negative” response. Hindpaw testing was performed bilaterally. Estimation of the mechanical withdrawal threshold by data fitting algorithm permitted the use of parametric statistics for analysis [37]. These data were analyzed as the difference between the fracture side and the contralateral untreated side, thus a negative value represents a reduction in the fracture hindpaw withdrawal threshold.
An incapacitance device (IITC Life Science, Woodland Hills, CA) was used to measure bilateral hindpaw weightbearing. The mice were manually held in a vertical position over the apparatus with the hindpaws resting on separate metal scale plates and the entire weight of the mouse was supported on the hindpaws. The duration of each measurement was 6 s and 6 consecutive measurements were taken at 10 s intervals. All 6 readings were averaged to calculate the bilateral hindpaw weight-bearing values. Right hindpaw (fracture side) weight-bearing data were analyzed as a ratio between the right hindpaw weight and the average of right and left hindpaw values ((2R/(R + L)) × 100%), thus a value less than 100% represents a decrease in weight bearing in the fracture limb.
2.4. Hindpaw temperature testing
The temperature of the bilateral hindpaws was measured using a fine wire thermocouple (Omega Engineering, Norwalk, CT) applied to the paw skin, as previously described previously [30]. The investigator held the wire using an insulating Styrofoam block. Three sites were tested over the dorsum of the hindpaw: the space between the first and second metatarsals (medial), the second and third metatarsals (central), and the fourth and fifth metatarsals (lateral). After a site was tested in one hindpaw the same site was immediately tested in the contralateral hindpaw. The testing protocol was medial dorsum right then left, central dorsum right then left, lateral dorsum right then left, medial dorsum left then right, central dorsum left then right, and lateral dorsum left then right. The six measurements for each hindpaw were averaged for the mean temperature. These data were analyzed as the difference between the fracture side and the contralateral untreated side, thus a positive value represents increased temperature in the fracture paw.
2.5. Hind paw thickness testing
A laser sensor technique was used to determine the dorsal-ventral thickness of the bilateral hind paws, as we have previously described [30]. For laser measurements each rat was briefly anesthetized with isoflurane and then held vertically so the hindpaw rested on a table top below the laser. The paw was gently held flat on the table with a small metal rod applied to the top of the ankle joint. Using optical triangulation, a laser with a distance-measuring sensor was used to determine the distance to the table top and to the top of the hindpaw at the midpoint of the third metatarsal and the difference was used to calculate the dorsal-ventral paw thickness. The measurement sensor device used in these experiments (Limab, Goteborg, Sweden) has a measurement range of 200 mm with a 0.01 mm resolution. These data were analyzed as the difference between the fracture side and the contralateral untreated side, thus a positive value represents increased paw thickness in the fracture paw.
2.6. B cell depletion/deficiency experiments in the fracture mouse CRPS model
This experiment examined the time course of post-fracture changes in hindpaw nociception and inflammation in wildtype (WT) mice and in transgenic muMT mice lacking mature B cells and immunoglobulin (Fig. 1). WT (n = 12) and muMT mice (n = 11) underwent baseline behavioral testing (measuring hindpaw von Frey allodynia, unweighting, warmth, and edema) and then, under isoflurane anesthesia, the distal tibia was fractured and casted. The cast was removed after 3 weeks of hindlimb immobilization and behavioral testing was repeated the next day. Additional behavioral testing was performed at 7, 10, 12, 15, 18, and 21 weeks after fracture. Another cohort of WT fracture mice were injected at 12 weeks post-fracture with anti-mouse CD20 antibody (200 ug/100ul, i.v., Genentech, South San Francisco, CA, n = 10) or vehicle (n = 10). Previously we demonstrated that CD20 antibody treatment completely eliminated CD220+ B cells in the blood and spleen for 2 weeks post-injection and that fracture muMT mice lacking B cells had no IgM deposition in the fracture hindpaw skin or sciatic nerve [29]. Hindpaw nociceptive testing (von Frey allodynia and unweighting) was performed prior to injection and at 1, 7, 14, 21, and 28 days after injection.
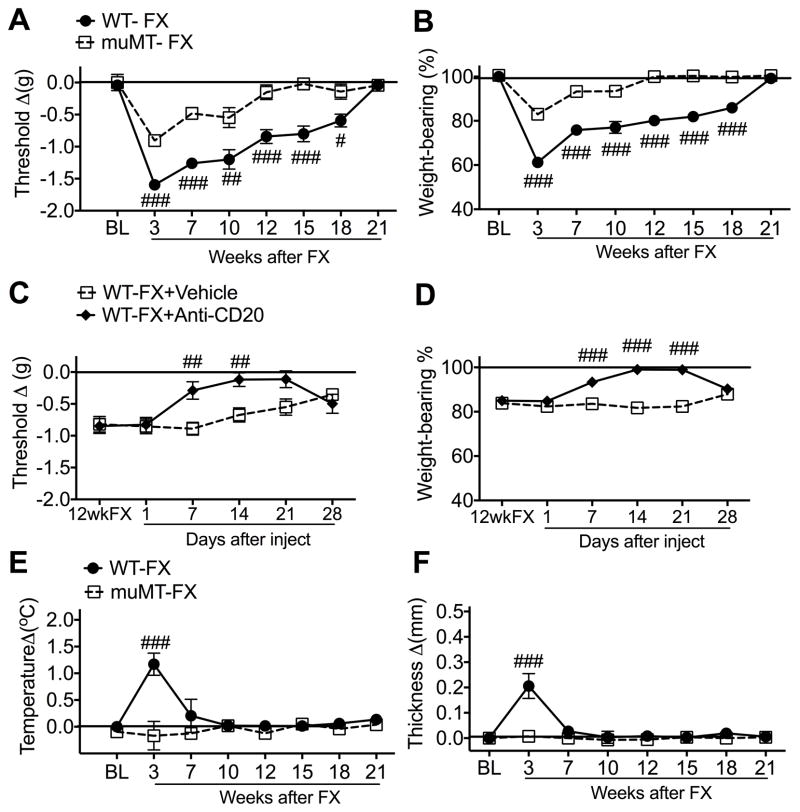
Fig. 1. Post-fracture (FX) nociceptive and vascular changes in wildtype (WT) and B cell deficient (muMT) mice.
WT and muMT mice underwent distal tibia FX with hind limb casting for 3 weeks, then the cast was removed and behavioral testing performed prior to FX (baseline, BL) and at 3, 7, 10, 12, 15, 18, and 21 weeks after FX. At 3 weeks post-FX the WT mice had developed hindpaw von Frey allodynia (A), unweighting (B), warmth (E), and edema (F), but muMT FX mice exhibited attenuated allodynia (A) and unweighting (B), and no warmth (E) or edema (D) after fracture. Allodynia and unweighting resolved in the muMT FX mice by 10 weeks post-fracture, but pain behaviors in the WT FX did not resolve until 21 weeks post-fracture. Another cohort of WT FX mice were injected with anti-mouse CD20 antibody (Anti-CD20, 200ug/100ul i.v.) or vehicle at 12 weeks post-FX (12wkFX). Previously we demonstrated that this treatment eliminates B cells and immunoglobulin in mice. Intravenous injection of anti-CD20 completely reversed post-FX hindpaw von-Frey mechanical allodynia (C) and unweighting (D) at 7–21 days post-injection. Measurements for (A), (C), (E), and (F) represent the difference between the FX side and contralateral paws, thus, a positive value represents an increase in temperature or thickness on the FX side; a negative value represents a decrease in mechanical nociceptive thresholds on the affected side. Measurements for (B) and (D) represent weight-bearing on the FX hind limb as a ratio to 50% of bilateral hind limb loading, thus, a percentage lower than 100% represents hind paw unweighting. A 2-way repeated measures analysis of variance was used to test the effects of FX and B-cell-deficient transgenic mice on the dependent variables, with Holm-Sidak test for post hoc contrasts. Data are expressed as mean values ± SEM. #P < 0.05, ## P < 0.01, and ### P < 0.001 for muMT-FX (n = 9–12) vs WT-FX (n = 9–11) and for WT-FX+AntiCD20 (n =10) vs WT-FX+Vehicle (n =10). WT: wildtype mice, MuMT: mice lacking B cells, FX: fracture, BL: baseline, 12wkFX: 12 weeks after fracture
2.7. Serum and immunoglobulin injection experiments in the fracture mouse CRPS model
This experiment examined the pronociceptive (hindpaw mechanical allodynia and unweighting) and inflammatory (hindpaw warmth and edema) effects of 3 week post-fracture wildtype (WT) mouse serum when injected into 3 week post-fracture WT and muMT mice (Fig. 2). Blood was collected by transcardial puncture in isoflurane anesthetized 3 week post-fracture wildtype (WT) mice. The blood was left undisturbed at room temperature for 60 min to allow clotting, then the blood samples were centrifuged at 1,500g for 15 min at 4°C and the serum supernatants were aliquoted and frozen at − 80°C. WT (n = 7) and muMT mice (n = 8) underwent baseline behavioral testing (measuring hindpaw von Frey allodynia, unweighting, warmth, and edema) and then, under isoflurane anesthesia, the distal tibia was fractured and casted. At 3 weeks post-fracture the casts were removed and behavioral testing was repeated the next day and then the mice were injected with the 3 week post-fracture WT mouse serum (500 ul, i.p.). Further behavioral testing was performed at 1, 7, 14, and 21 days after injection. Additional muMT mice (n = 10) underwent tibia fracture and casting and behavioral testing was performed at baseline and 3 weeks post-fracture, then the mice were injected with serum (500 ul, i.p.) from nonfractured WT control mice and behavioral testing was repeated at 1, 7, 14, and 21 days after serum injection.
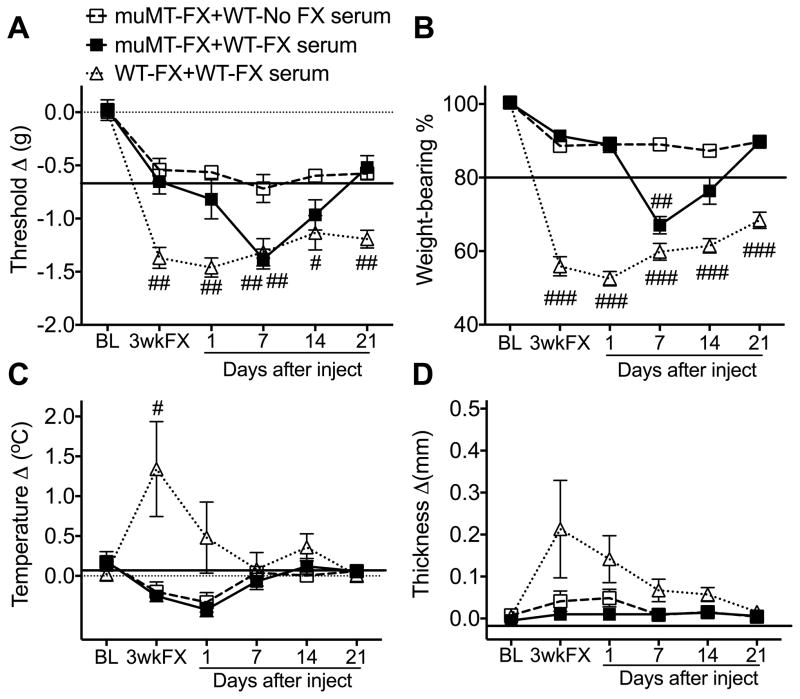
Fig. 2. Serum from wildtype (WT) fracture (FX) mice had pronociceptive effects in B cell deficient (muMT) FX mice.
When serum from 3 week post-FX WT mice was injected (500 ul, i.p.) into 3 week post-FX muMT mice, the mice gradually developed increased hindpaw von Frey allodynia (A) and unweighting (B) over the ensuing week, and consistent with the half-life of immunoglobulin, these pronociceptive effects resolved by 2 weeks post-injection. Injecting WT FX serum did not alter hindpaw temperature (C) or thickness (D) in the muMT FX mice. The pronociceptive effects of the FX serum were restricted to the FX limb and not observed in nonfractured mice (data not shown). At 3 weeks post-FX WT mice exhibited more robust hindpaw allodynia (A), unweighting (B), warmth (C), and edema (D) than muMT FX mice and when serum from 3 week post-FX WT mice was injected into the WT FX mice it had no pronociceptive or inflammatory effects (A–D). A 2-way repeated measures analysis of variance was performed followed by a Holm-Sidak test for post hoc contrasts. Data are expressed as mean values ± SEM. #P<.05, ##P < 0.01, ###P < 0.001 for μMT-FX+WT-FX serum (n = 8) or WT-FX+WT-FX serum (n = 7) vs μMT-FX+WT- No FX serum (n = 9–10). muMT: mice lacking B cells, FX: fracture, No FX serum: serum from WT mice that did not undergo FX, FX serum: serum from 3 week post-FX WT mice, BL: baseline, 3 wkFX: mice that are 3 weeks post-FX
In another experiment serum was collected from 3, 18, and 21 week post-fracture WT mice and from nonfractured control WT mice and the serum was injected into 3 week post-fracture muMT mice (Fig. 3). The muMT mice underwent baseline nociceptive behavioral testing (measuring hindpaw von Frey allodynia and unweighting), and then, under isoflurane anesthesia, the distal tibia was fractured and casted. At 3 weeks post-fracture the casts were removed and the next day nociceptive behavioral testing was repeated and the mice were injected with either 3 (n = 10), 18 (n = 10), 21 (n = 6) week post-fracture WT mouse serum (500 ul, i.p.) or serum from nonfracture control WT mice (n = 8). Further nociceptive behavioral testing was performed at 1, 7, 14, and 21 days after injection.
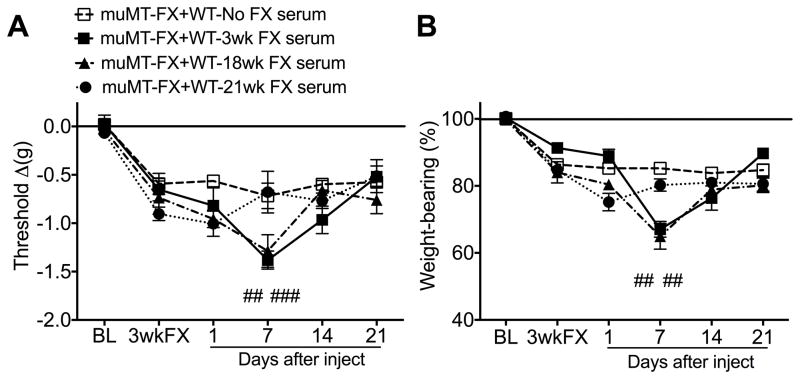
Fig. 3. The pronociceptive effects of wildtype (WT) mouse fracture (FX) serum resolved by 21 weeks post-FX.
WT mice exhibited unilateral hindpaw von Frey allodynia and unweighting at 3 weeks post-FX and these pain behaviors gradually resolved over the ensuing 21 weeks (Fig. 1). To test the hypothesis that pronociceptive autoantibodies persist in the serum of WT FX mice up to18, but not 21 weeks, sera from 3, 18, and 21 week post-FX WT mice were injected into 3 week post-FX muMT mice lacking B cells. Both 3 and 18 week post-FX WT mouse sera (500 ul i.p.) caused increased hindpaw allodynia (A) and unweighting (B) in 3 week post-FX muMT mice, but 21 week post-FX WT serum had no effect on allodynia or unweighting in 3 week post-FX muMT mice. A 2-way repeated measures analysis of variance was performed followed by a Holm-Sidak test for post hoc contrasts. Data are expressed as mean values ± SEM. ##P<0.01, and ###P<0.001 for muMT-FX+WT-3wk FX serum (n=7–10/cohort) and muMT-FX+WT-18wk FX serum (n=7–10/cohort) vs muMT-FX+WT-No FX serum (n=8/cohort) or muMT-FX+WT-21wk FX serum (n=6/cohort). muMT: mice lacking B cells, FX: fracture, No FX serum: serum from WT mice that did not undergo FX, FX serum: serum from 3 week post-FX WT mice, BL: baseline, 3 wkFX: mice that are 3 weeks post-FX
An additional experiment tested the pronociceptive effects of 3 week post-fracture WT mouse serum in 21 week and 29 week post-fracture WT mice with resolved allodynia and unweighting (Fig. 4). The WT fracture mice underwent baseline nociceptive behavioral testing (measuring hindpaw von Frey allodynia and unweighting) at 21 (n = 8/group) or 29 (n = 8/group) weeks post-fracture and then mice were injected with either 3 week post-fracture WT mouse serum (500 ul, i.p.) or serum from nonfracture control WT mice. Further nociceptive behavioral testing was performed at 1, 5, 7, 9, 14, and 21 days after injection.
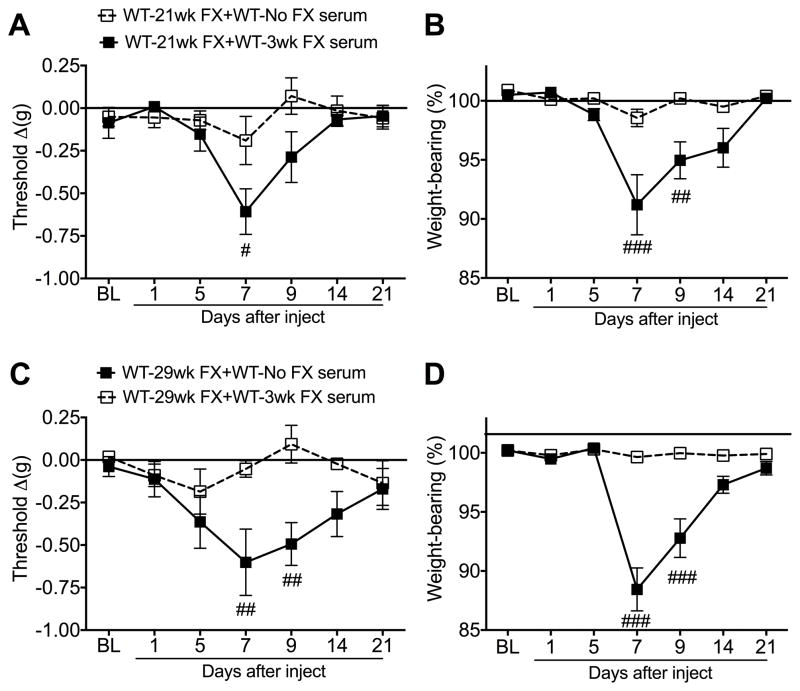
Fig. 4. Serum from 3 week post-fracture (FX) wildtype (WT) mice was pronociceptive in 21 and 29 week post-FX WT mice that had resolved allodynia and unweighting.
WT mice exhibited unilateral hindpaw von Frey allodynia and unweighting at 3 weeks post-FX and these pain behaviors gradually resolved over the ensuing 21 weeks (Fig. 1). Serum from 3 week post-FX WT mice was injected into 21 and 29 week post-FX WT mice to test the hypothesis that pronociceptive autoantigens persist in the FX hindlimb and corresponding lumbar cord after the resolution of post-FX pain behaviors. Injection of 3 week post-FX WT serum (500 ul, i.p.) caused a reoccurrence of the fracture limb hindpaw allodynia and unweighting in both the 21 (A,B) and 29 (C,D) week post-FX WT mice but had no effect in the contralateral limb or in control nonfractured mice (data not shown). A 2-way repeated measures analysis of variance was performed followed by a Holm-Sidak test for post hoc contrasts. Data are expressed as mean values ± SEM. #P<0.05, ##P<0.01, and ###P<0.001 for WT-21wk FX+WT-3wk FX serum (n=8/cohort) vs WT-21wk FX+WT-No FX serum (n=8/cohort) and WT-29wk FX+WT-3wk FX serum (n=8/cohort) vs WT-29wk FX+WT-No FX serum (n=8/cohort). WT: wildtype mice, FX: fracture, No FX serum: serum from WT mice that did not undergo FX, 3wk FX serum: serum from 3 week post-FX WT mice, BL: baseline
Another experiment was designed to identify the immunoglobulin isotype responsible for the pronociceptive effects of 3 week post-fracture WT serum in 3 week post-fracture muMT mice (Fig. 5). Under isoflurane anesthesia, transcardial puncture was performed in 3 week post-fracture WT mice and the serum was collected. The IgM from serum was extracted with a polypropylene column (BioRad, Hercules, CA) which was pre-packed with CaptureSelect™ IgM Affinity Matrix (Life Technologies, Carlsbad, CA), and a Slide-A-Lyzer Dialysis Cassettes (10K MWCO, Life Technologies, Carlsbad, CA). The bound IgM was eluted using 100mM glycine pH 3, the pH was adjusted to 7.4 using 1M Tris pH 8.0, and a Slide-A-Lyzer Dialysis Cassettes (10K MWCO, Life Technologies, Carlsbad, CA) was used to remove glycine from protein, and then quantified using a NanoDrop ND-1000 UV-Vis spectrophotometer (NanoDrop Technologies, Wilmington, DE). The recovery rate was 228 ug IgM/ml serum, which is virtually identical to the 220 ug IgM/ml serum concentration previously reported for C57BL/6 mice [25]. The dose of IgM used in the current study (500 ug, i.p.) was 4.4 fold greater than the amount of IgM that would be predicted in 500 ul of mouse serum (the volume of serum used in the injection studies described in Figs 2–4). The IgG from serum was extracted with CaptureSelect™ IgG Affinity Matrix (Life Technologies, Carlsbad, CA), the pH was adjusted to 7.4 using 1M Tris pH 8.0, and a Slide-A-Lyzer Dialysis Cassettes (10K MWCO, Life Technologies, Carlsbad, CA) was used to remove glycine from protein, and quantified using a NanoDrop ND-1000 UV-Vis spectrophotometer (NanoDrop Technologies, Wilmington, DE). The recovery rate was 1.2 mg IgG/ml serum, which is approximately half the 2.4 mg IgG/ml serum concentration reported for C57BL/6 mice [25]. The dose of IgG used in the current study (3.6 mg, i.p.) was 3 fold greater than the amount of IgG that would be predicted in 500 ul of WT fracture mouse serum. Prior investigators have used much lower doses of IgG (120ug/mouse) antibody to evoke autoimmune responses in mice [49,56], thus we were confident that a negative response to 3.6 mg of fracture mouse IgG would be physiologic and not attributable to insufficient dosage.
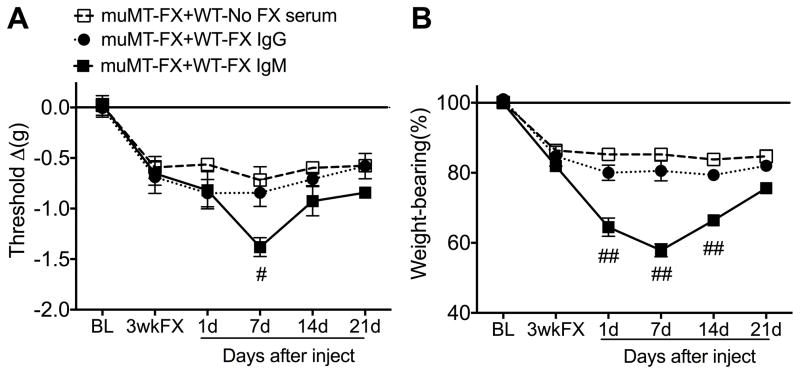
Fig. 5. Immunoglobulin M(IgM) pronociceptive effects in post-fracture (FX) muMT mice lacking B cells.
Injection of purified IgM (0.5 mg, i.p.) from wildtype (WT) 3 week post-FX mice gradually increased fracture limb hindpaw von Frey allodynia (A) and unweighting (B) in 3 week post-FX muMT mice, with significant effects at 7 days post-injection. IgM injection had no effect on nociceptive testing in the contralateral hindpaw (data not shown). IgG (5.0 mg, i.p.) injection had no effect. A 2-way repeated measures analysis of variance was performed followed by a Holm-Sidak test for post hoc contrasts. Data are expressed as mean values ± SEM. #P<0.05, and ##P<0.01 for muMT-FX+WT-FX IgM (n=9–11) vs muMT-FX+No FX-WT serum (n=9–11) or muMT-FX+WT-FX IgG (n=9–11). WT: wildtype mice, muMT: mice lacking B cells, FX: fracture, BL: baseline, 3wkFX: 3 week post-FX.
To test the pronociceptive effects of the immunoglobulin isotypes, muMT mice underwent baseline nociceptive behavioral testing (measuring hindpaw von Frey allodynia and unweighting), and then, under isoflurane anesthesia, the distal tibia was fractured and casted. At 3 weeks post-fracture the casts were removed and the next day nociceptive behavioral testing was repeated and the mice were injected with either IgM (500 ug, i.p., n = 11) or IgG (3.6 mg, i.p., n = 11) from 3 week post-fracture WT mice, or serum (500 ul, i.p.) from nonfracture control WT mice (n = 11). Further nociceptive behavioral testing was performed at 1, 7, 14, and 21 days after injection.
2.8. Enzyme immunoassay for albumin, IgM, and IgG
At 3 weeks post-fracture wildtype mice were euthanized and the fracture limb hindpaw skin, sciatic nerve, and corresponding L4,5 lumbar spinal cord tissues were collected and frozen immediately on dry ice (n = 9). Control tissues were collected from nonfractured wildtype mice (n = 11). Samples were cut into fine pieces in ice-cold phosphate buffered saline, pH 7.4, containing a cocktail of protease inhibitors (Roche Applied Science, Indianapolis, IN) and followed by homogenization using a Polytron device (Brinkmann Instruments, Westbury, NY). Homogenates were centrifuged at 12,000g for 15 min at 4 °C and supernatant fractions were frozen at −80°C until required for ELISA performance. An aliquot was subjected to protein assay (Bio-Rad, Hercules, CA) to normalize mediator levels. Albumin, IgM, and IgG protein levels were determined by using ELISA kits (GenWay Biotech, San Diego, CA) according to the manufacturer’s instructions. The results of these assays were confirmed by repeating each experiment twice (Fig. 6). Another ELISA study examined IgM protein levels in fracture limb hindpaw skin and sciatic nerve collected from 3, 7, 12, 18, 21, 23 week post-fracture wildtype mice and nonfracture wildtype controls (Fig. 7A,B, n = 8/group).
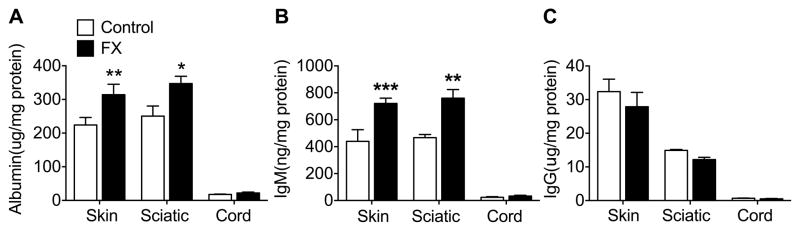
Fig. 6. IgM protein levels were elevated in hindpaw skin and sciatic nerve at 3 weeks post-fracture (FX) in the wildtype (WT) mice.
Enzyme immunoassays were used to measure albumin, IgM, and IgG protein levels in the FX limb hindpaw skin, sciatic nerve, and corresponding lumbar spinal cord. Compared with tissues collected from control nonfractured WT mice, both albumin (A) and IgM (B) protein levels were increased in the hindpaw skin and sciatic nerve after FX, but not in the spinal cord. Control mouse IgG protein levels were almost 80 and 40 fold greater in skin and sciatic nerve than IgM levels, but were unchanged after FX. Spinal cord levels of albumin, IgM and IgG were extremely low. A one-way analysis of variance was performed followed by a Holm-Sidak test for post hoc contrasts. Data are expressed as mean values ± SEM. *P<0.05, **P<0.01, ***P<0.001 FX (n = 7–8) vs Control (n = 7–8). Control: nonfractured wildtype mice, FX: 3 week post-fracture wildtype mice, Skin: hindpaw skin in FX limb, Sciatic: sciatic nerve in FX limb, Cord: lumbar spinal cord corresponding to FX limb
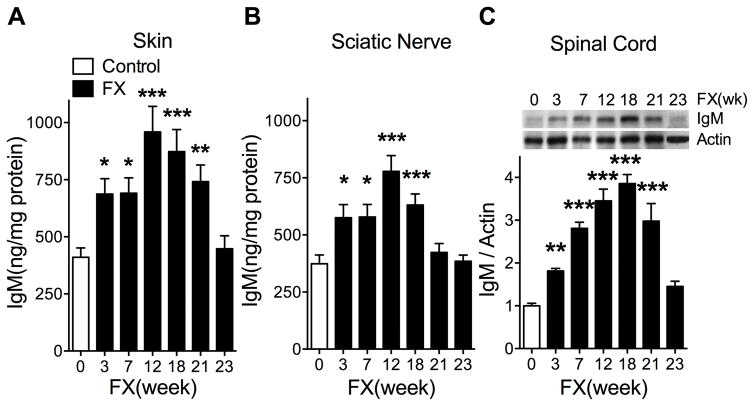
Fig. 7. Changes in IgM levels in the ipsilateral hindpaw skin, sciatic nerve, and lumbar spinal cord at 3, 7, 12, 18, 21, and 23 weeks after fracture (FX) in wildtype mice.
IgM levels were determined by enzyme immunoassay in skin (A) and sciatic nerve (B) and by western blot analysis in spinal cord (C), due to the low IgM levels observed in the spinal cord. After fracture there was a gradual increase in IgM levels in the hindpaw skin (A), sciatic nerve (B), and spinal cord (C), peaking at between 12 and 18 weeks post-fracture and then declining to control levels by 23 weeks post-fracture. Data were analyzed using a one-way analysis of variance followed by a Holm-Sidak test for post hoc contrasts, error bars indicate SEM. *P< 0.05, **P< 0.01, and ***P< 0.001 for fracture (FX, n=4–8) vs control (n=4–8). Control: nonfracture wildtype mice, FX: fracture wildtype mice, FX(week): weeks after fracture.
2.9. Western blot analysis for IgM
The L4–5 lumbar spinal cords ipsilateral to the fracture limb were harvested from 3, 7, 12, 18, and 21 weeks post-fracture wildtype mice and nonfracture wildtype controls (n = 4/group) and processed for IgM and β-actin western blot analyses as we have previously described [29]. IgM/Actin band intensity ratio was calculated to demonstrate the changes in spinal IgM levels at various time points after fracture (Fig. 7C).
2.10. Tissue processing and immunofluorescence confocal microscopy
This experiment was performed to identify the cellular localization of IgM autoantibody immunostaining in fracture limb hindpaw skin (Fig. 8). Three week post-fracture wildtype mice were euthanized and immediately perfused with 4% paraformaldehyde (PFA) in phosphate buffered saline (PBS), pH 7.4, via the ascending aorta and the hindpaw skin including sub-dermal layers was removed and post-fixed in 4% PFA for 2 h, and then the tissues were treated with 30% sucrose in PBS at 4°C before embedding in OCT (Sakura Finetek, Torrance, CA). Following embedding, 10-μm thick slices were made using a cryostat, mounted onto Superfrost microscope slides (Fisher Scientific, Pittsburg, PA), and stored at −80°C.
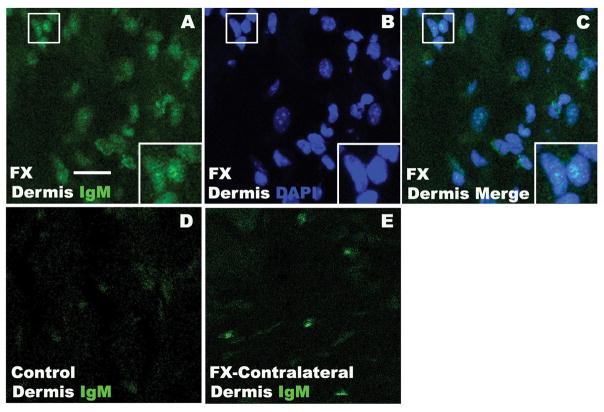
Fig. 8. Representative photomicrographs of IgM-antigen binding in 3 week post-fracture (FX) wildtype mouse hindpaw skin, using fluorescence labeled IgM purified from 3 week FX wildtype mouse serum.
IgM (green) immunostaining was observed in the dermis of FX mice (A). DAPI (blue) counterstain (B) showed that IgM labeling was diffusely distributed throughout the dermal cell nuclei (C). Small boxed regions in panels (A), (B), and (C) are shown enlarged in the lower right corner of the panels, respectively. Minimal IgM immunostaining was observed in control nonfracture hindpaw dermis (D) and in the contralateral hindpaw dermis after FX (E). These results support the hypothesis that fracture can induce the regional expression of dermal nuclear antigens in the fracture limb hindpaw skin, subsequently triggering B cells to secret IgM autoantibodies capable of binding to those nuclear antigens and initiating an antibody response. Scale bar = 20 um. FX: fracture wildtype mice, Control: nonfracture wildtype mice, FX-Contralateral: contralateral intact hindlimb of FX wildtype mice.
Under isoflurane anesthesia, transcardial puncture was performed in 3 week post-fracture wildtype mice and the serum was collected. The IgM from the serum was extracted with polypropylene column (BioRad, Hercules, CA) which was pre-packed with CaptureSelect™ IgM Affinity Matrix (Life Technologies, Carlsbad, CA), and a Slide-A-Lyzer Dialysis Cassettes (10K MWCO, Life Technologies, Carlsbad, CA) was used to remove glycine from protein, and quantified using a NanoDrop ND-1000 UV-Vis spectrophotometer (NanoDrop Technologies, Wilmington, DE). IgM labeling was performed using Alexa Fluor 488 NHS Ester (Life Technologies) with the dye-IgM molar ratio at 50:1 under around pH 7. The free dye was removed with a Slide-A-Lyzer Dialysis Cassettes (10K MWCO, Life Technologies, Carlsbad, CA). Hindpaw skin sections from control and 3 week post-fracture mice were incubated with IgM-488 at 1:500 dilution overnight at 4°C in PBS pH 7. After three washes, the sections were mounted with anti-fade mounting medium (Invitrogen). Images were visualized and captured using a confocal microscope (Zeiss LSM710 Upright multiple photon; Carl Zeiss, Jena, Germany). Control experiments included incubation of slices in primary and secondary antibody-free solutions, both of which led to low-intensity nonspecific staining patterns in preliminary experiments (data not shown).
2.11. Statistical analysis
Statistical analysis was performed using a two-way repeated measures ANOVA (Figs. 1–5) or a one-way ANOVA (Figs. 6 and 7) with Holm-Sidak multiple comparisons test for post-hoc contrasts. All data are presented as the mean ± standard error of the mean (SEM), and differences are considered significant at a P value less than 0.05 (Prism 5, GraphPad Software, San Diego, CA).
3. Results
3.1 Post-fracture nociceptive and vascular changes in wildtype (WT) and B cell deficient muMT mice
Figure 1 illustrates that at 3 weeks post-fracture WT mice exhibited unilateral hindpaw von Frey allodynia (A), unweighting (B), warmth (E), and edema (F). The muMT fracture mice lacking mature B cells and immunoglobulin also exhibited 3 weeks post-fracture hindpaw allodynia and unweighting, but to a lesser extent than the WT fracture mice, and failed to develop hindpaw warmth or edema. Hindpaw allodynia and unweighting pain behaviors resolved slowly over time, with muMT fracture mice resolving by 12 weeks post-fracture, and WT fracture mice resolving by 21 weeks post-fracture. Hindpaw warmth and edema resolve by 7 weeks post-fracture in the WT fracture mice. When anti-CD20 antibody (200ug/100ul, i.v.) was injected into 12 week post-fracture WT mice it completely reversed allodynia and unweighting within 7 days and this effect persisted for 21 days post-injection. We had previously demonstrated that this dose of anti-CD antibody completely depleted mature B cells in WT mice and partially reversed allodynia and unweighting in 3 week post-fracture WT mice [29]. Collectively, these data suggest that at 3 weeks post-fracture mature B cells are partially responsible for the hindpaw allodynia and unweighting observed in the WT fracture mice, but by 12 weeks post-fracture a switch occurred in the nociceptive mechanisms contributing to hindpaw allodynia and unweighting, with B cells becoming entirely responsible for the hindpaw nociceptive behaviors.
3.2 Serum from wildtype (WT) fracture mice had pronociceptive effects in muMT mice
When serum from 3 week post-fracture WT mice was injected into 3 week post-fracture muMT mice, the mice gradually developed increased hindpaw von Frey allodynia and unweighting over the ensuing week and, consistent with the half-life of immunoglobulin, these pronociceptive effects resolved by 2 weeks post-injection (Fig. 2). The pronociceptive effects of the serum were restricted to the fracture limb and were not observed in nonfractured mice (data not shown). When 3 week post-fracture WT mouse serum was injected into 3 week post-fracture WT fracture mice there was no effect on hindpaw allodynia, unweighting, warmth or edema. These data suggest that fracture induced autoantibodies in the serum of WT fracture mice have pronociceptive effects that are regionally restricted to the fracture hindpaw and not observed in control nonfracture WT mice. When WT fracture serum is injected into muMT fracture mice lacking mature B cells and immunoglobulin, there is an additional sensitizing effect on hindpaw allodynia and unweighting, but when WT fracture serum is injected into WT fracture mice there is no further sensitizing effect on the hindpaw allodynia and unweighting because WT fracture mice already express these pronociceptive autoantibodies.
3.3. The pronociceptive effects of WT fracture mouse serum resolved by 21 weeks post-fracture
When 3 week post-fracture muMT mice were injected with serum from 3 and 18 week post-fracture WT mice it caused a gradual increase in hindpaw allodynia and unweighting, but 21 week post-fracture serum had no pronociceptive effects (Fig. 3). These results support the hypothesis that the resolution of hindpaw allodynia and unweighting at 21 weeks post-fracture in the WT mice (Fig. 1) is due to the loss of pronociceptive autoantibodies at 21 weeks after fracture.
3.4. Serum from 3 week post-fracture WT mice was pronociceptive when injected into 21 and 29 week post-fracture WT mice
Wildtype fracture mice had no hindpaw allodynia or unweighting at 21 and 29 weeks after fracture, but hindpaw allodynia and unweighting were rekindled when these mice were injected with serum from 3 week post-fracture WT mice (Fig. 4). Injection of 3 week post-fracture WT serum had no effect on nociceptive testing in the contralateral intact limb in the 21 and 29 week post-fracture mice. These results suggest that pronociceptive autoantibodies are no longer being expressed in the 21 and 29 week post-fracture WT mice, but that these mice do continue to express fracture hindlimb restricted unique antigens that the pronociceptive autoantibodies must bind with to activate antigen-autoantibody-complement complexes capable of inducing nociceptive sensitization [23].
3.5. Immunoglobulin M (IgM) had pronociceptive effects in the 3 week post-fracture muMT mice
Injecting purified IgM from 3 week post-fracture WT mice into 3 week post-fracture muMT mice had the same pronociceptive effects as serum injection (Fig. 5). IgM injection had no effect on nociceptive testing in the contralateral intact limb in the 3 week post-fracture muMT mice. Injection of purified IgG from 3 week post-fracture WT mice into 3 week post-fracture muMT mice had no nociceptive effects and serum from nonfractured WT mice had no effect in the muMT fracture mice. These results indicate that IgM is the immunoglobulin isotype responsible for post-fracture autoantibody mediated pronociceptive effects.
3.6. IgM protein levels were elevated in fracture limb hindpaw skin and sciatic nerve
To detect if the immunoglobulin isotypes indicative of autoimmunity could be found in the fracture limb hindpaw skin, sciatic nerve, and corresponding L4/5 lumbar spinal cord tissue, ELISA assays were utilized to analyze albumin, IgM and IgG protein levels in nonfracture controls and 3 week post-fracture WT mice. Compared with nonfracture control tissues, both albumin and IgM levels were increased in fracture limb hindpaw skin and sciatic nerve, but not in the corresponding lumbar spinal cord (Fig. 6). IgG levels were unchanged after fracture. Spinal cord levels of albumen, IgM, and IgG were extremely low. Collectively these results suggest that only IgM immune complexes are present in skin and sciatic nerve at 3 weeks post-fracture in WT mice.
3.7. Post-fracture temporal changes in IgM protein levels in hindpaw skin, sciatic nerve, and spinal cord
The pronociceptive effects of fracture WT mouse serum in post-fracture muMT mice were mediated by IgM antibodies (Fig. 5) and were present only in serum collected between 3 and 18 weeks after fracture (Fig. 3). These pronociceptive effects were not observed with serum collected from 21 week post-fracture WT mice (Fig. 3), suggesting that IgM autoantibodies continued to be generated for up to 18 weeks after fracture. To test this hypothesis, IgM levels were measured in fracture limb hindpaw skin and sciatic nerve by ELISA, and in corresponding L4/5 lumbar spinal cord by western blot assay, at 0, 3, 7, 12, 18, 21, and 23 weeks after fracture in WT mice. Western blot assay was used for spinal cord tissues because of the extremely low levels of IgM present in spinal cord tissue and the greater sensitivity of the western assay, relative to ELISA. IgM levels began increasing at 3 weeks, peaked between 12 and 18 weeks, and began declining after that, reaching control levels by 23 weeks post-fracture (Fig. 7). These results support the hypothesis that IgM autoantibodies are expressed for 18 weeks after fracture, consistent with the duration of post-fracture nociceptive sensitization.
3.8. IgM autoantibody-antigen binding in fracture hindpaw skin was localized to dermal nuclei
Immunohistochemistry was utilized to identify the cellular location of IgM autoantibody-antigen binding in the fracture hindpaw skin of WT mice. IgM antibody purified from 3 week fracture WT mice was labeled with a fluorescent marker and used to immunostain antigens in hindpaw skin sections from 3 week fracture WT mice. DAPI counter staining was used to identify cell nuclei. IgM immunostaining was diffusely distributed throughout the dermal cell nuclei in fracture limb hindpaw skin, but only minimal staining was observed in the contralateral hindpaw skin sections and in nonfracture control hindpaw skin sections (Fig. 8). These results support the hypothesis that fracture can induce the regional expression of dermal nuclear antigens in the fracture limb hindpaw skin, subsequently triggering B cells to secret IgM autoantibodies capable of binding to those nuclear antigens and initiating an antibody response.
4. Discussion
Evidence from patients and animal models suggest that both sensory c-fibers and sympathetic neurons function aberrantly in CRPS [5,35,53]. Additional studies measuring interleukin 6 (IL-6), tumor necrosis factor alpha (TNF), and other pro-nociceptive mediators associated with the innate system of immunity, have demonstrated that these mediators are elevated in the skin blister fluid of CRPS patients [16,20,36], as is tryptase, a marker for mast cells capable of releasing a host of nociceptive mediators [22]. Using the CRPS fracture model we have shown that facilitated release of neuropeptides such as substance P and calcitonin gene-related peptide from sensory c-fibers [53], as well as norepinephrine released from sympathetic nerve terminals, lead to inflammation and pain sensitization by the activation of neuropeptide and adrenergic receptors on the surface of keratinocytes, vascular endothelial cells, mast cells, and microglia, causing the local production of high levels of inflammatory mediators such as interleukin 1 (IL-1), IL-6, TNF, C-C motif chemokine ligand 2 (CCL2), and nerve growth factor (NGF) [19,28,30–34,38,39,43,44].
Experimental maneuvers blocking these innate immune responses only partially normalize the nociceptive and vascular changes in the CRPS fracture model [14,30,33,38,39,54]. Similarly, CRPS clinical studies observe only partial therapeutic responses with treatments that inhibit innate immune mechanisms [6,21,24,48]. Recently we examined bilateral skin punch biopsies from 55 CRPS patients and demonstrated unilateral keratinocyte and mast cell proliferation, epidermal thickening, and keratinocyte inflammatory cytokine expression (IL-6, TNF) in early (< 3 month duration) CRPS affected skin [4]. This study also found that increased keratinocyte and mast cell proliferation gradually resolved over time, despite persistent pain symptoms. These results are consistent with skin blister fluid studies indicating a gradual resolution of cutaneous inflammatory mediator (IL-6, TNF) expression over time in CRPS affected skin [55]. These data support the hypothesis that post-traumatic neuropeptide signaling actives keratinocyte and mast cell mediated innate immune responses contributing to the development, but not the maintenance of CRPS, and we postulate that adaptive immunity plays a critical role in perpetuating CRPS.
At 3 weeks after fracture the muMT fracture mice exhibited a partial reduction in allodynia and unweighting, compared to WT fracture mice, suggesting that autoantibodies partially contribute to the nociceptive sensitization observed at the 3 week time point (Fig. 1). By week 12 nociceptive sensitization had resolved in the muMT fracture mice, but the WT fracture mice had residual, albeit resolving, allodynia and unweighting that was completely reversed after an anti-CD20 injection that blocked antibody production. The differing time courses for the resolution of pain behaviors in the WT and muMT fracture mice support the hypothesis that in the first 3 months after fracture nociceptive sensitization is mediated by both innate and adaptive immune mechanisms, but that more chronic sensitization is entirely dependent on B cell secreted IgM autoantibodies. Pain behaviors in the WT mice resolved by 21 weeks post-fracture, similar to the CRPS disease process, with the majority of CRPS patients undergoing spontaneous symptom resolution within a year of onset [3,40,41,57].
Direct support for the hypothesis that autoantibodies can trigger nociceptive sensitization was obtained by demonstrating that injection of 3 week WT fracture serum caused increased hindpaw allodynia and unweighting in 3 week muMT fracture mice (Fig. 2). Injecting fracture WT mouse serum had no effect on nociceptive thresholds in the contralateral intact hindlimb of the muMT fracture mice, suggesting that circulating autoantibodies alone are insufficient to cause CRPS-like changes and that neoantigens expressed in the fracture limb are required to form the antigen-antibody complexes that initiate CRPS-like changes. In addition, no pronociceptive effects were observed when 3 week WT fracture serum was injected into WT fracture mice already expressing pronociceptive autoantibodies (Fig. 2).
When serum from 18 week post-fracture WT mice was injected into 3 week muMT fracture mice, it induced increased nociceptive behaviors, but 21 week serum had no effect, suggesting that the loss of serum autoantibodies is crucial for the resolution of pain behaviors in the 21 week WT fracture mice (Fig. 3). Furthermore, 3 week WT fracture serum had pronociceptive effects when injected into 21 or 29 week post-fracture WT mice with resolved hindpaw allodynia and unweighting, consistent with the hypothesis that neoantigens are still being expressed in the fracture hindlimb but that autoantibodies are no longer being produced at 21 weeks after fracture (Fig. 4). In addition, IgM deposition in the ipsilateral hindpaw skin, sciatic nerve, and lumbar cord peaked at 12–18 weeks after fracture and then gradually diminished over time, returning to normal levels in the sciatic nerve by 21 weeks (Fig. 7).
Injecting purified IgM obtained from 3 week WT fracture serum into 3 week muMT fracture mice had the same pronociceptive effects as serum injection, but had no effect on nociceptive thresholds in the contralateral intact hindlimb (Fig. 5). Injection of purified IgG from 3 week WT fracture serum into 3 week muMT fracture mice had no pronociceptive effects. Furthermore, when IgM and IgG levels were measured in fracture hindlimb paw skin and sciatic nerve at 3 weeks after fracture, only IgM levels were elevated, suggesting that IgM, but not IgG, was initiating the formation of antibody-antigen-complement complexes in the hindpaw skin and sciatic nerve of the fracture limb (Fig. 6). These results support the hypothesis that IgM is the immunoglobulin isotype responsible for pronociception. The immunostaining localization of IgM-antigen complex deposition in the nuclei of the dermal hindpaw skin cells is another intriguing finding (Fig. 8). The presence of antinuclear antibodies can indicate an antibody response against self and is frequently observed in autoimmune diseases.
Witebsky’s criteria for an autoimmune disease include; 1) evidence of an autoimmune or inflammatory disorder from clinical clues, 2) demonstration of a specific antigen, and 3) reproduction of clinical features in recipient animals by passive transfer of pathogenic antibodies [45]. Current evidence suggests that CRPS fulfills the first criteria. Patients usually exhibit characteristics of an inflammatory disease, including localized pain, redness, swelling, and warmth [50]. Proliferation of keratinocytes and mast cells occur in the affected limb and proinflammatory cytokines are elevated in blister fluid and skin biopsies [4,16,20,36]. Small CRPS clinical trials have reported beneficial effects with anti-inflammatory medications such as corticosteroids and bisphosphonates [6,8,12,48]. In addition, CRPS displays a female predominance [9] and the incidence of CRPS may be higher in patients with chronic inflammatory disorders such as asthma [10], multiple sclerosis [42], and rheumatoid arthritis [2].
Regarding Witebsky’s second criteria requiring demonstration of a specific antigen, there is some intriguing evidence for the presence of autoantibodies in CRPS. Recently we demonstrated that fracture mouse sera (but not control sera) binds to keratin 16, elongation factor1-alpha 1, peripherin, annexin A2, and beta-enolase in fracture mouse skin and that all these antigens are up-regulated or redistributed to the cell membrane after fracture [47]. Additionally, fracture mouse and CRPS patient serum showed increased antibody binding to recombinant keratin 16 protein [47]. Experiments using immunohistochemical techniques and FACS analysis have identified sympathetic nervous system neurons as targets for serum antibodies from some CRPS patients [13,15,27]. Follow-up experiments using a cardiomyocyte preparation suggested that a majority of CRPS, but not healthy patients, had autoantibodies binding to and activating M-2 muscarinic and/or beta 2 adrenergic receptors [26]. Furthermore, a third of CRPS patients exhibit positive antinuclear antibody tests, a standard diagnostic test for autoimmune disease [11]. In addition, plasma exchange therapy was reported effective in reducing pain in 90% of chronic CRPS patients [1]. Together, these results demonstrate that autoantibodies are present in some CRPS patients, but further research is required to confirm a pronociceptive role for autoantibodies in CRPS.
Collectively, the results of the current study support the hypothesis that fracture with immobilization can induce the regionally restricted expression of dermal nuclear antigens in the fracture limb, subsequently triggering B cells to secret IgM antibodies capable of binding with those antigens and initiating a pronociceptive antibody response. The time course of this pronociceptive antibody response is limited in the mouse to 21 weeks post-fracture and a similar progression of innate and autoimmune processes may explain the spontaneous resolution of symptoms that occurs in most CRPS patients within a year of onset [3,40,41,57]. We postulate that CRPS patients usually regain immune tolerance over time and, while still making neoantigens in the injured limb, their adaptive immune system no longer identifies these antigens as “other”. An obvious concern would be that some patients fail to regain immune tolerance, resulting in chronic CRPS. Future studies looking at the pronociceptive effects of CRPS patient serum in the mouse fracture model could potentially confirm Witebsky’s third criteria for autoimmune disease, evidence that passive transfer of patient autoantibodies can evoke CRPS-like changes in animals. Pursuing autoimmunity as a contributor to CRPS could identify new components of the disease process, including novel mechanisms for activation of the adaptive immunity, the post-traumatic time course of the innate and adaptive pronociceptive immune responses, and discovery of new treatment approaches for this disabling condition.
Acknowledgments
This study was supported by the National Institutes of Health grants NS072143 and NS094438, and the Department of Veterans Affairs, Rehabilitation Research and Development Merit grant I01RX001475. The authors do not have financial or other relationships that might lead to conflict of interest.
References
- 1.Aradillas E, Schwartzman RJ, Grothusen JR, Goebel A, Alexander GM. Plasma Exchange Therapy in Patients with Complex Regional Pain Syndrome. Pain physician. 2015;18(4):383–94. [PubMed] [Google Scholar]
- 2.Beerthuizen A, Stronks DL, Van’t Spijker A, Yaksh A, Hanraets BM, Klein J, Huygen FJ. Demographic and medical parameters in the development of complex regional pain syndrome type 1 (CRPS1): prospective study on 596 patients with a fracture. Pain. 2012;153(6):1187–92. doi: 10.1016/j.pain.2012.01.026. [DOI] [PubMed] [Google Scholar]
- 3.Bickerstaff DR, Kanis JA. Algodystrophy: an under-recongnized complication of minor trauma. Br J Rheumatol. 1994;33:240–48. doi: 10.1093/rheumatology/33.3.240. [DOI] [PubMed] [Google Scholar]
- 4.Birklein F, Drummond PD, Li W, Schlereth T, Albrecht N, Finch PM, Dawson LF, Clark JD, Kingery WS. Activation of cutaneous immune responses in complex regional pain syndrome. J Pain. 2014;15(5):485–95. doi: 10.1016/j.jpain.2014.01.490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bruehl S. An update on the pathophysiology of complex regional pain syndrome. Anesthesiology. 2010;113(3):713–25. doi: 10.1097/ALN.0b013e3181e3db38. [DOI] [PubMed] [Google Scholar]
- 6.Brunner F, Schmid A, Kissling R, Held U, Bachmann LM. Biphosphonates for the therapy of complex regional pain syndrome I--systematic review. European journal of pain (London, England) 2009;13(1):17–21. doi: 10.1016/j.ejpain.2008.03.005. [DOI] [PubMed] [Google Scholar]
- 7.Chaplan SR, Bach FW, Pogrel JW, Chung JM, Yaksh TL. Quantitative assessment of tactile allodynia in the rat paw. Journal of neuroscience methods. 1994;53(1):55–63. doi: 10.1016/0165-0270(94)90144-9. [DOI] [PubMed] [Google Scholar]
- 8.Christensen K, Jensen EM, Noer I. The reflex dystrophy syndrome response to treatment with systemic corticosteroids. Acta Chir Scand. 1982;148:653–55. [PubMed] [Google Scholar]
- 9.de Mos M, de Bruijn AG, Huygen FJ, Dieleman JP, Stricker BH, Sturkenboom MC. The incidence of complex regional pain syndrome: a population-based study. Pain. 2007;129(1–2):12–20. doi: 10.1016/j.pain.2006.09.008. [DOI] [PubMed] [Google Scholar]
- 10.de Mos M, Huygen FJ, Dieleman JP, Koopman JS, Stricker BH, Sturkenboom MC. Medical history and the onset of complex regional pain syndrome (CRPS) Pain. 2008;139(2):458–66. doi: 10.1016/j.pain.2008.07.002. [DOI] [PubMed] [Google Scholar]
- 11.Dirckx M, Schreurs MW, de Mos M, Stronks DL, Huygen FJ. The prevalence of autoantibodies in complex regional pain syndrome type I. Mediators Inflamm. 2015;2015:718201. doi: 10.1155/2015/718201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dirckx M, Stronks DL, Groeneweg G, Huygen FJ. Effect of immunomodulating medications in complex regional pain syndrome: a systematic review. The Clinical journal of pain. 2012;28(4):355–63. doi: 10.1097/AJP.0b013e31822efe30. [DOI] [PubMed] [Google Scholar]
- 13.Dubuis E, Thompson V, Leite MI, Blaes F, Maihofner C, Greensmith D, Vincent A, Shenker N, Kuttikat A, Leuwer M, Goebel A. Longstanding complex regional pain syndrome is associated with activating autoantibodies against alpha-1a adrenoceptors. Pain. 2014;155(11):2408–17. doi: 10.1016/j.pain.2014.09.022. [DOI] [PubMed] [Google Scholar]
- 14.Gallagher JJ, Tajerian M, Guo T, Shi X, Li W, Zheng M, Peltz G, Kingery WS, Clark JD. Acute and chronic phases of complex regional pain syndrome in mice are accompanied by distinct transcriptional changes in the spinal cord. Molecular pain. 2013;9:40. doi: 10.1186/1744-8069-9-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Goebel A, Stock M, Deacon R, Sprotte G, Vincent A. Intravenous immunoglobulin response and evidence for pathogenic antibodies in a case of complex regional pain syndrome 1. Annals of neurology. 2005;57(3):463–4. doi: 10.1002/ana.20400. [DOI] [PubMed] [Google Scholar]
- 16.Groeneweg JG, Huygen FJ, Heijmans-Antonissen C, Niehof S, Zijlstra FJ. Increased endothelin-1 and diminished nitric oxide levels in blister fluids of patients with intermediate cold type complex regional pain syndrome type 1. BMC musculoskeletal disorders. 2006;7:91. doi: 10.1186/1471-2474-7-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Guo TZ, Offley SC, Boyd EA, Jacobs CR, Kingery WS. Substance P signaling contributes to the vascular and nociceptive abnormalities observed in a tibial fracture rat model of complex regional pain syndrome type I. Pain. 2004;108:95–107. doi: 10.1016/j.pain.2003.12.010. [DOI] [PubMed] [Google Scholar]
- 18.Guo TZ, Wei T, Li WW, Li XQ, Clark JD, Kingery WS. Immobilization contributes to exaggerated neuropeptide signaling, inflammatory changes, and nociceptive sensitization after fracture in rats. Journal of Pain. 2014 doi: 10.1016/j.jpain.2014.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Guo TZ, Wei T, Shi X, Li WW, Hou S, Wang L, Tsujikawa K, Rice KC, Cheng K, Clark DJ, Kingery WS. Neuropeptide deficient mice have attenuated nociceptive, vascular, and inflammatory changes in a tibia fracture model of complex regional pain syndrome. Molecular pain. 2012;8:85. doi: 10.1186/1744-8069-8-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Huygen FJ, De Bruijn AG, De Bruin MT, Groeneweg JG, Klein J, Zijlstra FJ. Evidence for local inflammation in complex regional pain syndrome type 1. Mediators Inflamm. 2002;11(1):47–51. doi: 10.1080/09629350210307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huygen FJ, Niehof S, Zijlstra FJ, van Hagen PM, van Daele PL. Successful treatment of CRPS 1 with anti-TNF. Journal of pain and symptom management. 2004;27(2):101–3. doi: 10.1016/j.jpainsymman.2003.12.006. [DOI] [PubMed] [Google Scholar]
- 22.Huygen FJ, Ramdhani N, van Toorenenbergen A, Klein J, Zijlstra FJ. Mast cells are involved in inflammatory reactions during Complex Regional Pain Syndrome type 1. Immunology letters. 2004;91(2–3):147–54. doi: 10.1016/j.imlet.2003.11.013. [DOI] [PubMed] [Google Scholar]
- 23.Jang JH, Clark JD, Li X, Yorek MS, Usachev YM, Brennan TJ. Nociceptive sensitization by complement C5a and C3a in mouse. Pain. 2010;148(2):343–52. doi: 10.1016/j.pain.2009.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kalita J, Vajpayee A, Misra UK. Comparison of prednisolone with piroxicam in complex regional pain syndrome following stroke: a randomized controlled trial. QJM : monthly journal of the Association of Physicians. 2006;99(2):89–95. doi: 10.1093/qjmed/hcl004. [DOI] [PubMed] [Google Scholar]
- 25.Klein-Schneegans AS, Kuntz L, Fonteneau P, Loor F. Serum concentrations of IgM, IgG1, IgG2b, IgG3 and IgA in C57BL/6 mice and their congenics at the lpr (lymphoproliferation) locus. Journal of autoimmunity. 1989;2(6):869–75. doi: 10.1016/0896-8411(89)90013-9. [DOI] [PubMed] [Google Scholar]
- 26.Kohr D, Singh P, Tschernatsch M, Kaps M, Pouokam E, Diener M, Kummer W, Birklein F, Vincent A, Goebel A, Wallukat G, Blaes F. Autoimmunity against the β 2 adrenergic receptor and muscarinic-2 receptor in complex regional pain syndrome. Pain. 2011;152:2690–700. doi: 10.1016/j.pain.2011.06.012. [DOI] [PubMed] [Google Scholar]
- 27.Kohr D, Tschernatsch M, Schmitz K, Singh P, Kaps M, Schafer KH, Diener M, Mathies J, Matz O, Kummer W, Maihofner C, Fritz T, Birklein F, Blaes F. Autoantibodies in complex regional pain syndrome bind to a differentiation-dependent neuronal surface autoantigen. Pain. 2009;143(3):246–51. doi: 10.1016/j.pain.2009.03.009. [DOI] [PubMed] [Google Scholar]
- 28.Li W, Shi X, Wang L, Guo T, Wei T, Cheng K, Rice KC, Kingery WS, Clark JD. Epidermal adrenergic signaling contributes to inflammation and pain sensitization in a rat model of complex regional pain syndrome. Pain. 2013;154(8):1224–36. doi: 10.1016/j.pain.2013.03.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li W-W, Guo T-Z, Shi X, Czirr E, Stan T, Sahbaie P, Wyss-Coray T, Kingery WS, Clark JD. Autoimmunity contributes to nociceptive sensitization in a mouse model of complex regional pain syndrome. Pain. 2014;155:2377–89. doi: 10.1016/j.pain.2014.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li W-W, Sabsovich I, Guo T-Z, Zhao R, Kingery WS, Clark JD. The role of enhanced cutaneous IL-1beta signaling in a rat tibia fracture model of complex regional pain syndrome. Pain. 2009;144:303–13. doi: 10.1016/j.pain.2009.04.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li WW, Guo TZ, Li XQ, Kingery WS, Clark JD. Fracture induces keratinocyte activation, proliferation, and expression of pro-nociceptive inflammatory mediators. Pain. 2010;151:843–52. doi: 10.1016/j.pain.2010.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li WW, Guo TZ, Liang D, Shi X, Wei T, Kingery WS, Clark JD. The NALP1 inflammasome controls cytokine production and nociception in a rat fracture model of complex regional pain syndrome. Pain. 2009;147:277–86. doi: 10.1016/j.pain.2009.09.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li WW, Guo TZ, Liang DY, Sun Y, Kingery WS, Clark JD. Substance P signaling controls mast cell activation, degranulation, and nociceptive sensitization in a rat fracture model of complex regional pain syndrome. Anesthesiology. 2012;116(4):882–95. doi: 10.1097/ALN.0b013e31824bb303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li WW, Guo TZ, Shi X, Sun Y, Wei T, Clark DJ, Kingery WS. Substance P spinal signaling induces glial activation and nociceptive sensitization after fracture. Neuroscience. 2015;310:73–90. doi: 10.1016/j.neuroscience.2015.09.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Marinus J, Moseley GL, Birklein F, Baron R, Maihofner C, Kingery WS, van Hilten JJ. Clinical features and pathophysiology of complex regional pain syndrome. The Lancet Neurology. 2011;10(7):637–48. doi: 10.1016/S1474-4422(11)70106-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Munnikes RJ, Muis C, Boersma M, Heijmans-Antonissen C, Zijlstra FJ, Huygen FJ. Intermediate stage complex regional pain syndrome type 1 is unrelated to proinflammatory cytokines. Mediators Inflamm. 2005;2005(6):366–72. doi: 10.1155/MI.2005.366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Poree LR, Guo TZ, Kingery WS, Maze M. The analgesic potency of dexmedetomidine is enhanced after nerve injury: a possible role for peripheral alpha2-adrenoceptors. Anesth Analg. 1998;87:941–48. doi: 10.1097/00000539-199810000-00037. [DOI] [PubMed] [Google Scholar]
- 38.Sabsovich I, Guo T-Z, Wei T, Zhao R, Li X, Clark DJ, Geis C, Sommer C, Kingery WS. TNF signaling contributes to the development of nociceptive sensitization in a tibia fracture model of complex regional pain syndrome type I. Pain. 2008;137:507–19. doi: 10.1016/j.pain.2007.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sabsovich I, Wei T, Guo TZ, Zhao R, Shi X, Li X, Yeomans DC, Klyukinov M, Kingery WS, Clark JD. Effect of anti-NGF antibodies in a rat tibia fracture model of complex regional pain syndrome type I. Pain. 2008;138(1):47–60. doi: 10.1016/j.pain.2007.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sandroni P, Benrud-Larson LM, McClelland RL, Low PA. Complex regional pain syndrome type I: incidence and prevalence in Olmsted county, a population-based study. Pain. 2003;103(1–2):199–207. doi: 10.1016/s0304-3959(03)00065-4. [DOI] [PubMed] [Google Scholar]
- 41.Sarangi PP, Ward AJ, Smith EJ, Staddon GE, Atkins RM. Algodystrophy and osteoporosis after tibial fractures. J Bone Joint Surg Br. 1993;75(3):450–2. doi: 10.1302/0301-620X.75B3.8496220. [DOI] [PubMed] [Google Scholar]
- 42.Schwartzman RJ, Gurusinghe C, Gracely E. Prevalence of complex regional pain syndrome in a cohort of multiple sclerosis patients. Pain physician. 2008;11(2):133–6. [PubMed] [Google Scholar]
- 43.Shi X, Guo TZ, Wei T, Li WW, Clark DJ, Kingery WS. Facilitated spinal neuropeptide signaling and upregulated inflammatory mediator expression contribute to postfracture nociceptive sensitization. Pain. 2015;156:1852–63. doi: 10.1097/j.pain.0000000000000204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shi X, Wang L, Clark JD, Kingery WS. Keratinocytes express cytokines and nerve growth factor in response to neuropeptide activation of the ERK1/2 and JNK MAPK transcription pathways. Regulatory peptides. 2013;186:92–103. doi: 10.1016/j.regpep.2013.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sommer C. Anti-autonomic nervous system antibodies in CRPS. Pain. 2011;152(12):2675–6. doi: 10.1016/j.pain.2011.07.008. [DOI] [PubMed] [Google Scholar]
- 46.Subbarao J, Stillwell GK. Reflex sympathetic dystrophy syndrome of the upper extremity: analysis of total outcome of management of 125 cases. Archives of physical medicine and rehabilitation. 1981;62(11):549–54. [PubMed] [Google Scholar]
- 47.Tajerian M, Hung V, Khan H, Lahey LJ, Sun Y, Birklein F, Krämer HH, Robinson WH, Kingery WS, Clark JD. Identification of KRT16 as a target of an autoantibody response in complex regional pain syndrome. Experimental neurology. 2017;287:14–20. doi: 10.1016/j.expneurol.2016.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tran DQ, Duong S, Bertini P, Finlayson RJ. Treatment of complex regional pain syndrome: a review of the evidence. Canadian journal of anaesthesia = Journal canadien d’anesthesie. 2010;57(2):149–66. doi: 10.1007/s12630-009-9237-0. [DOI] [PubMed] [Google Scholar]
- 49.Vargas ME, Watanabe J, Singh SJ, Robinson WH, Barres BA. Endogenous antibodies promote rapid myelin clearance and effective axon regeneration after nerve injury. Proceedings of the National Academy of Sciences of the United States of America. 2010;107(26):11993–8. doi: 10.1073/pnas.1001948107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Veldman PH, Reynen HM, Arntz IE, Goris RJ. Signs and symptoms of reflex sympathetic dystrophy: prospective study of 829 patients. Lancet (London, England) 1993;342(8878):1012–6. doi: 10.1016/0140-6736(93)92877-v. [DOI] [PubMed] [Google Scholar]
- 51.Wei T, Guo TZ, Li WW, Hou S, Kingery WS, Clark JD. Keratinocyte expression of inflammatory mediators plays a crucial role in substance P-induced acute and chronic pain. J Neuroinflammation. 2012;9:181. doi: 10.1186/1742-2094-9-181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wei T, Guo TZ, Li WW, Kingery WS, Clark JD. Acute versus chronic phase mechanisms in a rat model of CRPS. J Neuroinflammation. 2016;13:14. doi: 10.1186/s12974-015-0472-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wei T, Li WW, Guo TZ, Zhao R, Wang L, Clark DJ, Oaklander AL, Schmelz M, Kingery WS. Post-junctional facilitation of Substance P signaling in a tibia fracture rat model of complex regional pain syndrome type I. Pain. 2009;144(3):278–86. doi: 10.1016/j.pain.2009.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wei T, Sabsovich I, Guo TZ, Shi X, Zhao R, Li W, Geis C, Sommer C, Kingery WS, Clark DJ. Pentoxifylline attenuates nociceptive sensitization and cytokine expression in a tibia fracture rat model of complex regional pain syndrome. European journal of pain (London, England) 2009;13(3):253–62. doi: 10.1016/j.ejpain.2008.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wesseldijk F, Huygen FJ, Heijmans-Antonissen C, Niehof SP, Zijlstra FJ. Six years follow-up of the levels of TNF-alpha and IL-6 in patients with complex regional pain syndrome type 1. Mediators Inflamm. 2008;2008:469439. doi: 10.1155/2008/469439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Winer DA, Winer S, Shen L, Wadia PP, Yantha J, Paltser G, Tsui H, Wu P, Davidson MG, Alonso MN, Leong HX, Glassford A, Caimol M, Kenkel JA, Tedder TF, McLaughlin T, Miklos DB, Dosch HM, Engleman EG. B cells promote insulin resistance through modulation of T cells and production of pathogenic IgG antibodies. Nature medicine. 2011;17(5):610–7. doi: 10.1038/nm.2353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zyluk A. The natural history of post-traumatic reflex sympathetic dystrophy. Journal of hand surgery (Edinburgh, Scotland) 1998;23(1):20–3. doi: 10.1016/s0266-7681(98)80211-8. [DOI] [PubMed] [Google Scholar]