Abstract
Metformin, one of the most widely prescribed antidiabetic drugs in the world is being repurposed as a potential drug in cancer treatment. Epidemiological studies suggest that metformin exerts anticancer effects in diabetic patients with pancreatic cancer. However, at typical antidiabetic doses the bioavailability of metformin is presumably too low to exert antitumor effects. Thus, more potent analogs of metformin are needed in order to increase its anticancer efficacy. To this end, a new class of mitochondria-targeted metformin analogs (or mito-metformins) containing a positively-charged lipophilic triphenylphosphonium group, was synthesized and tested for their antitumor efficacy in pancreatic cancer cells. Results indicate that the lead compound, mito-metformin10, was nearly 1,000-fold more potent than metformin in inhibiting mitochondrial complex I activity, inducing reactive oxygen species (superoxide and hydrogen peroxide) that stimulate redox signaling mechanisms, including the activation of AMP kinase and inhibition of proliferation of pancreatic cancer cells. The potential use of the low-temperature EPR technique in assessing the role of mitochondrial complexes including complex I in tumor regression in response to metformin and mito-metformins in the in vivo setting is discussed.
Keywords: modified metformin, reactive oxygen species, redox signaling, mitochondrial metabolism, pancreatic cancer
Introduction
Metformin (Met, Figure 1) is one of the most prescribed antidiabetic drugs in the world.(1, 2) Metformin is a derivative of guanidine, an active component in the extracts from French lilac (G. officinalis), the plant that has been used for centuries to combat diabetes. A major advantage of metformin as a drug is that it is relatively safe and diabetic patients typically take daily several grams of the drug. Recently metformin is being repurposed as an antitumor drug due to epidemiological findings revealing an association between decreased incidence of pancreatic cancer and metformin use in diabetic individuals.(3, 4) However, at typical antidiabetic doses, the bioavailability of metformin and/or its uptake by cancer cells is probably too low to exert its antitumor effects in humans, although singificant antitumor activity was reported in the rodent models, at comparable plasma concentrations.(5, 6) It appears that the antineoplastic activity of metformin in humans will require much higher concentrations of the drug in the serum than what is typically administered in diabetics treated with metformin.(5) Thus, it is important to develop new and improved metformin analogs with increased bioavailability, resulting in more potent antitumor effects, although these analogs may show increased toxicity for routine treatment of diabetes. We hypothesized that attaching the triphenylphosphonium cation (TPP+) to metformin via an alkyl chain would greatly enhance its accumulation in mitochondria of cancer cells, leading to selective inhibition of proliferation of cancer cells.(7) Results obtained in this study substantiate the proposal that TPP+-modified metformins are more cytostatic to pancreatic cancer cells.
Figure 1.
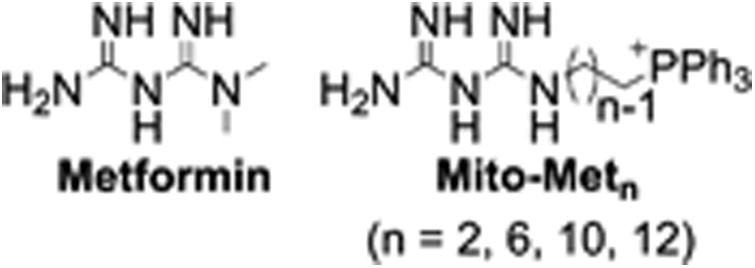
Chemical structures of metformin and mito-metformin analogs.
Metformin does not undergo metabolism like many other drugs but it is excreted out unchanged in the urine.(8) However, it induces extensive changes in the metabolic pathways and mitochondrial energy metabolism.(9) Although multiple mechanisms were proposed for metformin anticancer effects, mitochondria still remain as the major target of metformin, leading to inhibition of cell respiration, AMPK activation, mTOR inhibition, and inhibition of cell proliferation.(10, 11)
Recent research implicates a role for mitochondrial complex I inhibition in the antitumor effects of metformin.(12) Interestingly, cancers with mutations in mitochondrial genes encoding proteins of the mitochondrial complex I of the electron transport chain are more susceptible to biguanides such as metformin.(13) Thus, cancers with oxidative phosphorylation deficits are likely to be more sensitive to biguanides than normal tissues and consequently, patients with complex I mutated cancer phenotype may be more sensitive to metformin treatment.(14) Tumor microenvironment was proposed to be another factor for increased metformin potency in some tumor phenotypes.(15) More recently, metformin was reported to activate T cell-mediated immune response against cancer cells.(15-17)
We have recently shown that attaching a positively-charged lipophilic triphenylphosphonium group to a nitroxide, quinone, or a phenolic group via an aliphatic linker chain vastly enhanced their ability to target mitochondria, and that such mitochondria-targeted compounds (e.g., Mito-CP, Mito-Q and Mito-Vitamin-E) significantly induced antiproliferative and/or cytotoxic effects in tumor cells without markedly affecting noncancerous cells.(18-23) Similarly, we hypothesized that attaching a positively-charged lipophilic substituent will improve mitochondrial targeting of metformin (e.g., mito-metformins, Figure 1), thereby generating a new class of metformin analogs with enhanced antitumor potential. Results show that the mito-metformin analogs are much more potent (100 to 1,000-fold higher) than metformin in inhibiting human pancreatic adenocarcinoma cells (PDACs).(7)
Antiproliferative effects of metformin and mito-metformin10
The effect of mito-metformin10 and metformin on MiaPaCa-2 cells was investigated by monitoring colony formation and cell proliferation. As shown in Figure 2, in order to inhibit proliferation of pancreatic cancer cells, metformin was used at millimolar levels, whereas mito-metformin10 was used at micromolar concentrations. The IC50 values for inhibition of proliferation of human pancreatic cancer cells (MiaPaCa-2 and PANC-1) were more than 1,000-fold lower for Mito-Met10 (0.2 μM), as compared to Met (0.6-0.9 mM). MiaPaCa-2 cells which were treated with 0.5 or 1 millimolar concentration of metformin, or 0.5 to 1 micromolar concentration of mito-metformin, showed the same effects in colony formation and cell proliferation experiments. (7) The IC50 values for inhibiting 50% of colony formation for metformin and mito-metformin10 were calculated to be 1.3 mM and 1.1 μM, respectively. Thus, mito-metformin10 is ca. 1,000-fold more effective than metformin. The colony forming ability of the pancreatic cancers cells, while decreased by treatment with Mito-Met10, could be rescued be cell pre-treatment with compound C, an inhibitor of AMPK protein (Figure 2B). This indicates that AMPK signaling pathway is involved in the antiproliferative effects of Mito-Met10. Interestingly, Mito-Met10 showed a good selectivity towards pancreatic cancer cells, as compared to non-tumorigenic cells (Figure 2A), implicating a wider margin of therapeutic index for its use in vivo.
Figure 2.
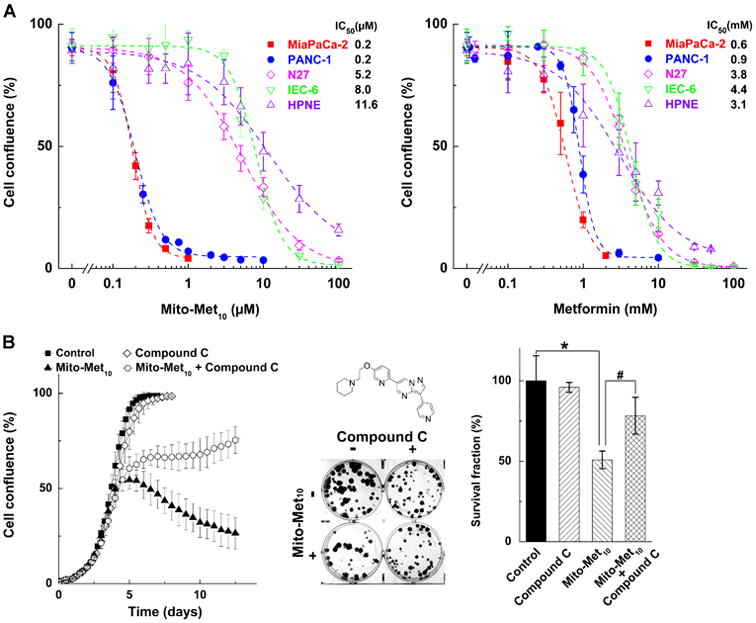
Effect of metformin and mito-metformin10 on PDAC proliferation. (A) Titration of pancreatic cancer (MiaPaCa-2 and PANC-1) and non-tumorigenic (N27, IEC-6 and HPNE) cells with Mito-Met10 (left panel) and Met (right panel). (B) Effect of Mito-Met10 on colony formation in MiaPaCa-2 cells and the rescue effects of AMPK inhibitor, compound C.(7)
Inhibition of pancreatic cancer cell respiration: complex I involvement
Metformin is weakly cationic, and weakly targets mitochondria. Metformin's antitumor effects were attributed in part to its ability to inhibit mitochondrial complex I-dependent cell respiration and ATP generation.(7, 12) In contrast to rotenone, a non-selective inhibitor of complex I in both normal and cancer cells, metformin reversibly and more selectively inhibits mitochondrial complex I in cancer cells.(12) As mito-metformin10 selectively accumulates into mitochondria, we compared the relative inhibitory effects of metformin and mito-metformin10 on complex I activity in MiaPaCa-2 cells. The real time monitoring of oxygen consumption rate (OCR) was used as a measure of mitochondrial function in intact cells. The mitochondrial respiration or OCR was monitored in MiaPaCa-2 cells treated with metformin and mito-metformin10 using the Seahorse XF extracellular flux analyzer.(7) Figure 3 shows the OCR measurement of mitochondrial complex I activity of MiaPaCa-2 cells pretreated for 24 h with metformin or mito-metformin10. The IC50 values for inhibition of mitochondrial complex I for metformin and mito-metformin10 were determined to be ca. 1.1 mM and 0.4 μM. These results show that the complex I inhibition may be mechanistically related to the antiproliferative effects of these compounds. Again, Mito-Met10 exhibited selectivity towards cancer cells in inhibiting mitochondrial complex I (Figure 3).
Figure 3.
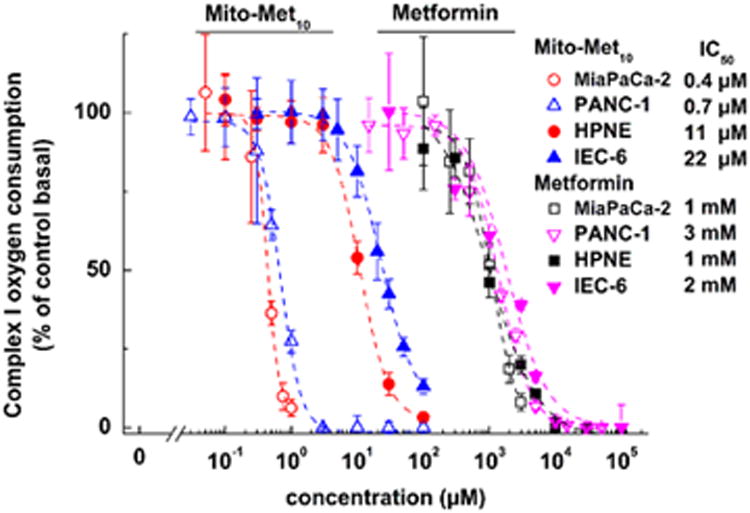
Effects of metformin and mito-metformin10 on mitochondrial complex I activity in pancreatic cancer (MiaPaCa-2 and PANC-1) and non-tumorigenic (HPNE and IEC-6) cells.(7)
Mito-metformin-mediated stimulation of reactive oxygen species and redox signaling
One of the consequences of inhibiting mitochondrial complex I is stimulation of one-electron reduction of oxygen to superoxide radical anion (O2•–) and other oxidants (hydrogen peroxide and higher oxidation heme species) derived from superoxide.(7) As mito-metformin10 treatment (at the concentration inhibiting proliferation) did not exert any cytotoxic effects, it is likely that reactive oxygen species (ROS) generated from mitochondria could be involved in redox signaling.(7) We used the cell-permeable probe, hydroethidine (HE), to detect superoxide by monitoring 2-OH-E+, the specific marker product of the reaction between superoxide and HE.(24-26) A marked increase in 2-OH-E+ formation was observed in mito-metformin10-treated MiaPaCa-2 cells (Fig. 4). Evidence for other one-electron oxidation species formed from mito-metformin10 interaction with mitochondrial proteins arose from studies demonstrating the formation of ethidium and a dimeric product of HE (E+ and E+-E+, Fig. 4) in isolated mitochondria and upon incubation of HE with heme proteins.(27, 28) Under similar experimental conditions, mito-metformin10 did not stimulate superoxide formation in noncancerous human pancreatic epithelial renin-expressing (HPNE) cells (Fig. 4).
Figure 4.
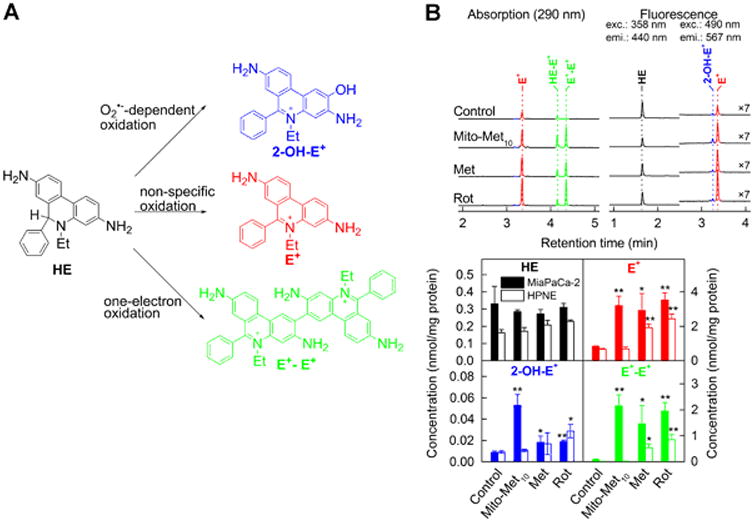
Intracellular superoxide stimulation by mito-metformin10. (A) Chemical scheme of superoxide-dependent and independent pathways of oxidation of hydroethidine (HE) probe. (B) Effect of Met (1 mM, 24 h), Mito-Met10 (1 μM, 24 h) or rotenone (ROT, 1 μM, 1 h) on HE oxidation in MiaPaCa-2 pancreatic cancer cells. Upper panel shows the HPLC chromatograms and lower panels shows the quantitative analysis of intracellular levels of HE and its oxidation products.(7)
We used the probe o-MitoPhB(OH)2 to detect H2O2 generated in mito-metformin-treated cells.(29-31) Results show that mito-metformin10 treatment of MiaPaCa-2 cells in the presence of o-MitoPhB(OH)2 led to an increase in o-MitoPhOH formation (Figure 5). Although this product can be formed from either H2O2, peroxynitrite, or hypochlorous acid reaction with o-MitoPhB(OH)2, the lack of detection of peroxynitrite- or hypochlorous acid-specific products in addition to o-MitoPhOH suggests that H2O2 was responsible for oxidation of o-MitoPhB(OH)2 to MitoPhOH.(29-31)
Figure 5.
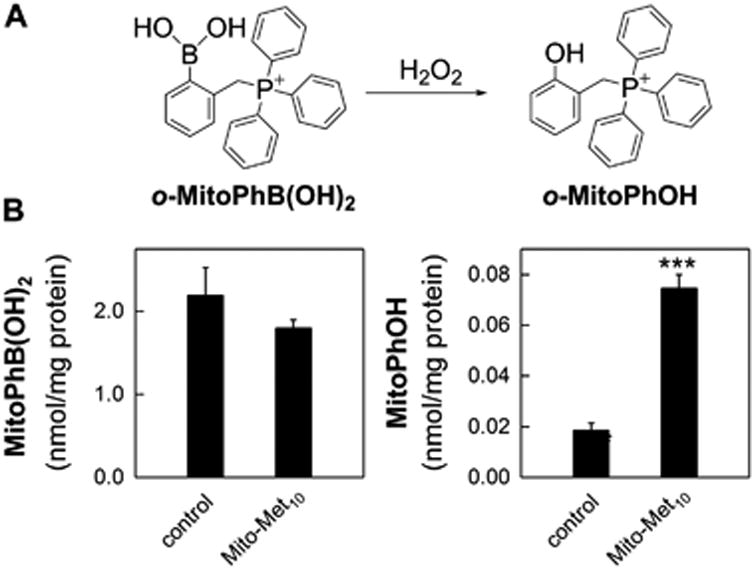
Hydrogen peroxide formation induced by mito-metformin10 in PDACs. (A) Chemical scheme of H2O2-dependent oxidation of the o-MitoPhB(OH)2 probe. (B) Intracellular levels of o-MitoPhB(OH)2 (left panel) and o-MitoPhOH (right panel) in MiaPaCa-2 pancreatic cancer cells treated for 24 h with Mito-Met10.
Mito-metformin10 activated adenosinemonophosphate (AMP)-activated protein kinase (AMPK) phosphorylation at a 1,000-fold lower concentration than metformin.(7) AMPK is a master regulator of cellular energy homeostasis and is activated via phosphorylation of its threonine-172 residue.(32) AMPK activation was linked to increased formation of hydrogen peroxide.(33, 34) Based on the results showing that mito-metformin10 stimulated mitochondrial superoxide, hydrogen peroxide and AMPK activation, we proposed that mito-metformin10-mediated antiproliferative effects are linked to inhibition of mitochondrial complex, enhanced ROS formation, and AMPK phosphorylation (Fig. 6). These observations have been recently confirmed by an independent study on antiproliferative effects of triphenylphosphonium-linked metformin derivatives against pancreatic cancer cells. (35)
Figure 6.
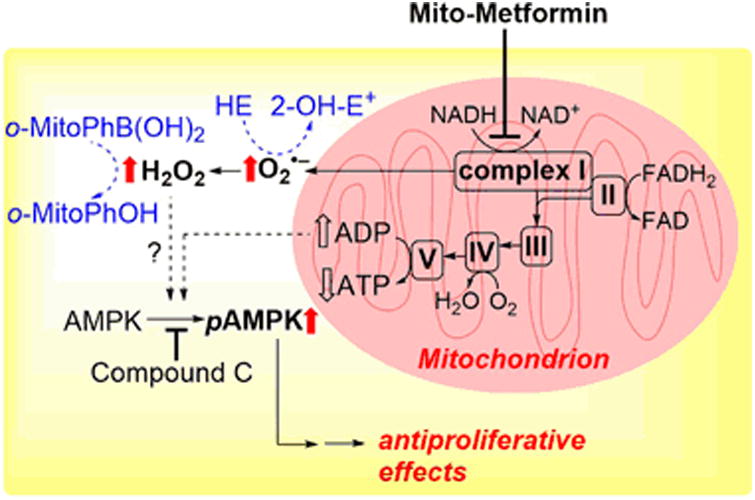
Proposed signaling pathway induced by mito-metformin10 in PDACs. Block arrows indicate the changes observed upon cell treatment with Mito-Met10.
Potential use of ex vivo low-temperature EPR in elucidating mitochondrial mechanism
Whereas the Seahorse technique can be used to measure the activities of mitochondrial complexes in cells, it is challenging to use this technique to measure mitochondrial activities in tissues. The low-temperature electron paramagnetic resonance (EPR) spectroscopy is uniquely capable of providing a “snapshot” of mitochondrial status in intact tissues.(36) To our knowledge, the EPR technique has not been used to monitor changes in the redox status of mitochondrial iron-sulfur complexes in cancer cells. The effect of mito-metformin10 and metformin on complex I activity in tumor tissues can be determined by performing EPR measurements at liquid helium temperatures. The signal at g=1.94 attributable to the reduced form of [2Fe-2S]+ center of mitochondrial complex I is monitored.(36, 37) Inhibition of this signal is equated to inhibition of complex I. In a recent publication, Bennett et al. discussed the use of low-temperature EPR in mitochondrial diseases.(38)
ROS generated from inhibiting complex I will react with other iron sulfur-containing proteins (e.g., mitochondrial and cytosolic aconitases), and the levels of oxidatively-inactivated aconitases can be monitored at g=2.03 and 2.01 EPR signals due to the [3Fe-4S]+ form of aconitase.(37, 39) Oxidatively-inactivated mitochondrial aconitase has a distinctly different EPR signal from oxidatively-inactivated cytosolic aconitase.(37, 39) Other signals from reduced and oxidized iron sulfur centers associated with downstream complexes I and II in the electron transport chain are also detectable using the low-temperature EPR. These experiments can be done in both preclinical and human tumor samples, and may provide additional mechanistic insights into response to therapy.
Conclusions
We have demonstrated that more potent analogs of metformin (a.k.a. mito-metformins) can be synthesized by attaching a mitochondria-targeting triphenylphosphonium group tethered to an alkyl side chain. Mito-metformin10 was determined to be the most potent analog tested in inhibiting mitochondrial respiration, ROS activation, AMPK phosphorylation, and inhibition of proliferation in pancreatic cancer cells. We propose that AMPK activation induced by complex I-mediated ROS signaling is responsible for the antiproliferative effects. More recently, hydrogen peroxide was reported to induce redox signaling via a redox-relay involving peroxiredoxins and STAT3.(40) One of the major targets of mito-metformins is the mitochondrial complex I in the electron transport chain. The exact mechanism by which metformin analogs inhibit complex I is not yet ascertained. It has been reported that the occurrence of mitochondrial complex I A/D transition could regulate ROS production.(41)The D-form of the enzyme has been shown to generate more O2•– than the A-form, and irreversible locking of complex I in the D-form may contribute to more O2•– formation.(41) Establishing this mechanism in the in vivo preclinical pancreatic cancer xenografts may be accomplished using the low-temperature EPR technique. This ex vivo EPR technique may also be extrapolated to analysis of human pancreatic tumors.
Acknowledgments
This work was supported by a grant from NIH (U01 CA178960) to M.D. and B.K. A.S. was supported by a grant from Polish National Science Centre No. 2015/18/E/ST4/00235.
Footnotes
Conflict of Interest: The authors declare that they have no conflict of interest.
Bibliography
- 1.Bailey C, Day C. Metformin: its botanical background. Pract Diabetes Int. 2004;21:115–117. [Google Scholar]
- 2.Thomas I, Gregg B. Metformin; a review of its history and future: from lilac to longevity. Pediatric Diabetes. 2017;18:10–16. doi: 10.1111/pedi.12473. [DOI] [PubMed] [Google Scholar]
- 3.Evans JM, Donnelly LA, Emslie-Smith AM, Alessi DR, Morris AD. Metformin and reduced risk of cancer in diabetic patients. BMJ (Clinical research ed) 2005;330:1304–1305. doi: 10.1136/bmj.38415.708634.F7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Heckman-Stoddard BM, Gandini S, Puntoni M, Dunn BK, Decensi A, Szabo E. Repurposing old drugs to chemoprevention: The case of metformin. Seminars in Oncology. 2016;43:123–133. doi: 10.1053/j.seminoncol.2015.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kordes S, Pollak MN, Zwinderman AH, Mathot RA, Weterman MJ, Beeker A, Punt CJ, Richel DJ, Wilmink JW. Metformin in patients with advanced pancreatic cancer: a double-blind, randomised, placebo-controlled phase 2 trial. The Lancet Oncology. 2015;16:839–847. doi: 10.1016/S1470-2045(15)00027-3. [DOI] [PubMed] [Google Scholar]
- 6.Chandel NS, Avizonis D, Reczek CR, Weinberg SE, Menz S, Neuhaus R, Christian S, Haegebarth A, Algire C, Pollak M. Are Metformin Doses Used in Murine Cancer Models Clinically Relevant? Cell Metab. 2016;23:569–570. doi: 10.1016/j.cmet.2016.03.010. [DOI] [PubMed] [Google Scholar]
- 7.Cheng G, Zielonka J, Ouari O, Lopez M, McAllister D, Boyle K, Barrios CS, Weber JJ, Johnson BD, Hardy M, Dwinell MB, Kalyanaraman B. Mitochondria-Targeted Analogues of Metformin Exhibit Enhanced Antiproliferative and Radiosensitizing Effects in Pancreatic Cancer Cells. Cancer research. 2016;76:3904–3915. doi: 10.1158/0008-5472.CAN-15-2534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Graham GG, Punt J, Arora M, Day RO, Doogue MP, Duong JK, Furlong TJ, Greenfield JR, Greenup LC, Kirkpatrick CM, Ray JE, Timmins P, Williams KM. Clinical pharmacokinetics of metformin. Clinical Pharmacokinetics. 2011;50:81–98. doi: 10.2165/11534750-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 9.Foretz M, Guigas B, Bertrand L, Pollak M, Viollet B. Metformin: From mechanisms of action to therapies. Cell Metabolism. 2014;20:953–966. doi: 10.1016/j.cmet.2014.09.018. [DOI] [PubMed] [Google Scholar]
- 10.Bridges H, Jones A, Pollak M, Hirst J. Effects of metformin and other biguanides on oxidative phosphorylation in mitochondria. The Biochemical journal. 2014;462:475–487. doi: 10.1042/BJ20140620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu X, I, Romero L, Litchfield LM, Lengyel E, Locasale JW. Metformin Targets Central Carbon Metabolism and Reveals Mitochondrial Requirements in Human Cancers. Cell Metabolism. 2016;24:728–739. doi: 10.1016/j.cmet.2016.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wheaton WW, Weinberg SE, Hamanaka RB, Soberanes S, Sullivan LB, Anso E, Glasauer A, Dufour E, Mutlu GM, Budigner GS, Chandel NS. Metformin inhibits mitochondrial complex I of cancer cells to reduce tumorigenesis. eLife. 2014;3:e02242. doi: 10.7554/eLife.02242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Birsoy K, Possemato R, Lorbeer FK, Bayraktar EC, Thiru P, Yucel B, Wang T, Chen WW, Clish CB, Sabatini DM. Metabolic determinants of cancer cell sensitivity to glucose limitation and biguanides. Nature. 2014;508:108–112. doi: 10.1038/nature13110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pollak M. Overcoming Drug Development Bottlenecks With Repurposing: Repurposing biguanides to target energy metabolism for cancer treatment. Nat Med. 2014;20:591–593. doi: 10.1038/nm.3596. [DOI] [PubMed] [Google Scholar]
- 15.Eikawa S, Nishida M, Mizukami S, Yamazaki C, Nakayama E, Udono H. Immune-mediated antitumor effect by type 2 diabetes drug, metformin. Proceedings of the National Academy of Sciences. 2015;112:1809–1814. doi: 10.1073/pnas.1417636112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Webb TJ, Carey GB, East JE, Sun W, Bollino DR, Kimball AS, Brutkiewicz RR, Flajnik M. Alterations in cellular metabolism modulate CD1d-mediated NKT-cell responses. Pathogens and Disease. 2016;74:ftw055–ftw055. doi: 10.1093/femspd/ftw055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Delmastro-Greenwood MM, Piganelli JD. Changing the energy of an immune response. American journal of clinical and experimental immunology. 2013;2:30–54. [PMC free article] [PubMed] [Google Scholar]
- 18.Cheng G, Zielonka J, McAllister D, Hardy M, Ouari O, Joseph J, Dwinell MB, Kalyanaraman B. Antiproliferative effects of mitochondria-targeted cationic antioxidants and analogs: Role of mitochondrial bioenergetics and energy-sensing mechanism. Cancer letters. 2015;365:96–106. doi: 10.1016/j.canlet.2015.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cheng G, Zielonka J, Dranka BP, McAllister D, Mackinnon AC, Jr, Joseph J, Kalyanaraman B. Mitochondria-targeted drugs synergize with 2-deoxyglucose to trigger breast cancer cell death. Cancer research. 2012;72:2634–2644. doi: 10.1158/0008-5472.CAN-11-3928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cheng G, Zielonka J, McAllister D, Tsai S, Dwinell MB, Kalyanaraman B. Profiling and targeting of cellular bioenergetics: inhibition of pancreatic cancer cell proliferation. British journal of cancer. 2014;111:85–93. doi: 10.1038/bjc.2014.272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cheng G, Zielonka J, McAllister DM, Mackinnon AC, Jr, Joseph J, Dwinell MB, Kalyanaraman B. Mitochondria-targeted vitamin E analogs inhibit breast cancer cell energy metabolism and promote cell death. BMC cancer. 2013;13:285. doi: 10.1186/1471-2407-13-285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dickey JS, Gonzalez Y, Aryal B, Mog S, Nakamura AJ, Redon CE, Baxa U, Rosen E, Cheng G, Zielonka J, Parekh P, Mason KP, Joseph J, Kalyanaraman B, Bonner W, Herman E, Shacter E, Rao VA. Mito-tempol and dexrazoxane exhibit cardioprotective and chemotherapeutic effects through specific protein oxidation and autophagy in a syngeneic breast tumor preclinical model. PloS one. 2013;8:e70575. doi: 10.1371/journal.pone.0070575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rao VA, Klein SR, Bonar SJ, Zielonka J, Mizuno N, Dickey JS, Keller PW, Joseph J, Kalyanaraman B, Shacter E. The antioxidant transcription factor Nrf2 negatively regulates autophagy and growth arrest induced by the anticancer redox agent mitoquinone. The Journal of biological chemistry. 2010;285:34447–34459. doi: 10.1074/jbc.M110.133579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhao H, Joseph J, Fales HM, Sokoloski EA, Levine RL, Vasquez-Vivar J, Kalyanaraman B. Detection and characterization of the product of hydroethidine and intracellular superoxide by HPLC and limitations of fluorescence. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:5727–5732. doi: 10.1073/pnas.0501719102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zielonka J, Kalyanaraman B. Hydroethidine- and MitoSOX-derived red fluorescence is not a reliable indicator of intracellular superoxide formation: another inconvenient truth. Free radical biology & medicine. 2010;48:983–1001. doi: 10.1016/j.freeradbiomed.2010.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zielonka J, Vasquez-Vivar J, Kalyanaraman B. Detection of 2-hydroxyethidium in cellular systems: a unique marker product of superoxide and hydroethidine. Nature protocols. 2008;3:8–21. doi: 10.1038/nprot.2007.473. [DOI] [PubMed] [Google Scholar]
- 27.Zielonka J, Hardy M, Kalyanaraman B. HPLC study of oxidation products of hydroethidine in chemical and biological systems: ramifications in superoxide measurements. Free Radical Biology and Medicine. 2009;46:329–338. doi: 10.1016/j.freeradbiomed.2008.10.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zielonka J, Srinivasan S, Hardy M, Ouari O, Lopez M, Vasquez-Vivar J, Avadhani NG, Kalyanaraman B. Cytochrome c-mediated oxidation of hydroethidine and mito-hydroethidine in mitochondria: Identification of homo- and heterodimers. Free Radical Biology and Medicine. 2008;44:835–846. doi: 10.1016/j.freeradbiomed.2007.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sikora A, Zielonka J, Adamus J, Debski D, Dybala-Defratyka A, Michalowski B, Joseph J, Hartley RC, Murphy MP, Kalyanaraman B. Reaction between peroxynitrite and triphenylphosphonium-substituted arylboronic acid isomers: Identification of diagnostic marker products and biological implications. Chemical research in toxicology. 2013;26:856–867. doi: 10.1021/tx300499c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zielonka J, Sikora A, Adamus J, Kalyanaraman B. Detection and differentiation between peroxynitrite and hydroperoxides using mitochondria-targeted arylboronic acid. Methods in Molecular Biology. 2015;1264:171–181. doi: 10.1007/978-1-4939-2257-4_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zielonka J, Zielonka M, Ver Plank L, Cheng G, Hardy M, Ouari O, Ayhan MM, Podsiadly R, Sikora A, Lambeth JD, Kalyanaraman B. Mitigation of NADPH Oxidase 2 Activity as a Strategy to Inhibit Peroxynitrite Formation. The Journal of biological chemistry. 2016;291:7029–7044. doi: 10.1074/jbc.M115.702787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hardie DG, Ashford ML. AMPK: regulating energy balance at the cellular and whole body levels. Physiology (Bethesda, Md) 2014;29:99–107. doi: 10.1152/physiol.00050.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mackenzie RM, I, Salt P, Miller WH, Logan A, Ibrahim HA, Degasperi A, Dymott JA, Hamilton CA, Murphy MP, Delles C, Dominiczak AF. Mitochondrial reactive oxygen species enhance AMP-activated protein kinase activation in the endothelium of patients with coronary artery disease and diabetes. Clinical science (London, England : 1979) 2013;124:403–411. doi: 10.1042/CS20120239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Quintero M, Colombo SL, Godfrey A, Moncada S. Mitochondria as signaling organelles in the vascular endothelium. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:5379–5384. doi: 10.1073/pnas.0601026103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Boukalova S, Stursa J, Werner L, Ezrova Z, Cerny J, Bezawork-Geleta A, Pecinova A, Dong L, Drahota Z, Neuzil J. Mitochondrial Targeting of Metformin Enhances Its Activity against Pancreatic Cancer. Molecular cancer therapeutics. 2016;15:2875–2886. doi: 10.1158/1535-7163.MCT-15-1021. [DOI] [PubMed] [Google Scholar]
- 36.Orme-Johnson NR, Hansen RE, Beinert H. Electron paramagnetic resonance-detectable electron acceptors in beef heart mitochondria. Reduced diphosphopyridine nucleotide ubiquinone reductase segment of the electron transfer system. The Journal of biological chemistry. 1974;249:1922–1927. [PubMed] [Google Scholar]
- 37.Chandran K, Aggarwal D, Migrino RQ, Joseph J, McAllister D, Konorev EA, Antholine WE, Zielonka J, Srinivasan S, Avadhani NG, Kalyanaraman B. Doxorubicin inactivates myocardial cytochrome c oxidase in rats: Cardioprotection by Mito-Q. Biophysical Journal. 2009;96:1388–1398. doi: 10.1016/j.bpj.2008.10.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bennett B, Helbling D, Meng H, Jarzembowski J, Geurts AM, Friederich MW, Van Hove JLK, Lawlor MW, Dimmock DP. Potentially diagnostic electron paramagnetic resonance spectra elucidate the underlying mechanism of mitochondrial dysfunction in the deoxyguanosine kinase deficient rat model of a genetic mitochondrial DNA depletion syndrome. Free Radical Biology and Medicine. 2016;92:141–151. doi: 10.1016/j.freeradbiomed.2016.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ghosh A, Chandran K, Kalivendi SV, Joseph J, Antholine WE, Hillard CJ, Kanthasamy A, Kanthasamy A, Kalyanaraman B. Neuroprotection by a mitochondria-targeted drug in a Parkinson's disease model. Free radical biology & medicine. 2010;49:1674–1684. doi: 10.1016/j.freeradbiomed.2010.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sobotta MC, Liou W, Stöcker S, Talwar D, Oehler M, Ruppert T, Scharf AND, Dick TP. Peroxiredoxin-2 and STAT3 form a redox relay for H2O2 signaling. Nat Chem Biol. 2015;11:64–70. doi: 10.1038/nchembio.1695. [DOI] [PubMed] [Google Scholar]
- 41.Babot M, Birch A, Labarbuta P, Galkin A. Characterisation of the active/de-active transition of mitochondrial complex I. Biochimica et Biophysica Acta (BBA) - Bioenergetics. 2014;1837:1083–1092. doi: 10.1016/j.bbabio.2014.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]


