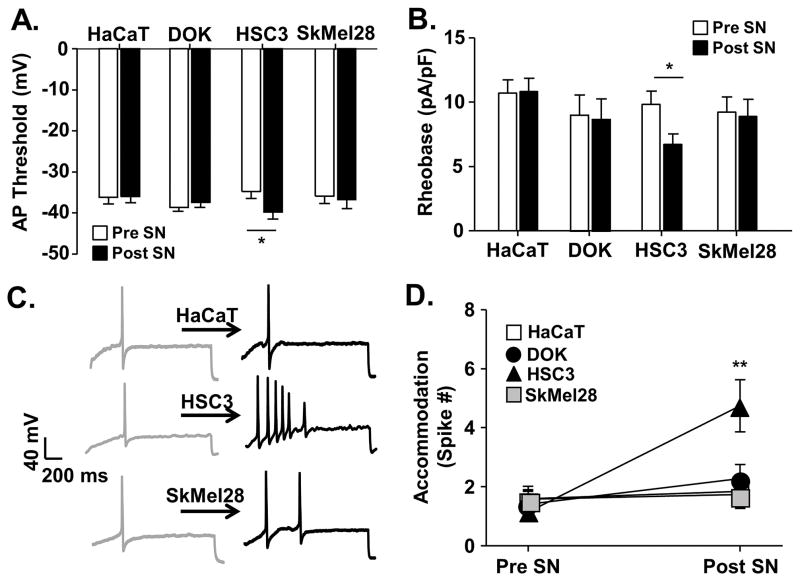
Figure 5. Oral cancer-secreted mediators induced neuronal excitability.
Whole cell current clamp electrophysiology was used to measure primary afferent neuron excitability following acute application of cell line supernatant. Application of HSC3 resulted in an increase in excitability parameters in retrogradely labeled (DiI+) dissociated trigeminal ganglia neurons (n = 5 mice, 24 neurons), whereas cell line supernatant from non-tumorigenic keratinocytes (HaCat, n = 4 mice, 19 neurons), precancerous dysplastic keratinocytes (DOK, n = 4 mice, 17 neurons), and melanoma (SkMel28, n = 4 mice, 12 neurons) did not. Data (A–C) assessed before (Pre SN) and after (Post SN) cell line supernatant application to tongue afferent neurons were pooled and plotted as means ± SE. There was a significant HSC3-induced decrease in rheobase (A) and AP threshold (B). Representative traces (C) illustrate action potentials evoked with a 250 millisecond ramp followed by a 500 millisecond sustained current injection protocol (accommodation) in single neurons at baseline and in response to cell line supernatant. Pooled data (D) demonstrate a significant increase in the number of spikes evoked after application of oral cancer cell line supernatant only. There was no difference in baseline excitability prior to cell line supernatant application; therefore, data was analyzed as a percent change from baseline using One-way ANOVA (* p < 0.05, ** p < 0.01).