Abstract
Purpose
Discussions between oncologists and advanced cancer patients (ACPs) may touch on the complex issue of clinical trial participation. Numerous initiatives have sought to improve the quality of these potentially-difficult conversations. However, we have limited data about what ACPs know about clinical research as they enter such discussions as, to date, such research has focused on the period following informed consent. This study examines ACPs’ understanding of clinical research in the treatment period before consent.
Methods
We conducted in-depth interviews with adult ACPs with limited treatment options at four clinics in an academic medical center. So as not to influence patients’ perspectives, interviewers probed patients’ knowledge of clinical research only if the patient first brought up the topic. 40–60 minute interviews were audio-recorded, transcribed, and analyzed thematically and via quantitative content analysis by an interdisciplinary team.
Results
Of 78 patients recruited, 56 (72%) spontaneously brought up the topic of clinical research during interview and are included in this analysis. Qualitative thematic analysis and quantitative content analysis revealed that patients’ knowledge varied in terms of (1) accuracy and (2) specificity (level of detail). ACPs who spoke with high specificity were not always accurate, and ACPs with accurate knowledge included both high- and low-specificity speakers.
Conclusions
ACPs’ knowledge of clinical research is variable. Patients who can discuss the technical details of their care may or may not understand the broader purpose and procedures of clinical trials. Understanding this variability is important for improving patient-provider communication about clinical research and supporting efforts to provide individualized care for ACPs.
Keywords: Decision Making, Biomedical Research, Patient Comprehension, Neoplasms, Metastatic disease, Academic Medical Centers
Discussions between advanced cancer patients (ACPs) and oncologists may include consequential and difficult discussions about clinical trials participation. The American Society of Clinical Oncology (ASCO) has recognized the challenges of communicating effectively about such complex topics [1–3], and has called on clinicians to uncover and address communication problems that hinder ACP understanding [1]. Communication skills training programs that serve these aims are widely available to oncologists [4–6] and embedded in graduate medical education [7]. Nevertheless, ACPs still struggle to understand the goals, procedures, and potential risks and benefits of clinical trials [8–11].
Incomplete or inaccurate understanding of clinical research is particularly troubling for ACPs, who become candidates for early-phase (EP) oncology trials as they exhaust standard therapies. In contrast to later-stage trials, EP trials are designed to investigate dosing and toxicity; they typically provide little to no therapeutic benefit and substantial side effects. Inadequate or inaccurate ACP knowledge in this circumstance consitutes an ethical problem, as it may compromise individuals’ ability to give informed consent. Many ACPs who have consented to EP trials, for example, express unrealistically positive expectations including the therapeutic misconception that such trials provide a pathway to cure [10, 12–18].
One way to improve provider-patient communication for early- and later-phase research would be for providers to better understand what patients know about clinical trials before trials recruitment begins. What do these patients already understand or misunderstand about clinical research when their provider first broaches the topic? At present, such data are largely unavailable. Scholarly understanding of patients’ knowledge about trials is gleaned primarily from surveys of patients who have already reviewed informed consent documents to participate in a clinical trial [8, 10, 19–24]. Moreover, clinicians do not discuss trials with all of their patients, which leaves an important group of patients outside of the focus of existing research [25]. Bridging this gap is difficult to do, as studying patients’ knowledge with surveys risks introducing ideas that are not part of their original baseline knowledge. Open-ended inquiry, in contrast, allows researchers to investigate patients’ understanding of clinical trials with minimal influence on patients’ exposure to these topics [26, 27].
This study used systematically-collected and -analyzed qualitative data to examine what ACPs who are poised to exhaust standard therapies know about clinical research. These analyses reveal the quality and types of information patients know or do not know about clinical trials as they approach a crucial yet understudied decision-point in their cancer journey.
Methods
Study design and setting
The data used here are drawn from a large study that used ethnography to examine advanced cancer patients’ understandings and decisions about early phase clinical trials. Ethnography is a qualitative, longitudinal method for investigating culture in which research subjects are encouraged to express beliefs in their own words [28]. To document the culture surrounding early phase trials our study cast a broad net, capturing information about patients’ illness experiences, treatment choices, and understandings of clinical research. The study was conducted in four cancer clinics (breast, colorectal, genitourinary, and melanoma) at a west coast academic medical center with an active clinical trials program.
Study sample and recruitment
Eligible patients were identified via an initial review of the medical record and subsequent consultation with the treating oncologist. Study inclusion criteria included capacity for meaningful communication in English, at least 18 years of age, diagnosis with metastatic cancer, no current or past enrollment in an early phase trial, and few or no available standard treatment options (assessed via review of the medical record and consultation with the treating oncologist). Recruitment was done using well-established ethnographic procedures and highly-trained ethnographic fieldworkers [28, 29]. For eligible patients, fieldworkers visited clinic on a day the patient was scheduled for a routine appointment with her/his oncologist. If the oncologist confirmed the patient’s eligibility and agreed to be observed, the oncologist introduced the fieldworker to the patient. The fieldworker described the purpose of the study and obtained oral consent to observe the visit. Though observations occurred in clinic settings, oncologists were not involved in data collection and fieldworkers were not involved in patient care.
Immediately following the visit, fieldworkers invited patients to participate in an in-depth interview, which was scheduled for a future date. At the time of the interview, fieldworkers described the study in detail, answered questions about the study, and obtained written informed consent for all study procedures. Informed consent was obtained from all individual participants included in the study. At the conclusion of the first interview, patients received a $35 gift card and were invited to participate in additional interviews and observations in the future. These recruitment procedures are also described in more detail elsewhere [30].
All study procedures were reviewed and approved by the appropriate IRB and the clinics in which they took place. In accordance with our protocol, we have taken steps to ensure the confidentiality of all who participated in this research, e.g., removing identifying details from published qualitative data.
Data collection
Fieldworkers collected multiple types of qualitative data. For each patient they conducted 2–5 direct observations of clinic visits and 1–4 in-depth interviews. The number of research contacts depended on patient availability and on whether patients felt well enough physically and emotionally to participate. Fieldworkers also conducted one in-depth interview and survey with a patient-identified caregiver when possible. Additionally, fieldworkers reviewed patients’ medical records and conducted quantitative surveys at multiple points in time.
This analysis examines data collected at the first (baseline) in-depth interview. Fieldworkers followed standard procedures for the interview. They used an in-depth semi-structured interview guide and conducted the interview as an open-ended conversation in order to capture ideas in the patient’s own words and to explore novel insights [29]. Interviews lasted approximately 60–90 minutes and were conducted at a location of the respondent’s choosing, such as the patient’s home, a private office at the medical center, or a semi-private space in the infusion center. Fieldworkers digitally recorded interviews and had them professionally transcribed verbatim.
Given the study’s aims, investigators developed study procedures that would not influence patients’ awareness or interest in clinical research. With IRB approval, fieldworkers described the study to patients as an examination of treatment decision-making in general rather than one that focused on clinical trials. If and when patients themselves raised the topic of clinical trials or research, fieldworkers then used open-ended questions to probe patients’ knowledge, experiences, and understandings of clinical research (e.g., “What does a Phase 1 trial mean to you?”; Online Resource 1). This article analyzes ACPs’ talk about clinical trials and research that occurred after patients themselves spontaneously introduced these topics during the baseline interview.
Data from patients who did not raise these topics in the baseline interview, and who therefore were not asked about their knowledge of clinical research, are not included in this analysis.
Data management and preparation
Data management occurred simultaneously with data collection so that emerging ideas, issues, and interpretations could inform ongoing fieldwork [31]. Utilizing standard ethnographic methods, fieldworkers discussed notable incidents from clinic observations and patient interviews at weekly meetings. These reports from the field informed ongoing fieldwork as well as the development and refinement of the project codes, codebook, and early analytic interpretation.
The study team entered the approximately 12,000 pages of interview transcripts and observational fieldnotes into an ATLAS.ti software database [32]. Trained analysts identified and coded patient discussions about trials or clinical research. ATLAS.ti facilitated the coding of transcripts and the systematic retrieval of data for analysis [33].
Data analysis
Analysts examined the ATLAS.ti database to identify all baseline interview transcripts in which patients discussed clinical trials or research. They extracted all baseline interview transcripts that included the codes “clinical trials” and “knowledge of the world of oncology” a code that indicated patients’ use of technical or biomedical language. They also extracted all baseline interview transcripts that contained the words “trial,” “experiment,” “study,” or “research.”
After extracting these interview transcripts, the study team conducted the analysis in two steps. First, they conducted a thematic qualitative analysis, a standard method used to uncover patterns in qualitative data [34, 35]. Multiple investigators (DD, LT, AR, PM) read and discussed the data to identify qualitative themes. Next, they conducted a confirmatory analysis of the qualitative themes by subjecting a sub-set of approximately one-third of the interviews to systematic quantitative analysis. The subset included ACPs with a range of experiences regarding engagement with clinical research [36]. Three investigators (DD, CJK, SG) independently evaluated each patient’s excerpts to discern 1) the number of facts mentioned by a patient about clinical trials or research; and 2) the overall specificity of the patient’s interview data on a 4-point scale (1-very general, 2-somewhat general, 3-somewhat specific, 4-very specific; see Table 2 for definitions). In most cases investigators agreed about the number of facts and the degree of specificity of each excerpt. When they did not, the investigators discussed the different scores until a consensus emerged. Members of the study team with clinical experience and expertise (DD, AR, PM) independently reviewed and assessed whether each fact was accurate (yes/no) to produce an accuracy ratio (accurate facts/total facts) for each transcript. This exercise also produced a high degree of consensus, and the investigators discussed and resolved disagreements at an in-person meeting.
Table 2.
Exemplar excerpts illustrating variation in patient understanding1
| Variations in accuracy, from most to least accurate |
|
Participant 1 (Accuracy: 100%, Specificity: 3.0) INT: Okay. So what does that term mean to you - Phase One? P1: Phase One means they’re still trying to figure out the dosage and safety, but they told me that it’s almost Phase Two because now they figure it out. They had it for three years to kind of figure out what’s the best dosage to be used. So I came there just with their optimal dosage... |
|
Participant 2 (Accuracy: 66%,
Specificity: 2.4) INT: And are you getting it as part of a study for the drug company or is it just a regular -- P2: You know, I think I can’t remember. ‘Cause I recall Dr. [ ] saying there was a study but I also recall her saying that the study involved taking the Multiplar drug with the Nexavar. And I think my blood tests were too poor for that. So I’m not sure if I’m in a study or not. INT: It doesn't sound like it would bother you though if it turned out that this was part of a study. P2: You know, life is a study. I mean, when you say that, it’s more about people worrying about who the information gets released to. And once again, I don’t -- You really have to explain to me someone that would have a problem and why they would have a problem with -- I mean, I understand the problem with trying to get insurance and not wanting them to know, and that’s pretty much what I call, that’s on the edge of being fraudulent anyway, both you and the insurance company. But yeah, I mean, I’m all about open information. Once again, I don’t see holding back anything. Just hinders society, hinders the individual. |
|
Participant 3 (Accuracy: 40%, Specificity: 4.0) INT: ... One of the things we’re actually very interested in in this study is the new thing and how patients think about the upsides or downsides of getting the new thing and particularly around early phase clinical trials. And it sounds like you’ve probably thought about these issues a lot. I’m curious what your thoughts are. P3: Right. Well early phase clinical trials, I wouldn't be in an early phase because I don't know if I’m getting the drug or not. I would be in a late phase, 2 or 3, clinical trial. I’ve not been in a clinical trial because I’ve been fortunate enough to get the benefits of a clinical trial after, whether it’s FDA approved or not. So those have been my two experiences. With the XGEVA, I got it before it was FDA approved and with the, certainly now there’s a drug I’m on, the Afinitor, and Afinitor is what I’ve been on since December. My markers - it’s a tricky thing with markers because my markers said my circulating tumor cells have always been really low, like zero to five. My CEA is not high but my CA1530 is always very high but as Dr. [ ] said, we don’t have a baseline so we don’t know. |
| Variations in specificity, from most to least specific |
|
Participant 4 (Specificity: 3.8, Accuracy: 89%) P4: At Johns Hopkins it was all cardiac. I worked in the Cardiology Department, so it was everything from basic research to clinical trials with patients coming in. And at Scripps Clinic it was all basic research, no patients. INT: Okay. So I’m going to ask you a little bit about your understanding of the trial that you’re currently on. What phase is it in, do you know? P4: I don't know. Phase I maybe for this drug INT: Okay. So tell me what Phase I trial means to you. P4: That they’ve got a well in this case, a combination of drugs that seems to be working and they’re moving it and it’s a carryon from the original B-RAF study. And the MEC appears to have put a second roadblock to cell division and I think it was January that they started this phase. So it may be just a carryon from the first one or it may be a new phase of a new study. So my understanding is that they’re bringing patients in, putting them on, testing us all the time to see how we’re doing with the drug combination. INT: Okay. So would you say the purpose of the trial is to determine how well the drug works or is it to do something else? P4: I think it’s to determine the efficacy of the drug and how it affects the patients, what kind of side effects are there and what they need to do to minimize the side effects. |
|
Participant 5 (Specificity: 2.2, Accuracy: 93%) INT: Okay. So sometimes people talk about trials being in phases. Do you recall there being mention of a phase for that? P5: I don’t. Doesn't mean it wasn't but I don’t remember. INT: That’s fine. And who was running the trial? Does that sound familiar? P5: A lady named Dr. [ ]... [referring to director of EP clinic]. P5: We never did because the genes, mine were not the kind that they could turn back on. They were either absent or I don't know what else their problem was, mutated in some way so that they were not the kind that can be awakened by that program. |
|
Participant 6 (Specificity: 1.0, Accuracy: n/a) INT: Okay. The trial that you’re thinking about doing, do you know what phase it’s in? P6: I don’t. INT: Okay. Do you know what it means when they say trials are phased? P6: No. |
INT= Interviewer;
P[number] = Participant identification
Results
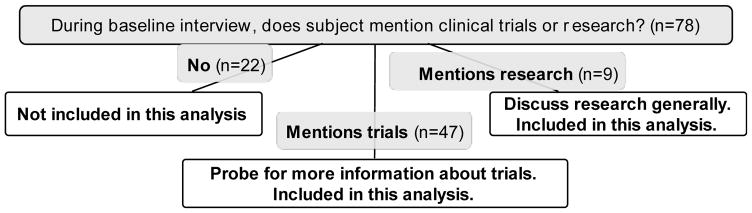
Fieldworkers conducted observations in 4 clinics over 18 months. Approximately 75% of observed ACPs were deemed eligible, and 80% of eligible patients (60% of those observed) provided contact information. Of the 125 who provided contact information, 20 proved unreachable, 16 declined to participate in an interview, and 7 died before they could be interviewed. Additional in-depth review of patient medical records identified 4 patients who participated in the study but were actually ineligible due to prior experience on early phase trials. These patients were removed from the study analysis. In total, 78 patients (62% of those who provided contact information) completed a baseline interview and were observed in clinic; they comprise our Advanced Cancer Cohort (ACC; Table 1). Most patients went on to participate in additional study interviews and observations as well (total means of 1.9 and 2, respectively). Fifty-six (72%) patients in the ACC spontaneously mentioned clinical trials (n = 47) or clinical research (n = 9) during their baseline interview (Figure 1), as described below.
Table 1.
Characteristics of the Advanced Cancer Cohort (N = 78)
| n | % | |
|---|---|---|
| Cancer Site | 17 | 22 |
| Breast | 25 | 32 |
| Genitourinary | 23 | 29 |
| Gastrointestinal | 13 | 17 |
| Melanoma | ||
| Gender | ||
| Male | 47 | 60 |
| Female | 31 | 40 |
| Age | ||
| 28–40 | 3 | 4 |
| 41–55 | 27 | 35 |
| 56–65 | 20 | 25 |
| 66–75 | 24 | 31 |
| 76+ | 4 | 5 |
| Race/Ethnicity* | ||
| White | 50 | 64 |
| Non-White | 20 | 26 |
| Missing | 8 | 10 |
| Marital status* | ||
| Married | 47 | 60 |
| Not Married | 23 | 30 |
| Missing | 8 | 10 |
| Educational level* | ||
| BA+ | 45 | 58 |
| <BA | 25 | 32 |
| Missing | 8 | 10 |
Eight patients did not complete the survey portion of the interview in which information for these 3 characteristics was obtained.
Figure 1.
Mentions of clinical research in baseline interviews of the Advanced Cancer Cohort (ACC)
Characterizing patient understanding
Shortly after data collection began, fieldworkers observed variation in the degree to which study participants understood the purposes and procedures of clinical research. Fieldworkers noted in their weekly reports and in discussion at the research team meetings that some patients spoke with great accuracy about clinical research while others revealed significant misconceptions even about trials in which they participated. Moreover, they noted that some patients spoke in general terms about clinical research while others described it in more specific terms (e.g., the procedures of a given study; the types of therapeutic strategies under investigation; how therapies in development compared to existing and approved treatments).
After completing data collection and coding, the research team systematically investigated these variations in understanding in the qualitative data. Their search of the ATLAS.ti database yielded 152 pages of baseline interview excerpts in which patients discussed clinical trials or research (n = 56; these data are used for all following analyses). Thematic analysis confirmed that variation in the accuracy and specificity of ACPs’ accounts of clinical trials and research were prevalent in the data. Table 2 shows illustrative examples. A patient who spoke with a high degree of accuracy, for example, correctly described the purpose of Phase 1 trials as “trying to figure out the dosage and safety” (Participant 1), whereas one who demonstrated low accuracy asserted incorrectly that Phase 1 trials employ randomization and placebos (Participant 3). Similarly, a patient who communicated with high specificity mentioned precise terms that were specific to cancer treatment (e.g., a B-RAF study, MEC chemotherapy; Participant 4), whereas patients who communicated with low specificity included no detailed information (Participant 6) or only widely-known health-related terms that are not specific to cancer care (e.g., “genes,” “mutation”; Participant 5).
Study investigators then conducted confirmatory quantitative content analysis of approximately one-third of these interviews (n=19), described above. The number of facts in patients’ excerpts ranged from 0 to 11, with a mean of 4.4. Specificity scores ranged from 1 (very general) to 4 (very specific) with a mean score of 2.3. The accuracy scores for excerpts that had one or more facts ranged from 40% – 100% with a mean of 85%. These findings supported the interpretive insights derived from the qualitative review. These results are summarized in Online Resource 2.
Examination of the specificity and accuracy of the information communicated by patients revealed four distinct patterns of patient knowledge, which we illustrate here with excerpts from Table 2. One group of patients demonstrated both accuracy and specificity in understanding. Patients in this group communicated specific information about clinical research and trials and this information was quite accurate. For example, asked what the term “phase 1” means to her, Participant 1 used specific concepts (e.g., dosage, Phase 2, optimal dosage) in her factually correct answer: “Phase 1 means they’re still trying to figure out the dosage and safety, but they told me that it’s almost Phase 2 because now they figure it out. They had it for three years to kind of figure out what’s the best dosage to be used. So I came there just with their optimal dosage...” Participant 4 used specific technical terms in her accurate description of the trial she had joined: “[The study is] a carryon from the original B-RAF study. And the MEC appears to have put a second roadblock to cell division…”
A second group of patients spoke of clinical research using mostly vague, non-specific terms and less accurate information or no information. For example, Participant 2 used general, non-technical language for nearly all of his extended discussion of trials participation, and said “I’m not sure if I’m in a study or not…You know, life is a study.” Participant 6 provided no factual information about the trial she was considering:
Interviewer: Do you know what phase it’s in?
Participant: I don’t.
Interviewer: Okay. Do you know what it means when they say trials are phased?
Participant: No.
A third group of patients communicated highly-specific information about clinical trials and research, but the information they discussed was mostly inaccurate. For example, Participant 3 used many technical terms in her discussion (“CEA,” “CA1530,” “baseline”), but incorrectly asserted that early phase trials employ randomization: “Well early phase clinical trials, I wouldn't be in an early phase because I don't know if I’m getting the drug or not…My markers said my circulating tumor cells have always been really low, like zero to five. My CEA is not high but my CA1530 is always very high but as Dr. [ ] said, we don’t have a baseline so we don’t know.”
A fourth group of patients used general terms to discuss trials and research, yet did so with a high degree of accuracy. Participant 5, for example, used almost no specific scientific language, yet he was accurate in describing why he was ineligible for a trial: “My [genes] were not the kind that they could turn back on. They were either absent or…mutated in some way so that they were not the kind that can be awakened by that program.”
Discussion
Helping advanced cancer patients understand the goals, procedures, and potential risks and benefits of clinical research participation has been a longstanding challenge [12, 37, 38]. To date, researchers have focused on what patients learn from the consent forms they review during recruitment [8, 23, 24]. These studies are important, but they exclude patients who never initiate trials recruitment, e.g. the vast majority of all cancer patients. These studies also do not capture what patients know before recruitment baseline knowledge that arguably establishes a foundation for patients’ openness to and perception of clinical trial offers.
Using qualitative data, this study provides insights into how ACPs make sense of clinical trials and research. We found that some ACPs were able to discuss clinical research in accurate and specific detail, but others included few specifics or were inaccurate. Our ACP sample was relatively well-educated, which suggests that inaccurate or insufficient understanding of clinical research occurs even among patients with high health literacy 9, 39]. Our results also suggest that the specificity and accuracy of patients’ knowledge was often linked but not necessarily so. These findings regarding advance cancer patients’ baseline trials knowledge has implications for providers and clinical researchers.
These results remind providers of the value of teaching purposively about clinical research opportunities. Many cancer patients do not understand fundamental information about cancer clinical trials before or after they consent [8–10, 23, 24]. Our qualitative data suggest that some patients can accurately describe specific details of a study to which they have been recruited and thus sound well-informed without understanding the fundamentals of clinical research and their role in it. Providers who are attuned to this complex knowledge pattern can communicate purposively with their patients. They should consider focusing any discussions of clinical trials on the fundamental ways in which clinical research proceeds and their patients’ role in it. They may want to defer discussion of complex or technical study details until they are confident that patients appreciate the broader context in which the specific study takes place. Providers could consider incorporating teach-back or similar techniques to assess patients’trials knowledge. And they should consider whether patients could be better served by introducing the possibility of clinical research participation early in the therapeutic relationship [8, 40].
Contemporary informed consent practices typically emphasize study-specific details about trial procedures, benefits, and risks [41, 42]. Our results suggest that clinician-researchers should ensure that consent practices communicate the general purpose and nature of research participation not merely the technical procedures of an individual study. This recommendation is consistent with recently-adopted changes to the Federal Common Rule governing consent for clinical research [43]. The revised Common Rule mandates that informed consent for clinical research not merely provide lists of isolated facts but facilitate a prospective subject’s understanding of the reasons one might want to participate in research. While no specific evidence was cited in the Federal Register to justify this provision of the Rule, the results of this study support this policy change. The results we present here provide a starting point for future research on how to implement the Common Rule’s approach “emphasizing efforts to foster understanding overall” of clinical research participation.
Finally, these findings provide a starting point for future research on how accuracy and specificity about trials are distributed across patient populations; how they change over time; and how they correlate with characteristics such as age, education, socio-economic status, and health literacy. These findings point to a need to explore how patients achieve accurate understanding of the fundamentals, as distinct from the specifics, of clinical research. Such information would be useful for characterizing the “optimal content” of clinicians’ conversations with ACPs to promote informed patient choice [1]. It would also further our understanding of the phenomenon of therapeutic misconception and aid attempts to develop effective interventions to combat it [13, 14].
This study has several limitations. As in most ethnographic studies, these findings cannot be generalized. These results highlight important new questions, and ongoing research must explore their breadth and generalizability. For example, this study’s sample size is insufficient to examine how knowledge of clinical research varies with education, income or race/ethnicity; future studies should examine these important questions, as noted above. A second limitation is that we examined data from a self-selected group of ACPs who spontaneously discussed the issue of clinical research during a baseline interview. It is possible that data derived from highly-structured interviews would differ from what we obtained using a semi-structured protocol. However, the interview approach we used was necessary for the overall goals of our ethnographic study. During planned future analysis of the complete qualitative dataset, we will assess similarities and differences between baseline and follow-up interviews. A third limitation is that we conducted this study at an academic medical center, which serves a disproportionately highly-educated, high-income patient population. This limits the direct generalizability of our results. However, less-advantaged patients face even greater barriers to obtaining specific and accurate knowledge of clinical trials and research. As such, our results in this highly-advantaged population may actually understate the importance of these issues for advanced cancer patients more generally. Finally, our identification and interpretation of patients’ expressions of knowledge could have been vulnerable to observation bias, but we took numerous steps to minimize this risk (e.g., employing codes to retrieve data, triangulating interpretations across two analytic approaches, convening a transdisciplinary research team).
In summary, this study documents new dimensions of variation in advanced cancer patients’ knowledge about clinical trials and research. It is widely appreciated that more can be done to enhance patients’ understanding of care options and clinical research. Thisstudy points to ways in which enhanced understanding might be achieved.
Supplementary Material
Acknowledgments
RESEARCH SUPPORT
The research for this publication was sponsored by the National Cancer Institute (NCI) award #R01 CA152195.
Footnotes
Ethical approval: “All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.”
PREVIOUS PRESENTATION
Poster at the ASCO Annual Meeting, July 2014
DISCLAIMERS
None
References
- 1.Peppercorn JM, Smith TJ, Helft PR, et al. American society of clinical oncology statement: Toward individualized care for patients with advanced cancer. J Clin Oncol. 2011;29:755–760. doi: 10.1200/JCO.2010.33.1744. [DOI] [PubMed] [Google Scholar]
- 2.Jenkins V, Solis-Trapala I, Langridge C, et al. What oncologists believe they said and what patients believe they heard: an analysis of phase I trial discussions. J Clin Oncol. 2011;29:61–8. doi: 10.1200/JCO.2010.30.0814. [DOI] [PubMed] [Google Scholar]
- 3.Wall L, Farmer ZL, Webb MW, et al. Description of the types and content of phase 1 clinical trial consent conversations in practice. Clin Trials. 2015;12:567–574. doi: 10.1177/1740774515601679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pham AK, Bauer MT, Balan S. Closing the patient-oncologist communication gap: a review of historic and current efforts. J Cancer Educ. 2014;29:106–13. doi: 10.1007/s13187-013-0555-0. [DOI] [PubMed] [Google Scholar]
- 5.Barth J, Lannen P. Efficacy of communication skills training courses in oncology: a systematic review and meta-analysis. Ann Oncol. 2011;22:1030–40. doi: 10.1093/annonc/mdq441. [DOI] [PubMed] [Google Scholar]
- 6.Back AL, Arnold RM, Baile WF, et al. Efficacy of Communication Skills Training for Giving Bad News and Discussing Transitions to Palliative Care. Arch Intern Med. 2007;167:453. doi: 10.1001/archinte.167.5.453. [DOI] [PubMed] [Google Scholar]
- 7.Accreditation Council for Graduate Medical Education. ACGME Common Program Requirements. Chicago, IL: 2016. [Google Scholar]
- 8.Miller JD, Kotowski MR, Comis RL, et al. Measuring Cancer Clinical Trial Understanding. Health Commun. 2011;26:82–93. doi: 10.1080/10410236.2011.527624. [DOI] [PubMed] [Google Scholar]
- 9.Biedrzycki BA. Research Information Knowledge, Perceived Adequacy, and Understanding in Cancer Clinical Trial Participants. Oncol Nurs Forum @BULLET. 2011;38 doi: 10.1188/11.ONF.E291-E296. [DOI] [PubMed] [Google Scholar]
- 10.Pawlowski J, Malik Lbc, Mahalingam Dbc. Advanced cancer patients understanding and perceptions of phase i clinical trials. Cancer Invest. 2015;33:490–495. doi: 10.3109/07357907.2015.1069833. [DOI] [PubMed] [Google Scholar]
- 11.Pentz RD, White M, Harvey RD, et al. Therapeutic misconception, misestimation, and optimism in participants enrolled in phase 1 trials. Cancer. 2012;118:4571–8. doi: 10.1002/cncr.27397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Weeks JC, Catalano PJ, Cronin A, et al. Patients’ expectations about effects of chemotherapy for advanced cancer. N Engl J Med. 2012;367:1616–25. doi: 10.1056/NEJMoa1204410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Miller M. Phase I Cancer Trials: A Collusion of Misunderstanding. Hastings Cent Rep. 2000;30:34. doi: 10.2307/3527646. [DOI] [PubMed] [Google Scholar]
- 14.Roberts TG, Goulart BH, Squitieri L, et al. Trends in the Risks and Benefits to Patients With Cancer Participating in Phase 1 Clinical Trials. JAMA. 2004;292:2130. doi: 10.1001/jama.292.17.2130. [DOI] [PubMed] [Google Scholar]
- 15.Jenkins VA, Anderson JL, Fallowfield LJ. Communication and informed consent in phase 1 trials: a review of the literature from January 2005 to July 2009. Support Care Cancer. 2010;18:1115–21. doi: 10.1007/s00520-010-0836-7. [DOI] [PubMed] [Google Scholar]
- 16.Godskesen T, Nygren P, Nordin K, et al. Phase 1 clinical trials in end-stage cancer: patient understanding of trial premises and motives for participation. Support Care Cancer. 2013;21:3137–42. doi: 10.1007/s00520-013-1891-7. [DOI] [PubMed] [Google Scholar]
- 17.Bruce JY, Hlubocky FJ, Daugherty CK. 4 “Was it worth it?” A pilot study of advanced cancer patients’ retrospective perceptions of benefit from Phase 1 trial participation. J Investig Med. 2007;55:S347. [Google Scholar]
- 18.Fortier I, Burton PR, Robson PJ, et al. Quality, quantity and harmony: the DataSHaPER approach to integrating data across bioclinical studies. Int J Epidemiol. 2010;39:1383–93. doi: 10.1093/ije/dyq139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Joffe S, Cook EF, Cleary PD, et al. Quality of informed consent in cancer clinical trials: a cross-sectional survey. Lancet. 2001;358:1772–1777. doi: 10.1016/S0140-6736(01)06805-2. [DOI] [PubMed] [Google Scholar]
- 20.Olver IN, Buchanan L, Laidlaw C, Poulton G. The adequacy of consent forms for informing patients entering oncological clinical trials. Ann Oncol. 1995;6:867–70. doi: 10.1093/oxfordjournals.annonc.a059352. [DOI] [PubMed] [Google Scholar]
- 21.Jefford M, Mileshkin L, Matthews J, et al. Satisfaction with the decision to participate in cancer clinical trials is high, but understanding is a problem. Support Care Cancer. 2011;19:371–379. doi: 10.1007/s00520-010-0829-6. [DOI] [PubMed] [Google Scholar]
- 22.Biedrzycki Ba. Decision making for cancer clinical trial participation: a systematic review. Oncol Nurs Forum. 2010;37:387–99. doi: 10.1188/10.ONF.E387-E399. [DOI] [PubMed] [Google Scholar]
- 23.Agrawal M, Grady C, Fairclough DL, et al. Patients’ decision-making process regarding participation in phase I oncology research. J Clin Oncol. 2006;24:4479–84. doi: 10.1200/JCO.2006.06.0269. [DOI] [PubMed] [Google Scholar]
- 24.Sanchini V, Reni M, Calori G, et al. Informed consent as an ethical requirement in clinical trials: an old, but still unresolved issue. An observational study to evaluate patient’s informed consent comprehension. J Med Ethics. 2014;40:269–75. doi: 10.1136/medethics-2012-101115. [DOI] [PubMed] [Google Scholar]
- 25.Joseph G, Dohan D. Diversity of participants in clinical trials in an academic medical center: the role of the “Good Study Patient?”. Cancer. 2009;115:608–15. doi: 10.1002/cncr.24028. [DOI] [PubMed] [Google Scholar]
- 26.Fernandez JW, Herzfeld M. In search of meaningful methods. In: Bernard HR, Gravlee CC, editors. Handb Methods Cult Anthropol. 2. Rowman & Littlefield; Lanham, MD: 2014. pp. 55–96. [Google Scholar]
- 27.Lambert H, McKevitt C. Anthropology in health research: from qualitative methods to multidisciplinarity. Bmj. 2002;325:210–213. doi: 10.1136/bmj.325.7357.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Atkinson P, Coffey A, Delamont S, et al. Handbook of ethnography. Sage; 2001. [Google Scholar]
- 29.Emerson RM, Fretz RI, Shaw LL. Writing Ethnographic Fieldnotes, Second Edition (Google eBook) University of Chicago Press; 2011. [Google Scholar]
- 30.Dunn LB, Wiley J, Garrett S, et al. Interest in Initiating an Early Phase Clinical Trial: Results of a Longitudinal Study of Advanced Cancer Patients. Psychooncology. 2016 doi: 10.1002/pon.4179. [DOI] [PubMed] [Google Scholar]
- 31.Strauss AL, Corbin JM. Basics of qualitative research: grounded theory procedures and techniques. Sage Publications; 1990. [Google Scholar]
- 32.Weitzman EA. Analyzing qualitative data with computer software. Health Serv Res. 1999;34:1241–63. [PMC free article] [PubMed] [Google Scholar]
- 33.Weitzman EA, Miles MB. Choosing software for qualitative data analysis: An overview. Field methods. 1995;7:1–5. [Google Scholar]
- 34.Boyatziz R. Transforming qualitative information: Thematic analysis and code development. Sage; Thousand Oaks, CA: 1998. [Google Scholar]
- 35.Guest G, MacQueen KM. Applied Thematic Analysis. Sage; Thousand Oaks, CA: 2011. [Google Scholar]
- 36.Patton MQ. Qualitative evaluation and research methods. 2. Sage Publications, Inc; 1990. [Google Scholar]
- 37.Friis LS, Elverdam B, Schmidt KG. The patient’s perspective A qualitative study of acute myeloid leukaemia patients’ need for information and their information-seeking behaviour. Support Care Cancer. 2003;11:162–170. doi: 10.1007/s00520-002-0424-6. [DOI] [PubMed] [Google Scholar]
- 38.Hack TF, Degner LF, Parker PA, et al. The communication goals and needs of cancer patients: A review. Psychooncology. 2005;14:831–845. doi: 10.1002/pon.949. [DOI] [PubMed] [Google Scholar]
- 39.Koh HK, Brach C, Harris LM, Parchman ML. A proposed “health literate care model” would constitute a systems approach to improving patients’ engagement in care. Health Aff (Millwood) 2013;32:357–67. doi: 10.1377/hlthaff.2012.1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kao CY, Aranda S, Krishnasamy M, Hamilton B. Interventions to improve patient understanding of cancer clinical trial participation: a systematic review. Eur J Cancer Care (Engl) 2017;26:e12424. doi: 10.1111/ecc.12424. [DOI] [PubMed] [Google Scholar]
- 41.Flory J, Emanuel E. Interventions to improve research participants’ understanding in informed consent for research: a systematic review. JAMA. 2004;292:1593–601. doi: 10.1001/jama.292.13.1593. [DOI] [PubMed] [Google Scholar]
- 42.Malik L, Kuo J, Yip D, Mejia A. How well informed is the informed consent for cancer clinical trials? Clin Trials. 2014;11:686–688. doi: 10.1177/1740774514548734. [DOI] [PubMed] [Google Scholar]
- 43.Federal Policy for the Protection of Human Subjects. Fed Regist. 2017;82:7149–7274. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.