Abstract
Background
Inflammasome-mediated neuroinflammation may cause secondary injury following traumatic brain injury (TBI) in children. The pattern recognition receptors NLRP1 and NLRP3 are essential components of their respective inflammasome complexes. We sought to investigate whether NLRP1 and/or NLRP3 abundance is altered in children with severe TBI.
Methods
Cerebrospinal fluid (CSF) from children (n=34) with severe TBI (Glasgow coma scale score [GCS] ≤8) who had externalized ventricular drains placed for routine care was evaluated for NLRP1 and NLRP3 at 0–24h, 25–48h, 49–72h, and >72h post-TBI and was compared to infection-free controls that underwent lumbar puncture to rule out CNS infection (n=8). Patient age, sex, initial GCS, mechanism of injury, treatment with therapeutic hypothermia, and 6 month Glasgow outcome score (GOS) were collected.
Results
CSF NLRP1 was undetectable in controls and detected in 2 TBI patients and at only <24h post-TBI. CSF NLRP3 levels were increased in TBI patients compared with controls at all time points, p<0.001. TBI patients ≤ 4 years of age had higher peak NLRP3 levels vs. patients > 4 (15.50 [3.65–25.71] vs. 3.04 [1.52–8.87] ng/mL, respectively; p=0.048). Controlling for initial GCS in multivariate analysis, peak NLRP3 > 6.63ng/mL was independently associated with poor outcome at 6 months.
Conclusions
In the first report of NLRP1 and NLRP3 in childhood neurotrauma we found that CSF NLRP3 is elevated in children with severe TBI and independently associated with younger age and poor outcome. Future studies correlating NLRP3 with other markers of inflammation and response to therapy are warranted.
Keywords: NLRP3, NLRP1, Inflammasome, Traumatic brain injury, Biomarker, Neuroinflammation, Secondary Brain Injury, Pediatric
INTRODUCTION
Traumatic brain injury (TBI) accounts for over 60,000 hospitalizations in children per year in the U.S. alone and is a major cause of trauma-related morbidity and mortality1,2. The neuroinflammatory response to TBI, activated by the release of host-derived proteins termed danger associated molecular patterns (DAMPs), may lead to delayed neuronal death and impact recovery. Several studies indicate a critical role for the inflammasome complex in initiating the immune response to DAMPs after brain trauma3,4. Multiple inflammasome complexes have been described within the central nervous system (CNS) including NACHT domain-, Leucine rich repeat-, and PYD-containing Protein 1 (NLRP1) and NLRP3. The inflammasome complex amplifies cleavage of pro-caspase-1 to caspase-1, which then cleaves pro-interleukin (IL)-1β and pro-IL-18 to the active cytokines 5–9. Caspase-1 and IL-1β are rapidly activated after TBI and protein levels remain elevated through 3 days post-injury 10. Adults with severe TBI were found to have detectable cerebrospinal fluid (CSF) levels of NLRP1 and caspase-1 which correlated with functional outcome at 5 months post-TBI 5. In children with severe TBI, caspase-1 levels are increased in CSF after TBI, particularly in patients ≤ 4 years old 11.
NLRP1 inflammasome complex formation and lesion volume in a TBI model are reduced by antibody to the inflammasome adaptor protein apoptosis-associated speck-like protein containing a CARD (ASC) 7. In another TBI model, NLRP3 and inflammasome complexes were found to be increased in the pericontusional cortex following injury 9.
To date, there have been no studies of the NLRP1 or NLRP3 inflammasome proteins after childhood trauma. We hypothesized that the NLRP1 and NLRP3 would be increased after severe TBI in children and detectable in CSF compared with uninjured control subjects. and that NLRP1 and NLRP3 would be associated with clinical variables and outcome (Glasgow Outcome Scale [GOS] score). We believe that these studies could provide evidence for this innate immune system mechanism within such patients, justifying evaluation of therapies targeting inflammasome-mediated neuroinflammation.
MATERIALS AND METHODS
Patients
All of the study procedures outlined below, including the collection of CSF from children with severe TBI, data collection from the medical records and interventions as part of the parent studies, were approved by the Institutional Review Board of the University of Pittsburgh. Children (age ≤ 18 years at the time of injury) with severe TBI (Glasgow Coma Scale score (GCS) ≤ 8) who had an externalized ventricular drain (EVD) placed as part of their standard care were eligible for inclusion in this study (n = 34). CSF samples were collected at four time points after injury: <24h, 25–48h, 49–72h, and >72h. Once collected, CSF was centrifuged to remove cellular debris and stored at −80°C for batch analysis. CSF from children undergoing lumbar puncture to rule out CNS infection, but ultimately shown to be infection-free, was used as control (n = 8).
Management of children with severe TBI at our institution has been previously described and is in accordance with the most recent evidence-based guidelines12. A subset of children included in this study were enrolled in a randomized, controlled trial of early therapeutic hypothermia (32–33°C for 48h) after severe TBI (NCT00222742)13. The following variables were recorded: age at the time of injury, patient sex, race, GCS, mechanism of injury, diagnosis of abusive head trauma by the hospital’s Child Advocacy Center, use of therapeutic hypothermia, and GOS at 6 months after injury.
Enzyme-Linked Immunosorbent Assay (ELISA)
Commercially available ELISA kits for NLRP1 and NLRP3 (MyBioSource, San Diego, CA) with detection ranges of 18.75 – 1200 pg/mL and 0.156 – 10 ng/mL, respectively, were used for this analysis. All procedures were performed in accordance with the manufacturer’s instructions. Specifically, standards and CSF samples (100 μL) were loaded into wells and incubated for 2 hours at 37°C. The primary biotin-antibody was added to each well and incubated at 37°C for 1 hour. Following a buffer wash, the secondary antibody and detector horseradish peroxidase (HRP)-avidin was added to all wells for a 1-hour incubation at 37°C. The chromogenic substrate - 3,3′,5,5′-Tetramethylbenzidine (TMB) - was then applied, the plate was covered in foil, and then incubated at 37 degrees for 30 min. Lastly, the stop solution was added and the optical density was measured using a microplate reader set to 450 nm with a correction wavelength of 540 nm. A standard curve was created and the concentrations of NLRP1 and NLRP3 from individual samples were determined.
Statistical Analysis
Demographic information among cases versus controls was examined using the Fisher exact test and student’s t-test for categorical and continuous variables, respectively. Trends in NLRP1 and NLRP3 across the four time points were analyzed using repeated measures ANOVA. Age and GOS variables were a priori dichotomized to facilitate analysis with respect to NLRP3 level. Further analysis between NLRP3 and demographic data was completed using the Mann-Whitney U Test. A receiver operating characteristic (ROC) curve was generated for peak NLRP3 level with relationship to outcome. Youden’s J statistic was calculated for optimal NLRP3 cutoff to predict poor outcome and this was used in univariate and multivariate logistic regression analysis. Multivariate analysis was performed to determine associations between peak NLRP3 and 6 month GOS adjusting for GCS and demographic variables with p<0.1 in univariate analysis. All NLRP3 values are provided as median [interquartile range] or mean [±SEM] with two-sided p < 0.05 considered statistically significant. Calculations were performed with GraphPad Prism (GraphPad Software, Inc., La Jolla, CA) and SPSS version 23 (IBM Corp, Armonk, NY).
RESULTS
Thirty-four children who suffered severe TBI were included in the study, contributing a total of 122 CSF samples (Table 1). Median GCS was 7 (range: 3–14). Motor vehicle accident (MVA) was the most common mechanism of injury (29.4%), followed by pedestrian accidents (11.8%), abusive head trauma (AHT 17.6%), falls (14.7%), and other mechanisms (26.5%). Sixteen patients (47%) were part of the Cool Kids trial. Of these, 7 (43.75%) were randomized to therapeutic hypothermia. All other patients received standard care. Most of the case population (91.2%) identified as Caucasian. These cases were compared to 8 controls which had similar mean age (7.68 years [±0.88] vs 10.69 years [±2.18] respectively, p=0.160) and sex (67.6% vs 75.0% male, p=1.00) across groups.
Table 1.
Demographic Data for Children with Severe Traumatic Brain Injury and Controls
| TBI | Control | |
|---|---|---|
| N | ||
| All | 34 | 8 |
| ≤ 4y | 12 (35.3) | 2 (25) |
| 5–18y | 22 (64.7) | 6 (75) |
| Age, years | 7.68 ± 2.18 | 10.69 ± 0.89 |
| Male | 23 (67.6) | 6 (75) |
| Race | ||
| Caucasian | 31 (91.2) | |
| African American | 1 (2.9) | |
| Other | 2 (5.9) | |
| GCS | 7 [3–14] | |
| Mechanism of injury | ||
| MVA | 10 (29.4) | |
| Pedestrian vs Vehicle | 4 (11.8) | |
| Abusive Head Trauma | 6 (17.6) | |
| Fall | 5 (14.7) | |
| Other | 9 (26.5) | |
| GOS | ||
| 5 (good) | 16 (47.1) | |
| 4 (moderate disability) | 11 (32.4) | |
| 3 (severe disability) | 3 (8.8) | |
| 2 (vegetative) | 0 (0) | |
| 1 (death) | 4 (11.8) |
Data presented as mean ± SE, n (%), median[range] as appropriate
NLRP1 was detectable in a pilot study of 2 out of 18 patients (11%) with severe TBI at 0–24h and in each case returned to undetectable levels by 24–48h. Additionally, none of the 8 control patients had detectable levels of NLRP1 in their CSF. Given the lack of NLRP1 elevation after pediatric trauma in the initial samples, additional analysis of NLRP1 was not performed.
Twenty-six patients had CSF measured at all four time frames and 8 patients had CSF measured at 1 – 3 time frames. NLRP3 was detectable in all 122 CSF samples tested from cases (Table 2) and in all 8 controls. Among the 26 cases with four NLRP3 measurements, the concentration of NLRP3 was significantly higher than controls at all four time frames (0–24h: 5.75 ng/mL [1.56 – 21.55], 25–48h: 1.87 ng/mL [0.82 – 4.86], 49–72h: 2.43 ng/mL [0.75 – 6.57], >72h: 4.12 ng/mL [1.71 – 9.74], controls: 0.33 ng/mL [0.26 – 0.47]; p < 0.001 at all time points) (Figure 1). We determined the minimum NLRP3 value for each patient across the 4 time frames and compared those values to controls and found the group median was 1.15 ng/mL [0.70 – 2.91] vs. 0.33 ng/mL [0.26 – 0.47] in cases vs. controls, respectively, p<0.001.
Table 2.
Mean and Peak NLRP3 levels by Demographics in Children with Severe TBI
| Mean NLRP3 Level | Peak NLRP3 Level | |
|---|---|---|
| All Children with Severe TBI | 2.65 [1.73–11.37] | 4.76 [1.70–18.50] |
| Age | ||
| ≤ 4 years | 9.00 [1.73–11.37] | 15.50 [3.65–25.71] |
| > 4 years | 2.12 [1.06–5.27] | 3.04 [1.52–8.87] |
| Sex | ||
| Male | 2.58 [1.35–11.65] | 6.10 [1.53–21.16] |
| Female | 3.64 [1.17–5.26] | 4.11 [2.20–17.59] |
| Mechanism of Injury | ||
| MVA | 3.96 [1.89–14.45] | 13.63 [2.96–27.75] |
| Pedestrian vs. Vehicle | 1.36 [0.91–7.08] | 2.49 [1.01–14.06] |
| Abusive Head Trauma | 1.86 [1.02–9.09] | 6.26 [1.50–17.99] |
| Fall | 4.02 [0.99–10.10] | 5.40 [1.87–19.78] |
| Other | 2.71 [1.06–4.98] | 2.94 [1.40–9.16] |
| GOS | ||
| Favorable Outcome (4–5) | 1.91 [1.09–8.77] | 3.49 [1.53–17.59] |
| Unfavorable Outcome (1–3) | 6.09 [3.84–9.37] | 8.59 [7.16–26.36] |
Data presented as median [IQR]
MVA, motor vehicle accident; GOS, Glasgow Outcome Scale
Figure 1. CSF concentration of NLRP3 over time after traumatic brain injury in children.
CSF NLRP3 concentration was determined at all four time points in 26 cases, and at a single time point in 8 controls. The CSF levels of NLRP3 in children with severe TBI were significantly increased at all four time points vs. controls. The temporal trend fit a quadratic regression line (p<0.001). (*p < 0.001 vs. control).
NLRP3 levels showed a significant change over time. The most common temporal pattern was a peak within the first 24 hours after TBI, decreased between 25–48, then increased at samples taken from 48–72h and > 72h. Repeated measures ANOVA revealed this to be a significant quadratic trend, p<0.001, rather than a linear relationship.
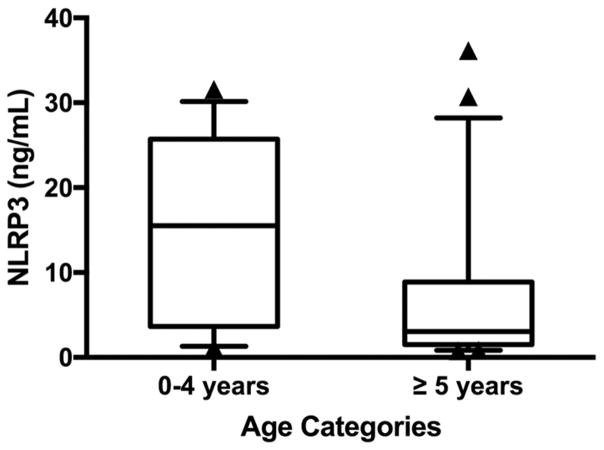
Younger patients (≤ 4 years) had a higher peak NLRP3 level (group median 15.50 ng/mL [3.65–25.71]) compared with older patients (age > 4 years) (group median 3.04 ng/mL [1.52–8.87], p=0.048) (Figure 2).
Figure 2. Maximum NLRP3 level by age.
Younger children (age 0–4 years) had a higher peak NLRP3 level of 15.50 ng/mL [3.65–25.71] compared to older patients (age ≥ 5 years) at 3.04 ng/mL [1.52–8.87], p=0.048.
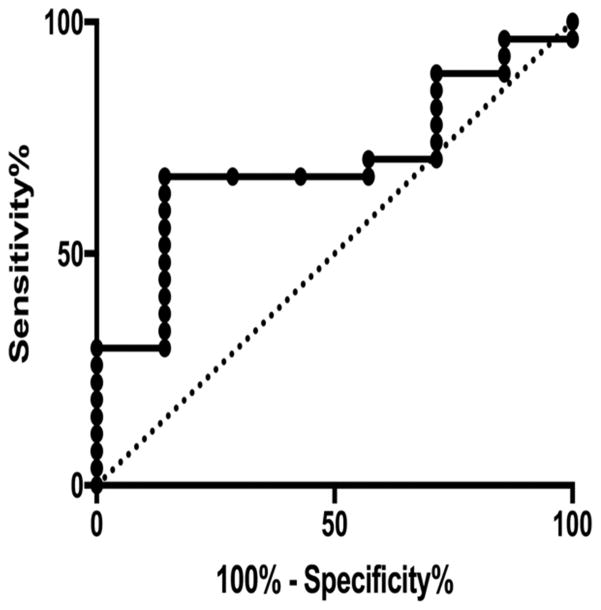
The 6 month GOS was dichotomized into favorable outcome (GOS 1–2) vs unfavorable outcome (GOS 3–5). A ROC curve was generated for peak NLRP3 concentration prediction of 6 month GOS with an AUC of 0.69. The optimal NLRP3 cutoff (defined as that which maximizes the sum of sensitivity and specificity, or equivalently, Youden’s J statistic) was 6.63 ng/mL, which gave a sensitivity of 0.67 and specificity of 0.86 (Figure 3). TBI patients with an NLRP3 level > 6.63 ng/mL at any time point were at higher risk for poor outcome (RR 7.6 95%CI: [1.40–45.62], p=0.013). Univariate regression analysis of age, gender, GCS, and mechanism of injury did not yield significant associations of these variables to CSF NLRP3. CSF NLRP3 level > 6.63 ng/mL was independently associated with GOS when controlling for initial GCS to adjust for injury severity (Table 3; OR 10.34, 95%CI: [1.04–102.78], p=0.046).
Figure 3. ROC curve of peak NLRP3 level for prediction of 6 month dichotomized GOS.
ROC curves were generated for peak NLRP3 level. The AUC was 0.72, indicating moderate predictive value. Youden’s J statistic determined the optimal NLRP3 cutoff was 6.63 ng/mL, which gave a sensitivity of 0.71 and specificity of 0.77.
Table 3.
Associations Between Increased Cerebrospinal Fluid NLRP3a and Clinical Variables
| Univariate | Multivariate | |||
|---|---|---|---|---|
| p | OR | [95% CI] | p | |
| Age | 0.283 | |||
| Sex | 0.400 | |||
| GCS | 0.185 | 1.17 | [0.81–1.68] | 0.401 |
| Mechanism | 0.335 | |||
| GOS at 6 months | 0.031 | 10.34 | [1.04 – 102.78] | 0.046 |
Increased NLRP3 defined as > 6.63 ng/mL
CI, confidence interval; OR, odds ratio; GCS, Glasgow Coma Sale score; GOS, Glasgow Outcome Scale score
DISCUSSION
We found the CSF concentration of NLRP3 is elevated in children with severe TBI. In addition, we observed age-related differences, with higher peak NLRP3 level measured in children ≤ 4y. Lastly, a peak NLRP3 > 6.63ng/mL was independently associated with poor outcome when controlling for injury severity. NLRP1, in contrast, was detected in only 11% of children with severe TBI. In these two patients, NLRP1 was found elevated in the CSF at ≤ 24h and was undetectable on subsequent measurements. This is the largest study to date in patients with severe TBI that has measured NLRP1 or NLRP3 and the only study of these inflammasome components in children.
NLRP1 and NLRP3 are members of the NOD-like receptor (NLR) protein family of cytosolic pattern recognition receptors (PRR), activated by various infectious triggers or in response to sterile danger signals such as uric acid, ATP, or oxidative stress 4,6,14. Activation of NLRP1 or NLRP3 results in assembly of the inflammasome, a multiprotein complex most often composed of a PRR such as NLRP1 or NLRP3, the adaptor protein ASC, and caspase-1. The inflammasome amplifies activation of caspase-1, thereby increasing subsequent processing of pro-IL-1β and pro-IL-18 into active forms, and may result in the pro-inflammatory pathway of regulated necrosis called pyroptosis 4,15. The NLRP1 inflammasome, the first inflammasome described, is classically activated by the Bacillus anthracis toxin 8,16. The NLRP3 inflammasome has been shown to respond to a wide variety of triggers, including pathogen associated molecular patterns (PAMPs) and DAMPs 6,8,17 that may be released in response to TBI or subsequent infection.
Studies in experimental brain injury models and in adults with moderate to severe TBI have suggested a role for the NLRP1 and NLRP3 inflammasomes in mediating post-traumatic neuroinflammation. Adamczak et al. 5 and de Rivero Vaccari et al. 7 performed the only previous studies to measure NLRP1 in patients with TBI, both of which were performed in adults with moderate to severe injury. Increased levels of ASC, caspase-1, and NLRP1 were detected and associated with unfavorable outcome at 5 months. NLRP1 and NLRP3 are intracellular PRRs, however de Rivero Vaccari et al. 18 showed evidence of NLRP1 in exosomes isolated from the CSF of patients with TBI. In contrast to the evidence supporting the role of NLRP1 in patients with TBI, NLRP3 expression has previously only been shown in in vivo TBI models. In the study by Liu et al. 9 increased expression of NLRP3, ASC, and caspase-1 mRNA was detected by 6 hours after fluid percussion injury and increased protein concentration of NLRP3 and cleaved Caspase-1 were measured in brain homogenates by 24 hours. Immunoprecipitation studies showed protein-protein interactions between NLRP3 and ASC suggesting formation of the inflammasome complex. It is possible that some or all of the NLRP3 we detected in CSF of children with severe TBI was present in extracellular vesicles (EVs) including exosomes, and also possible that NLRP1 was infrequently detected in our study because it was confined to EVs. This could allow for cell-cell communication and activation, or conversely could be used to limit NLRP1 or NLRP3 activation in cells.
Of significant interest in our study is the association of age ≤ 4 y with higher peak NLRP3 concentration. Although epidemiologic studies of TBI have generally shown that pediatric patients have better outcomes than adults with similar severity of injury, the youngest children tend to have worse outcomes than other pediatric patients19. Children ≤ 4 y may be at particular risk for poor outcome due to a higher incidence of abusive head trauma, delayed presentation, hypoxic injury, or seizures 20–22. Several clinical investigations have demonstrated a more robust neuroinflammatory response to head trauma in young children and this may also impact outcomes. For example, Newell et al. 23 examined CSF levels of ferritin, a marker of macrophage activation, in pediatric patients with severe TBI and found an association between high ferritin and age ≤ 4 y. Similarly, Satchell et al. 11 found an increased concentration of caspase-1 in the CSF of children ≤ 4 years after severe TBI. The latter study is of particular relevance, as caspase-1 activation is highly amplified by the inflammasome complex and therefore may be due to an increase in NLRP3 production and inflammasome activation.
We observed that peak CSF NLRP3 > 6.63 ng/mL significantly increased the risk of poor outcome, even when controlling for TBI severity as measured by GCS. Previous studies of the cytokine IL-1β, which is activated by the inflammasome and increases after TBI in adults and children 24–28, have shown mixed results as a predictor of functional outcome. Antagonism of IL-1β signaling has been supported in pre-clinical TBI models29–31 and a randomized controlled phase II trial of IL-1 receptor antagonist in adults with severe TBI showed the drug entered the CNS, was not associated with adverse events, and altered the neuroinflammatory response. Direct inhibitors of inflammasome-mediated inflammation have been developed and may be more effective in regulating pro-inflammatory pathways, as they will also be expected to limit IL-18 activation, secretion, and pyroptotic cell death32–35. Examples of therapies shown to inhibit NLRP3 include ketogenic diet 36, the small molecule MCC95037{Coll, 2015 #4;Coll, 2015 #4}, minocycline, and glyburide38 that are being tested in TBI and other CNS injuries including stroke, subarachnoid hemorrhage, and spinal cord injury. Combined with predictive information, these potential therapies could be useful outside the initial resuscitation and 24 hours of hospitalization, allowing physicians to deliver targeted therapy for the patients at highest risk of poor outcome.
In this study, NLRP3 was measured at 4 time points to reveal temporal trends. NLRP3 levels changed significantly over time, and fit a quadratic regression curve indicating that NLRP3 levels were high on post-injury day 1, lower on day 2, and increased again on post-injury day 3 or 4. Although the initial increase in NLRP3 may be due to traumatic lysis or necrotic cell death, the late rise in NLRP3 levels seen after 48 hours may represent cellular stress from elevated ICP, infection, or other triggers leading to inflammasome activation.
Limitations in this investigation include the fact that pediatric TBI is a heterogeneous disease and that we were unable to evaluate the effects of various injury patterns and mechanisms of injury. Also we have only included those patients with severe injury defined as GCS ≤ 8 who had EVD placement and treatment according to a predefined protocol. We did not measure other inflammatory or cell death biomarkers such as IL-1 or IL-18 that have been used as markers of inflammasome activation.
CONCLUSIONS
In conclusion, this investigation demonstrates that there is a significant and sustained increase in CSF NLRP3 concentrations in pediatric patients with severe TBI compared to controls. This increase is most pronounced in younger patients and a peak NLRP3 level > 6.63 ng/mL is associated with poor outcome. While these data are consistent with inflammasome activation in the largest number of TBI patients to-date, larger studies using targeted interventions are needed to confirm a role for inflammasome-mediated neuroinflammation after TBI.
Acknowledgments
Support: NINDS grants R01 NS38620 (RC) and U01 NS081041 (MB); NICHD T32 HD40686 (DS, JW); and NIH UL1-TR-000005 (DW).
Footnotes
Ethical Approval: All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
References
- 1.Coronado VG, Xu L, Basavaraju SV, et al. Surveillance for traumatic brain injury-related deaths--United States, 1997–2007. MMWR Surveill Summ. 2011;60:1–32. [PubMed] [Google Scholar]
- 2.Moreau JF, Fink EL, Hartman ME, et al. Hospitalizations of children with neurologic disorders in the United States. Pediatr Crit Care Med. 2013;14:801–10. doi: 10.1097/PCC.0b013e31828aa71f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fink KB, Andrews LJ, Butler WE, et al. Reduction of post-traumatic brain injury and free radical production by inhibition of the caspase-1 cascade. Neuroscience. 1999;94:1213–8. doi: 10.1016/s0306-4522(99)00345-0. [DOI] [PubMed] [Google Scholar]
- 4.Martinon F, Burns K, Tschopp J. The inflammasome: a molecular platform triggering activation of inflammatory caspases and processing of proIL-beta. Mol Cell. 2002;10:417–26. doi: 10.1016/s1097-2765(02)00599-3. [DOI] [PubMed] [Google Scholar]
- 5.Adamczak S, Dale G, de Rivero Vaccari JP, Bullock MR, Dietrich WD, Keane RW. Inflammasome proteins in cerebrospinal fluid of brain-injured patients as biomarkers of functional outcome: clinical article. J Neurosurg. 2012;117:1119–25. doi: 10.3171/2012.9.JNS12815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.de Rivero Vaccari JP, Dietrich WD, Keane RW. Activation and regulation of cellular inflammasomes: gaps in our knowledge for central nervous system injury. J Cereb Blood Flow Metab. 2014;34:369–75. doi: 10.1038/jcbfm.2013.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.de Rivero Vaccari JP, Lotocki G, Alonso OF, Bramlett HM, Dietrich WD, Keane RW. Therapeutic neutralization of the NLRP1 inflammasome reduces the innate immune response and improves histopathology after traumatic brain injury. J Cereb Blood Flow Metab. 2009;29:1251–61. doi: 10.1038/jcbfm.2009.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Latz E, Xiao TS, Stutz A. Activation and regulation of the inflammasomes. Nat Rev Immunol. 2013;13:397–411. doi: 10.1038/nri3452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu HD, Li W, Chen ZR, et al. Expression of the NLRP3 inflammasome in cerebral cortex after traumatic brain injury in a rat model. Neurochem Res. 2013;38:2072–83. doi: 10.1007/s11064-013-1115-z. [DOI] [PubMed] [Google Scholar]
- 10.Frugier T, Morganti-Kossmann MC, O’Reilly D, McLean CA. In situ detection of inflammatory mediators in post mortem human brain tissue after traumatic injury. J Neurotrauma. 2010;27:497–507. doi: 10.1089/neu.2009.1120. [DOI] [PubMed] [Google Scholar]
- 11.Satchell MA, Lai Y, Kochanek PM, et al. Cytochrome c, a biomarker of apoptosis, is increased in cerebrospinal fluid from infants with inflicted brain injury from child abuse. J Cereb Blood Flow Metab. 2005;25:919–27. doi: 10.1038/sj.jcbfm.9600088. [DOI] [PubMed] [Google Scholar]
- 12.Kochanek PM, Carney N, Adelson PD, et al. Guidelines for the acute medical management of severe traumatic brain injury in infants, children, and adolescents--second edition. Pediatr Crit Care Med. 2012;13(Suppl 1):S1–82. doi: 10.1097/PCC.0b013e31823f435c. [DOI] [PubMed] [Google Scholar]
- 13.Adelson PD, Wisniewski SR, Beca J, et al. Comparison of hypothermia and normothermia after severe traumatic brain injury in children (Cool Kids): a phase 3, randomised controlled trial. Lancet Neurol. 2013;12:546–53. doi: 10.1016/S1474-4422(13)70077-2. [DOI] [PubMed] [Google Scholar]
- 14.Savage CD, Lopez-Castejon G, Denes A, Brough D. NLRP3-Inflammasome Activating DAMPs Stimulate an Inflammatory Response in Glia in the Absence of Priming Which Contributes to Brain Inflammation after Injury. Front Immunol. 2012;3:288. doi: 10.3389/fimmu.2012.00288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bergsbaken T, Fink SL, Cookson BT. Pyroptosis: host cell death and inflammation. Nat Rev Microbiol. 2009;7:99–109. doi: 10.1038/nrmicro2070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fink SL, Bergsbaken T, Cookson BT. Anthrax lethal toxin and Salmonella elicit the common cell death pathway of caspase-1-dependent pyroptosis via distinct mechanisms. Proc Natl Acad Sci U S A. 2008;105:4312–7. doi: 10.1073/pnas.0707370105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhou R, Yazdi AS, Menu P, Tschopp J. A role for mitochondria in NLRP3 inflammasome activation. Nature. 2011;469:221–5. doi: 10.1038/nature09663. [DOI] [PubMed] [Google Scholar]
- 18.de Rivero Vaccari JP, Brand F, 3rd, Adamczak S, et al. Exosome-mediated inflammasome signaling after central nervous system injury. J Neurochem. 2016;136(Suppl 1):39–48. doi: 10.1111/jnc.13036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Luerssen TG, Klauber MR, Marshall LF. Outcome from head injury related to patient’s age. A longitudinal prospective study of adult and pediatric head injury. J Neurosurg. 1988;68:409–16. doi: 10.3171/jns.1988.68.3.0409. [DOI] [PubMed] [Google Scholar]
- 20.Christian CW, Block R Committee on Child A, Neglect American Academy of P. Abusive head trauma in infants and children. Pediatrics. 2009;123:1409–11. doi: 10.1542/peds.2009-0408. [DOI] [PubMed] [Google Scholar]
- 21.Shein SL, Bell MJ, Kochanek PM, et al. Risk factors for mortality in children with abusive head trauma. J Pediatr. 2012;161:716–22. e1. doi: 10.1016/j.jpeds.2012.03.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kemp AM, Joshi AH, Mann M, et al. What are the clinical and radiological characteristics of spinal injuries from physical abuse: a systematic review. Arch Dis Child. 2010;95:355–60. doi: 10.1136/adc.2009.169110. [DOI] [PubMed] [Google Scholar]
- 23.Newell E, Shellington DK, Simon DW, et al. Cerebrospinal Fluid Markers of Macrophage and Lymphocyte Activation After Traumatic Brain Injury in Children. Pediatr Crit Care Med. 2015;16:549–57. doi: 10.1097/PCC.0000000000000400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shiozaki T, Hayakata T, Tasaki O, et al. Cerebrospinal fluid concentrations of anti-inflammatory mediators in early-phase severe traumatic brain injury. Shock. 2005;23:406–10. doi: 10.1097/01.shk.0000161385.62758.24. [DOI] [PubMed] [Google Scholar]
- 25.Chiaretti A, Genovese O, Aloe L, et al. Interleukin 1beta and interleukin 6 relationship with paediatric head trauma severity and outcome. Childs Nerv Syst. 2005;21:185–93. doi: 10.1007/s00381-004-1032-1. discussion 94. [DOI] [PubMed] [Google Scholar]
- 26.Helmy A, Carpenter KL, Menon DK, Pickard JD, Hutchinson PJ. The cytokine response to human traumatic brain injury: temporal profiles and evidence for cerebral parenchymal production. J Cereb Blood Flow Metab. 2011;31:658–70. doi: 10.1038/jcbfm.2010.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hutchinson PJ, O’Connell MT, Rothwell NJ, et al. Inflammation in human brain injury: intracerebral concentrations of IL-1alpha, IL-1beta, and their endogenous inhibitor IL-1ra. J Neurotrauma. 2007;24:1545–57. doi: 10.1089/neu.2007.0295. [DOI] [PubMed] [Google Scholar]
- 28.Perez-Barcena J, Ibanez J, Brell M, et al. Lack of correlation among intracerebral cytokines, intracranial pressure, and brain tissue oxygenation in patients with traumatic brain injury and diffuse lesions. Crit Care Med. 2011;39:533–40. doi: 10.1097/CCM.0b013e318205c7a4. [DOI] [PubMed] [Google Scholar]
- 29.Tehranian R, Andell-Jonsson S, Beni SM, et al. Improved recovery and delayed cytokine induction after closed head injury in mice with central overexpression of the secreted isoform of the interleukin-1 receptor antagonist. J Neurotrauma. 2002;19:939–51. doi: 10.1089/089771502320317096. [DOI] [PubMed] [Google Scholar]
- 30.Clausen F, Hanell A, Bjork M, et al. Neutralization of interleukin-1beta modifies the inflammatory response and improves histological and cognitive outcome following traumatic brain injury in mice. Eur J Neurosci. 2009;30:385–96. doi: 10.1111/j.1460-9568.2009.06820.x. [DOI] [PubMed] [Google Scholar]
- 31.Clausen F, Hanell A, Israelsson C, et al. Neutralization of interleukin-1beta reduces cerebral edema and tissue loss and improves late cognitive outcome following traumatic brain injury in mice. Eur J Neurosci. 2011;34:110–23. doi: 10.1111/j.1460-9568.2011.07723.x. [DOI] [PubMed] [Google Scholar]
- 32.Sifringer M, Stefovska V, Endesfelder S, et al. Activation of caspase-1 dependent interleukins in developmental brain trauma. Neurobiol Dis. 2007;25:614–22. doi: 10.1016/j.nbd.2006.11.003. [DOI] [PubMed] [Google Scholar]
- 33.Yatsiv I, Morganti-Kossmann MC, Perez D, et al. Elevated intracranial IL-18 in humans and mice after traumatic brain injury and evidence of neuroprotective effects of IL-18-binding protein after experimental closed head injury. J Cereb Blood Flow Metab. 2002;22:971–8. doi: 10.1097/00004647-200208000-00008. [DOI] [PubMed] [Google Scholar]
- 34.Schmidt OI, Morganti-Kossmann MC, Heyde CE, et al. Tumor necrosis factor-mediated inhibition of interleukin-18 in the brain: a clinical and experimental study in head-injured patients and in a murine model of closed head injury. J Neuroinflammation. 2004;1:13. doi: 10.1186/1742-2094-1-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Helmy A, Guilfoyle MR, Carpenter KL, Pickard JD, Menon DK, Hutchinson PJ. Recombinant human interleukin-1 receptor antagonist in severe traumatic brain injury: a phase II randomized control trial. J Cereb Blood Flow Metab. 2014;34:845–51. doi: 10.1038/jcbfm.2014.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Youm YH, Nguyen KY, Grant RW, et al. The ketone metabolite beta-hydroxybutyrate blocks NLRP3 inflammasome-mediated inflammatory disease. Nat Med. 2015;21:263–9. doi: 10.1038/nm.3804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Coll RC, Robertson AA, Chae JJ, et al. A small-molecule inhibitor of the NLRP3 inflammasome for the treatment of inflammatory diseases. Nat Med. 2015;21:248–55. doi: 10.1038/nm.3806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lamkanfi M, Mueller JL, Vitari AC, et al. Glyburide inhibits the Cryopyrin/Nalp3 inflammasome. J Cell Biol. 2009;187:61–70. doi: 10.1083/jcb.200903124. [DOI] [PMC free article] [PubMed] [Google Scholar]