Abstract
The microaerophylic organism Propionibacterium acnes has shown consistent association with prostate cancer (PC). Studies linking circumcision with reduced PC further support anaerobes involvement as circumcision reduces anaerobe colonisation on the glans penis. A 1988 study linked anaerobes with PC but considered them as opportunists in necrotic tumour. A hypothesis that a “Helicobacter-like” process causes PC justified this pilot study. Active surveillance patients were enrolled. Post-prostate massage urine samples were screened using the Matrix-Assisted Laser Desorption/Ionization Time-of-Flight (MALDI-TOF) technique for bacterial identification after culture in anaerobic and aerobic conditions. 8 out of 18 patients (41%) had either obligate anaerobic (n = 5) or microaerophilic (n = 4, one of whom also had anaerobes) organisms identified. None of 10 control samples contained obligate anaerobes. Although mean PSA was 63% higher in those with low oxygen tolerating bacteria, two high outliers resulted in this difference being non-significant. Given the substantially higher proportion of PC patients with organisms growing in a low concentration of oxygen when combined with previous studies compared to controls, the degree of significance was as high as smoking 5–9 cigarettes a day and needs further investigation. Translational research in trials combining Vitamin D and aspirin have begun as part of such investigation.
Introduction
There is considerable amount of evidence that chronic inflammation is a concomitant of cancer development in most sites, including prostate cancer1. It is unclear whether it is a precursor/promotor of cancer development2,3, just a manifestation of a response to the disorderly development that occurs as the cancer progresses4 or a mixture of both as has been suggested by the hypothesis of cancer as a wound that does not heal5,6. Linkage between low oxygen-tolerating organisms and malignant transformation has been established with evidence for the association of the microaerophylic bacterium, Helicobacter.pylori, in stomach cancer7,8. More limited is evidence that Vitamin D deficiency is a promoting factor for this cancer type9,10. In prostate cancer there is no well accepted pathogen consistently associated with this malignancy nor the inflammation associated with it despite multiple studies, many of which have provided early suggestions but failed to be confirmed on subsequent studies11.
However, recent studies have suggested that infection with Propionibacterium.acnes (PA), a known sun-sensitive12 microaerophylic bacterium associated with acne, has shown a more consistent association with prostate cancer13–18. Studies linking circumcision with reduced risk of prostate cancer19–21 is further supportive evidence of the role of such organisms in prostate cancer aetiology as randomised trials of circumcision have demonstrated reduced anaerobe colonisation of the glans penis after circumcision22. The uncircumcised glans penis has direct access to the prostate through the urethra23 which is relatively anoxic. Although there is one report from 1988 linking anaerobes with prostate cancer24 and one study demonstrating that antibiotics can clear them from the prostate25, most workers have until recently considered them as opportunists colonising necrotic tumour tissue rather than being considered as actual causative factors. Due to a long-held view that a “Helicobacter-like” process could be involved in causation of prostate cancer26–29, this pilot study was undertaken to screen for aerobic and anaerobic bacteria by culturing small aliquots of urine samples obtained following a “mini-prostate massage” done for PCA3 testing. The use of a commercial MALDI-TOF (Matrix-Assisted Laser Desorption/Ionization Time-of-Flight) mass spectrometry device allowed for the rapid and accurate identification of bacteria.
Results
Clinical demographics of participants
Table 1 lists age, clinical and Gleason Grading pathology staging and PSA levels of 18 patients selected for study while undergoing a period of surveillance without having had any surgical, radiation or drug treatment. Table 2 lists the diagnosis in the group of non-prostate cancer controls undergoing microbiological urine testing for non-malignant causes who were selected for having adequate surplus urine for anaerobic culture.
Table 1.
Clinical characteristics of prostate cancer patients.
| Specimen Identifier | AGE | PSA | T stage | Gleason Grade |
|---|---|---|---|---|
| 01 ML | 58 | 1.29 | T1, N0, M0 | G 3 + 3 |
| 02 PH | 57 | 4.54 | T2, N0, M0 | G 3 + 3 |
| 03 PO | 60 | 7.3 | T1, N0, M0 | G 3 + 3 |
| 04 HS | 72 | 6.1 | T2, N0, M0 | G 3 + 3 |
| 05 FJD | 63 | 5.08 | T2, N0, M0 | G 3 + 3 |
| 06 PK | 74 | 10.7 | T3a, N0, M0 | G 3 + 4 |
| 07 PW | 69 | 3.22 | T1, N0, M0 | G 3 + 3 |
| 08 JL | 69 | 12 | T2, N0, M0 | G 3 + 4 |
| 09 CO | 55 | 6.7 | T2, N0, M0 | G 3 + 4 |
| 10 SP | 66 | 12 | T1, N0, M0 | G 3 + 3 |
| 11 AH | 69 | 11 | T1, N0, M0 | G 3 + 3 |
| 12 ST | 62 | 26 | T2, N0, M0 | G 4 + 5 |
| 13 PL | 61 | 7.4 | T3a, N0, M0 | G 4 + 3 |
| 14 AB | 75 | 9.52 | T1, N0, M0 | G 3 + 4 |
| 15 AO | 52 | 4.84 | T1, N0, M0 | G 3 + 3 |
| 16 WP | 85 | 26 | T2, N0, M0 | G 3 + 4 |
| 17 SR | 65 | 5.36 | T1, N0, M0 | G 3 + 3 |
| 18 KP | 69 | 7.5 | T1, N0, M0 | G 3 + 3 |
Table 2.
Control “normal” males.
| Specimen Identifier | Age | Diagnosis |
|---|---|---|
| 01 MA | 85 | Under investigation |
| 02 OSt-L | 77 | Pre-op |
| 03 EC | 81 | Routine blood |
| 04 MC | 80 | Pre-op |
| 05 RP | 77 | Respiratory arrest |
| 06 MLu | 70 | Post op |
| 07 BM | 87 | Under investigation |
| 08 RS | 76 | A&E attendance |
| 09 GA | 65 | ITU |
| 10 KD | 72 | Cardiac arrest |
Oxygen dependence of organisms detected after prostate massage in prostate cancer patients
Table 3 summarises microbiology findings and shows that 5 of 18 (27.8%) had obligate anaerobe (4/5 having Peptoniphilus harei) and 4 (1 of who also had pure anaerobe) of 18 (22%) had microaerophylic bacteria, i.e., 8/18 (44%) had one or other type of low oxygen-tolerating organisms in post-prostate massage urine. This compares to 0 of 10 “normals” having obligate anaerobes.
Table 3.
Oxygen dependence of organisms detected after prostate massage in study patients.
| Patient code | DATE | Aerobic and facultatively anaerobic Organisms isolated | Obligate anaerobic Organisms isolated | Microaerophylic Organisms isolated |
|---|---|---|---|---|
| 01 ML | 6/8/15 | Corynebacterium amycolatum Staph haemolyticus | Nil | Nil |
| 02 PH | 6/8/15 | Strep sp, Staph haemolyticus & C. glucuronydictum | Peptoniphilus harei Veillonella montpenellerensis | Nil |
| 03 PO | 10/8/15 | Strep sp, Staph hominis & Dermabacter hominis | Nil | Actinomyces neuii |
| 04 HS | 11/8/15 | Aerococcus urinae, staph epidermidis, Staph simulans Corynebacterium tuberculostearicum | Fusobacterium nucleatum Fusobacterium gondiaformans Peptoniphilus harei Actinobaculum schaali?i | Nil |
| 05 FJD | 27/8/15 | Nil | Nil | Nil |
| 06 PK | 27/8/15 | Enterococcus faecalis | Nil | Nil |
| 07 PW | 3/9/15 | Staph epidermidis & Staph haemolyticus | Nil | Nil |
| 08 JL | 27/8/15 | Enterococcus faecalis, Staph epiderm & Micrococcus luteus | Nil | Brevibacterium casei |
| 09 CO | 10/9/15 | Nil | Nil | Nil |
| 10 SP | 10/9/15 | Strep agalactiae, Gardnerella sp Strep pneumoniae, Strep anginosus | Nil | Nil |
| 11 AH | 10/9/15 | Staph epiderm, Staph hominis Dermabacter hominis C. tuberculostearicum | Nil | Nil |
| 12 ST | 10/9/15 | Staph capitis, Staph hominis Strep anginosus Acinetobacter radioresistens | Peptoniphilus harei | Nil |
| 13 PL | 10/9/15 | C. tuberculostearicum Strep agalactiae, Strep angino Dermobacter hominis | Veillonella parvula Actinobaculum schaalii | Actinomyces turicencis Brevibacterium paucvorans |
| 14 AB | 17/9/15 | Staph epiderm, Citrobacter koseri Strep pneumoniae | Nil | Nil |
| 15 AO | 17/9/15 | Staph hominis Staph haemolyticus Strep anginosus | Nil | Nil |
| 16 WP | 17/9/15 | C. amycolatum Strep anginosus Dermobacter hominis Staph epidermis | Peptostreptococcus anaerobius, Peptoniphilus harei Finegoldia magna | Nil |
| 17 SR | 17/9/15 | Aerococcus urinae, E. faecalis Staph haem, Staph hominis, M luteus, Strep pneuminiae Kocuria rhizophilia | Nil | Actinomyces neuii |
| 18 PK | 17/9/15 | Staph epidermidis, Staph capitis, Staph haemolyticus E. faecalis | Nil | Nil |
PSA and type of bacterial isolate
Figure 1 shows results of dot plot distribution comparing the mean PSA of those urines containing obligate anaerobe or microaerophylic bacteria (mean PSA 11.7 range 4.5–26) and those with no such organism (mean PSA 7.2 range 1.29–12). Although the mean PSA level was 63% higher in the patients with low oxygen-tolerating bacteria, the small numbers and two high outliers of 26 ng/m (both positive for Peptoniphilus harei) result in the difference in the medians only being 7.4 vs 7.1 and the distribution being non-significant in Wilcoxon rank sum test (p value = 0.423).
Figure 1.
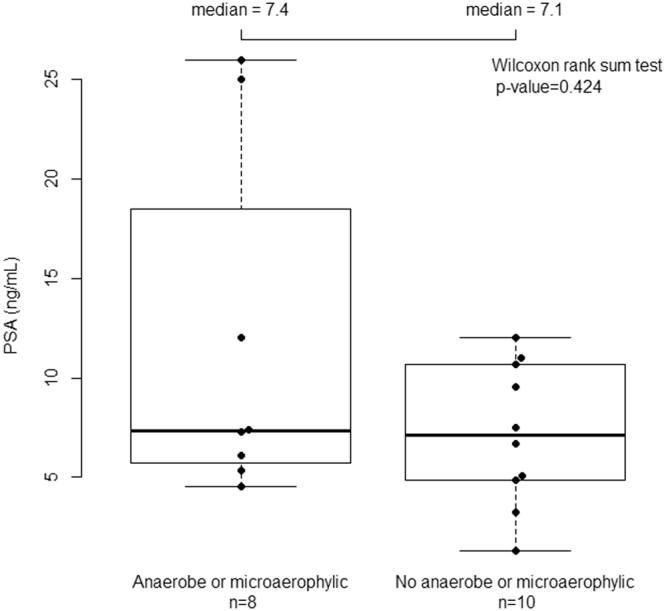
Shows a dot plot distribution of PSA in 18 Active Surveillance patients comparing combined group of obligate anaerobe & microaerophilic positive patients and those without such bacteria.
Discussion
It has been known since the study of Cooper et al. 198824 that anaerobes are present more frequently in the tissue biopsies of malignant than benign prostates and that this frequency was higher than contemporary reports of urine cultures of normal subjects30. Recently there has been a cluster of reports showing a higher frequency of the microaerophylic organism P. acnes in malignant compared to benign prostates13–18 and reports that circumcision both reduces anaerobe contamination of the glans22 and reduces the risk of prostate cancer19–21. As a consequence, interest in both circumcision and these classes of bacteria has increased. This is particularly so since the cohort study of prostate cancer risk and circumcision by Spence et al.20. This study involved 1590 French Canadian residents and 1618 electoral registry controls. Although it failed to show any significant protection in the majority white population (OR 0.93 95% CI 0.80–1.12) except in men circumcised after the age 36 and non-significantly in the 26% of their men circumcised at birth (OR 0.86 95% CI 0.72–1.04), there was significant reduction (OR 0.44 95% CI 0.19–0.86) in the small subgroup of black men. More significant in respect of the results of our study was the fact that although there was no association in Spence et al’s study with history of an STD, there was an association with a history of prostatitis. Perhaps the most important observation from this study was the observation that circumcision after the age of 36 gave protection. Clearly as an unpredicted observation it needs to be repeated but based on the observation contained in our report and the evidence on the role of phimosis in development of penile cancer31, the easiest way to recruit people to a study that clarified whether anaerobes do contribute to the raised PSA would be to target patients with raised PSA presenting with phimosis and measure PSA before and after surgery.
Although our study is small and lacks adequate matched contemporaneous controls, these preliminary results certainly are different from those obtained from normal urine by others using earlier techniques30 as well as our small series of “normal” controls, admittedly without prostate massage. However, pooling this limited literature data base of 27 prostate cancers and 551 “controls” (Table 4) produces a highly statistically significant difference (Pearson's χ² = 70.304 (df = 1, p < 2.2e-16) with Yates' continuity correction), providing justification for a prospective study including age- and sex-matched control samples from routine health care procedures such as cardiac stents and hip replacements and patients with benign prostatic hyperplasia (BPH).
Table 4.
Summary reports of anaerobes in normal urines & tissue from patients with BPH vs with prostate cancer.
| No of cases studied | Positive anaerobes | |
|---|---|---|
| Normal Urine30 | 517 | 5.9% |
| BPH tissue #24 | 24 | 0% |
| Control “normal” urines (Bhudia et al. this series) | 10 | 0% |
| Prostate Cancer tissue ##24 | 9 | 67% # vs ## P = 0.001 Fishers exact test |
| Prostate Cancer urines (Bhudia et al. this series) | 18 | 44% |
| TOTAL “CONTROLS” | 551 | 5.5% |
| TOTAL PROSTATE CANCER | 27 | 52% Pearson's χ² = 70.3 (df = 1, p < 2.2e-16) with Yates' continuity correction |
In this small number of cases studied, no case was found with either P. acnes or H. pylori (the microaerophylic organism proven to have malignant association with stomach cancer2) nor the facultative anaerobe, Streptoccocus gallolyticus, (associated with colon cancer32,33) although there was one case (Tables 3, 4) associated with one of the Fusobacterium species, an anaerobe recently reported to be associated with pancreatic cancer34. Given the increasing numbers of cancers reported to have an association with anaerobes, they do give more support for the concept that, given the known hypoxic state of cancers, such organisms could indeed play a role either accelerating progression35 or in addition playing a role in the induction as proven in stomach cancer. In this respect prostate cancer is possibly a particularly informative cancer to begin study of the mechanism involved given the data demonstrating reduced long-term sun exposure having a more clear-cut association (Table 5) with increased risk of cancer28,36,37, than spot Vitamin D levels38. As puberty is a time when males prove particularly sensitive to P. acnes and as with breast and cervix cancer there is some evidence linking early puberty with prostate cancer risk27,39, it could be that population-based studies of PSA levels in pubertal males29 might give further evidence that could go some way to support the hypothetical interpretation of these data. This is that a 40–60 year life time of urban living with sub-clinical Vitamin D-induced, macrophage-mediated, non-antigen-specific immune deficiency40 is as oncogenic in terms of the non-smoking related cancers as smoking between five to nine cigarettes a day over 40–60 years is a cause of lung cancer41 (Table 5). An even more fruitful area for research is suggested by the observation of Head et al (Table 5) who demonstrated a 2.5 odds ratio for increased death from cancer at 10–15 years of follow up of civil servants taking sick leave for a psychiatric cause42,43. Many such people would be likely to rise with the sun, return home at sunset, take lunch at their desks and suffer from “non-seasonal-SAD” (seasonal affective disorder) because, whatever sun they were exposed to, did not follow the normal seasonal variation. Further population-based studies of “CBT-light” in such patients44 could be very informative with serial monitoring of mood and anonymised screening of the bloods of recruited patients for PSA. Clearly such a study (limited to adults over the age of 40 given data that such individual with PSA greater than the median level of age corrected PSA had a 3.75 HR of developing prostate cancer45) would only be justified if a non-surgical approach had been shown to normalise PSA long-term.
Table 5.
Comparison of anaerobes and prostate/cervix ca risk and imprecise measures of sun exposure vs more precise lung ca risk of low dose tobacco.
| No of studies | Actual or Median level of risk | |
|---|---|---|
| Geographic study of PC risk low vs high sun exposure55 | 8 | 1.36 (range 1.01–1.73) |
| Clinical Questionnaire PC risk low vs high sun exposure55 | 8 | 1.13 (range 1.0–1.41) |
| Bacterial vaginosis & Ca Cervix56 | 19 | 1.51 (1.24–1.83) |
| HPV, BV and abnormal cytology vs BV alone vs BV absent57 | 1 | 3.82 vs 2.91 vs 1.00 |
| 10 yr cancer mortality post a psychiatric sick note vs no note in civil service58 | 1 | 2.49 (1.33 to 4.68) |
| Male death Lung cancer from smoking 1–4 cigarettes a day59 | 1 | 2.79 ((0.94 to 8.28) |
| Presence of anaerobes in prostate & PC risk see Table 4 for actual data | 1 | Odds ratio* (95% C.I.) = 17.8 (7. 6, 42.0); p-value= 3.88e-10 * Odds ratio was computed by median-unbiased estimation (exact CI). |
| Male death Lung cancer from smoking 5–9 cigarettes a day59 | 1 | 11.1 (6.94–20.44) |
Given laboratory studies in mice that demonstrated that this class of bacteria can be controlled therapeutically by treatment with Vitamin D46 and the continuing amounts of data supporting the link between Vitamin D or sun exposure deficiency with increased risk of human cancer, trials of Vitamin D combined with anti-inflammatory agents such as aspirin aiming to reduce tumour progression (see http://www.isrctn.com/ISRCTN91422391 and https://www.provent.org.uk) could provide evidence to support the above interpretation. The increasing recognition of the role of Vitamin D in enhancing non-antigen-specific macrophage anti-pathogen responses40,47 provides an understandable mechanism. However, given the similarities in bacterial flora between what we have found and those in bacterial vaginosis, recently increasingly accepted as promoting HPV-induced cervix cancer (Table 5)48,49, it is likely for long-term control that treatment of both the patient and his partner may be necessary with selective use of appropriate anti-anaerobe antibiotic if response does not occur to Vitamin D alone. However, given the increasing problems with widespread antibiotic resistance and recent reports of successful use of E. coli vaccines in women with recurrent urinary tract infection50, it is likely that such a non-antibiotic approach may be preferable51.
To conclude, MALDI-TOF enabled rapid and accurate identification of bacteria present in the post-prostate massage urine. A higher proportion of organisms growing in a low concentration of oxygen were found in prostate cancer samples than in previous normal studies and in the small number of “control” urines tested.
Given the association of cervical cancer with bacterial vaginosis and increased knowledge about how organisms such as Helicobacter pylori induce stomach cancer and association of Streptoccocus gallolyticus with colon cancer, larger scale studies in prostate cancer patients are required using techniques such as MALDI-TOF or Metagenomic sequencing52 to rapidly identify bacteria which have hitherto been problematic to identify.
Given the increasing number of indications that the first event in the initiation of prostate cancer may be occurring in the early years of puberty, there is a need to find ways to better assess annual sun exposure to quantify the effect of the 30–40 years of chronic sun deficiency in modern urban environments.
Methods
Patient selection
The patients selected attended the prostate cancer clinic at Barts Hospital (Barts Health NHS Trust) and were patients on follow up or planned for active surveillance. Two medical students as part of an approved audit (ref no. 6227) identified and collected clinical and biochemical information of suitable patients. The consultant in charge took informed consent for donation of post-prostate examination urine collection under City & London East ethics approval (ref no. 09/H0704/4+5 date 16/07/2014). The students then undertook delivery to the laboratory and observed the screening procedure which was undertaken in microbiology in accordance with the approved protocol. In addition, a selection of surplus urines from routine urine requests from age-matched men, were screened for obligate anaerobes only as well, although they had not had prostate massage.
Microbiology
1.5 ml of urine was centrifuged at 16,000 g for 2 minutes. The supernatant was then discarded and 10 ul of the deposit plated on Columbia Horse Blood agar (Thermofisher, Basingstoke, UK) and Fastidious anaerobic agar (Thermofisher, Basingstoke, UK). Plates were incubated for 72h at 35˚C in air + 5% CO2 or anaerobically in an atmosphere of 90% N2, 5% H2 and 5% CO2. Resultant colonies were identified by MALDI-TOF using a Bruker Maldi Biotyper mass spectrometer53,54. All data generated and analysed during this study are included in this published article.
Acknowledgements
We are grateful to Professor Nick Lemoine Director of Barts Cancer Institute for supporting the study and reviewing the draft manuscript, to Professor Frances Balkwill, laboratory Lead, Centre for Cancer and Inflammation, Barts Cancer Institute for reviewing the draft manuscript, to Professor Jack Cuzick for statistical support and reviewing the manuscript, to Mr Greg Shaw, Mr John Hines and Karen Wilkinson in Barts Health Specialist Prostate Cancer Clinic for selecting the patients and taking informed consent, to Barts Charity for a grant to Mark Wilks for purchase of the Matrix-Assisted Laser Desorption/Ionization Time-of-Flight (MALDI-TOF) equipment and to Orchid-Cancer Charity whose funding have supported Professor Tim Oliver’s continued exploration of this hypothesis for the last 21 years.
Author Contributions
R.B. identified and collected clinical and biochemical information of suitable patients undergoing post-prostate examination urine collection and undertook delivery to the laboratory and observed the screening procedure, helped with the data analysis and editing of drafts of the manuscript, O.A. identified and collected clinical and biochemical information of suitable patients undergoing post-prostate examination urine collection, A.A. was responsible for statistical analysis of the data, A.W. processed the samples through culture and then selection for the MALDI-TOF, M.W. suggested using the MALDI-TOF, for the study and analysed the results, T.O. conceived the study and supervised the students during the active part of recruitment and prepared the first draft and the re-drafting and responding to subsequent modifications suggested by the reviewers. All authors reviewed and approved the final version prior to submission.
Competing Interests
MW was in receipt of Barts and The London Charity Equipment Grant for provision of MOLDI-TOF (Grant reference number 486/1193).
Footnotes
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.De Marzo AM, et al. Inflammation in prostate carcinogenesis. Nat Rev Cancer. 2007;7:256–269. doi: 10.1038/nrc2090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Forman D. Helicobacter-pylori and gastric cancer. Scand J Gastoenterology. 1996;31:48–51. doi: 10.3109/00365529609094533. [DOI] [PubMed] [Google Scholar]
- 3.Forman D, et al. Geographic association of Helicobacter pylori antibody prevalence and gastric cancer mortality in rural China. Int J Cancer. 1990;46:608–611. doi: 10.1002/ijc.2910460410. [DOI] [PubMed] [Google Scholar]
- 4.Kulbe H, et al. A dynamic inflammatory cytokine network in the human ovarian cancer microenvironment. Cancer Res. 2012;72:66–75. doi: 10.1158/0008-5472.CAN-11-2178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Balkwill, F. & Mantovani, A. Inflammation and cancer: back to Virchow? Lancet357, 539-545, doi:10.1016/S0140-6736(00)04046-0 (2001). [DOI] [PubMed]
- 6.Crusz, S. M. & Balkwill, F. R. Inflammation and cancer: advances and new agents. Nat Rev Clin Oncol, 10.1038/nrclinonc.2015.105 (2015). [DOI] [PubMed]
- 7.Raei N, Behrouz B, Zahri S, Latifi-Navid S. Helicobacter pylori Infection and Dietary Factors Act Synergistically to Promote Gastric Cancer. Asian Pac J Cancer Prev. 2016;17:917–921. doi: 10.7314/APJCP.2016.17.3.917. [DOI] [PubMed] [Google Scholar]
- 8.Corbinais C, et al. ComB proteins expression levels determine Helicobacter pylori competence capacity. Scientific reports. 2017;7:41495. doi: 10.1038/srep41495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kwon HJ, et al. Vitamin D(3) upregulated protein 1 deficiency promotes N-methyl-N-nitrosourea and Helicobacter pylori-induced gastric carcinogenesis in mice. Gut. 2012;61:53–63. doi: 10.1136/gutjnl-2011-300361. [DOI] [PubMed] [Google Scholar]
- 10.Hosoda K, et al. Identification and characterization of a vitamin D(3) decomposition product bactericidal against Helicobacter pylori. Scientific reports. 2015;5:8860. doi: 10.1038/srep08860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hrbacek J, Urban M, Hamsikova E, Tachezy R, Heracek J. Thirty years of research on infection and prostate cancer: no conclusive evidence for a link. A systematic review. Urol Oncol. 2013;31:951–965. doi: 10.1016/j.urolonc.2012.01.013. [DOI] [PubMed] [Google Scholar]
- 12.Agak GW, et al. Propionibacterium acnes Induces an IL-17 Response in Acne Vulgaris that Is Regulated by Vitamin A and Vitamin D. J Invest Dermatol. 2014;134:366–373. doi: 10.1038/jid.2013.334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cohen RJ, Shannon BA, McNeal JE, Shannon T, Garrett KL. Propionibacterium acnes associated with inflammation in radical prostatectomy specimens: a possible link to cancer evolution? J Urol. 2005;173:1969–1974. doi: 10.1097/01.ju.0000158161.15277.78. [DOI] [PubMed] [Google Scholar]
- 14.Alexeyev O, et al. Association between the presence of bacterial 16S RNA in prostate specimens taken during transurethral resection of prostate and subsequent risk of prostate cancer (Sweden) Cancer Causes Control. 2006;17:1127–1133. doi: 10.1007/s10552-006-0054-2. [DOI] [PubMed] [Google Scholar]
- 15.Shannon BA, Cohen RJ, Garrett KL. Polymerase chain reaction-based identification of Propionibacterium acnes types isolated from the male urinary tract: evaluation of adolescents, normal adults and men with prostatic pathology. BJU Int. 2006;98:388–392. doi: 10.1111/j.1464-410X.2006.06273.x. [DOI] [PubMed] [Google Scholar]
- 16.Sutcliffe S, Giovannucci E, Isaacs WB, Willett WC, Platz EA. Acne and risk of prostate cancer. Int J Cancer. 2007;121:2688–2692. doi: 10.1002/ijc.23032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cavarretta I, et al. The Microbiome of the Prostate Tumor Microenvironment. Eur Urol. 2017 doi: 10.1016/j.eururo.2017.03.029. [DOI] [PubMed] [Google Scholar]
- 18.Manzoor, M. A. P. & Rekha, P. D. Prostate cancer: Microbiome - the 'unforeseen organ'. Nat Rev Urol, 10.1038/nrurol.2017.97 (2017). [DOI] [PubMed]
- 19.Wright JL, Lin DW, Stanford JL. Circumcision and the risk of prostate cancer. Cancer. 2012;118:4437–4443. doi: 10.1002/cncr.26653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Spence AR, Rousseau MC, Karakiewicz PI, Parent ME. Circumcision and prostate cancer: a population-based case-control study in Montreal, Canada. BJU Int. 2014;114:E90–98. doi: 10.1111/bju.12741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Prabalan N, Singian E, Jarjanazi H, Paganini-Hill A. Male circumcision and Prostate Cancer: a meta-analysis. Prostate Cancer and Prostatic Diseases. 2015;18:352–357. doi: 10.1038/pcan.2015.34. [DOI] [PubMed] [Google Scholar]
- 22.Price LB, et al. The effects of circumcision on the penis microbiome. PLoS One. 2010;5:e8422. doi: 10.1371/journal.pone.0008422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lai FC, Kennedy WA, 2nd, Lindert KA, Terris MK. Effect of circumcision on prostatic bacterial colonization and subsequent bacterial seeding following transrectal ultrasound-guided prostate biopsies. Techniques in urology. 2001;7:305–309. [PubMed] [Google Scholar]
- 24.Cooper, P. N., Millar, M. R. & Godwin, P. G. Anaerobes and carcinoma of the prostate. Br Med J (Clin Res Ed)296, 466–467 (1988). [DOI] [PMC free article] [PubMed]
- 25.Magri V, Restelli A, Marras E, Perletti G. A severely symptomatic case of anaerobic chronic bacterial prostatitis successfully resolved with moxifloxacin therapy. Anaerobe. 2010;16:206–209. doi: 10.1016/j.anaerobe.2009.10.008. [DOI] [PubMed] [Google Scholar]
- 26.Oliver R. in Cancer Surveys. 1995;23:309–310. [PubMed] [Google Scholar]
- 27.Oliver JC, Oliver RT, Ballard RC. Influence of circumcision and sexual behaviour on PSA levels in patients attending a sexually transmitted disease (STD) clinic. Prostate Cancer Prostatic Dis. 2001;4:228–231. doi: 10.1038/sj.pcan.4500535. [DOI] [PubMed] [Google Scholar]
- 28.Oliver T, Lorinez A, Cuzick J. Prostate cancer prevention by short-term anti-androgens: the rationale behind design of pilot studies. Recent Results Cancer Res. 2009;181:195–205. doi: 10.1007/978-3-540-69297-3_18. [DOI] [PubMed] [Google Scholar]
- 29.Oliver RT. Circumcision and/or vaccination against human papillomavirus in the male to prevent infection with human immunodeficiency virus: an early surrogate endpoint for the later prevention of penile, prostate, anal and oral cancer? BJU Int. 2009;104:753–755. doi: 10.1111/j.1464-410X.2009.08596.x. [DOI] [PubMed] [Google Scholar]
- 30.Geckler RW, Standiford HC, Calia FM, Kramer HC, Hornick RB. Anaerobic bacteriuria in a male urologic outpatient population. J Urol. 1977;118:800–802. doi: 10.1016/S0022-5347(17)58199-9. [DOI] [PubMed] [Google Scholar]
- 31.Larke NL, Thomas SL, Dos Santos Silva I, Weiss HA. Male circumcision and penile cancer: a systematic review and meta-analysis. Cancer Causes Control. 2011 doi: 10.1007/s10552-011-9785-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Krishnan S, Eslick GD. Streptococcus bovis infection and colorectal neoplasia: a meta-analysis. Colorectal Dis. 2014;16:672–680. doi: 10.1111/codi.12662. [DOI] [PubMed] [Google Scholar]
- 33.Takamura, N., Kenzaka, T., Minami, K. & Matsumura, M. Infective endocarditis caused by Streptococcus gallolyticus subspecies pasteurianus and colon cancer. BMJ case reports2014, 10.1136/bcr-2013-203476 (2014). [DOI] [PMC free article] [PubMed]
- 34.Mitsuhashi K, et al. Association of Fusobacterium species in pancreatic cancer tissues with molecular features and prognosis. Oncotarget. 2015;6:7209–7220. doi: 10.18632/oncotarget.3109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Danesh J, Newton R, Beral V. Epidemiology. A human germ project? Nature. 1997;389(21):23–24. doi: 10.1038/37879. [DOI] [PubMed] [Google Scholar]
- 36.Gilbert R, et al. Predictors of 25-hydroxyvitamin D and its association with risk factors for prostate cancer: evidence from the prostate testing for cancer and treatment study. Cancer Causes Control. 2012;23:575–588. doi: 10.1007/s10552-012-9919-8. [DOI] [PubMed] [Google Scholar]
- 37.Bonilla C, et al. Using genetic proxies for lifecourse sun exposure to assess the causal relationship of sun exposure with circulating vitamin d and prostate cancer risk. Cancer Epidemiol Biomarkers Prev. 2013;22:597–606. doi: 10.1158/1055-9965.EPI-12-1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gilbert, R. et al. Associations of circulating and dietary vitamin D with prostate cancer risk: a systematic review and dose-response meta-analysis. Cancer Causes Control, 10.1007/s10552-010-9706-3 (2011). [DOI] [PubMed]
- 39.Bonilla C, et al. Pubertal development and prostate cancer risk: Mendelian randomization study in a population-based cohort. BMC medicine. 2016;14:66. doi: 10.1186/s12916-016-0602-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liu PT, et al. Toll-like receptor triggering of a vitamin D-mediated human antimicrobial response. Science. 2006;311:1770–1773. doi: 10.1126/science.1123933. [DOI] [PubMed] [Google Scholar]
- 41.Doll R, Peto R, Wheatley K, Gray R, Sutherland I. Mortality in relation to smoking: 40 years' observations on male British doctors. BMJ. 1994;309:901–911. doi: 10.1136/bmj.309.6959.901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Head J, et al. Diagnosis-specific sickness absence as a predictor of mortality: the Whitehall II prospective cohort study. BMJ. 2008;337:a1469. doi: 10.1136/bmj.a1469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Oliver, T. Whitehall sickness absence and Cancer: ?Due to Sunlight deficiency http://www.bmj.com/content/337/bmj.a1469?tab=responses337 (2008). <http://www.bmj.com/content/337/bmj.a1469/reply#bmj_el_202937?sid=096094ef-71f6-4d52-b473-c5f4eb3e97dc>.
- 44.Richards, D. A. et al. Cost and Outcome of Behavioural Activation versus Cognitive Behavioural Therapy for Depression (COBRA): a randomised, controlled, non-inferiority trial. Lancet, 10.1016/S0140-6736(16)31140-0 (2016). [DOI] [PMC free article] [PubMed]
- 45.Fang J, et al. Low levels of prostate-specific antigen predict long-term risk of prostate cancer: results from the Baltimore Longitudinal Study of Aging. Urology. 2001;58:411–416. doi: 10.1016/S0090-4295(01)01304-8. [DOI] [PubMed] [Google Scholar]
- 46.Basson A. Vitamin D and Crohn's disease in the adult patient: a review. JPEN. Journal of parenteral and enteral nutrition. 2014;38:438–458. doi: 10.1177/0148607113506013. [DOI] [PubMed] [Google Scholar]
- 47.Ho S, Pothoulakis C, Koon HW. Antimicrobial peptides and colitis. Current pharmaceutical design. 2013;19:40–47. doi: 10.2174/13816128130108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zozaya M, et al. Bacterial communities in penile skin, male urethra, and vaginas of heterosexual couples with and without bacterial vaginosis. Microbiome. 2016;4:16. doi: 10.1186/s40168-016-0161-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Xiao B, et al. Predictive value of the composition of the vaginal microbiota in bacterial vaginosis, a dynamic study to identify recurrence-related flora. Scientific reports. 2016;6:26674. doi: 10.1038/srep26674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bauer, H. W. et al. A long-term, multicenter, double-blind study of an Escherichia coli extract (OM-89) in female patients with recurrent urinary tract infections. Eur Urol47, 542-548; discussion 548, 10.1016/j.eururo.2004.12.009 (2005). [DOI] [PubMed]
- 51.Huttner A, et al. Safety, immunogenicity, and preliminary clinical efficacy of a vaccine against extraintestinal pathogenic Escherichia coli in women with a history of recurrent urinary tract infection: a randomised, single-blind, placebo-controlled phase 1b trial. The Lancet infectious diseases. 2017;17:528–537. doi: 10.1016/S1473-3099(17)30108-1. [DOI] [PubMed] [Google Scholar]
- 52.Smelov V, et al. Metagenomic sequencing of expressed prostate secretions. J Med Virol. 2014;86:2042–2048. doi: 10.1002/jmv.23900. [DOI] [PubMed] [Google Scholar]
- 53.Lagier JC, et al. Microbial culturomics: paradigm shift in the human gut microbiome study. Clinical microbiology and infection : the official publication of the European Society of Clinical Microbiology and Infectious Diseases. 2012;18:1185–1193. doi: 10.1111/1469-0691.12023. [DOI] [PubMed] [Google Scholar]
- 54.Haigh JD, et al. Rapid identification of bacteria from bioMerieux BacT/ALERT blood culture bottles by MALDI-TOF MS. Br J Biomed Sci. 2013;70:149–155. doi: 10.1080/09674845.2013.11669949. [DOI] [PubMed] [Google Scholar]
- 55.Oliver, T., Blackburn, L. & Chinegwundoh, F. in Seventh AACR Conference on The Science of Health Disparities in Racial/Ethnic Minorities and the Medically Underserved. Abstract nr B89. (Cancer Epidemiol Biomarkers Prev).
- 56.Gillet E, et al. Bacterial vaginosis is associated with uterine cervical human papillomavirus infection: a meta-analysis. BMC Infect Dis. 2011;11:10. doi: 10.1186/1471-2334-11-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Peres AL, et al. Molecular analysis and conventional cytology: association between HPV and bacterial vaginosis in the cervical abnormalities of a Brazilian population. Genetics and molecular research : GMR. 2015;14:9497–9505. doi: 10.4238/2015.August.14.13. [DOI] [PubMed] [Google Scholar]
- 58.Head J, et al. Diagnosis-specific sickness absence as a predictor of mortality: the Whitehall II prospective cohort study. BMJ. 2008;337:a1469. doi: 10.1136/bmj.a1469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bjartveit K, Tverdal A. Health consequences of smoking 1-4 cigarettes per day. Tob Control. 2005;14:315–320. doi: 10.1136/tc.2005.011932. [DOI] [PMC free article] [PubMed] [Google Scholar]


