Abstract
The purpose of the study was to compare the two mucin secretogogues, diquafosol (DQS) and rebamipide (RBM), for the treatment of dry eye syndrome (DES) in office workers. Dry eye patients using computers for >4 h/day were randomly assigned treatment with either DQS or RBM. Main outcomes measures included changes in tear film break-up time (TBUT) and subjective symptoms assessed by the Dry Eye-Related Quality of Life Score (DEQS). The subjects had scheduled examinations at 0 and 4 weeks, and the examinations at 2 and 8 weeks were optional. Changes in keratoconjunctival fluorescein score and a patient satisfaction questionnaire were also recorded. Both groups showed significant improvements in the DEQS scores at 2, 4, and 8 weeks following the initiation of the study. Both groups showed significant increases in the TBUT at 2 and 4 weeks. No significant difference was found between the DQS and RBM groups at any time periods. Patients reported more comfort with the use of DQS compared with the use of RBM. No local or systemic side effects were noted. The results of the present study indicated that both DQS and RBM were effective for the treatment of DES in office workers.
Introduction
Dry eye syndrome (DES) is a common eye disorder with an increasing incidence that affects more than 10 million people in Japan1. A recent report by the Asia Dry Eye Society defines DES as follows: “Dry eye is a multifactorial disease characterized by unstable tear film causing a variety of symptoms and/or visual impairment, potentially accompanied by ocular surface damage”2. Although DES primarily affects females and the elderly, it is also a concern among young, active workers. A recent study reported that more than half of Japanese office workers suffered from DES, and the use of a computer for >4 h/day was a risk factor for DES3. In addition, studies have shown that DES is associated with general health problems and decreased productivity, including a decreased quality of sleep, depression, and impaired subjective happiness4–13.
DES has been primarily treated using artificial tears and antiinflammatory therapies14–16. Two kinds of mucin secretogogues, diquafosol sodium [3% Diquas® ophthalmic solution (DQS), Santen Pharmaceutical, Osaka, Japan; chemical name, tetrasodium P1,P4-bis(5′-uridyl) tetraphosphate] and rebamipide [2% Mucosta® ophthalmic suspension (RBM), Otsuka Pharmaceutical, Tokyo, Japan; chemical name, (2RS)-2-(4-chlorobenzoylamino)-3- (2-oxo-1,2-dihydroquinolin-4-yl) propanoic acid] have recently become commercially available in Japan. While both formulations increase tear film mucins, they have different mechanisms of action. DQS is a purinergic P2Y2 receptor agonist that increases mucin expression and its secretion from goblet cells in mice17, rats18, rabbits19, and humans20. RBM is a mucoprotective drug, and it has been used for the treatment of gastoric/duodenal ulcers in Japan. RBM has been shown to increase the production of mucins from corneal or conjunctival tissues either in cultured cells21 or in animal models22,23. Unlike DQS, RBM has been shown to increase the goblet cell numbers24,25. Uchino Y recently reported that RBM increased MUC 16 protein synthesis in human corneal epithelial cells26. In addition, RBM has been shown to have antiinflammatory effects27–29. Both drugs have been reported to be effective for various kinds of DES including Sjogren’s syndrome, non-Sjogren’s aqueous-deficient DES, and DES with tear film instability30–41. However, there has been no large-scale prospective report that compared the efficacy and safety of these drugs. In the present study, we conducted a prospective, randomized, clinical trial to determine the efficacy and safety of DQS and RBM for the treatment of DES in office workers that used computers.
Results
Participants
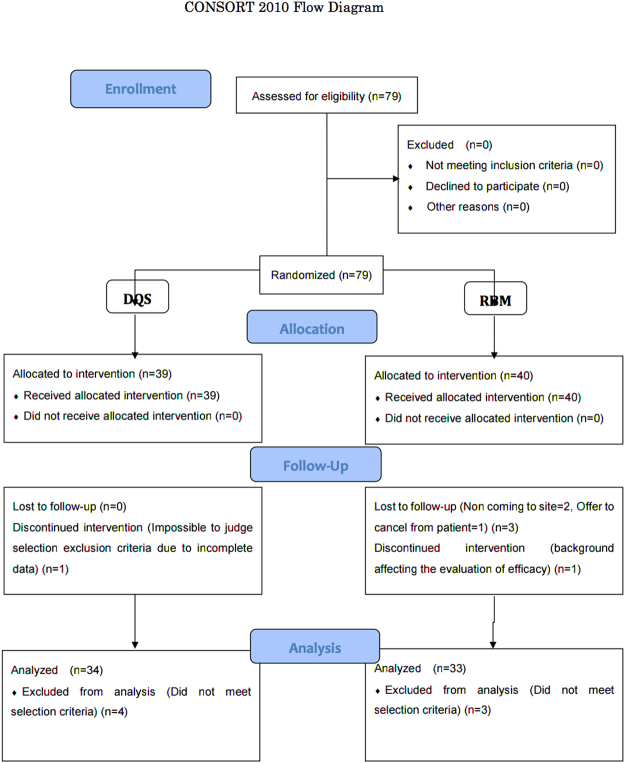
A diagram of participant flow is shown in Fig. 1. Of the 79 workers enrolled, 12 dropped out of the study due to the following: 7 workers did not come for the follow-up, 3 workers discontinued the study due to self-judgment, 1 worker moved to another place, and 1 worker required ophthalmic surgery (chalazion excision).
Figure 1.
CONSORT (Consolidated Standards of Reporting Trials) 2010 flow diagram.
Table 1 shows a demographic profile of the workers in the study. There were 20 males and 47 females with a total mean age of 40.0 ± 10.2 years. Thirty-four and 33 eyes were allocated to the DQS and RBM groups, respectively. There was no significantly different parameter between the two groups (Table 1).
Table 1.
Demographic profile of subjects.
| Diquafosol (n = 34) | Rebamipide (n = 33) | P value | ||
|---|---|---|---|---|
| Age (years, mean±SD) | 38.2 ± 9.7 | 41.5 ± 9.6 | 0.17 | |
| Sex (male:female) | 10:24 | 10:23 | 1.00 | |
| Mean computer use time | 4–6 h/day | 9 | 7 | 0.44 |
| 6–8 h/day | 10 | 8 | ||
| >8 h/day | 15 | 18 | ||
| Other eye Drops used | None | 29 | 28 | 1.00 |
| Artificial tears | 0 | 3 | ||
| Hyaluronates | 3 | 2 | ||
| Others | 2 | 0 | ||
| BSCVA | 1.23 ± 0.15 | 1.18 ± 0.10 | 0.11 | |
| Mean TBUT (s) | 3.45 ± 1.02 | 3.20 ± 0.84 | 0.28 | |
| Schirmer’s value (mm/5 min) | 13.7 ± 9.50 | 15.2 ± 10.7 | 0.56 | |
| Mean QOL score | 44.7 ± 20.2 | 48.6 ± 18.0 | 0.41 | |
BSCVA, best spectacle-corrected visual acuity; TBUT, tear film beak-up time; QOL; quality of life (determined by the Dry Eye-Related Quality of Life Score test).
Changes in subjective symptoms
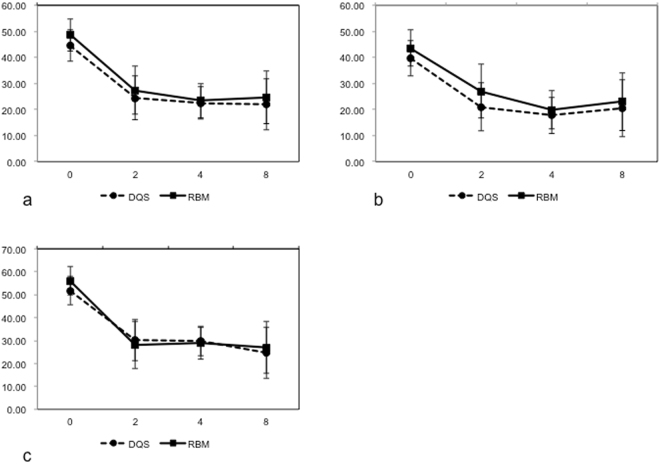
Figure 2A shows the changes in total subjective symptoms of the DEQS and RBM groups. Both groups showed significant improvements in symptoms at 2, 4, and 8 weeks following the initial treatments (P < 0.0001 at all time points). No significant difference was noted between DQS and RBM groups throughout the observation period.
Figure 2.
Changes in subjective symptoms in diquafosol and rebamipide groups assessed by the Dry Eye-Related Quality of Life Scores: (a) changes in the total score, (b) changes in the ophthalmic symptoms, and (c) changes in the quality of life-related scores.
Figures 2B,C shows the changes in ocular symptoms and daily life-related symptoms. In ocular symptoms, both DQS and RBM groups showed significant improvements in symptoms at 2, 4, and 8 weeks following the initial treatments (P < 0.0001 at all time points). In daily life-related symptoms, both groups showed significant improvements in symptoms at 2 (P < 0.0001 and P = 0.0009 in the DQS and RBM groups, respectively), 4 (P < 0.0001 in both groups), and 8 weeks (P = 0.0004 and 0.0002 in the DQS and RBM groups, respectively) following the initial treatments. No significant difference was found between the two groups throughout the observation periods.
Changes in the TBUT and fluorescein scores
Both the DQS and RBM groups showed significant prolongation of TBUT at 2 and 4 weeks following initiation of the treatment, compared with pretreatment values (Fig. 3). Only the RBM group showed a significant increase in the TBUT at week 8 (P = 0.022). No significant difference was noted between the DQS and RBM groups at any period.
Figure 3.
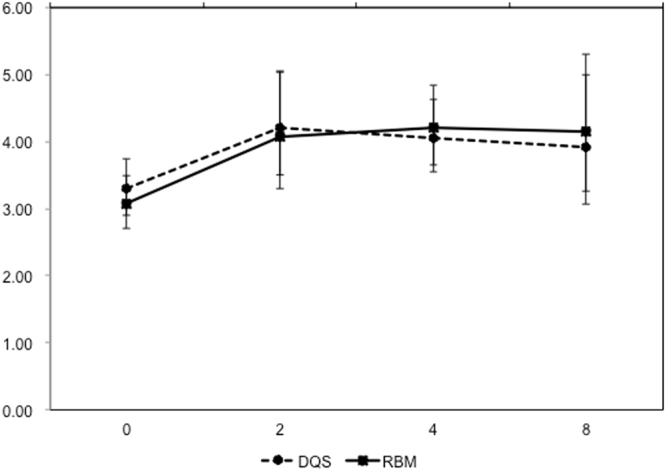
Changes in tear film break-up times.
The RBM group showed significant decreases in fluorescein scores at 2 (P = 0.0023), 4 (P = 0.0054), and 8 (P = 0.044) weeks, but not in the DQS groups compared with week 0. There was no significant difference between the DQS and RBM groups during any study period (Fig. 4). No local or systemic side effects were noted in both groups.
Figure 4.
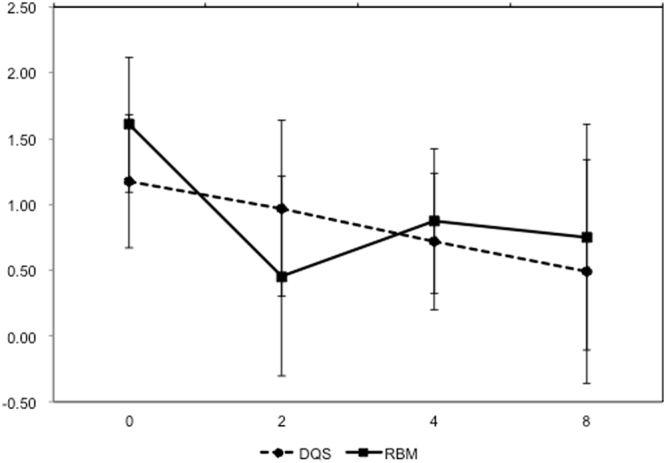
Changes in fluorescein staining scores.
Questionnaire
The results of the questionnaire are shown in Table 2. Twenty-eight and 23 responses were obtained from the DQS and RBM groups, respectively. The workers reported that comfort was better in the DQS group compared with the RBM group (P = 0.042). There was no significant difference between the two groups regarding “easy to use” and “willing to use more.”
Table 2.
Patients’ preferences for diquafosal and rebamipide eye drops using the questionnaire.
| Questionnaire | Diquafosol (n = 34) | Rebamipide (n = 33) | P value |
|---|---|---|---|
| Comfort | |||
| Very good | 5 (17.9%) | 1 (4.3%) | 0.042 |
| Good | 6 (21.4%) | 5 (21.7%) | |
| Fair | 16 (57.1%) | 11 (47.8%) | |
| Bad | 1 (3.6%) | 6 (26.1%) | |
| Very bad | 0 (0%) | 0 (0%) | |
| Easy to use | |||
| Very good | 5 (17.9%) | 1 (4.3%) | 0.62 |
| Good | 10 (35.7%) | 14 (60.9%) | |
| Fair | 11 (39.3%) | 5 (21.7%) | |
| Bad | 2 (7.1%) | 2 (8.7%) | |
| Very bad | 0 (0%) | 1 (4.3%) | |
| Willing to use more | |||
| Very positive | 3 (10.7%) | 1 (4.3%) | 0.20 |
| Positive | 13 (46.4%) | 8 (34.8%) | |
| Fair | 8 (28.6%) | 9 (39.1%) | |
| Negative | 4 (14.3%) | 5 (21.7%) | |
| Very negative | 0 (0%) | 0 (0%) | |
Factor analyses
The results of the factor analyses are shown in Table 3. There were two factors (factors 3 and 4) that showed a relatively strong association with the use of eye drops. For factor 3, “dryness” needed improvement, usefulness (i.e., comfort, easy to use, willing to use more) was preferred in the DQS group, and “heavy feeling” needed improvement in the RBM group. The results of factor 4 showed that the epithelial damage responded to DQS treatment in workers with limited computer times, for users of other DES eye drops for more than 2 weeks, and for workers with low Schirmer’s test values. DEQS scores, the presence of photophobia, and worsening of DES symptoms while reading improved more in workers in the RBM group who had characteristics of the above workers.
Table 3.
The results of the factor analyses.
| Factors | Factor1 | Factor2 | Factor3 | Factor4 | Factor5 |
|---|---|---|---|---|---|
| Drugs | −0.176 | 0.164 | 0.439 | −0.425 | 0.339 |
| Sex | −0.249 | 0.042 | −0.162 | 0.025 | −0.718 |
| Age | −0.065 | 0.149 | −0.035 | −0.125 | −0.089 |
| Mean duration of computer work | −0.231 | −0.091 | −0.102 | −0.478 | −0.206 |
| Use of other dry eye medications | 0.029 | 0.196 | −0.002 | 0.414 | −0.311 |
| Corrected visual acuity | 0.069 | 0.080 | −0.234 | 0.258 | 0.703 |
| Schirmer’s I test | 0.037 | −0.061 | 0.228 | −0.583 | 0.040 |
| Comfort for eye drop use | −0.154 | −0.017 | 0.806 | −0.210 | 0.034 |
| Easy to use | 0.014 | −0.296 | 0.667 | −0.134 | −0.206 |
| Willing to use more | 0.028 | −0.211 | 0.813 | 0.057 | −0.113 |
| Δ log(BUT) | 0.166 | −0.214 | −0.114 | −0.027 | 0.559 |
| Δ Staining scores | −0.113 | −0.133 | 0.062 | −0.417 | −0.192 |
| Δ DEQS score (total) | 0.921 | 0.050 | −0.061 | 0.483 | 0.179 |
| Δ DEQS score (ocular symptom) | 0.922 | 0.045 | 0.021 | 0.200 | 0.237 |
| Δ DEQS score (QOL) | 0.753 | 0.046 | −0.119 | 0.638 | 0.098 |
| Δ Foreign body sensation | 0.601 | 0.221 | 0.000 | −0.056 | 0.344 |
| Δ Dry sensation | 0.574 | 0.102 | 0.388 | 0.087 | 0.221 |
| Δ Ocular pain | 0.819 | −0.110 | −0.084 | 0.320 | 0.000 |
| Δ Eye strain | 0.680 | −0.210 | 0.183 | 0.093 | 0.099 |
| Δ Heavy feeling | 0.604 | 0.141 | −0.375 | 0.120 | 0.069 |
| Δ Eye redness | 0.232 | 0.595 | −0.209 | 0.111 | 0.056 |
| Δ Difficulty in eye open | 0.660 | 0.269 | −0.115 | 0.247 | 0.504 |
| Δ Blurring | 0.388 | 0.346 | −0.157 | 0.209 | 0.111 |
| Δ Photophobia | 0.639 | −0.211 | −0.116 | 0.531 | 0.081 |
| Δ Newspaper reading | 0.403 | −0.207 | −0.120 | 0.727 | −0.230 |
| Δ TV watching | 0.602 | −0.068 | −0.119 | −0.190 | −0.182 |
*Factors with strong association were shown in bold.
Discussion
Office workers are exposed to numerous risk factors for DES. Several studies have reported that prolonged computer use is associated with the development of DES. A recent study also reported that sedentary behavior and the resulting metabolic syndrome were risk factors for DES5,8. Furthermore, many office workers use soft contact lenses and/or are exposed to air-conditioning, which are both considered to be risk factors for DES. DES effects have been shown to decrease the productivity of office workers42. In a recent clinical study that examined 672 Japanese office workers who used computers, 65 workers (11.6%) were diagnosed with DES and 303 workers (54.0%) were diagnosed with probable DES according to the Japanese Dry Eye Criteria43. Most of the subjects with probable DES had a decreased TBUT (<5 s), positive symptoms without measurable ocular surface epitheliopathy, and decreased tear secretion (i.e., a short TBUT-type DES). Decreased TBUT is an important parameter for DES in office workers, so this parameter was chosen as one of the main outcomes in the study. Overall, 44 eyes were categorized as short-BUT-type DES.
Ocular surface mucins have been implicated in the pathogenesis of DES. Uchino et al. reported that the MAC5AC concentration in tears was significantly lower in office workers that used computers for long periods of time compared with short-term computer users44. Both DQS and RBM are mucin secretogogues; thus, they are expected to be effective for the treatment of DES in office workers. Since it became available in Japan in 2011, DQS ophthalmic solution has been used and proven effective for treating various types of DES, including aqueous-deficient, short TBUT-type DES and postoperative DES following cataract or laser in situ keratomileusis34,36,45–49. Due to its unique properties for mucous tissue protection, RBM has been widely used for the treatment of gastric and duodenal ulcers. As eye drops, RBM was launched in the Japanese market in 2012 for the treatment of DES. It has been reported to increase the production of mucin-like substances in the cornea and conjunctiva, increase the number of goblet cells, suppress expression of cytokines, and attenuate tumor necrosis factor-α-induced barrier disruption in the corneal epithelium21,23,28. Although both DQS and RBM promote mucin secretion in tears, there have been many reports indicating their own unique mechanisms of action. DQS increases tear film quantity for up to 30 min following instillation in normal human subjects, which was much longer than artificial tears and hyaluronates50. Due to its mechanism of action, DQS is considered to be useful for aqueous-deficient DES. In addition, DQS upregulates the expression of secretory mucins (e.g., MAC5AC) and membrane-bound mucins (e.g., MUC1, MUC4, and MUC16) that are responsible for the wettability of the ocular surface epithelium. Recent studies also reported that P2Y2 receptors are expressed in meibomian glands; Arita et al. reported the potential usefulness of DQS for the treatment of obstructive meibomian gland dysfunction51. In contrast, RBM has been shown to be effective for various ocular surface diseases other than DES, including allergic conjunctivitis, filamentary keratitis, conjunctivochalasis, lid wiper epitheliopathy, and superior limbic keratoconjunctivitis52–54. The underlying mechanism is not totally clear; however, there are two major hypotheses that explain RBM`s unique properties. One is that RBM has anti-inflammatory and antioxidant properties23. The second mechanism is that RBM increases the number of goblet cells24. These two mechanisms are consistent with RBM being a potential treatment for ocular surface diseases related to persistent inflammation and friction between the ocular surface epithelium and lid margins. RBM also increased the expression of membrane-bound mucins in vitro 55.
The results of the present study indicated that both DQS and RBM were effective in alleviating DES symptoms and prolonging TBUT. The effects were found as early as 2 weeks. Factor analyses indicated that DQS was more effective in workers using computers for long periods of time or in eyes with decreased Schirmer’s test values. These findings are consistent with the mechanism of action of DQS, involving promotion of aqueous secretion from the conjunctival epithelium. In addition, recent studies reported that DQS is effective for contact lens users with DES49,56. Although contact lens users were excluded from the present study, these observations may expand the usefulness of DQS in office workers. RBM was also an effective treatment for eyes with ocular surface epithelial damage (Fig. 4). It is notable that some workers preferred using DQS rather than RBM. RBM is a single dose unit drug that is a white emulsion, so some patients may have had ambivalent feelings about using such an emulsion in their eye. Because DES patients need to apply eye drops over a long period, a comfortable eye drop generally may be selected to continue the therapy.
There were some limitations in the present study. First, as previously mentioned, we did not include contact lens wearers in the study, although many workers with DES in the office wear disposable soft contact lenses, so the efficacy of DQS and RBM for contact lens wearers should be investigated in the future. In addition, we did not study the long-term effects of the drugs. Because both drugs improved DES symptoms and signs when used over an extended period of time, continuous use of the drugs would further increase their efficacy32,37. Also, the present study did not elucidate the mechanism of action why DQS and RBM were effective for the DES in office workers as no cytological/biological studies including impression cytology or mucin measurements. Further studies are needed to determine the long-term effects.
In summary, in a prospective, randomized, clinical study, we found that DQS and RBM were both effective for alleviating irritating symptoms and prolonging the TBUT of DES in office workers. Because the drugs have different mechanisms of action and usability, they may be used differently based on the subtypes of DES and the patients’ preferences.
Methods
Study design and participants
This was an open-labeled, prospective, randomized, multicenter clinical trial involving one university hospital and six private clinics in the Tokyo metropolitan area. The protocol adhered to the principles of the Declaration of Helsinki. Written informed consent were obtained by all subjects after explaining the purpose and potential risks of the study. The study was approved by the internal review board of each participating hospital/clinic, and the clinical trial was registered in the Clinical Trial Registry of the University Hospital Medical Information Network (UMIN-CTR; UMIN000012742) with the date of registration (01/01/2014). The study was approved by the internal review board of the Tokyo Dental College (I-13-12). The study was also approved by the internal review board of the IRB in the Keishokai Medical Corporation that manages Ryogoku Eye Clinic, Shinjuku Eye Clinic, and Iidabashi Eye Clinic in October 2013. Other participating clinics including Shimazaki Eye Clinic, Ichikawa Shapo Eye Clinic, and Smile Eye Clinic charged the IRB of the Keishokai Medical Cooperation with the deliberation of the ethical validity of the study protocol.
Inclusion criterion included: 1) DES or suspected DES according to the criteria of the Japan Dry Eye Society57, 2) in the Dry Eye-Related Quality of Life Score (DEQS), a general condition score >4 and any of the scores regarding bothersome ocular symptoms >3, 3) full-time office workers who used computers an average of >4 h/day, and 4) workers 20–60 years of age. The following workers were excluded: users of either DQS or RBM within 2 weeks of the study initiation, users of systemic medications that can influence tear/ocular surface conditions, pregnant females, workers with active ocular surface disorders other than DES, workers with abnormal eyelids or blinking, workers with a history of ocular surgeries, workers using punctual plugs, and contact lens wearers. There were 5 subjects in each group who have been using artificial tears at the time of initiation of the study, and they were instructed to continue using the eye drops throughout the study period without changing the frequency.
The diagnostic criteria of the Japan Dry Eye Society are: 1) positive symptoms, 2) qualitative or quantitative disturbance of the tear film (Schirmer I test equal or less than 5 mm/5 min or BUT equal or less than 5 sec), 3) keratoconjunctival epithelial damage (staining score greater than 3 points). The presence of all criteria renders a diagnosis of definite dry eye and the presence of two out of the three criteria renders a diagnosis of probable dry eye57. In the present study, 3 and 10 eyes in the DQS and RBM groups, respectively, were “definite dry eye”, while 31 and 23 eyes in the DQS and RBM groups, respectively, were “probable dry eye”. Vast majority of the eyes with “probable dry eye” had positive symptoms and BUT with equal or less than 5 seconds, while keratoconjunctival staining scores with less than 3 points (30 and 23 eyes in the DQS and RBM groups, respectively). The workers were randomly divided into two groups using an envelope method, with use of DQS eye drops six times/day or RBM eye drops four times/day. If workers had been using other eye drops, they continued to use them during the study period at the same frequency. The workers had scheduled examinations at 0 and 4 weeks after the initiation of treatments. Examinations at 2 and 8 weeks were optional.
Previous studies reported that DQS or RBM improved subjective symptom scores 30–60% in DES patients33,36. We assumed that clinically meaningful changes in symptoms were 20%, with a 30% standard deviation. A sample size of 40 eyes (80 eyes in total) in each group was chosen to provide at least 80% power to detect a 20% difference in rejection rates using a one-sided α-error level of 0.05.
Examinations
The primary outcome of the study included changes in subjective symptoms assessed by the DEQS, and changes in tear film break-up time (TBUT). The DEQS is a validated questionnaire consisting of 15 items and two subscales involving the Impact on Daily Life and Bothersome Ocular Symptoms58; six items in the questionnaire pertain to bothersome ocular symptoms and nine items consider impacts on daily life. All patients first answered the frequency of symptoms and disability, and then answered the degree of each item. The calculated summary score ranged from 0–100, with a higher score representing a greater disability. In addition to the DEQS questionnaire, the patients’ preferences for drug treatment were examined in a different set of questionnaires consisting of three questions: 1) Were the eye drops comfortable? 2) Were the eye drops easy to use? 3) Are you willing to continue using the eye drops? The answer to each question was graded on a scale using five degrees.
The TBUT was chosen as the main outcome because impaired tear film stability is considered to be the major mechanism that causes dry eye and/or visual impairment2. The TBUT was measured after instillation of a minimum amount of preservative-free 1% fluorescein dye. The time until the first dry spot appeared was recorded, and the average of three measurements was used in subsequent analyses. The second and third outcome measures included the patients’ preference using the questionnaire and the ocular surface damage assessed by fluorescein staining scores. Staining scores were evaluated using van Bijsterveld scoring in which the staining intensity was measured semiquantitatively using a range from 0–3 in the cornea and nasal/temporal bulbar conjunctiva (a maximum of 9 points)59. To evaluate secretion of tears, the standard Schirmer’s test without topical anesthesia was performed more than 30 min after determining the TBUT or the vital stain scores to avoid affecting the two tests. Cutoff values were determined according to the Japanese Dry Eye Criteria. Local or systemic side effects were also recorded.
Data collection and statistical analysis
Clinical data were sent to the data center, and the eligibility of the subjects for this study were checked by a masked examiner. Continuous background variables were summarized using the mean and standard deviations, and the t-test was used for a comparison between treatment groups. Categorical background variables were summarized using count and percentages, and Fisher’s exact test and the Cochran–Mantel–Haenszel test were used for their comparisons. To evaluate changes in outcome variables and to compare treatment groups in treated eyes, mixed effects models were used with each time point, treatment, and interaction as fixed effects, with the patients as a random effect.
To describe the structural relationships among the treatment, background variables, and outcomes, factor analyses were used with the principal component method and Promax rotation. Fifty-four patients and 28 variables, including sex, age, mean computer work time, other eye drops for DES, visual acuity, Schirmer’s test as a background variable, questionnaire results, and changes in DEQS as outcomes were used in the analyses.
Acknowledgements
The authors thank Biostatistical Research Company for the statistical analyses. The English in this document has been checked by at least two professional editors, both native speakers of English. For a certificate, please see: http://www.textcheck.com/certificate/eQ19Xr, This study was registered in the Clinical Trial Registry of the University Hospital Medical Information Network (UMIN-CTR; UMIN000012742) with the date of registration (01/01/2014). The study was approved by the internal review board of the Tokyo Dental College (I-13-12). The study was also approved by the internal review board of the IRB in the Keishokai Medical Corporation that manages Ryogoku Eye Clinic, Shinjuku Eye Clinic,and Iidabashi Eye Clinic in October 2013. Other participating clinics including Shimazaki Eye Clinic, Ichikawa Shapo Eye Clinic, and Smile Eye Clinic charged the IRB of the Keishokai Medical Cooperation with the deliberation of the ethical validity of the study protocol.
Author Contributions
J.S. created the original idea of the study, and wrote the main manuscript. S.D., M.S., and M.S. contributed to the refinements of the protocol. S.D., M.S., K.F., M.S., M.I., and T.O. contributed to the collection of data. All authors reviewed the manuscript.
Competing Interests
This study was supported by a grant from Santen Pharmaceutical Company. Personal financial interests: J.S. and D.S. received financial supports from Santen Pharmaceutical Company and Otsuka Pharmaceutical Company for reimbursement for attending symposia. J.S. also received financial supports from Santen Pharmaceutical Company and Otsuka Pharmaceutical Company for his scientific advisory board.
Footnotes
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Uchino M, et al. Prevalence and risk factors of dry eye disease in Japan: Koumi study. Ophthalmology. 2011;118:2361–2367. doi: 10.1016/j.ophtha.2011.05.029. [DOI] [PubMed] [Google Scholar]
- 2.Tsubota, K. et al. New Perspectives on Dry Eye Definition and Diagnosis: A Consensus Report by the Asia Dry Eye Society. Ocul Surf (2016). [DOI] [PubMed]
- 3.Uchino M, et al. Prevalence of dry eye disease and its risk factors in visual display terminal users: the Osaka study. Am J Ophthalmol. 2013;156:759–766. doi: 10.1016/j.ajo.2013.05.040. [DOI] [PubMed] [Google Scholar]
- 4.Ayaki M, et al. Sleep and mood disorders in dry eye disease and allied irritating ocular diseases. Sci Rep. 2016;6:22480. doi: 10.1038/srep22480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kawashima M, et al. Decreased tear volume in patients with metabolic syndrome: the Osaka study. Br J Ophthalmol. 2014;98:418–420. doi: 10.1136/bjophthalmol-2013-303953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kawashima M, et al. Associations between subjective happiness and dry eye disease: a new perspective from the Osaka study. PLoS One. 2015;10:e0123299. doi: 10.1371/journal.pone.0123299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kawashima M, et al. The association of sleep quality with dry eye disease: the Osaka study. Clin Ophthalmol. 2016;10:1015–1021. doi: 10.2147/OPTH.S99620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kawashima M, et al. The Association between Dry Eye Disease and Physical Activity as well as Sedentary Behavior: Results from the Osaka Study. J Ophthalmol. 2014;2014:943786. doi: 10.1155/2014/943786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Labbe A, et al. Dry eye disease, dry eye symptoms and depression: the Beijing Eye Study. Br J Ophthalmol. 2013;97:1399–1403. doi: 10.1136/bjophthalmol-2013-303838. [DOI] [PubMed] [Google Scholar]
- 10.Li M, Gong L, Sun X, Chapin WJ. Anxiety and depression in patients with dry eye syndrome. Curr Eye Res. 2011;36:1–7. doi: 10.3109/02713683.2010.519850. [DOI] [PubMed] [Google Scholar]
- 11.Szakats, I., Sebestyen, M., Nemeth, J., Birkas, E. & Purebl, G. The Role of Health Anxiety and Depressive Symptoms in Dry Eye Disease. Curr Eye Res, 1–6 (2015). [DOI] [PubMed]
- 12.Uchino M, et al. Dry eye disease and work productivity loss in visual display users: the Osaka study. Am J Ophthalmol. 2014;157:294–300. doi: 10.1016/j.ajo.2013.10.014. [DOI] [PubMed] [Google Scholar]
- 13.Wan, K. H., Chen, L. J. & Young, A. L. Depression and anxiety in dry eye disease: a systematic review and meta-analysis. Eye (Lond) (2016). [DOI] [PMC free article] [PubMed]
- 14.Stevenson D, Tauber J, Reis BL. Efficacy and safety of cyclosporin A ophthalmic emulsion in the treatment of moderate-to-severe dry eye disease: a dose-ranging, randomized trial. The Cyclosporin A Phase 2 Study Group. Ophthalmology. 2000;107:967–974. doi: 10.1016/S0161-6420(00)00035-X. [DOI] [PubMed] [Google Scholar]
- 15.McDonald CC, Kaye SB, Figueiredo FC, Macintosh G, Lockett CA. randomised, crossover, multicentre study to compare the performance of 0.1% (w/v) sodium hyaluronate with 1.4% (w/v) polyvinyl alcohol in the alleviation of symptoms associated with dry eye syndrome. Eye (Lond) 2002;16:601–607. doi: 10.1038/sj.eye.6700169. [DOI] [PubMed] [Google Scholar]
- 16.Shimmura S, et al. Sodium hyaluronate eyedrops in the treatment of dry eyes. Br J Ophthalmol. 1995;79:1007–1011. doi: 10.1136/bjo.79.11.1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kojima T, et al. The effects of 3% diquafosol sodium application on the tear functions and ocular surface of the Cu,Zn-superoxide dismutase-1 (Sod1)-knockout mice. Mol Vis. 2014;20:929–938. [PMC free article] [PubMed] [Google Scholar]
- 18.Choi KE, Song JS, Kang B, Eom Y, Kim HM. Immediate Effect of 3% Diquafosol Ophthalmic Solution on Tear MUC5AC Concentration and Corneal Wetting Ability in Normal and Experimental Keratoconjunctivitis Sicca Rat Models. Curr Eye Res. 2017;42:666–671. doi: 10.1080/02713683.2016.1233986. [DOI] [PubMed] [Google Scholar]
- 19.Fujihara T, Murakami T, Nagano T, Nakamura M, Nakata K. INS365 suppresses loss of corneal epithelial integrity by secretion of mucin-like glycoprotein in a rabbit short-term dry eye model. J Ocul Pharmacol Ther. 2002;18:363–370. doi: 10.1089/10807680260218524. [DOI] [PubMed] [Google Scholar]
- 20.Shigeyasu C, Hirano S, Akune Y, Yamada M. Diquafosol Tetrasodium Increases the Concentration of Mucin-like Substances in Tears of Healthy Human Subjects. Curr Eye Res. 2015;40:878–883. doi: 10.3109/02713683.2014.967871. [DOI] [PubMed] [Google Scholar]
- 21.Takeji Y, Urashima H, Aoki A, Shinohara H. Rebamipide increases the mucin-like glycoprotein production in corneal epithelial cells. J Ocul Pharmacol Ther. 2012;28:259–263. doi: 10.1089/jop.2011.0142. [DOI] [PubMed] [Google Scholar]
- 22.Urashima H, Okamoto T, Takeji Y, Shinohara H, Fujisawa S. Rebamipide increases the amount of mucin-like substances on the conjunctiva and cornea in the N-acetylcysteine-treated in vivo model. Cornea. 2004;23:613–619. doi: 10.1097/01.ico.0000126436.25751.fb. [DOI] [PubMed] [Google Scholar]
- 23.Ohguchi T, et al. The effects of 2% rebamipide ophthalmic solution on the tear functions and ocular surface of the superoxide dismutase-1 (sod1) knockout mice. Invest Ophthalmol Vis Sci. 2013;54:7793–7802. doi: 10.1167/iovs.13-13128. [DOI] [PubMed] [Google Scholar]
- 24.Kase S, Shinohara T, Kase M. Effect of topical rebamipide on human conjunctival goblet cells. JAMA Ophthalmol. 2014;132:1021–1022. doi: 10.1001/jamaophthalmol.2014.431. [DOI] [PubMed] [Google Scholar]
- 25.Kase S, Shinohara T, Kase M, Ishida S. Effect of topical rebamipide on goblet cells in the lid wiper of human conjunctiva. Exp Ther Med. 2017;13:3516–3522. doi: 10.3892/etm.2017.4390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Uchino Y, Woodward AM, Argueso P. Differential effect of rebamipide on transmembrane mucin biosynthesis in stratified ocular surface epithelial cells. Exp Eye Res. 2016;153:1–7. doi: 10.1016/j.exer.2016.10.007. [DOI] [PubMed] [Google Scholar]
- 27.Fukuda K, Ishida W, Tanaka H, Harada Y, Fukushima A. Inhibition by rebamipide of cytokine-induced or lipopolysaccharide-induced chemokine synthesis in human corneal fibroblasts. Br J Ophthalmol. 2014;98:1751–1755. doi: 10.1136/bjophthalmol-2014-305425. [DOI] [PubMed] [Google Scholar]
- 28.Tanaka H, et al. Rebamipide increases barrier function and attenuates TNFalpha-induced barrier disruption and cytokine expression in human corneal epithelial cells. Br J Ophthalmol. 2013;97:912–916. doi: 10.1136/bjophthalmol-2012-302868. [DOI] [PubMed] [Google Scholar]
- 29.Ueta M, Sotozono C, Yokoi N, Kinoshita S. Rebamipide suppresses PolyI:C-stimulated cytokine production in human conjunctival epithelial cells. J Ocul Pharmacol Ther. 2013;29:688–693. doi: 10.1089/jop.2012.0054. [DOI] [PubMed] [Google Scholar]
- 30.Gong L, et al. A randomised, parallel-group comparison study of diquafosol ophthalmic solution in patients with dry eye in China and Singapore. Br J Ophthalmol. 2015;99:903–908. doi: 10.1136/bjophthalmol-2014-306084. [DOI] [PubMed] [Google Scholar]
- 31.Igarashi T, et al. Improvements in Signs and Symptoms of Dry Eye after Instillation of 2% Rebamipide. J Nippon Med Sch. 2015;82:229–236. doi: 10.1272/jnms.82.229. [DOI] [PubMed] [Google Scholar]
- 32.Kinoshita S, et al. Rebamipide (OPC-12759) in the treatment of dry eye: a randomized, double-masked, multicenter, placebo-controlled phase II study. Ophthalmology. 2012;119:2471–2478. doi: 10.1016/j.ophtha.2012.06.052. [DOI] [PubMed] [Google Scholar]
- 33.Kinoshita S, et al. A Randomized, Multicenter Phase 3 Study Comparing 2% Rebamipide (OPC-12759) with 0.1% Sodium Hyaluronate in the Treatment of Dry Eye. Ophthalmology. 2013;120:1158–1165. doi: 10.1016/j.ophtha.2012.12.022. [DOI] [PubMed] [Google Scholar]
- 34.Lee JH, Song IS, Kim KL, Yoon SY. Effectiveness and Optical Quality of Topical 3.0% Diquafosol versus 0.05% Cyclosporine A in Dry Eye Patients following Cataract Surgery. J Ophthalmol. 2016;2016:8150757. doi: 10.1155/2016/8150757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Matsumoto Y, Ohashi Y, Watanabe H, Tsubota K. & Diquafosol Ophthalmic Solution Phase 2 Study, G. Efficacy and safety of diquafosol ophthalmic solution in patients with dry eye syndrome: a Japanese phase 2 clinical trial. Ophthalmology. 2012;119:1954–1960. doi: 10.1016/j.ophtha.2012.04.010. [DOI] [PubMed] [Google Scholar]
- 36.Shimazaki-Den S, Iseda H, Dogru M, Shimazaki J. Effects of diquafosol sodium eye drops on tear film stability in short but type of dry eye. Cornea. 2013;32:1120–1125. doi: 10.1097/ICO.0b013e3182930b1d. [DOI] [PubMed] [Google Scholar]
- 37.Takamura E, Tsubota K, Watanabe H, Ohashi Y, Group DOSPS. A randomised, double-masked comparison study of diquafosol versus sodium hyaluronate ophthalmic solutions in dry eye patients. Br J Ophthalmol. 2012;96:1310–1315. doi: 10.1136/bjophthalmol-2011-301448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tauber J, et al. Double-masked, placebo-controlled safety and efficacy trial of diquafosol tetrasodium (INS365) ophthalmic solution for the treatment of dry eye. Cornea. 2004;23:784–792. doi: 10.1097/01.ico.0000133993.14768.a9. [DOI] [PubMed] [Google Scholar]
- 39.Wu D, Chen WQ, Li R, Wang Y. Efficacy and safety of topical diquafosol ophthalmic solution for treatment of dry eye: a systematic review of randomized clinical trials. Cornea. 2015;34:644–650. doi: 10.1097/ICO.0000000000000429. [DOI] [PubMed] [Google Scholar]
- 40.Yamaguchi M, et al. Real-world assessment of diquafosol in dry eye patients with risk factors such as contact lens, meibomian gland dysfunction, and conjunctivochalasis: subgroup analysis from a prospective observational study. Clin Ophthalmol. 2015;9:2251–2256. doi: 10.2147/OPTH.S96540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yokoi N, Sonomura Y, Kato H, Komuro A, Kinoshita S. Three percent diquafosol ophthalmic solution as an additional therapy to existing artificial tears with steroids for dry-eye patients with Sjogren’s syndrome. Eye (Lond) 2015;29:1204–1212. doi: 10.1038/eye.2015.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Miljanovic B, Dana R, Sullivan DA, Schaumberg DA. Impact of dry eye syndrome on vision-related quality of life. Am J Ophthalmol. 2007;143:409–415. doi: 10.1016/j.ajo.2006.11.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yokoi N, et al. Importance of tear film instability in dry eye disease in office workers using visual display terminals: the Osaka study. Am J Ophthalmol. 2015;159:748–754. doi: 10.1016/j.ajo.2014.12.019. [DOI] [PubMed] [Google Scholar]
- 44.Uchino Y, et al. Alteration of tear mucin 5AC in office workers using visual display terminals: The Osaka Study. JAMA Ophthalmol. 2014;132:985–992. doi: 10.1001/jamaophthalmol.2014.1008. [DOI] [PubMed] [Google Scholar]
- 45.Baek, J., Doh, S.H. & Chung, S.K. The Effect of Topical Diquafosol Tetrasodium 3% on Dry Eye After Cataract Surgery. Curr Eye Res, 1–5 (2016). [DOI] [PubMed]
- 46.Kaido M, Uchino M, Kojima T, Dogru M, Tsubota K. Effects of diquafosol tetrasodium administration on visual function in short break-up time dry eye. J Ocul Pharmacol Ther. 2013;29:595–603. doi: 10.1089/jop.2012.0246. [DOI] [PubMed] [Google Scholar]
- 47.Koh S, Inoue Y, Sugmimoto T, Maeda N, Nishida K. Effect of rebamipide ophthalmic suspension on optical quality in the short break-up time type of dry eye. Cornea. 2013;32:1219–1223. doi: 10.1097/ICO.0b013e318294f97e. [DOI] [PubMed] [Google Scholar]
- 48.Toda I, et al. Combination therapy with diquafosol tetrasodium and sodium hyaluronate in patients with dry eye after laser in situ keratomileusis. Am J Ophthalmol. 2014;157:616–622 e611. doi: 10.1016/j.ajo.2013.11.017. [DOI] [PubMed] [Google Scholar]
- 49.Yamaguchi M, et al. Clinical usefulness of diquafosol for real-world dry eye patients: a prospective, open-label, non-interventional, observational study. Adv Ther. 2014;31:1169–1181. doi: 10.1007/s12325-014-0162-4. [DOI] [PubMed] [Google Scholar]
- 50.Yokoi N, Kato H, Kinoshita S. Facilitation of tear fluid secretion by 3% diquafosol ophthalmic solution in normal human eyes. Am J Ophthalmol. 2014;157:85–92 e81. doi: 10.1016/j.ajo.2013.09.009. [DOI] [PubMed] [Google Scholar]
- 51.Arita R, et al. Topical diquafosol for patients with obstructive meibomian gland dysfunction. Br J Ophthalmol. 2013;97:725–729. doi: 10.1136/bjophthalmol-2012-302668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Itakura H, Kashima T, Itakura M, Akiyama H, Kishi S. Topical rebamipide improves lid wiper epitheliopathy. Clin Ophthalmol. 2013;7:2137–2141. doi: 10.2147/OPTH.S54511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Takahashi Y, Ichinose A, Kakizaki H. Topical rebamipide treatment for superior limbic keratoconjunctivitis in patients with thyroid eye disease. Am J Ophthalmol. 2014;157:807–812 e802. doi: 10.1016/j.ajo.2013.12.027. [DOI] [PubMed] [Google Scholar]
- 54.Ueta M, Sotozono C, Koga A, Yokoi N, Kinoshita S. Usefulness of a new therapy using rebamipide eyedrops in patients with VKC/AKC refractory to conventional anti-allergic treatments. Allergol Int. 2014;63:75–81. doi: 10.2332/allergolint.13-OA-0605. [DOI] [PubMed] [Google Scholar]
- 55.Itoh S, Itoh K, Shinohara H. Regulation of human corneal epithelial mucins by rebamipide. Curr Eye Res. 2014;39:133–141. doi: 10.3109/02713683.2013.834939. [DOI] [PubMed] [Google Scholar]
- 56.Shigeyasu C, Yamada M, Akune Y, Fukui M. Diquafosol for Soft Contact Lens Dryness: Clinical Evaluation and Tear Analysis. Optom Vis Sci. 2016;93:973–978. doi: 10.1097/OPX.0000000000000877. [DOI] [PubMed] [Google Scholar]
- 57.Uchino M, et al. The features of dry eye disease in a Japanese elderly population. Optom Vis Sci. 2006;83:797–802. doi: 10.1097/01.opx.0000232814.39651.fa. [DOI] [PubMed] [Google Scholar]
- 58.Sakane Y, et al. Development and validation of the Dry Eye-Related Quality-of-Life Score questionnaire. JAMA Ophthalmol. 2013;131:1331–1338. doi: 10.1001/jamaophthalmol.2013.4503. [DOI] [PubMed] [Google Scholar]
- 59.van Bijsterveld OP. Diagnostic tests in the Sicca syndrome. Arch Ophthalmol. 1969;82:10–14. doi: 10.1001/archopht.1969.00990020012003. [DOI] [PubMed] [Google Scholar]