Abstract
One of the hallmarks of the tumour microenvironment is hypoxia resulting from increased oxygen consumption by proliferative cancer cells and altered vasculature. Hypoxic tension initiates various cellular signals and can drive epithelial to mesenchymal transition (EMT), a process important in cancer progression. In this study, using the antioxidant N-acetylcysteine (NAC), we show that hypoxia-induced reactive oxygen species (ROS) in MDA-MB-468 breast cancer cells, selectively regulate hypoxia-induced increases in N-cadherin and SERPINE1, two proteins involved in cell adhesion. Treatment of cells with NAC also attenuated hypoxia-mediated activation of EGFR, but did not have any effect on hypoxia-mediated induction of HIF1α. Exogenous hydrogen peroxide phenocopied the effects of hypoxia on N-cadherin and SERPINE1 expression and EGFR activation, suggesting its possible involvement in these hypoxia-mediated events. Reflective of their effect on cell adhesion proteins and EGFR (associated with migratory phenotypes), NAC also reduced cell migration under hypoxic conditions, a crucial event in metastasis. Our findings suggest a selective role for redox signalling in the regulation of specific components of the responses to hypoxia and induction of EMT in breast cancer cells. This study provides new evidence supporting the potential of targeting ROS as a therapeutic strategy for the control of breast cancer metastasis.
Introduction
Tumours rapidly exhaust the local oxygen supply creating a hypoxic environment1. This hypoxic microenvironment around cancer cells can promote invasion and metastasis as well as resistance to radiation therapy and anti-cancer drugs1,2. Cancer cells also have increased levels of reactive oxygen species (ROS) production compared to normal cells, which may contribute to tumour progression and metastasis3–6. ROS also play critical roles in the regulation of signal transduction pathways in a range of cellular processes and are increased by hypoxia in a number of cell types7–9. ROS increase in response to hypoxia occurs via the transfer of electrons from ubisemiquinone to molecular oxygen at the Q0 site of the mitochondrial complex III10,11.
Several groups have shown that hypoxia induces epithelial to mesenchymal transition (EMT) in breast cancer cells12–15, a process important in tumour metastasis16. During EMT, cancer cells acquire features of mesenchymal-like cells including enhanced migratory and invasive abilities, changes in cellular adhesion, remodelling of the extracellular matrix, and increased resistance to stress and apoptosis17,18. ROS can induce EMT, however, the specificity of their action in the regulation of particular signalling pathways or EMT markers is dependent on the cellular context and type of tissue and is not fully understood19–22. MDA-MB-468 cells are a commonly used model in the study of EMT in triple-negative breast cancer (TNBC)23–25, a type of breast cancer associated with high aggressiveness, poor prognosis and limited treatment options26,27. The EMT inducible MDA-MB-468 breast cancer cells are a PTEN mutant cell line with high levels of EGFR expression28,29. These features are also associated with metastasis and poor survival in TNBC patients30,31.
In this study we investigated the role of hypoxia-induced ROS increases in bestowing mesenchymal properties to breast cancer cells. To achieve this goal we defined the effects of ROS scavenging in the induction of EMT markers, activation of hypoxia-induced signalling pathways, and migration of breast cancer cells, and further attempted to understand the molecular mechanisms involved.
Results
Hypoxia increases the intracellular levels of reactive oxygen species in MDA-MB-468 cells
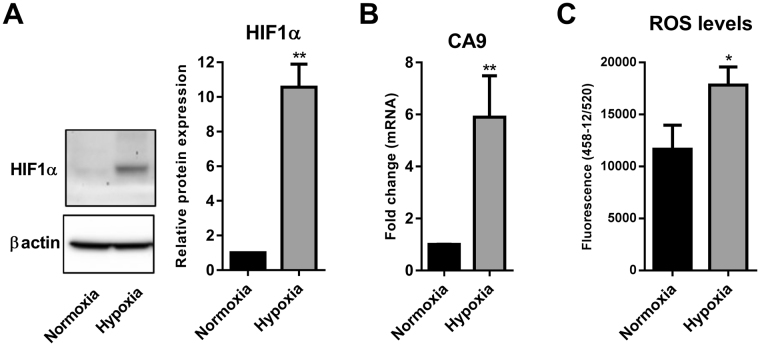
The induction of hypoxia (1% O2) in MDA-MB-468 cells was confirmed by quantifying the levels of the master regulator of hypoxia responses, hypoxia inducible factor 1- alpha (HIF1α)32 and an endogenous marker of hypoxic cells, carbonic anhydrase-9 (CA9)33. HIF-1α is stabilized via inhibition of prolyl hydroxylase domain (PHD) enzymes in the absence of oxygen, a process that can occur within a few hours of hypoxic exposure32. Given the rapid increase in HIF1α protein levels through hypoxia-mediated stabilization of HIF1α via inhibition of PHD enzymes32, and the time for gene transcription, HIF1α protein levels and target mRNA levels were assessed at 6 h and 24 h, respectively. Hypoxia significantly increased the protein levels of HIF1α (Fig. 1A) and mRNA levels of CA9 (Fig. 1B). We then assessed the intracellular levels of reactive oxygen species (ROS) using the DCF-DA assay. Exposure of MDA-MB-468 cells to hypoxia also resulted in a significant increase in intracellular ROS levels measured by DCF fluorescence (Fig. 1C). These results demonstrated the induction of hypoxic responses and the up-regulation of intracellular ROS in MDA-MB-468 breast cancer cells.
Figure 1.
Hypoxia increases intracellular ROS levels. (A) Representative cropped immunoblot (left) and densitometry analysis (right) of HIF1α protein levels in MDA-MB-468 cells exposed to hypoxia (6 h) compared to normoxic cells (full-lenght immunoblot is shown in the Supplementary Fig. S2). (B) CA9 mRNA levels in normoxic and hypoxic (24 h) cells were assessed using real time RT-PCR. (C) Intracellular levels of ROS measured by DCF-DA assay in cells exposed to hypoxia (12 h) compared to control cells (remained in normoxia). Graphs represent the mean ± SD for three independent experiments. *p < 0.05, **p < 0.01 (unpaired t-test).
ROS specifically controls the hypoxia-induced mRNA expression of N-cadherin and SERPINE1 but not other EMT markers
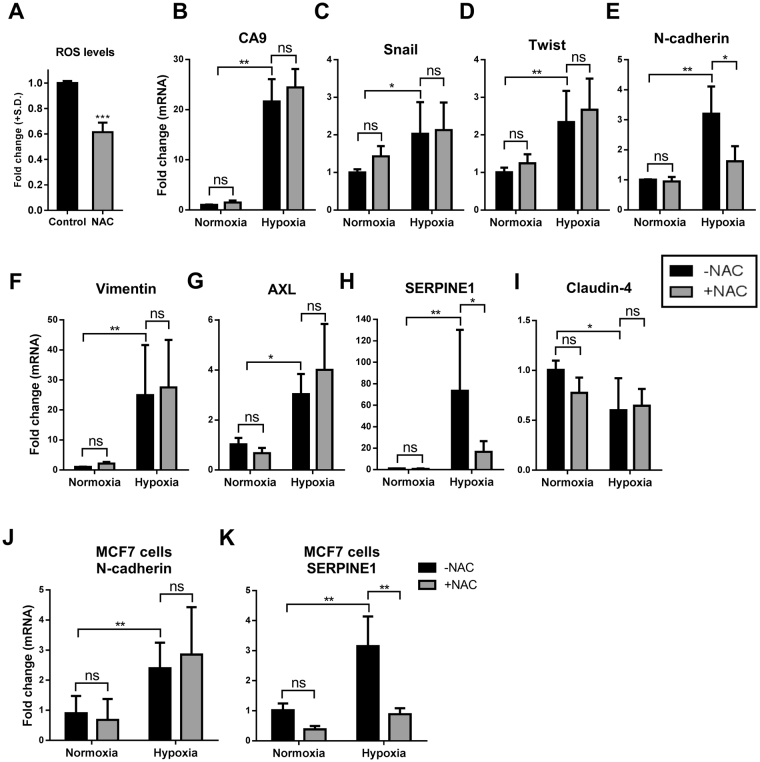
ROS can alter gene expression34,35. Our previous studies show a remodelling in gene transcription 24 h post hypoxia12,36. Given the up-regulation of ROS by hypoxia, the effect of ROS on the expression levels of some of the key genes associated with hypoxia responses was explored using the antioxidant and ROS scavenging agent NAC37, reducing hypoxic ROS levels by around 40% in MDA-MB-468 cells (Fig. 2A). The up-regulation of the hypoxia marker CA9 by hypoxia was not sensitive to NAC treatment (Fig. 2B). We then explored the effect of NAC on the levels of classic EMT markers, Snail, Twist, N-cadherin and vimentin (Fig. 2C–F). All of these EMT markers were significantly up-regulated by hypoxia, however, NAC treatment only attenuated hypoxia-mediated increases in N-cadherin (Fig. 2E). NAC had no effect on N-cadherin expression under normoxic conditions (Fig. 2E). The NAC sensitivity of two recently identified hypoxia-induced EMT markers (AXL and SERPINE124), was then explored. AXL and SEPRPINE1 were significantly up-regulated with hypoxia by approximately 3- and 60-fold, respectively (Fig. 2G,H). NAC treatment did not have any effect on the expression levels of the AXL receptor tyrosine kinase (Fig. 2F), however, it had a dramatic inhibitory effect on hypoxia-induced increases in SERPINE1 (~75% reduction, Fig. 2H). Similar to N-cadherin mRNA regulation, NAC had no significant effect on SERPINE1 expression under normoxic conditions (Fig. 2H). Assessment of the expression of the epithelial marker Claudin-4 showed its down-regulation by hypoxia, however, this was not altered by NAC treatment (Fig. 2I). These results indicate that redox signalling is involved in the promotion of specific elements of EMT including hypoxia-induced increases in N-cadherin and SERPINE1 but not other EMT markers in MDA-MB-468 breast cancer cells. Hypoxic up-regulation of N-cadherin and SERPINE1 was not HIF1α dependent as siRNA silencing of HIF1α did not reduce this up-regulation (Supplementary Fig. S1). The effect of NAC on the hypoxia-induced expression of N-cadherin and SERPINE1 was also assessed in the luminal A breast cancer cell line MCF7. NAC had no effect on hypoxia-induced increases in N-cadherin, however, it again significantly reduced the expression of SERPINE1 induced by hypoxia in MCF7 cells (Fig. 2J and K).
Figure 2.
The effect of NAC treatment on mRNA levels of a panel of genes associated with EMT and hypoxic responses. (A) Intracellular levels of ROS measured by DCF-DA assay in cells exposed to hypoxia (12 h) in the presence and absence of NAC (10 mM). Graph represents the mean ± SD relative to the control (hypoxia with no NAC) for three independent experiments. ***p < 0.0001 (unpaired t-test). Post serum-reduction MDA-MB-468 breast cancer cells were treated with or without NAC (10 mM) and exposed to hypoxia for 24 h. The effect of NAC on (B) CA9, (C) Snail, (D) Twist, (E) N-cadherin, (F) vimentin, (G) AXL, (H) SERPINE1 and claudin-4 (I) was assessed using real time RT-PCR. ns = not significant (p > 0.05), *p < 0.05, **p < 0.01 (two-way ANOVA, with Tukey’s multiple comparisons test). Graphs represent the mean ± SD for three independent experiments. The effect of NAC on the expression of (J) N-cadherin and (K) SERPINE1 in hypoxia and control normoxia was assessed using real time RT-PCR. ns = not significant (p > 0.05), **p < 0.01 (two-way ANOVA, with Tukey’s multiple comparisons test). Graphs represent the mean ± SD for three independent experiments.
ROS mediate the activation of hypoxia-induced EGFR but not HIF1α
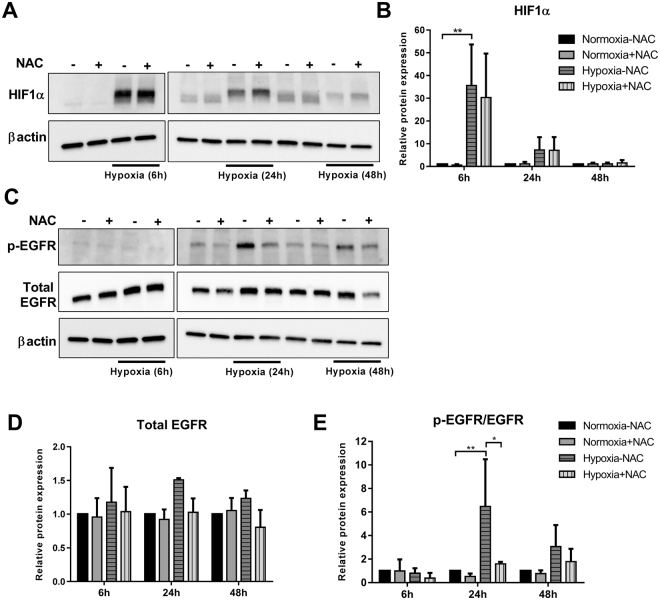
Given the redox modulation of hypoxia-induced gene expression of specific EMT markers, we sought to explore the involvement of ROS in the activation of two key hypoxia-associated signalling pathways HIF1α32 and EGFR38 at different hypoxic time points. Exposure of cells to 6 h hypoxia significantly increased the protein levels of HIF1α, however, NAC treatment did not have any effect on this induction (Fig. 3A and B). At 24 h hypoxia, there was a trend towards up-regulation of HIF1α levels with hypoxia that was also insensitive to ROS scavenging. Elevated HIF1α levels were not maintained at 48 h hypoxia (Fig. 3A and B).
Figure 3.
Effect of NAC on hypoxia-induced HIF1α and EGFR signalling. MDA-MB-468 cells were treated with NAC (10 mM) and exposed to hypoxia for 6 h, 24 h and 48 h. (A) Representative cropped immunoblot and (B) densitometry analysis of HIF1α protein levels (normalised to β-actin). (C) Representative cropped immunoblot and densitometry analysis of (D) total EGFR (normalised to β-actin) and phosphorylated EGFR (p-EGFR) (Y1173) levels (normalised to β-actin and total EGFR) (full-lenght immunoblots are shown in the Supplementary Fig. S2). *p < 0.01, **p < 0.001 (two-way ANOVA, with Tukey’s multiple comparisons test). All densitometry data presented are the mean of three independent experiments ± SD.
Hypoxia, at any time point tested, did not significantly affect the total EGFR levels, and NAC treatment also did not change these levels (Fig. 3C and D). Maximal EGFR phosphorylation at Y1173 (the major EGFR signalling site39) was evident at 24 h and was significantly reduced by NAC. This increase was redox dependant as treatment with NAC significantly decreased (~70%) the hypoxia-induced EGFR phosphorylation (Fig. 3C and E). At 48 h there was a trend towards reduction of both total and activated EGFR by NAC, however, this was not statistically significant. These findings provide further evidence of the specificity in the redox modulation of cellular signals associated with hypoxia.
Hypoxia-induced N-cadherin and SERPINE1 expression and EGFR activation are not sensitive to Trolox and Tempol
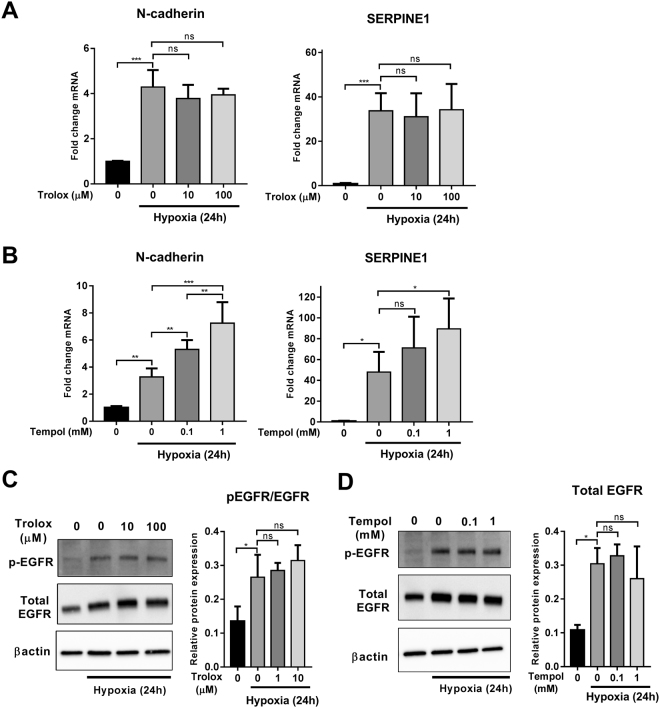
To understand the detailed mechanism of redox regulation of hypoxia-induced N-cadherin and SERPINE1 expression and EGFR activation, we treated cells with Trolox and TEMPOL, two ROS scavengers with distinct mechanisms of action. Trolox, a water-soluble vitamin E analog, inhibits lipid peroxidation by scavenging peroxyl radicals (ROO•)40. TEMPOL, on the other hand, is a superoxide dismutase (SOD) mimetic that attenuates superoxide anions41. Neither Trolox (10 and 100 µM) nor Tempol (0.1 and 1 mM) inhibited the induction of N-cadherin and SERPINE1 mRNA expression (Fig. 4A and B) and EGFR phosphorylation by hypoxia (Fig. 4C and D). Tempol appeared to enhance the mRNA levels of N-cadherin and SERPINE1 in a concentration dependent manner (Fig. 4B). Tempol, in addition to its superoxide anion scavenging, can also increase hydrogen peroxide (H2O2) levels42. This led us to investigate the effect of H2O2 on the expression of N-cadherin and SERPINE1 and phosphorylation of EGFR.
Figure 4.
Effect of Trolox and Tempol on hypoxia-induced N-cadherin and SERPINE1 expression and EGFR activation. MDA-MB-468 cells were treated with Trolox (10 and 100 µM) and Tempol (0.1 and 1 mM) and placed under hypoxic conditions for 24 h. The mRNA levels of N-cadherin and SERPINE1 following Trolox (A) and Tempol (B) treatments were assessed using real time RT-PCR. Levels of EGFR phosphorylation (normalised to β-actin and total EGFR) following Trolox (C) and Tempol (D) treatments were assessed using immunoblotting (representative cropped immunoblots are shown in the figure; full-lenght immunoblots are shown in the Supplementary Fig. S2). ns = not significant (p > 0.05), *p < 0.05, **p < 0.01, ***p < 0.001 (one-way ANOVA, with Tukey’s multiple comparisons test). Graphs represent the mean ± SD for three independent experiments.
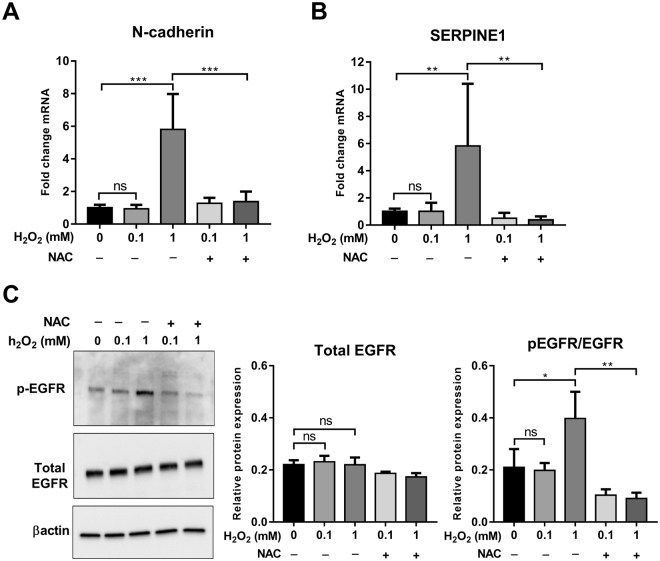
Hydrogen peroxide induces N-cadherin and SERPINE1 mRNA expression and EGFR phosphorylation
H2O2 is a non-radical ROS molecule43 that is induced by hypoxia44,45. We explored the effect of exogenous H2O2 treatment on the expression of N-cadherin and SERPINE1 and the activation of EGFR. Treatment of MDA-MB-468 cells with H2O2 (1 mM) in normoxic conditions resulted in a significant increase in the mRNA levels of N-cadherin and SERPINE1 (Fig. 5A and B) and EGFR phosphorylation (Fig. 5C), and this increase was attenuated by NAC. The ability of H2O2 to phenocopy the observed effects of hypoxia suggests that H2O2 may have an important role in the regulation of N-cadherin and SERPINE1 expression and EGFR activation in the hypoxia model.
Figure 5.
Effect of H2O2 on the expression of N-cadherin and SERPINE1 and the phosphorylation of EGFR. Post serum reduction (24 h), MDA-MB-468 cells were treated with H2O2 (0.1 and 1 mM) in the presence or absence of NAC (10 mM). The mRNA levels of N-cadherin (A) and SERPINE1 (B) were assessed using real time RT-PCR. (C) Representative cropped immunoblot (left) and densitometry analysis (right) of total EGFR (normalised to β-actin) and pEGFR (normalised to β-actin and total EGFR) (full-lenght immunoblots are shown in the Supplementary Fig. S2). ns = not significant (p > 0.05), *p < 0.05, **p < 0.01, ***p < 0.001 (one-way ANOVA, with Tukey’s multiple comparisons test). Graphs represent the mean ± SD for three independent experiments.
ROS contribute to the migration of MDA-MB-468 breast cancer cells under hypoxic conditions
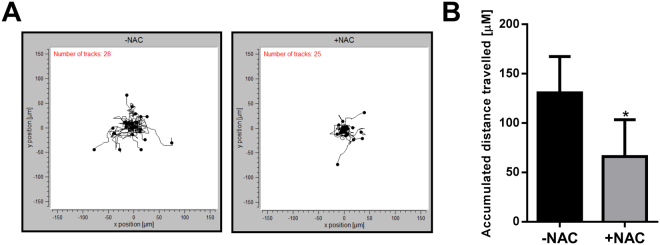
Hypoxia can induce cell migration in a variety of cancer cell models46–49. Given the role of ROS in the regulation of N-cadherin and SERPINE1 (involved in matrix remodelling and cell migration50,51) and the effects of ROS on hypoxia-induced activation of the pro-migratory receptor EGFR52,53, we sought to explore the effects of NAC on the migration of MDA-MB-468 breast cancer cells during hypoxia. EMT results in a transition from collective to single cell motility and this is considered to be a crucial step in the course of tumour progression and metastasis54,55. Therefore, in the context of EMT, it is important to assess the migration of single cells. We used a collagen-based model and individual cell tracking system to assess and quantify the migration of MDA-MB-468 breast cancer cells. In this single-cell migration assay, the accumulative distance of individual cells migrated over a course of 12 h post exposure to hypoxia (24 h) is assessed. NAC treatment significantly decreased the migration of cells exposed to hypoxia (Fig. 6). This finding reveals the importance of ROS in bestowing cells with more migratory characteristics, which is an important consequence of EMT in breast cancer cells18.
Figure 6.
ROS scavenging with NAC reduces cell migration under hypoxic conditions. MDA-MB-468 cells were treated with NAC and exposed to hypoxia. Cell migration was analysed from 24 h to 36 h under hypoxic conditions. (A) Spatial plot of all cells from representative wells from the same experiment and (B) quantitative analysis of the mean accumulated distance travelled by cells from three independent experiment (duplicate wells) treated with NAC compared to the control cells without NAC treatment. *p < 0.05, (paired t-test).
Discussion
In this study we demonstrated that hypoxia resulted in mRNA up-regulation of the hypoxic marker CA933 and an up-regulation in HIF1α protein levels, as well as an increase in intracellular ROS levels in MDA-MB-468 breast cancer cells. Hypoxia also resulted in an elevation in the mesenchymal markers vimentin, N-cadherin, Snail, Twist, AXL and SERPINE1. The thiol-containing ROS scavenger NAC was used to investigate the role of redox in hypoxia-associated cellular responses. NAC is a United States Food and Drug Administration (FDA)-approved drug for the treatment of chronic obstructive lung disease (COPD)56 and acetaminophen (paracetamol) overdose57. It is also a synthetic precursor of intracellular cysteine and glutathione, which has free radical scavenging properties either directly by redox transitions of thiols, or indirectly via increasing intracellular glutathione levels58. NAC has anti-tumorigenic effects on tumours of the breast59, lung60 and also in Kaposi’s Sarcoma61. Herein we observed that ROS attenuation with NAC specifically reduced N-cadherin and SERPINE1 mRNA levels induced by hypoxia. N-cadherin and SERPINE1 are both involved in cell-cell adhesion. N-cadherin is a predominantly mesenchymal homotypic cell adhesion protein found in adherens junctions that contributes to the induction of EMT and metastasis62. SERPINE1, also known as plasminogen activator inhibitor type-1 (PAI-1), is an important regulatory protein involved in the extracellular matrix reorganisation and cell adhesion63, and is up-regulated in EMT in both EGF and hypoxia models of EMT24. Matrix remodelling is a crucial step for acquisition of more migratory phenotypes64. Assessment of the effect of NAC treatment on the expression of hypoxia-mediated N-cadherin and SERPINE1 in estrogen receptor positive (ER+), luminal-like MCF7 breast cancer cells65, identified a redox dependence in the regulation of SERPINE1 but not N-cadherin expression. These data suggest that hypoxia can mediate increases in N-cadherin using different molecular pathways. The potential molecular subtype specific dependence of this difference on ER expression status and/or the basal molecular phenotype should be the focus of future studies.
NAC did not show any effect on levels of classic EMT markers, Snail, Twist, AXL and vimentin suggesting the specificity of the redox signal in the regulation of distinctive aspects of EMT. This may suggest the existence of multiple regulators of the EMT phenotype. Indeed, we recently reported that the calcium permeable ion channel TRPM7 promotes epidermal growth factor (EGF)-induced EMT by selectively regulating vimentin protein expression36,66. Indeed, there is increasing evidence suggesting a reciprocal interplay between calcium and ROS signalling in fine tuning cellular signalling networks67.
We also investigated the effect of ROS scavenging with NAC on the levels of HIF1α protein and EGFR phosphorylation, two of the key hypoxia-associated signalling pathways32,38. NAC had no significant effect on the protein levels of the hypoxia master regulator, HIF1α, under normoxic or hypoxic conditions. In contrast, in human hepatoma 3B, fibrosarcoma, and epithelial lung cells HIF1α stabilisation during hypoxia is redox sensitive11,68,69. However, studies by Chua et al. in human osteosarcoma found that hypoxic HIF1α stabilisation occurs independently of mitochondrial ROS70 such as we observed in our model. Clearly the cellular context and tissue specific role of ROS in responses to hypoxia is important. Our studies did identify a critical role for ROS with hypoxia-mediated activation of EGFR. EGFR is overexpressed in TNBC, where its inhibition can induce a change from a mesenchymal to a more epithelial phenotype71. The two tested ROS scavengers, Trolox (scavenger of peroxyl radicals40) and Tempol (scavenger of superoxide anions41), did not attenuate the hypoxia-induced expression of N-cadherin and SEPRINE1 and phosphorylation of EGFR at the tested concentrations, suggesting the non-involvement of these free radicals in the observed hypoxic events. Instead, N-cadherin and SEPRINE1 mRNA levels were significantly up-regulated by increasing concentrations of Tempol. This effect could have been due to elevated H2O2 levels since induction of H2O2 is a property of Tempol in addition to its role as a superoxide scavenger42. Indeed, treatment of cells with H2O2 in normoxia phenocopied the effect of hypoxia on the up-regulation of N-cadherin and SEPRINE1 expression and EGFR phosphorylation, suggesting the involvement of H2O2 in these hypoxia-mediated events. H2O2 is a stable diffusable non-radical oxidant and is involved in many biological processes such as signal transduction, cell differentiation and proliferation72. In the context of our studies (showing H2O2 induction of SERPINE1 and N-cadherin mRNA and EGFR phosphorylation), it is interesting to note that H2O2 can also regulate the activity of transcription factors73 and protein phosphorylation via cysteine oxidation74 in other models. Future studies could assess the role of potential transcription factors in the up-regulation of ROS-mediated SERPINE1 and N-cadherin. Given our findings of a link between redox signalling and the induction of N-cadherin and SERPINE1 and EGFR activation, and since each of these proteins are involved in the promotion of cell migration50,51,75, we investigated the effect of ROS scavenging on the migration of MDA-MB-468 cells. A collagen-based cell migration model revealed that cells treated with NAC showed significantly less motility under hypoxic conditions. Transition from collective to single cell migration is a consequence of EMT, and is an important step towards tumour invasion and metastasis54,55. Metastasis is responsible for about 90% of human cancer death, and is influenced by the tumour microenvironment76, which is often low in O2 and high in pro-oxidants77. Our studies provide further evidence for a role of ROS in contributing to breast cancer progression particularly in the context of the hypoxic tumour microenvironment. This work also shows that there are specific events induced by hypoxia that occur independently of ROS, including specific EMT processes associated with vimentin, Snail and Twist induction. Since these events may be critical to aspects of disease progression including therapeutic resistance78 any therapeutic targeting of metastatic progression associated with hypoxia will require an approach encompassing the targeting of redox-dependent and redox-independent processes. Indeed, our results may help explain why the protective effects against metastasis of ROS scavengers has shown varied success in vivo and in clinical studies79,80.
In conclusion, in this study we have demonstrated a role for redox signalling in the promotion of hypoxia-induced changes in specific EMT markers and in the regulation of specific hypoxic cellular responses. We have also defined a critical role for ROS in MDA-MB-468 breast cancer cell migration under hypoxic conditions. Further studies are warranted to explore the mechanism of the regulation of specific hypoxia-associated cellular responses by ROS.
Materials and Methods
Cell culture
MDA-MB-468 and MCF7 breast cancer cells12 were cultured in Dulbecco’s Modified Eagle’s Medium (DMEM; D6546 Sigma- Aldrich, St Louis, MO, USA) supplemented with 10% foetal bovine serum (FBS) and 4 mM L-glutamine and maintained in a humidified incubator (37 °C with 5% CO2). For the induction of hypoxia, 24 h post seeding, cells were serum reduced (0.5% FBS, 24 h) and placed in a Sanyo MCO-18M multi-gas incubator with 1% O2 for the indicated time points (see figure legends). Time points were selected based on preliminary data and literature reports of hypoxia mediated changes in this and/or a similar model12,36,81. Control cells (normoxia) were kept in the humidified incubator with 21% O2 for the matching time points. MDA-MB-468 and MCF7 cells were cultured for less than 10 passages (5–6 weeks) and were regularly screened for mycoplasma contamination (MycoAlert; Lonza Basel, Switzerland). Cell line STR profiling was confirmed by QIMR Berghofer Medical Research Institute, Brisbane, Australia using the StemElite ID Profiling Kit (Promega, Madison, WI, USA).
Antioxidants and hydrogen peroxide treatments
The three antioxidants used in this study were purchased from Sigma-Aldrich and were as follow: N-Acetyl-L-cysteine (NAC) (Cat# A7250), Trolox (( ± )-6-Hydroxy-2,5,7,8-tetramethylchromane-2-carboxylic acid) (Cat# 238813) and Tempol (4-Hydroxy-2,2,6,6-tetramethylpiperidine 1-oxyl) (Cat# 176141). Hydrogen peroxide (H2O2) was purchased from Cell Biolabs as one of the components of the OxiSelectTM Intracellular ROS Assay Kit (STA-342). All the antioxidants and H2O2 were added to MDA-MB-468 cells in serum reduced-media (0.5% FBS) and incubated with cells for 24 h prior to RNA or protein isolation.
Real time RT-PCR
Total RNA from cells was isolated and purified using the RNeasy Plus Mini Kit (Qiagen, Hilden, Germany). Omniscript RT Kit (205111, Qiagen) was used for the reverse transcription of the purified RNA. The target cDNA was amplified under universal cycling conditions in a StepOnePlusTM instrument (Applied Biosystems) using TaqMan® gene expression assays and the TaqMan® Fast Universal PCR Master Mix (Applied Biosystems, Carlsbad, CA, USA). The relative target quantity was determined using the comparative CT (ΔΔCT) method and normalised to 18 S ribosomal RNA.
Immunoblotting
Protein samples were resolved on NuPAGE Novex 4–12% Bis-Tris Gradient Gel (Invitrogen, Waltham, MA, USA) by SDS gel electrophoresis and transferred to polyvinylidene difluoride membrane (PVDF). Primary antibodies used in this study were as follows: 1:1000 dilution of EGFR (2232) and phospho-EGFR (Tyr 1173) (4407) antibodies from Cell Signalling Technology (Danvers, MA, USA), 1:500 dilution of HIF1α antibody from BD Bioscience (Bedford, MA, USA; 610959), 1:10,000 dilution of β-actin antibody from Sigma-Aldrich (A5441). Horseradish peroxidase-conjugated goat anti-mouse (170–6516) and goat anti-rabbit (170–6515) secondary antibodies were purchased from Bio-Rad (Hercules, CA, USA) and used at a 1:10,000 dilution. Primary antibodies were incubated with PVDF membranes overnight at 4 °C (with the exception of β-actin antibody - 1 h at room temperature). Secondary antibodies were incubated for 1 h at room temperature. Protein bands were detected with chemiluminescence using SuperSignalTM West Dura Substrate (34075, Thermo Fisher Scientific, Waltham, MA, USA) and visualised using a Bio-Rad VersaDoc Imaging System or a Bio-Rad ChemiDoc Imaging System. The intensity of bands was quantified with Image Lab V 5.2.1 software (Bio-Rad) and normalised to β-actin controls. Phosphorylated proteins were then normalised to their respective total protein level.
Measurement of intracellular ROS
Intracellular ROS levels was measured using the OxiSelectTM Intracellular ROS Assay Kit (STA-342, Cell Biolabs, San Diego, CA, USA), which uses a cell-permeable fluorogenic 2′, 7′-dichlorodihydrofluorescin diacetate (DCFH-DA) that after being diffused into cells is deacetylated by cellular esterases to non-fluorescent 2′, 7′-dichlorodihydrofluorescin (DCFH), which is oxidised to highly fluorescent 2′, 7′-dichlorodihydrofluorescin (DCF) by ROS. The fluorescent intensity is proportional to the intracellular ROS levels within the cytosol82. Cells were seeded at 2 × 105 per well in 96-well plates. Post seeding (24 h), cells were serum reduced for a further 24 h and then placed in a hypoxic incubator (or remained in the normoxic incubator for the control group) for 12 h. For the measurement of intracellular ROS, cells were gently washed twice with phosphate-buffered saline (PBS) and then incubated with 10 µM DCFH-DA for 30 min at 37 °C. The fluorescent intensity was measured using a FLUOstar Omega Microplate Reader (BMG LABTECH, Ortenberg, Germany) with excitation at 485–512 nm and emission at 520 nm. Fluorescence background was subtracted from all samples.
Cell migration assay
Collagen matrices were prepared for the assessment of cell motility. Cell culture plates (96-well) were coated with 50 µL of a collagen mixture containing 10X PBS (8% v/v), DMEM (24% v/v), collagen type I from bovine skin (final concentration 2 mg/mL; C4243, Sigma-Aldrich), brought to physiological pH with 1 M NaOH. Collagen gels were formed by incubating plates at 37 °C, 5% CO2 for 1 h. Cells were then seeded at a very low density (to ensure the assessment of single cell movement) of 500 cells per well on top of the collagen gel. After serum reduction (0.5% FBS) for 24 h, cells were treated with NAC (10 mM) and placed on the stage of a JuLiTM Stage Live Cell Imaging System (NanoEnTek Inc. Seoul, South Korea) housed in a hypoxia incubator. Bright field images from the centre of wells were taken every 15 min using a 4X objective. Assessment of cell motility was performed 24 h post exposure to hypoxia for a period of 12 h (between 24 h and 36 h in hypoxia). Strict criteria were implemented in the exclusion of cells for quantification of their motility. Exclusion criteria included the removal of cells that died, divided or moved in or out of the field of view during the time of assessment. After implementing these exclusion criteria, all cells within the field of view (ranging from 17 to 48 cells), were selected for assessment of their migration during the 12 h period. Cells were individually tracked using the Manual Tracking plug-in of ImageJ 1.49q software (NIH, Bethesda, MD, https://imagej.nih.gov/ij/). The position of cells in each frame taken every 15 min was recorded. Chemotaxis and Migration Tool V2.0 (Ibidi, Munich, Germany) was used for the illustration of cell movements and calculation of accumulated distance travelled by each cell.
Statistical analysis
GraphPad Prism version 6.05 for Windows (GraphPad Software Inc, La Jolla, CA, USA) was used for statistical analysis. Statistical tests used are described in each figure legend. Experiments were performed in triplicate and data are presented as mean ± SD.
Electronic supplementary material
Acknowledgements
The research was supported by the National Health and Medical Research Council (NHMRC; project grants 569645 and 1022263) and the Cancer Council Queensland (1042819). G.R.M is supported by the Mater Foundation. E.W.T. was funded in part by the EMPathy Breast Cancer Network of the National Breast Cancer Foundation (CG-10-04), Australia. The Translational Research Institute is supported by a grant from the Australian Government.
Author Contributions
I.A. performed and interpreted the experiments and wrote the manuscript. R.M.P. conducted experiments and edited the manuscript. E.W.T., S.J.R.-T. and G.R.M. helped to conceive the study, interpreted the results and edited the manuscript.
Competing Interests
S. J. R.-T. and G. R. M. are scientific collaborators with QUE-Oncology Inc. All other authors declare no potential conflict of interest.
Footnotes
Electronic supplementary material
Supplementary information accompanies this paper at 10.1038/s41598-017-15474-7.
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Semenza GL. Hypoxia-inducible factors: mediators of cancer progression and targets for cancer therapy. Trends Pharmacol Sci. 2012;33:207–214. doi: 10.1016/j.tips.2012.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Semenza GL. The hypoxic tumor microenvironment: A driving force for breast cancer progression. Biochim Biophys Acta (2015). [DOI] [PMC free article] [PubMed]
- 3.Liou GY, Storz P. Reactive oxygen species in cancer. Free Radic Res. 2010;44:479–496. doi: 10.3109/10715761003667554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Storz P. Reactive oxygen species in tumor progression. Front Biosci. 2005;10:1881–1896. doi: 10.2741/1667. [DOI] [PubMed] [Google Scholar]
- 5.Wu WS. The signaling mechanism of ROS in tumor progression. Cancer Metastasis Rev. 2006;25:695–705. doi: 10.1007/s10555-006-9037-8. [DOI] [PubMed] [Google Scholar]
- 6.Szatrowski TP, Nathan CF. Production of Large Amounts of Hydrogen-Peroxide by Human Tumor-Cells. Cancer Res. 1991;51:794–798. [PubMed] [Google Scholar]
- 7.Poli G, Leonarduzzi G, Biasi F, Chiarpotto E. Oxidative stress and cell signalling. Curr Med Chem. 2004;11:1163–1182. doi: 10.2174/0929867043365323. [DOI] [PubMed] [Google Scholar]
- 8.Aslan M, Ozben T. Oxidants in receptor tyrosine kinase signal transduction pathways. Antioxid Redox Signal. 2003;5:781–788. doi: 10.1089/152308603770380089. [DOI] [PubMed] [Google Scholar]
- 9.Li L, Cheung SH, Evans EL, Shaw PE. Modulation of Gene Expression and Tumor Cell Growth by Redox Modification of STAT3. Cancer Res. 2010;70:8222–8232. doi: 10.1158/0008-5472.CAN-10-0894. [DOI] [PubMed] [Google Scholar]
- 10.Bell EL, et al. The Qo site of the mitochondrial complex III is required for the transduction of hypoxic signaling via reactive oxygen species production. J Cell Biol. 2007;177:1029–1036. doi: 10.1083/jcb.200609074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chandel NS, et al. Reactive oxygen species generated at mitochondrial complex III stabilize hypoxia-inducible factor-1alpha during hypoxia: a mechanism of O2 sensing. J Biol Chem. 2000;275:25130–25138. doi: 10.1074/jbc.M001914200. [DOI] [PubMed] [Google Scholar]
- 12.Azimi, I. et al. Altered purinergic receptor-Ca signaling associated with hypoxia-induced epithelial-mesenchymal transition in breast cancer cells. Mol Oncol (2015). [DOI] [PMC free article] [PubMed]
- 13.Lester RD, Jo M, Montel V, Takimoto S, Gonias SL. uPAR induces epithelial-mesenchymal transition in hypoxic breast cancer cells. J Cell Biol. 2007;178:425–436. doi: 10.1083/jcb.200701092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hugo, H. J. et al. Direct repression of MYB by ZEB1 suppresses proliferation and epithelial gene expression during epithelial-to-mesenchymal transition of breast cancer cells. Breast Cancer Res15 (2013). [DOI] [PMC free article] [PubMed]
- 15.Jo M, et al. Reversibility of Epithelial-Mesenchymal Transition (EMT) Induced in Breast Cancer Cells by Activation of Urokinase Receptor-dependent Cell Signaling. J Biol Chem. 2009;284:22825–22833. doi: 10.1074/jbc.M109.023960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Polyak K, Weinberg RA. Transitions between epithelial and mesenchymal states: acquisition of malignant and stem cell traits. Nat Rev Cancer. 2009;9:265–273. doi: 10.1038/nrc2620. [DOI] [PubMed] [Google Scholar]
- 17.Kalluri R, Weinberg RA. The basics of epithelial-mesenchymal transition. J Clin Investig. 2009;119:1420–1428. doi: 10.1172/JCI39104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Thiery JP, Sleeman JP. Complex networks orchestrate epithelial-mesenchymal transitions. Nat Rev Mol Cell Biol. 2006;7:131–142. doi: 10.1038/nrm1835. [DOI] [PubMed] [Google Scholar]
- 19.Cannito S, et al. Epithelial-Mesenchymal Transition: From Molecular Mechanisms, Redox Regulation to Implications in Human Health and Disease. Antioxid Redox Signal. 2010;12:1383–1430. doi: 10.1089/ars.2009.2737. [DOI] [PubMed] [Google Scholar]
- 20.Giannoni E, Parri M, Chiarugi P. EMT and Oxidative Stress: A Bidirectional Interplay Affecting Tumor Malignancy. Antioxid Redox Signal. 2012;16:1248–1263. doi: 10.1089/ars.2011.4280. [DOI] [PubMed] [Google Scholar]
- 21.Cichon MA, Radisky DC. ROS-induced epithelial-mesenchymal transition in mammary epithelial cells is mediated by NF-kappa B-dependent activation of Snail. Oncotarget. 2014;5:2827–2838. doi: 10.18632/oncotarget.1940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Radisky DC, et al. Rac1b and reactive oxygen species mediate MMP-3-induced EMT and genomic instability. Nature. 2005;436:123–127. doi: 10.1038/nature03688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lo HW, et al. Epidermal growth factor receptor cooperates with signal transducer and activator of transcription 3 to induce epithelial-mesenchymal transition in cancer cells via up-regulation of TWIST gene expression. Cancer Res. 2007;67:9066–9076. doi: 10.1158/0008-5472.CAN-07-0575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cursons J, et al. Stimulus-dependent differences in signalling regulate epithelial-mesenchymal plasticity and change the effects of drugs in breast cancer cell lines. Cell communication and signaling: CCS. 2015;13:26. doi: 10.1186/s12964-015-0106-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Davis, F. M. et al. Induction of epithelial-mesenchymal transition (EMT) in breast cancer cells is calcium signal dependent. Oncogene (2013). [DOI] [PMC free article] [PubMed]
- 26.Ricciardi, G. R. R. et al. Prognostic markers in triple-negative breast cancer (TNBC): The role of androgen receptor, e-cadherin, and Ki67. J Clin Oncol33 (2015).
- 27.Dent R, et al. Triple-negative breast cancer: Clinical features and patterns of recurrence. Clin Cancer Res. 2007;13:4429–4434. doi: 10.1158/1078-0432.CCR-06-3045. [DOI] [PubMed] [Google Scholar]
- 28.Stemke-Hale K, et al. An integrative genomic and proteomic analysis of PIK3CA, PTEN, and AKT mutations in breast cancer. Cancer Res. 2008;68:6084–6091. doi: 10.1158/0008-5472.CAN-07-6854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.deFazio A, Chiew YE, Sini RL, Janes PW, Sutherland RL. Expression of c-erbB receptors, heregulin and oestrogen receptor in human breast cell lines. Int J Cancer. 2000;87:487–498. doi: 10.1002/1097-0215(20000815)87:4<487::AID-IJC5>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 30.Phuah SY, et al. Triple-negative breast cancer and PTEN (phosphatase and tensin homologue) loss are predictors of BRCA1 germline mutations in women with early-onset and familial breast cancer, but not in women with isolated late-onset breast cancer. Breast Cancer Res. 2012;14:R142. doi: 10.1186/bcr3347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Changavi AA, Shashikala A, Ramji AS. Epidermal Growth Factor Receptor Expression in Triple Negative and Nontriple Negative Breast Carcinomas. J Lab Physicians. 2015;7:79–83. doi: 10.4103/0974-2727.163129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Weidemann A, Johnson RS. Biology of HIF-1alpha. Cell Death Differ. 2008;15:621–627. doi: 10.1038/cdd.2008.12. [DOI] [PubMed] [Google Scholar]
- 33.Olive PL, et al. Carbonic anhydrase 9 as an endogenous marker for hypoxic cells in cervical cancer. Cancer Res. 2001;61:8924–8929. [PubMed] [Google Scholar]
- 34.Kunsch C, Medford RM. Oxidative stress as a regulator of gene expression in the vasculature. Circ Res. 1999;85:753–766. doi: 10.1161/01.RES.85.8.753. [DOI] [PubMed] [Google Scholar]
- 35.Jain M, et al. Mitochondrial Reactive Oxygen Species Regulate Transforming Growth Factor-beta Signaling. J Biol Chem. 2013;288:770–777. doi: 10.1074/jbc.M112.431973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Davis FM, et al. Induction of epithelial-mesenchymal transition (EMT) in breast cancer cells is calcium signal dependent. Oncogene. 2014;33:2307–2316. doi: 10.1038/onc.2013.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zafarullah M, Li WQ, Sylvester J, Ahmad M. Molecular mechanisms of N-acetylcysteine actions. Cell Mol Life Sci. 2003;60:6–20. doi: 10.1007/s000180300001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Misra, A., Pandey, C., Sze, S. K., Thanabalu, T. Hypoxia Activated EGFR Signaling Induces Epithelial to Mesenchymal Transition (EMT). PLoS ONE7 (2012). [DOI] [PMC free article] [PubMed]
- 39.Kondratov KA, Chernorudskiy AL, Amosova AP, Kornilova ES. Termination of tyrphostin AG1478 application results in different recovery of EGF receptor tyrosine residues 1045 and 1173 phosphorylation in A431 cells. Cell Biol Int. 2010;34:81–87. doi: 10.1042/CBI20090159. [DOI] [PubMed] [Google Scholar]
- 40.Nicolescu AC, Li Q, Brown L, Thatcher GRJ. Nitroxidation, nitration, and oxidation of a BODIPY fluorophore by RNOS and ROS. Nitric Oxide-Biol Ch. 2006;15:163–176. doi: 10.1016/j.niox.2006.01.010. [DOI] [PubMed] [Google Scholar]
- 41.Muscoli C, et al. On the selectivity of superoxide dismutase mimetics and its importance in pharmacological studies. Br J Pharmacol. 2003;140:445–460. doi: 10.1038/sj.bjp.0705430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chen YF, Cowley AW, Zou AP. Increased H2O2 counteracts the vasodilator and natriuretic effects of superoxide dismutation by tempol in renal medulla. Am J Physiol-Reg I. 2003;285:R827–R833. doi: 10.1152/ajpregu.00636.2002. [DOI] [PubMed] [Google Scholar]
- 43.Trachootham D, Alexandre J, Huang P. Targeting cancer cells by ROS-mediated mechanisms: a radical therapeutic approach? Nature Reviews Drug Discovery. 2009;8:579–591. doi: 10.1038/nrd2803. [DOI] [PubMed] [Google Scholar]
- 44.Vergara R, Parada F, Rubio S, Perez FJ. Hypoxia induces H2O2 production and activates antioxidant defence system in grapevine buds through mediation of H2O2 and ethylene. J Exp Bot. 2012;63:4123–4131. doi: 10.1093/jxb/ers094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Klemm DJ, et al. Reduction of Reactive Oxygen Species Prevents Hypoxia-induced CREB Depletion in Pulmonary Artery Smooth Muscle Cells. J Cardiovasc Pharmacol. 2011;58:181–191. doi: 10.1097/FJC.0b013e31821f2773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Joseph JV, et al. Hypoxia enhances migration and invasion in glioblastoma by promoting a mesenchymal shift mediated by the HIF1 alpha-ZEB1 axis. Cancer Lett. 2015;359:107–116. doi: 10.1016/j.canlet.2015.01.010. [DOI] [PubMed] [Google Scholar]
- 47.Hongo K, et al. Hypoxia enhances colon cancer migration and invasion through promotion of epithelial-mesenchymal transition. J Surg Res. 2013;182:75–84. doi: 10.1016/j.jss.2012.08.034. [DOI] [PubMed] [Google Scholar]
- 48.Cronin, P. A., Wang, J. H., Redmond, H. P. Hypoxia increases the metastatic ability of breast cancer cells via upregulation of CXCR4. BMC Cancer10 (2010). [DOI] [PMC free article] [PubMed]
- 49.Evensen NA, et al. Hypoxia promotes colon cancer dissemination through up-regulation of cell migration-inducing protein (CEMIP) Oncotarget. 2015;6:20723–20739. doi: 10.18632/oncotarget.3978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Czekay RP, et al. PAI-1: An Integrator of Cell Signaling and Migration. Int J Cell Biol. 2011;2011:562481. doi: 10.1155/2011/562481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Shih W, Yamada S. N-cadherin-mediated cell-cell adhesion promotes cell migration in a three-dimensional matrix. J Cell Sci. 2012;125:3661–3670. doi: 10.1242/jcs.103861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Holsken A, et al. EGFR Signaling Regulates Tumor Cell Migration in Craniopharyngiomas. Clin Cancer Res. 2011;17:4367–4377. doi: 10.1158/1078-0432.CCR-10-2811. [DOI] [PubMed] [Google Scholar]
- 53.Maretzky, T. et al. Migration of growth factor-stimulated epithelial and endothelial cells depends on EGFR transactivation by ADAM17. Nat Commun2 (2011). [DOI] [PMC free article] [PubMed]
- 54.Giampieri S, et al. Localized and reversible TGFbeta signalling switches breast cancer cells from cohesive to single cell motility. Nat Cell Biol. 2009;11:1287–1296. doi: 10.1038/ncb1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Friedl P. Prespecification and plasticity: shifting mechanisms of cell migration. Curr Opin Cell Biol. 2004;16:14–23. doi: 10.1016/j.ceb.2003.11.001. [DOI] [PubMed] [Google Scholar]
- 56.Millea PJ. N-Acetylcysteine: Multiple Clinical Applications. Am Fam Physician. 2009;80:265–269. [PubMed] [Google Scholar]
- 57.Brok, J., Buckley, N., Gluud, C. Interventions for paracetamol (acetaminophen) overdose. Cochrane Database Syst Rev (2006). [DOI] [PubMed]
- 58.Sun SY. N-acetylcysteine, reactive oxygen species and beyond. Cancer Biol Ther. 2010;9:109–110. doi: 10.4161/cbt.9.2.10583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Agarwal A, Munoz-Najar U, Klueh U, Shih SC, Claffey KP. N-acetyl-cysteine promotes angiostatin production and vascular collapse in an orthotopic model of breast cancer. Am J Pathol. 2004;164:1683–1696. doi: 10.1016/S0002-9440(10)63727-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.D’Agostini F, Bagnasco M, Giunciuglio D, Albini A, De Flora S. Inhibition by oral N-acetylcysteine of doxorubicin-induced clastogenicity and alopecia, and prevention of primary tumors and lung micrometastases in mice. Int J Oncol. 1998;13:217–224. doi: 10.3892/ijo.13.2.217. [DOI] [PubMed] [Google Scholar]
- 61.Albini A, et al. Inhibition of angiogenesis-driven Kaposi’s sarcoma tumor growth in nude mice by oral N-acetylcysteine. Cancer Res. 2001;61:8171–8178. [PubMed] [Google Scholar]
- 62.Huber MA, Kraut N, Beug H. Molecular requirements for epithelial-mesenchymal transition during tumor progression. Curr Opin Cell Biol. 2005;17:548–558. doi: 10.1016/j.ceb.2005.08.001. [DOI] [PubMed] [Google Scholar]
- 63.Andreasen PA. PAI-1 - A potential therapeutic target in cancer. Curr Drug Targets. 2007;8:1030–1041. doi: 10.2174/138945007781662346. [DOI] [PubMed] [Google Scholar]
- 64.Bonnans C, Chou J, Werb Z. Remodelling the extracellular matrix in development and disease. Nat Rev Mol Cell Biol. 2014;15:786–801. doi: 10.1038/nrm3904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Holliday, D. L., Speirs, V. Choosing the right cell line for breast cancer research. Breast Cancer Res13 (2011). [DOI] [PMC free article] [PubMed]
- 66.Azimi I, Monteith GR. Plasma membrane ion channels and epithelial to mesenchymal transition in cancer cells. Endocr-Relat Cancer. 2016;23:R517–R525. doi: 10.1530/ERC-16-0334. [DOI] [PubMed] [Google Scholar]
- 67.Gorlach A, Bertram K, Hudecova S, Krizanova O. Calcium and ROS: A mutual interplay. Redox Biol. 2015;6:260–271. doi: 10.1016/j.redox.2015.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Pan Y, et al. Multiple factors affecting cellular redox status and energy metabolism modulate hypoxia-inducible factor prolyl hydroxylase activity in vivo and in vitro. Mol Cell Biol. 2007;27:912–925. doi: 10.1128/MCB.01223-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Schroedl C, McClintock DS, Budinger GRS, Chandel NS. Hypoxic but not anoxic stabilization of HIF-1 alpha requires mitochondrial reactive oxygen species. Am J Physiol-Lung C. 2002;283:L922–L931. doi: 10.1152/ajplung.00014.2002. [DOI] [PubMed] [Google Scholar]
- 70.Chua YL, et al. Stabilization of hypoxia-inducible factor-1alpha protein in hypoxia occurs independently of mitochondrial reactive oxygen species production. J Biol Chem. 2010;285:31277–31284. doi: 10.1074/jbc.M110.158485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ueno NT, Zhang DW. Targeting EGFR in Triple NegativeBreast Cancer. J Cancer. 2011;2:324–328. doi: 10.7150/jca.2.324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Gough, D. R., Cotter, T. G. Hydrogen peroxide: a Jekyll and Hyde signalling molecule. Cell Death Dis2 (2011). [DOI] [PMC free article] [PubMed]
- 73.Marinho HS, Real C, Cyrne L, Soares H, Antunes F. Hydrogen peroxide sensing, signaling and regulation of transcription factors. Redox Biol. 2014;2:535–562. doi: 10.1016/j.redox.2014.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Rhee SG, Bae YS, Lee SR, Kwon J. Hydrogen peroxide: a key messenger that modulates protein phosphorylation through cysteine oxidation. Sci STKE. 2000;2000:pe1. doi: 10.1126/stke.2000.18.pe1. [DOI] [PubMed] [Google Scholar]
- 75.Brandt BH, et al. c-erbB-2/EGFR as dominant heterodimerization partners determine a motogenic phenotype in human breast cancer cells. FASEB J. 1999;13:1939–1949. doi: 10.1096/fasebj.13.14.1939. [DOI] [PubMed] [Google Scholar]
- 76.Reddy BY, et al. The Microenvironmental Effect in the Progression, Metastasis, and Dormancy of Breast Cancer: A Model System within Bone Marrow. Int J Breast Cancer. 2012;2012:721659. doi: 10.1155/2012/721659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Whiteside TL. The tumor microenvironment and its role in promoting tumor growth. Oncogene. 2008;27:5904–5912. doi: 10.1038/onc.2008.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Singh A, Settleman J. EMT, cancer stem cells and drug resistance: an emerging axis of evil in the war on cancer. Oncogene. 2010;29:4741–4751. doi: 10.1038/onc.2010.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Sayin, V. I. et al. Antioxidants Accelerate Lung Cancer Progression in Mice. Sci Transl Med6 (2014). [DOI] [PubMed]
- 80.Piskounova E, et al. Oxidative stress inhibits distant metastasis by human melanoma cells. Nature. 2015;527:186−+. doi: 10.1038/nature15726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kim JH, et al. Expression of endothelial cell-specific molecule-1 regulated by hypoxia inducible factor-1alpha in human colon carcinoma: impact of ESM-1 on prognosis and its correlation with clinicopathological features. Oncol Rep. 2012;28:1701–1708. doi: 10.3892/or.2012.2012. [DOI] [PubMed] [Google Scholar]
- 82.Oksvold MP, Skarpen E, Widerberg J, Huitfeldt HS. Fluorescent histochemical techniques for analysis of intracellular signaling. J Histochem Cytochem. 2002;50:289–303. doi: 10.1177/002215540205000301. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.