Abstract
Background: Treatment of hereditary tyrosinemia type 1 with nitisinone and phenylalanine and tyrosine restricted diet has largely improved outcome, but the best blood sampling time for assessment of metabolic control is not known.
Aim: To study diurnal and day-to-day variation of phenylalanine and tyrosine concentrations in tyrosinemia type 1 patients.
Methods: Eighteen tyrosinemia type 1 patients aged >1 year (median age 7.9 years; range 1.6–20.7) were studied. Capillary blood samples were collected 4 times a day (T1: pre-breakfast, T2: pre-midday meal, T3: before evening meal, and T4: bedtime) for 3 days. Linear mixed-effect models were used to investigate diurnal and day-to-day variation of both phenylalanine and tyrosine.
Results: The coefficients of variation of phenylalanine and tyrosine concentrations were the lowest on T1 (13.8% and 14.1%, respectively). Tyrosine concentrations did not significantly differ between the different time points, but phenylalanine concentrations were significantly lower at T2 and T3 compared to T1 (50.1 μmol/L, 29.8 μmol/L, and 37.3 μmol/L, respectively).
Conclusion: Our results indicated that for prevention of too low phenylalanine and too high tyrosine concentrations, measurement of phenylalanine and tyrosine pre-midday meal would be best, since phenylalanine concentrations are the lowest on that time point. Our results also indicated that whilst blood tyrosine concentrations were stable over 24 h, phenylalanine fluctuated. Day-to-day variation was most stable after an overnight fast for both phenylalanine and tyrosine. Therefore, in tyrosinemia type 1 patients the most reliable time point for measuring phenylalanine and tyrosine concentrations to enable interpretation of metabolic control is pre-breakfast.
Introduction
Hereditary tyrosinemia type 1 (McKusick 27670, HT1) is a rare inherited metabolic disease caused by fumarylacetoacetate hydrolase (FAH) deficiency in the liver and kidney. FAH is the final enzyme in tyrosine catabolism. Without treatment, patients develop liver failure, hepatocellular carcinoma, porphyria-like neurological episodes, renal tubulopathy, and cardiomyopathy (Mayorandan et al. 2014). Increased blood and urine succinylacetone (SA) are diagnostic indicators (de Laet et al. 2013).
Treatment of HT1 consists of the combination of prescription of NTBC (2-(2 nitro-4-3 trifluoro-methylbenzoyl)-1, 3-cyclohexanedione) and dietary restriction of phenylalanine (Phe) and tyrosine (Tyr) (de Laet et al. 2013). NTBC has led to remarkable improvement in the outcome of HT1 (Larochelle et al. 2012; de Laet et al. 2013). It inhibits the enzyme 4-hydroxyphenylpyruvate dioxygenase at a stage before the metabolic defect, but increases tyrosine concentrations as a consequence, necessitating a diet restriction of tyrosine and its indispensable precursor phenylalanine. Monitoring and adjustment of treatment is based on measurements of NTBC, SA, and phenylalanine and tyrosine concentrations (de Laet et al. 2013).
Neurocognitive delay is reported in HT1 (Masurel-Paulet et al. 2008; de Laet et al. 2011; Bendadi et al. 2014; van Vliet et al. 2014), suggesting a relation with both low phenylalanine and high tyrosine concentrations (de Laet et al. 2011; van Vliet et al. 2014) and/or Phe:Tyr ratios (de Laet et al. 2011). Therefore, regular and reliable measurement of both phenylalanine and tyrosine concentrations could be important. However, what is the predictive value of a single concentration of phenylalanine and tyrosine in treated HT1 patients taken at a specific time point during the day?
Two studies have paid attention to the variation of phenylalanine and tyrosine concentrations in HT1 patients (Wilson et al. 2000; Daly et al. 2012). The first was an intervention study in five HT1 patients and one tyrosinemia type II patient showing that phenylalanine concentrations decreased during the day in five patients (Wilson et al. 2000). The second study was a retrospective study in eleven HT1 patients showing lower blood phenylalanine concentrations in the afternoon (Daly et al. 2012). Notwithstanding of clear interest, these studies leave the predictability of a single blood sample of phenylalanine and tyrosine in a 24 h time period and from day-to-day to be studied.
Therefore, the aim of this prospective study was to investigate diurnal and day-to-day variation of phenylalanine and tyrosine concentrations in HT1 patients treated with NTBC and dietary restriction of phenylalanine and tyrosine. The results should enable reliable recommendations about the optimal timing of phenylalanine and tyrosine for safe dietary changes in HT1.
Methods
Subjects
Patients aged ≥1 year with HT1 were recruited. Recruitment took place at the University Medical Center Groningen (UMCG), Beatrix Children’s Hospital (the Netherlands), and Birmingham Children’s Hospital (United Kingdom). Inclusion criteria included: (1) treatment with NTBC (Orfadin, Swedish Orphan International AB, Stockholm, Sweden); (2) tyrosine restricted diet and phenylalanine, tyrosine free L-amino supplements; (3) good metabolic control defined as having 60% of the tyrosine concentration measurements <500 μmol/L in the 12 months to entering the study. Patients with liver transplants, co-morbidities, or additional phenylalanine supplements were excluded. Patient characteristics are shown in Table 1. Written informed consent was obtained from all participants and/or caregivers. The medical ethics committee of the UMCG provided approval. At Birmingham Children’s Hospital a favorable ethical opinion was obtained from the local research ethics committee.
Table 1.
Patient characteristics
| No. | M/F | Age | Weight | Average of 3 days | ||
|---|---|---|---|---|---|---|
| Total protein (g) | Natural protein (g) | Protein substitute (g) | ||||
| 1 | M | 15.3 | 74.7 | 96 | 36 | 60 |
| 2 | M | 15 | 57 | 92 | 32 | 60 |
| 3 | M | 11.2 | 27.7 | 75 | 15 | 60 |
| 4 | F | 10.5 | 31 | 74 | 14 | 60 |
| 5 | M | 9.8 | 24 | 86 | 26 | 60 |
| 6 | M | 8.1 | 26.9 | 79 | 19 | 60 |
| 7 | F | 8 | 31.2 | 66 | 16 | 50 |
| 8 | M | 7.9 | 45.2 | 86 | 26 | 60 |
| 9 | F | 7.5 | 21.4 | 59 | 14 | 45 |
| 10 | M | 7.3 | 23.2 | 66 | 26 | 40 |
| 11 | M | 6.5 | 21.4 | 56 | 11 | 45 |
| 12 | F | 3.1 | 12.4 | 35 | 17 | 18 |
| 13 | F | 2.2 | 10 | 34 | 13 | 21 |
| 14 | F | 1.6 | 11.5 | 35 | 5 | 30 |
| 15 | M | 7.2 | 31 | 47 | 13 | 34 |
| 16 | M | 6.4 | 24.5 | 36 | 12 | 24 |
| 17 | M | 20.7 | 58 | 88 | 28 | 60 |
| 18 | M | 13.8 | 59 | 63 | 20 | 44 |
No. number
Assessment of Dietary Intake
The participants continued their usual tyrosine restricted diet including phenylalanine and tyrosine free L-amino acid supplements. Participants or caregivers kept a written record of all food, drinks, and L-amino acid supplements during the 3 days of the study period. The nutritional analysis of food intake of the Dutch participants was calculated using a hospital computer program based on the Dutch food composition database (NEVO 2010) (Westenbrink and Jansen-van der Vliet 2010). The nutritional analysis of food intake of the British participants was calculated using the Microdiet computer program based on McCance and Widdowson’s The Composition of Foods series, with supplementary analysis data provided by manufacturers and added to the database.
Body weight measurements were performed by participants or caregivers at home 1 week before the start of the study, at the beginning of the study, and the day after the study to monitor that a stable body weight was maintained.
Blood Sampling
All participants or caregivers collected blood spot samples from finger punctures on filter paper (Grade TFN 179 g/m2. Sartorius. Göttingen, Germany) and stored at −20°C till analysis. This was performed 4 times a day (pre-breakfast: 7–8 a.m., pre-midday meal: 12–1 p.m., before evening meal: 5–6 p.m. and bedtime) for 3 days with 12 samples per participant. Phenylalanine and tyrosine concentrations of all blood specimens were analyzed by LC-MS/MS in the laboratory of Metabolic Diseases of the UMCG in Groningen following a standardized protocol taking into account differences such as the spot size and localization of the punch (Holub et al. 2006; Lawson et al. 2016).
Statistical Analysis
Descriptive statistics were applied for baseline characteristics. The normality of the data was assessed by analyzing the P–P plots. Data were represented as median and range. For categorical data, proportions are shown. To investigate the mean difference of phenylalanine and tyrosine concentrations between the separate time points (diurnal variation) and to account for intra individual correlations between multiple measurements, a linear mixed-effect model was applied. With this repeated measures analysis, all available data points were used.
The coefficient of variation (CV) of tyrosine and phenylalanine per subject per time point was used as a measure of day-to-day variation. To investigate the difference in CV of both phenylalanine and tyrosine between the separate measurement moments, linear mixed-effect models were applied. In both linear mixed-effect models the individual variation in daily intercept per subject was accounted for by estimating a random effect. Two sided significance tests were used (α < 0.05). Statistical analyses were performed using IBM SPSS statistical software, version 22.0 (IMB Corporation, Armonk, NY, USA).
A blood phenylalanine concentration <30 μmol/L was considered clinically suboptimal as well as a blood tyrosine >400 μmol/L.
Results
Patient Characteristics
Eighteen participants (12, 66.7% males) were included with a median age of 7.9 years (range 1.6–20.7). Five patients were recruited from UMCG. Two participants were diagnosed by newborn screening, the others after development of clinical symptoms. Thirteen patients were recruited from Birmingham Children’s Hospital. Four patients diagnosed due to previous family diagnosis, and two diagnosed via screening (incidental findings). All others were diagnosed after development of clinical symptoms. Nitisinone was prescribed in a median dose of 0.95 (range 0.65–2) mg/kg/day.
Blood Concentrations
Before assessing the diurnal and day-to-day variation, we investigated blood tyrosine and phenylalanine concentrations during the study period in relation to the current treatment recommendations for HT1 patients. In total we had 215 samples (18 subjects × 12 blood spots = 216, 1 missing value). Blood tyrosine concentrations were within the aimed range 200–400 μmol/L in 128 (59.5%) of the samples. Tyrosine concentrations were <200 μmol/L in 9 (4.2%) samples (range 135–181), ≥400 μmol/L in 78 (36.3%) samples (range 400–791), and ≥600 μmol/L in 6 (2.8%) samples (range 600–791). Phenylalanine concentrations were <20 μmol/L in 20 (9.3%) samples (range 8–19), <30 μmol/L in 57 (26.5%) samples (range 8–29), <40 μmol/L in 108 (50.2%) samples (range 8–39), and ≥40 μmol/L in 107 (48.8%) samples (range 40–143).
Diurnal and Day-to-Day Variation of Blood Tyrosine Concentrations
To determine the optimal time point for measuring blood tyrosine concentrations to indicate metabolic control, both the diurnal variation and the CV per time point as a measure of day-to-day variation of blood tyrosine concentrations were assessed by a linear mixed-effect model. Mean tyrosine concentrations (μmol/L) per time point per subject are shown graphically in Fig. 1a.
Fig. 1.
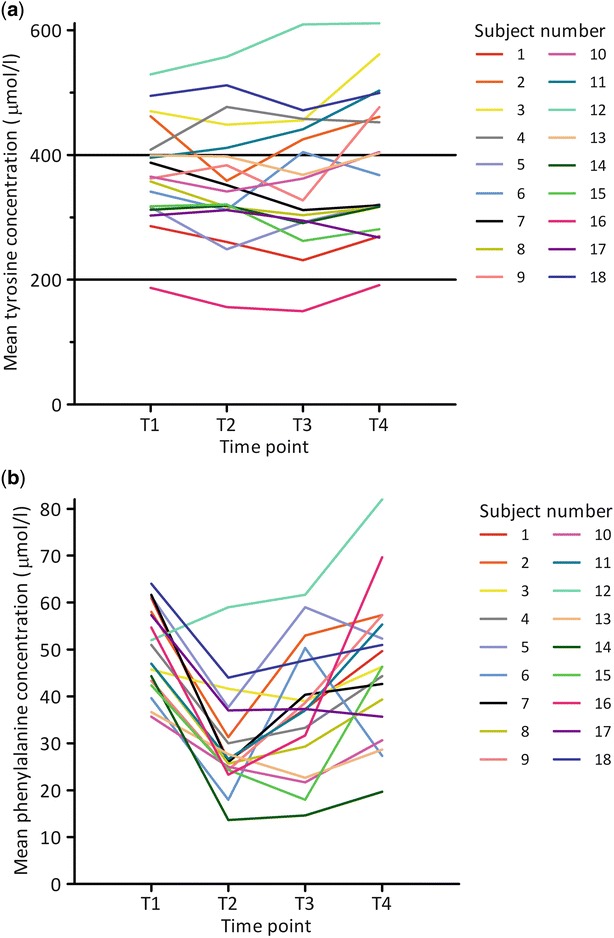
Mean tyrosine concentrations (μmol/L) (a) and mean phenylalanine concentrations (μmol/L) (b) per time point per subject. The black horizontal lines indicate the upper and lower reference value of tyrosine (de Laet et al. 2013). T1 = pre-breakfast, T2 = pre-midday meal, T3 = before evening meal, T4 = bedtime
Assessing diurnal variation, the linear mixed-effect model for predicting tyrosine concentrations showed that the tyrosine concentrations were expected to be 371.9 μmol/L at T1. Tyrosine concentrations were lower at T2 and T3 compared to T1 with 13.9 μmol/L and 13.1 μmol/L, respectively. Tyrosine concentrations at T4 were expected to be 18.2 μmol/L higher compared to T1. Differences in tyrosine concentrations were not significant (Table 2). In post-hoc analysis it was shown that tyrosine concentrations at T2 and T3 were significantly lower than at T4 (p = 0.002) with, respectively, 32.1 and 31.3 μmol/L (data not shown).
Table 2.
Results of mixed-effect model for predicting tyrosine en phenylalanine concentrations in blood in tyrosinemia type 1 patients
| Variable | Tyrosine (μmol/L) | Phenylalanine (μmol/L) | ||||
|---|---|---|---|---|---|---|
| ß | 95% CI | p | ß | 95% CI | p | |
| Intercept | 371.9 | 341.4; 402.4 | <0.001 | 50.1 | 45.9; 54.3 | <0.001 |
| Time point | ||||||
| T1 before breakfast | Reference value | Reference value | ||||
| T2 before lunch | −13.9 | −33.6; 5.8 | 0.165 | −20.3 | −25.2; −15.5 | <0.001 |
| T3 before dinner | −13.1 | −32.7; 6.5 | 0.188 | −12.8 | −17.6; −7.9 | <0.001 |
| T4 before bedtime | 18.2 | −1.4; 37.8 | 0.068 | −3.7 | −8.6; 1.1 | 0.130 |
CI confidence interval, β effect differences in μmol/L compared to T1 (intercept)
The linear mixed-effect model for predicting the day-to-day variation of tyrosine concentrations showed that the CV of tyrosine was expected to be 13.8% at T1. CV of tyrosine concentrations on T2 is expected to be the same as on T1. CVs of tyrosine concentrations at T3 and T4 were expected to be 1.6% and 3.1% higher than at T1, respectively. None of these differences were significant (Table 3).
Table 3.
Results of mixed-effect model for predicting the day-to-day variation of tyrosine en phenylalanine concentrations in blood in tyrosinemia type 1 patients
| Variable | CV tyrosine (%) | CV phenylalanine (%) | ||||
|---|---|---|---|---|---|---|
| ß | 95% CI | p | ß | 95% CI | p | |
| Intercept | 13.8 | 9.8; 17.9 | <0.001 | 14.1 | 7.3; 20.8 | <0.001 |
| Time point | ||||||
| T1 before breakfast | Reference value | Reference value | ||||
| T2 before lunch | 0.0 | −4.6; 4.6 | 1.000 | 11.0 | 1.5; 20.5 | 0.024 |
| T3 before dinner | 1.6 | −3.0; 6.1 | 0.488 | 6.5 | −3.0; 16.0 | 0.177 |
| T4 before bedtime | 3.1 | −1.5; 7.6 | 0.184 | 15.2 | 5.7; 24.7 | 0.002 |
CV coefficient of variation, CI confidence interval, β effect differences in μmol/L compared to T1 (intercept)
Diurnal and Day-to-Day Variation of Blood Phenylalanine Concentrations
To determine the optimal time point for measuring blood phenylalanine concentrations to indicate metabolic control, both the diurnal variation and the CV per time point as a measure of day-to-day variation of blood phenylalanine concentrations were assessed by a linear mixed-effect model. Mean phenylalanine concentrations (μmol/L) per time point per subject are shown graphically in Fig. 1b.
Assessing diurnal variation, the linear mixed-effect model for predicting phenylalanine concentrations showed that the phenylalanine concentrations at T2 and T3 were expected to be significantly lower (p < 0.001) than at T1 with 20.3 μmol/L and 12.8 μmol/L, respectively. Phenylalanine concentrations at T4 were expected to be 3.7 μmol/L lower than at T1; this difference was not significant (Table 2).
The linear mixed-effect model for predicting the day-to-day variation of phenylalanine concentrations showed that the CV of phenylalanine was expected to be 14.1% on T1. CV of phenylalanine concentrations at T2 and T4 are expected to be 11.0 and 6.5% higher than on T1. These differences are significantly different (p = 0.024 and p = 0.002, respectively). CV of phenylalanine at T3 is expected to be 6.5% higher than at T1; this difference was not significant (Table 3).
Discussion
This is the first study investigating the optimal time to take blood samples to reliably monitor metabolic control in HT1 patients. Addressing the optimal time of blood sampling includes two discussions: (1) the optimal time point to detect a clinically relevant phenylalanine and/or tyrosine concentration; (2) the most reliable time of measurement giving the least day-to-day variation. The clinically most optimal moment for blood sampling may be the moment where phenylalanine concentrations are the lowest and tyrosine concentrations are the highest. Our results indicated that for that purpose measurement of phenylalanine and tyrosine pre-midday meal would be best, since phenylalanine concentrations are the lowest on that time point. However, our results also indicated that whilst blood tyrosine concentrations were stable over 24 h, phenylalanine fluctuated. Day-to-day variation was most stable after an overnight fast for both phenylalanine and tyrosine. For comparison, in healthy individuals amino acids such as phenylalanine and tyrosine concentrations rise during the day due to intake and decrease during the night (Maher et al. 1984; Farquhar et al. 1985). In contrast, in patients with phenylketonuria (PKU) phenylalanine and tyrosine concentrations decrease rather than increase during the day with meals and nett-protein synthesis, while they tend to increase during periods of fasting in which there is a nett-catabolism of natural protein as shown for phenylalanine in (PKU) (Farquhar et al. 1985; van Spronsen et al. 1993). This difference is due to the fact that the intake of phenylalanine and tyrosine is decreased in the diet of the patients.
Methodological Issues
Before addressing the results in more detail, the following methodological issues are discussed. First, we did not study patients with clinically proven deficiencies of amino acids as all our patients showed acceptable growth curves and also met the Dutch recommendations for total protein intake for patients who use amino acid supplementation as part of daily protein intake. Second, amino acid analysis was done in blood spots. Measurements of amino acids in blood spots are influenced by factors such as humidity, hematocrit, and size and location of punches in the blood spot (Holub et al. 2006; Lawson et al. 2016). Patients’ hematocrit was not measured, but can be considered normal as no illness or trauma occurred in our patients during the time of the study. Size and location of punches in the blood spot was standardized, so that the influence of such blood spot related issues can be regarded to be as minimal as possible. Third, the study was performed at home to maintain normal patient routine and not in a research environment in which intake is standardized. As a consequence we had to assume that participants followed the study protocol in the correct manner. However, we instructed each participant/caregiver before study commencement and maintained regular contact during the study period and all participants/caregivers returned blood samples according to protocol. Fourth, we did measure in blood spots in a protocolled way rather than in plasma or serum. The advantage is that the discussion on differences between measurement in blood spot versus plasma as shown in various articles is not of importance for this study. On the other hand, we do not know exactly how to translate our data and conclusions for measurements in plasma, knowing that the debate on the comparison between blood spot and plasma is ongoing (Gregory et al. 2007; Kand’ár and Zakova 2009; De Silva et al. 2010; Mo et al. 2013; Groselj et al. 2015).
Optimal Metabolic Control Is Less Clear for Blood Phenylalanine than Blood Tyrosine
Thus far, there is no clear consensus about the lower acceptable limit for blood phenylalanine. The lower end of the reference values are between 26 and 46 μmol/L, also depending on age (Pasquali and Longo 2014). Concentrations <20 μmol/L being considered to be very low (Wilson et al. 2000). It is often advised to keep phenylalanine plasma concentrations >40 μmol/L (Chakrapani et al. 2012). This is why we reported phenylalanine levels <20, <30, <40, and ≥40 μmol/L. For tyrosine, upper target concentrations are reported as 400, 500, 600 μmol/L, while it also is advised to keep tyrosine plasma concentrations between 200 and 400 μmol/L (Wilson et al. 2000; Chakrapani et al. 2012; de Laet et al. 2013). The advised concentrations are based on plasma while phenylalanine and tyrosine concentrations of this study are measured in blood spots. Therefore, we reported concentrations between <200, between 200 and 400, ≥400 and ≥600 μmol/L. To decide on clinical significance of a variation in phenylalanine and/or tyrosine concentrations we decided to define a variation significant when for phenylalanine one of the samples showed a result of <30 μmol/L, while the others were >30 μmol/L, while for tyrosine one of the samples showed a result of >400 μmol/L when the others were <400 μmol/L.
Day-to-Day Variation Is Comparable for Phenylalanine and Tyrosine, While Diurnal Variation Only Plays a Role for Phenylalanine
When considering the optimal time of blood sampling in HT1 patients, this includes two discussions: (1) the most reliable time of measurement giving the least day-to-day variation; and (2) the optimal time point to detect a clinically relevant phenylalanine and/or tyrosine concentration. With respect to the first issue, our study demonstrated that CVs of both phenylalanine and tyrosine concentrations were the lowest at T1 (13.8% and 14.1%, respectively). With respect to the second issue, phenylalanine concentrations were both statistically and clinically significantly lower at T2 and T3 compared to T1, showing levels <30 μmol/L. This indeed is of clinical importance since too low phenylalanine concentrations in PKU are related to impaired growth and development (Rouse 1966; Smith et al. 1990; Teissier et al. 2012), and possibly also to physical and mental development in HT1 (de Laet et al. 2011; van Vliet et al. 2014).
In contrast, we found the highest tyrosine levels at T4, being 18.1 μmol/L higher than at T1, but still within an acceptable range of variation. Therefore, this difference is not considered clinically significant.
Clinical Implications Regarding the Optimal Timing of Blood Sampling
Until now, little attention has been paid to the optimal timing of blood sampling for metabolic control in HT1 and leaving us without recommendation. Most knowledge on the most reliable time of phenylalanine and tyrosine as metabolic control is available from PKU in which phenylalanine is much higher and tyrosine lower (Blau et al. 2010). In PKU, phenylalanine also shows a decrease during the day, being the highest just before breakfast, and tyrosine showing large variation during the day (van Spronsen et al. 1993; van Spronsen et al. 1996; MacDonald et al. 1998). In HT1 our data add to the data of Wilson et al. (2000) and Daly et al. (2012) showing that phenylalanine can decrease during the day, while tyrosine is more or less stable. So, when concluding on the most optimal time for blood sampling for metabolic control in practice, the exact moment does not matter when tyrosine is regarded. In contrast for phenylalanine, we have to choose between the moment that is the most consistent (pre-breakfast) and the moment that may give the largest clue to a clinically significant low level (pre-midday meal). As consistency is very important, we would advise to choose for blood spot sampling prior to breakfast taking into account that it is important to take a decrease into account that may easily be around 20 μmol/L. Considering the clinical consequences of (cerebral) phenylalanine deficiency, we suggest that in HT1 the fasting lower target reference for phenylalanine concentrations in blood spots should be at least 50 μmol/L with the basic thought that phenylalanine concentrations should not be lower than 30 μmol/L during the day. If there is any suggestion of clinical phenylalanine deficiency (e.g., impaired growth, impaired growth of nails and hair), a second blood sample taken later on the same day after a meal may be warranted to check blood phenylalanine concentrations. If phenylalanine concentrations are consistently low, phenylalanine supplementation should be added to maintain blood concentrations within target range.
Conclusion
In conclusion, the most reliable moment for measuring metabolic control in HT1 patients in regular patient care is in a fasting state prior to breakfast. This study also confirms that phenylalanine concentrations may drop significantly post breakfast by an average of 20.3 μmol/L. Therefore, we recommend that metabolic control in HT1 patients is measured in fasting state, and that the fasting lower target reference for phenylalanine concentrations in blood spots should be at least 50 μmol/L. Future research should investigate whether the diurnal and day-to-day variation of blood phenylalanine and tyrosine concentrations is different in HT1 patients receiving phenylalanine supplementation.
Acknowledgement
This study was made possible by a grant of “Stichting Joris” (Dutch Tyrosinemia Foundation).
Take Home Message
We recommend blood sampling for metabolic control in tyrosinemia type I pre-breakfast.
Compliance with Ethics Guidelines
Conflict of Interest
Esther van Dam has received advisory board fees from Biomarin and Merck Serono.
Anne Daly has received research funding and lecture fees from Nutricia and Vitaflo.
Gineke Venema-Liefaard has no conflicts of interest to declare.
Margreet van Rijn has received research grants, consultancy fees, and advisory board fees from Merck Serono and Nutricia Research, speaker honoraria from Merck Serono, Nutricia Research and Orphan Europe, and expert testimony fees from Merck Serono.
Terry G. J. Derks has received speaker honoraria from Danone, Nutricia, Recordati Rare Diseases and Vitaflo, research grants from Sigma Tau and Vitaflo, and consultancy and advisory board fees from Dimension Therapeutics.
Patrick J. McKiernan has received advisory board fees and speaker honoraria from Sobi.
M. Rebecca Heiner-Fokkema has no conflicts of interests to declare.
Anita MacDonald received research funding and lecture fees from Merck Serono, Nutricia, and Vitaflo, and advisory board fees from Arla Foods, Biomarin, Merck Serono and Nutricia.
Francjan J. van Spronsen has received research grants, advisory board fees, and speaker honoraria form Merck Serono, Nutricia Research and Sobi, speaker fees from Vitaflo, and advisory board fees from Arla Foods and Biomarin.
Informed Consent
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki.
Declaration of 1975, as revised in 2000. Informed consent was obtained from all patients and/or their parents/caregivers before being included in the study.
Details of the Contributions of the Authors
Esther van Dam collected data, drafted the initial manuscript, revised the manuscript, and approved the final manuscript as submitted.
Anne Daly included patients in Birmingham, collected data, co-wrote the manuscript, and approved the final manuscript as submitted.
Gineke Venema-Liefaard included patients in Groningen, collected data, critically reviewed the manuscript, and approved the final manuscript as submitted.
Margreet van Rijn co-wrote the manuscript and approved the final manuscript as submitted.
Terry G. J. Derks critically reviewed the manuscript and approved the final manuscript as submitted.
Patrick J. McKiernan critically reviewed the manuscript and approved the final manuscript as submitted.
M. Rebecca Heiner-Fokkema was responsible for the biochemical analyses, critically reviewed the manuscript, and approved the final manuscript as submitted.
Anita MacDonald included patients in Birmingham, collected data, co-wrote the manuscript, and approved the final manuscript as submitted.
Francjan J. van Spronsen initiated the study and wrote the study draft, co-wrote the manuscript, and approved the final manuscript as submitted.
Competing Interests
All authors have indicated that they have no financial relationships relevant to this article to disclose.
Contributor Information
Esther van Dam, Email: e.van.dam@umcg.nl.
Collaborators: Matthias R. Baumgartner, Marc Patterson, Shamima Rahman, Verena Peters, Eva Morava, and Johannes Zschocke
References
- Bendadi F, de Koning TJ, Visser G, et al. Impaired cognitive functioning in patients with tyrosinemia type I receiving nitisinone. J Pediatr. 2014;164:398–401. doi: 10.1016/j.jpeds.2013.10.001. [DOI] [PubMed] [Google Scholar]
- Blau N, van Spronsen FJ, Levy HL. Phenylketonuria. Lancet. 2010;376:1417–1427. doi: 10.1016/S0140-6736(10)60961-0. [DOI] [PubMed] [Google Scholar]
- Chakrapani A, Gissen P, McKiernan P. Disorders of tyrosine metabolism. In: Saudubray J, van den Berghe G, Walter JH, editors. Inborn metabolic diseases-diagnosis and treatment. Berlin: Springer; 2012. pp. 265–276. [Google Scholar]
- Daly A, Gokmen-Ozel H, MacDonald A, Preece MA, Davies P, Chakrapani A, McKiernan P. Diurnal variation of phenylalanine concentrations in tyrosinaemia type 1: should we be concerned? J Hum Nutr Diet. 2012;25:111–116. doi: 10.1111/j.1365-277X.2011.01215.x. [DOI] [PubMed] [Google Scholar]
- de Laet C, Munoz VT, Jaeken J, et al. Neuropsychological outcome of NTBC-treated patients with tyrosinaemia type 1. Dev Med Child Neurol. 2011;53:962–964. doi: 10.1111/j.1469-8749.2011.04048.x. [DOI] [PubMed] [Google Scholar]
- de Laet C, Dionisi-Vici C, Leonard JV, et al. Recommendations for the management of tyrosinaemia type 1. Orphanet J Rare Dis. 2013;8:8. doi: 10.1186/1750-1172-8-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Silva V, Oldham CD, May SW. L-phenylalanine concentration in blood of phenylketonuria patients: a modified enzyme colorimetric assay compared with amino acid analysis, tandem mass spectrometry, and HPLC methods. Clin Chem Lab Med. 2010;48:1271–1279. doi: 10.1515/cclm.2010.271. [DOI] [PubMed] [Google Scholar]
- Farquhar DL, Steven F, Westwood A. Preliminary report on inverse diurnal variation of phenylalanine: implications in maternal phenylketonuria. Hum Nutr Appl Nutr. 1985;39:224–226. [PubMed] [Google Scholar]
- Gregory CO, Yu C, Singh RH. Blood phenylalanine monitoring for dietary compliance among patients with phenylketonuria: comparison of methods. Genet Med. 2007;9:761–765. doi: 10.1097/GIM.0b013e318159a355. [DOI] [PubMed] [Google Scholar]
- Groselj U, Murko S, Zerjav Tansek M, Kovac J, Trampus Bakija A, Repic Lampret B, Battelino T. Comparison of tandem mass spectrometry and amino acid analyzer for phenylalanine and tyrosine monitoring – implications for clinical management of patients with hyperphenylalaninemia. Clin Biochem. 2015;48:14–18. doi: 10.1016/j.clinbiochem.2014.09.014. [DOI] [PubMed] [Google Scholar]
- Holub M, Tuschl K, Ratschmann R, et al. Influence of hematocrit and localisation of punch in dried blood spots on levels of amino acids and acylcarnitines measured by tandem mass spectrometry. Clin Chim Acta. 2006;373:27–31. doi: 10.1016/j.cca.2006.04.013. [DOI] [PubMed] [Google Scholar]
- Kand’ár R, Zakova P. Determination of phenylalanine and tyrosine in plasma and dried blood samples using HPLC with fluorescence detection. J Chromatogr B Analyt Technol Biomed Life Sci. 2009;877:3926–3929. doi: 10.1016/j.jchromb.2009.09.045. [DOI] [PubMed] [Google Scholar]
- Larochelle J, Alvarez F, Bussieres JF, et al. Effect of nitisinone (NTBC) treatment on the clinical course of hepatorenal tyrosinemia in Quebec. Mol Genet Metab. 2012;107:49–54. doi: 10.1016/j.ymgme.2012.05.022. [DOI] [PubMed] [Google Scholar]
- Lawson AJ, Bernstone L, Hall SK. Newborn screening blood spot analysis in the UK: influence of spot size, punch location and haematocrit. J Med Screening. 2016;23:7–16. doi: 10.1177/0969141315593571. [DOI] [PubMed] [Google Scholar]
- MacDonald A, Rylance GW, Asplin D, Hall SK, Booth IW. Does a single plasma phenylalanine predict quality of control in phenylketonuria? Arch Dis Child. 1998;78:122–126. doi: 10.1136/adc.78.2.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maher TJ, Glaeser BS, Wurtman RJ. Diurnal variations in plasma concentrations of basic and neutral amino acids and in red cell concentrations of aspartate and glutamate: effects of dietary protein intake. Am J Clin Nutr. 1984;39:722–729. doi: 10.1093/ajcn/39.5.722. [DOI] [PubMed] [Google Scholar]
- Masurel-Paulet A, Poggi-Bach J, Rolland MO, et al. NTBC treatment in tyrosinaemia type I: long-term outcome in French patients. J Inherit Metab Dis. 2008;31:81–87. doi: 10.1007/s10545-008-0793-1. [DOI] [PubMed] [Google Scholar]
- Mayorandan S, Meyer U, Gokcay G, et al. Cross-sectional study of 168 patients with hepatorenal tyrosinaemia and implications for clinical practice. Orphanet J Rare Dis. 2014;9:107. doi: 10.1186/s13023-014-0107-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mo XM, Li Y, Tang AG, Ren YP. Simultaneous determination of phenylalanine and tyrosine in peripheral capillary blood by HPLC with ultraviolet detection. Clin Biochem. 2013;46:1074–1078. doi: 10.1016/j.clinbiochem.2013.05.047. [DOI] [PubMed] [Google Scholar]
- Pasquali M, Longo N. Amino acids. In: Blau N, Duran M, Gibson KM, Dionisi-Vici C, editors. Physician’s guide to the diagnosis, treatment, and follow-up of inherited metabolic diseases. Berlin: Springer; 2014. pp. 749–759. [Google Scholar]
- Rouse BM. Phenylalanine deficiency syndrome. J Pediatr. 1966;69:246–249. doi: 10.1016/S0022-3476(66)80327-X. [DOI] [PubMed] [Google Scholar]
- Smith I, Beasley MG, Ades AE. Intelligence and quality of dietary treatment in phenylketonuria. Arch Dis Child. 1990;65:472–478. doi: 10.1136/adc.65.5.472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teissier R, Nowak E, Assoun M, et al. Maternal phenylketonuria: low phenylalaninemia might increase the risk of intra uterine growth retardation. J Inherit Metab Dis. 2012;35:993–999. doi: 10.1007/s10545-012-9491-0. [DOI] [PubMed] [Google Scholar]
- van Spronsen FJ, van Rijn M, van Dijk T, Smit GP, Reijngoud DJ, Berger R, Heymans HS. Plasma phenylalanine and tyrosine responses to different nutritional conditions (fasting/postprandial) in patients with phenylketonuria: effect of sample timing. Pediatrics. 1993;92:570–573. [PubMed] [Google Scholar]
- van Spronsen FJ, van Dijk T, Smit GP, van Rijn M, Reijngoud DJ, Berger R, Heymans HS. Large daily fluctuations in plasma tyrosine in treated patients with phenylketonuria. Am J Clin Nutr. 1996;64:916–921. doi: 10.1093/ajcn/64.6.916. [DOI] [PubMed] [Google Scholar]
- van Vliet D, van Dam E, van Rijn M, et al. Infants with tyrosinemia type 1: should phenylalanine be supplemented? JIMD Rep. 2014;18:117. doi: 10.1007/8904_2014_358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westenbrink S, Jansen-van der Vliet M (2010) NEVO-tabel Nederlands voedingsbestand 2010
- Wilson CJ, Van Wyk KG, Leonard JV, Clayton PT. Phenylalanine supplementation improves the phenylalanine profile in tyrosinaemia. J Inherit Metab Dis. 2000;23:677–683. doi: 10.1023/A:1005666426079. [DOI] [PubMed] [Google Scholar]


