Abstract
Feasible, sensitive and clinically relevant outcome measures are of extreme importance when designing clinical trials. For paediatric mitochondrial disease, no robust end point has been described to date. The aim of this study was to select the domains of daily physical activity, which can be measured by 3D accelerometry, that could serve as sensitive end points in future clinical trials in children with mitochondrial disorders.
In this exploratory observational study, 17 patients with mitochondrial disease and 16 age- and sex-matched controls wore 3D accelerometers at the upper leg, upper arm, lower arm and chest during one weekend. Using the raw data obtained by the accelerometers, we calculated the following outcome measures: (1) average amount of counts per hour the sensors were worn; (2) the maximal intensity; (3) the largest area under the curve during 30 min and (4) categorized activities lying, standing or being dynamically active. Measuring physical activity during the whole weekend was practically feasible in all participants. We found good face validity by visually correlating the validation videos and activity diaries to the accelerometer data-graphs. Patients with mitochondrial disorders had significantly lower peak intensity and were resting more, compared to their age- and sex-matched peers.
Finally, we suggest domains of physical activity that could be included when measuring daily physical activity in children with mitochondrial disorders, preferably using more user-friendly devices. These include peak activity parameters for the arms (all patients) and legs (ambulatory patients). We recommend using or developing devices that measure these domains of physical activity in future clinical studies.
Electronic supplementary material
The online version of this chapter (doi:10.1007/8904_2016_35) contains supplementary material, which is available to authorized users.
Keywords: 3D accelerometry, Children, Daily physical activity, Mitochondrial disease, Outcome measures
Introduction
Since lack of energy and fatigue are among the most burdensome complaints experienced by children with mitochondrial disease and their parents (Koene et al. 2013b), this symptom should be covered in future clinical trials. However, testing fatigue or fatigability in this paediatric population is challenging, since many children are not able to rate their fatigue using one of the widely used self-report fatigue questionnaires (Swanink et al. 1995; Gordijn et al. 2011) because of intellectual disabilities and most endurance tests (e.g. cycle ergometry (LeMura et al. 2001) are not feasible or too burdensome for children with mitochondrial disorders. Besides, measuring performance in a laboratory situation may not always reflect the disabilities experienced in daily life since the performance in daily life may differ from the abilities of the child (Abel et al. 2003; Beenakker et al. 2005; Parreira et al. 2010).
In a recent review of all published studies in mitochondrial disease, an international expert panel recommended (amongst others) to use validated and clinically meaningful end points (Pfeffer et al. 2013). Only very few studies investigating outcome measures in paediatric mitochondrial disease have been published and most of the outcome measures studied are not generally applicable among children with mitochondrial disease. In a small group of children with non-proven mitochondrial disease, Martens et al. (2014) found lower physical activity level compared to healthy peers.
We hypothesized that measuring daily physical activity at home is a clinically relevant outcome measure and a good reflection of the fatigue experienced by children with mitochondrial disease in daily life. Physical activity is defined as “any bodily movement produced by skeletal muscle contraction that results in caloric expenditure” and includes sports, hobbies, playing, walking, cycling and activities of daily living (Caspersen et al. 1985). Physical activity has many domains, including the type, intensity, frequency and duration of the physical activity. It is currently not known which of these aspects is most affected in patients with mitochondrial disorders.
Daily physical activity in a home situation can be measured by using 3D accelerometry (Bjornson 2005; McDonald et al. 2005; Capio et al. 2010; Clanchy et al. 2011a, b; Jeannet et al. 2011; Koene et al. 2013a). Many commercially available activity monitors that are based on accelerometry measure only general domains of movement, such as the total amount of body activity, step count and position. Such monitors provide only the calculated parameters, and not the raw acceleration data. In this study, we aimed to select more detailed domains of physical activity that can be measured by accelerometry in future clinical trials. For this study, we selected an accelerometer (MOX) that provides raw accelerations in 3D, so we could design a tailored analysis.
Methods
This is an exploratory, observational study, aiming to select the domains of physical activity that should be measured by accelerometry in future clinical trials in patients with mitochondrial disease. The domains of physical activity were selected based on deviation from healthy age- and sex-matched peers. Before selecting these domains, the feasibility and face validity of the 3D accelerometer in this population was studied.
Accelerometer
For this study, we used a MOX accelerometer (MOX sensor, model MMOXX1.01, Maastricht Instruments BV, The Netherlands), that measures accelerations (Range ± 6G) in three degrees of freedom with a sample frequency of 25 Hz. The acceleration data was filtered with a Butterworth 0.025–7.5 Hz 4th-order high-pass filter to remove noise and movement artefacts. This accelerometer provides raw data which can be used for tailored analyses.
A set of four or five sensors was used. The accelerometers were attached to the chest, dominant lower arm and upper arm and to the leg using an attachment band (limbs) or a top (chest; Fig. 1). If the patient used a wheelchair, a fifth accelerometer was attached to the wheelchair. The wheelchair sensor was used to indicate passive moments of the child.
Fig. 1.
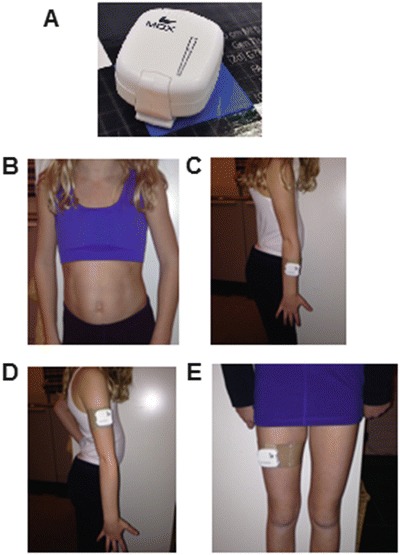
The accelerometer localizations. (a) The MOX-accelerometer is approximately 4.5 × 4.0 × 1.4 cm in size and weighs 27 g; (b) Attachment of the accelerometer to chest; (c) to the lower (dominant) arm; (d) the upper arm and (e) the (dominant) upper leg
To estimate the amount of daily activity, various parameters were calculated from the acceleration data of each sensor, by using Matlab procedures that were developed and validated beforehand (Meijer et al. 2014). The first parameter is the activity counts, which was calculated by integrating the acceleration over 1-minute episodes and summing this outcome over all three axes. A constant acceleration of 1G (gravitational constant) over 1 min corresponds with 1,000 counts (Meijer et al. 2014). We used the following outcome measures: (1) average amount of counts per hour the sensors were worn (average counts (total amount of counts measured with the sensor/worn h; counts/h), also referred to activity level); (2) the maximal intensity (maximal amount of counts per min (counts/min)) and (3) the largest area under the curve (AUC) during 30 min (largest AUC during 1/2 h (counts)). The second outcome measure is an activity classification which categorizes the performed activities per second into lying, standing or being dynamically active. Lying and standing are classified depending on the gravitational angle acting on the posterior-anterior and cranial-caudal axes. Being dynamically active is classified when the integration of the acceleration over 1-second episodes is above a pre-defined threshold.
Study Protocol
Study Protocol for Patients
Patients were recruited at the Radboud Centre for Mitochondrial Medicine. Patients aged 4–18 years old with a confirmed mitochondrial disease, either based on pathological mutations in mtDNA or nuclear DNA or on mitochondrial dysfunction in fresh muscle as measured by routine biochemistry as applied in our centre (Rodenburg 2011), were eligible for inclusion. This group includes the full clinical spectrum of mitochondrial disorders, ranging from patients with exercise intolerance only to wheelchair bound patients with severe movement disorders. Exclusion criteria: (1) expected by the treating physician that travelling to the hospital would be too burdensome to the patient; (2) fever; (3) epilepsia continua; or (4) altered state of consciousness compared to normal at the time of inclusion. The number of children was determined by the number of children that could be included in the same season.
Patients were assessed at the outpatient clinic of the Radboud Center for Mitochondrial Medicine (RCMM) on Fridays. An experienced paediatric physiotherapist performed the Gross Motor Function Measure-88 (GMFM). After the two tests, parents were instructed how to attach the accelerometers. Wearing the sensors, patients were – if possible – instructed to follow a validation protocol (standardized activities, including waving, throwing a ball, lying down, sitting, standing, walking and running). In case of limited physical abilities, the position (orientation) of the arm, leg and chest was changed passively, if possible at low, middle and high velocity (intensity). The patient was videotaped with a synchronized camera during all tests, to be able to correlate specific movements (e.g. raising an arm, walking, movement disorders or epilepsy) to the data obtained by the accelerometer. By correlating these video images with the accelerometer data-graphs (correspondence of orientation and intensity for each sensor) we determined the face validity of the measurements in a laboratory situation.
After completion of the validation protocol, patients were asked to wear the sensors over the weekend, while the parents completed an activity diary. Parents were asked to complete the diary with the exact timing and a description of the activity (e.g. 12:36–13:18: Lunch, independently eating bread with knife and fork). For practical reasons (complexity of data analysis and logistics), we chose to only measure for 2 days, instead of the recommended 7 days (Cain et al. 2013). Patients were asked to wear the accelerometers at all waking hours, with the exception of bathing, showering and swimming. The reported activities were also correlated to the accelerometer data-graphs (correspondence of the intensity of the movements during the described activities) to determine face validity of the measurements at home.
On Monday, the parents were interviewed by phone for the feasibility and comfort of the accelerometers with a self-made questionnaire. Moreover, the parents were interviewed with the Pediatric Evaluation of Disability Inventory (PEDI), measuring the performance and capability in the activities of daily life (Custers et al. 2002; Vos-Vromans et al. 2005). The PEDI and the GMFM were used to determine the correlation between activity parameters and gross motor function (capability) and functional abilities (performance).
Study Protocol for Healthy Controls
The healthy controls were recruited at two regular schools in the surroundings of Nijmegen. Healthy controls were eligible for inclusion when they were healthy and aged between 4 and 18 years. Exclusion criteria: (1) confirmed diagnosis of Attention Deficit and Hyperactivity Disorder (ADHD); (2) symptoms of exercise intolerance, fatigue or muscle problems or (3) the child was under regular surveillance of a paediatrician. Controls were sex- and age-matched to a single patient.
Healthy controls were instructed in their home-environment in the same weekend as the age- and sex-matched patient. The attachment and localization of the accelerometers was the same as the patient protocol. Validation, using the validation protocol and videotaping, was also similar to the patient protocol. Healthy controls were also instructed to wear the accelerometer during waking hours and to keep an activity diary. On Monday, the feasibility and comfort of the accelerometers was evaluated.
Analyses
Feasibility
Feasibility was tested using the parent reported complications of wearing the accelerometers and the quantity of the data obtained (% of subjects; the time the device collected data as a percentage of the intended measurement period (Saturday 0:00 to Sunday 23:59) and the time the device collected data as a percentage of the time the sensor was worn). Only patients in whom more than one sensor failed were excluded from the analyses. For the patients in which one sensor failed, only the available data are presented.
Face Validity
Face validity was assessed by visually correlating the videos with the obtained accelerometer data-graphs (correspondence of orientation and intensity for each sensor) during the validation protocol in each subject. Subsequently, the data from the diaries was correlated to the accelerometer data-graphs (correspondence of the intensity of the movements during the described activities). Only when the video images and described activities clearly did not correlate to the data-graphs, the data were excluded from the analyses. We assessed whether the percentage of dynamic activity and the total leg activity was lower in non-ambulatory children compared to ambulatory children. Finally, the functional abilities, assessed by the GMFM and the PEDI were correlated to the measurements.
Patients Versus Controls
We compared patients and their age- and sex-matched controls on each of the above-mentioned variables.
Subgroup Analyses
Based on the molecular finding in each patients, we’ve created three subgroups: (1) genetically confirmed primary mitochondrial disease; (2) genetically confirmed secondary mitochondrial disease (patients with a mutation in a non-mitochondrial gene with biochemically proven mitochondrial dysfunction, either with or without a proven link to mitochondrial processes) and (3) biochemically confirmed mitochondrial dysfunction.
Statistical Analyses
Because of the relatively small number of subjects included in our study, we used non-parametric tests to assess differences and correlations. We used a p-value of 0.05 for statistical significance; because of the exploratory character of this study, we did not use adjust critical p-values using the Bonferroni method. Missing data were not replaced. All analyses were performed using IBM’s SPSS statistics software packages, version 20.0.0.1. Correlation coefficients were interpreted in accordance with the guidelines provided at the BMJ website (http://www.bmj.com/about-bmj/resources-readers/publications/statistics-square-one/11-correlation-and-regression).
Ethics
This study was approved by the regional Medical Research Ethics Committee (MREC NL50560.091.14). In accordance with the Helsinki agreement, written informed consent was obtained from participant’s legal guardian and, where indicated, the participant.
Results
Study Population
Seventeen patients and 16 healthy age- and sex-matched controls were included in this study from February to May 2015. One healthy control withdrew his consent 1 day before the measurement would start and no other age- and sex-matched control was available for that weekend. The groups were comparable with respect to age, sex, BMI and sports- and highest education of parents, but – as expected – differed significantly with respect to height, weight, time spent at sports and the level of education of the child (Supplementary Table 1). There was a wide variability in the genetic, biochemical, clinical and functional abilities in the children with mitochondrial disease (Supplementary Table 2).
Feasibility
All participants, including patients with severe mental retardation, tolerated wearing the accelerometers for the duration of the measurement. The full study protocol was completed by 29 children (88% of total study population): 3 participants temporarily removed the sensors: 2 removed the top to ventilate after exercise and because of a party, 1 did not attach the chest-sensor on Sunday because of discomfort of the top and 1 boy lost his upper leg sensor during outdoor playing. Five sensors failed to record any data and the batteries of one sensor failed during the measurements (18% of all participants; 4% of all measurements). Due to these technical issues, 6% of the total measured time and 8% of the time the sensors were worn was missing in 6 participants (4 patients and 2 healthy controls). One healthy control had to be excluded from the analyses because he lost his upper leg sensor and his upper arm sensor failed to record any data. Most subjects wore their sensors from the moment they awoke to the moment they undressed for bed; three participants removed all sensors after dinner (averagely sensors were worn 94% of the woken time). The time the sensors were taken off because of swimming, showering or bathing was 1.9%. The time the sensors were not worn because of lack of understanding or lack of motivation was 8.7%.
Face Validity
For all patients and healthy controls, the movements (orientation, intensity) at the videos corresponded to the acceleration data that was visualized in graphs (Fig. 2). Dynamic activity (i.e. walking) and the activity of arms and legs was higher in ambulatory compared to non-ambulatory patients. For these analyses, we excluded a boy who was not able to walk but had excellent abilities to move (on his buttock), but not to walk, from these analyses since we could not define in which group he belonged. We found a (very) strong and significant correlation between the motor abilities as measured with the GMFM and the resting percentage (ρ = −0.82), the largest amount of activity of the leg during half an hour (ρ = 0.65) and the peak-activity of the lower arm (ρ = 0.67; all p < 0.0001). The score on the mobility domain of the PEDI (functional abilities) also correlated very strongly with the resting percentage (ρ = −0.87) and strongly with the largest amount of activity of the leg during half an hour (ρ = 0.70) and the peak-activity of the leg (ρ = 0.60; all p < 0.0001; Supplementary Table 3).
Fig. 2.

Raw data. (a) Raw data representing the orientation and activity of the sensors during the validation protocol (patient 17). (b, c) Raw data representing the physical activity of two representative patients during the validation protocol in the laboratory. (c, d) Raw data representing the physical activity of one day for a matched couple. The couple (couple 3) with the most striking difference was selected, the healthy control is in panel (c) and panel (d) represents the patient
Patients Versus Controls
We observed no difference in any of the activity variables between all children (including both healthy controls and patients) aged 12 years and older and children under 12 years of age (p = 0.005–0.815). We also observed no difference in any of the activity variables between patients under or above 12 years of age (p = 0.05–0.79). The same accounts for the difference between boys and girls (p = 0.05–1.00 and p = 0.005–1.00, respectively).
Almost all activity variables were significantly lower in patients with mitochondrial disorders compared to their age- and sex-matched controls (Table 1). We saw no difference in the quantity of arm activity between patients and controls. When comparing individual results, all but one patient had higher percentages of rest during the weekend, except for one girl matched to a healthy girl who had to study for her final exams during the whole weekend (couple 2 in Supplementary Fig. 1).
Table 1.
Average activity over the weekend for children with mitochondrial disease compared to age- and sex-matched controls (including subgroup analyses for genetically confirmed primary mitochondrial disease, genetically confirmed secondary mitochondrial disease and biochemically confirmed mitochondrial dysfunction)
| Patients (n = 17) | Healthy controls (n = 15) | Patients with genetically confirmed primary mitochondrial disease (n = 9) | Matched controls (n = 7) | Patients with genetically confirmed secondary mitochondrial disease (n = 4) | Matched controls (n = 4) | Patients with biochemically confirmed mitochondrial dysfunction (n = 8) | Matched controls (n = 7) | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Median | Range | Median | Range | p-value for difference between patients and healthy controls | Median | Range | Median | Range | p-value for difference between patients and matched controls | Median | Range | Median | Range | p-value for difference between patients and matched controls | p-value for difference between patients in this group and genetically confirmed primary mitochondrial disease patients | Median | Range | Median | Range | p-value for difference between patients and matched controls | p-value for difference between patients in this group and genetically confirmed primary mitochondrial disease patients | |
| Wear time (h/day) | 10 | (8–13) | 12 | (6–15) | 0.004 | 9 | (8–13) | 12 | (9–14) | 0.1 | 11 | (10–12) | 12 | (10–15) | 0.6 | 0.2 | 10 | (8–12) | 12 | (6–14) | 0.03 | 0.6 |
| Average % rest | 86 | (44–99) | 60 | (2–89) | <0.001 | 75 | (44–98) | 59 | (51–73) | 0.07 | 98 | (86–99) | 57 | (32–83) | 0.03 | 0.04 | 86 | (63–92) | 69 | (47–89) | 0.2 | 0.5 |
| Average % standing | 4 | (0–23) | 18 | (7–48) | 0.004 | 15 | (0–22) | 21 | (13–30) | 0.05 | 0 | (0–1) | 18 | (9–48) | 0.03 | 0.02 | 4 | (3–23) | 12 | (7–36) | 0.2 | 0.5 |
| Average % dynamic activity | 13 | (1–50) | 18 | (5–33) | 0.02 | 13 | (2–35) | 20 | (9–27) | 0.1 | 7 | (1–50) | 22 | (9–27) | 0.5 | 0.6 | 9 | (5–17) | 17 | (5–33) | 0.2 | 1.0 |
| Average counts upper leg (1,000 counts/h) | 9.4 | (3.1–29.6) | 18.0 | (7.0–27.2 | 0.008 | 10.9 | (5.1–29.6) | 15.0 | (10.2–22.0) | 0.2 | 4.7 | (3.1–17.4) | 17.1 | (11.7–26.8) | 0.1 | 0.2 | 11.7 | (7.3–15.0) | 15.8 | (7.0–27.1) | 0.5 | 1.0 |
| Average counts upper arm (1,000 counts/h) | 15.7 | (11.3–36.2) | 19.0 | (10.5–30.2) | 0.3 | 18.8 | (14.2–32.2) | 17.5 | (14.5–25.9) | 0.9 | 12.9 | (11.2–36.3) | 22.4 | (15.7–30.2) | 0.3 | 0.2 | 15.7 | (13.8–21.8) | 17.8 | (10.5–22.9) | 0.9 | 0.5 |
| Average counts lower arm (1,000 counts/h) | 21.5 | (16.1–41.8) | 27.1 | (15.3–37.1) | 0.3 | 25.3 | (18.5–40.3) | 26.1 | (18.5–34.7) | 1.0 | 20.1 | (16.1–41.8) | 31.3 | (23.0–37.1) | 0.3 | 0.5 | 20.5 | (17.8–30.3) | 23.9 | (14.0–29.8) | 0.9 | 0.4 |
| Average counts wheelchair (1,000 counts/h) | 1.3 | (0.7–1.4) | NA | NA | 1.1 | (0.8–1.3) | NA | 1.1 | (0.7–1.4) | NA | 1.4 | (1.3–1.5) | NA | |||||||||
| Maximal intensity upper leg (1,000 counts/min) | 1.0 | (0.5–2.6) | 1.9 | (0.9–2.6 | <0.001 | 1.1 | (0.7–2.6) | 1.8 | (1.2–2.4) | 0.1 | 0.8 | (0.5–1.0) | 1.8 | (1.1–2.4) | 0.03 | 0.2 | 1.2 | (0.9–1.6) | 2.3 | (0.9–2.6) | 0.1 | 1.0 |
| Maximal intensity upper arm (1,000 counts/min) | 1.0 | (0.8–1.9) | 1.6 | (0.9–2.1) | <0.001 | 1.0 | (0.8–1.9) | 1.7 | (1.1–1.9) | 0.02 | 0.9 | (0.8–1.2) | 1.4 | (1.1–1.9) | 0.06 | 0.4 | 1.1 | (0.8–1.5) | 1.6 | (0.9–2.1) | 0.2 | 0.9 |
| Maximal intensity lower arm (1,000 counts/min) | 1.2 | (0.9–2.2) | 1.9 | (1.0–2.5) | <0.001 | 1.1 | (1.0–2.2) | 1.9 | (1.3–2.5) | 0.03 | 1.1 | (1.0–1.4) | 1.8 | (1.5–2.4) | 0.03 | 0.5 | 1.4 | (0.9–1.8) | 1.9 | (1.0–2.5) | 0.3 | 0.5 |
| Largest AUC during 1/2 h upper leg (1,000 counts) | 10.4 | (6.4–37.3) | 26.8 | (11.4–66.2) | <0.001 | 18.9 | (8.8–37.3) | 27.5 | (16.3–30.1) | 0.2 | 7.2 | (6.4–17.3) | 30.3 | (14.7–41.8) | 0.06 | 0.04 | 12.5 | (9.9–21.6) | 24.0 | (11.4–66.2) | 0.1 | 0.8 |
| Largest AUC during ½ h upper arm (1,000 counts) | 15.8 | (1.1–27.3) | 25.3 | (11.7–368) | 0.003 | 15.7 | (14.0–27.3) | 26.2 | (16.3–30.1) | 0.05 | 12.9 | (11.1–25.1) | 25.5 | (22.9–36.8) | 0.1 | 0.2 | 17.9 | (12.0–23.0) | 20.7 | (11.0–30.7) | 0.8 | 0.7 |
| Largest AUC during 1/2 h lower arm (1,000 counts) | 21.2 | (16.0–34.8) | 30.8 | (16.7–43.4) | 0.004 | 21.9 | (18.9–34.8) | 30.8 | (19.3–40.0) | 0.1 | 18.7 | (16.0–29.3) | 32.3 | (27.1–43.4) | 0.06 | 0.3 | 25.6 | (16.7–28.5) | 25.2 | (16.7–40.6) | 0.5 | 0.8 |
A level of p = 0.05 was used for significance; significant values are indicated in bold
AUC area under the curve, min minutes
Subgroup Analyses
When comparing patients with genetically confirmed primary mitochondrial disease to their peers, we found a similar pattern of differences compared to all patients versus their matched controls. Only the maximal intensity of the upper- and lower arm and the largest AUC during half an hour for the upper arm reached significance in this small group. Patients with genetically confirmed secondary mitochondrial dysfunction differed significantly in their % of rest and standing, as well as their maximal intensity for the upper leg and lower arm. For patients with biochemically confirmed mitochondrial dysfunction, only the wear time differed significantly from their healthy peers, although the median peak activities were all (not significantly) lower. When comparing the latter two groups to the genetically confirmed primary mitochondrial disease patients, patients with genetically confirmed secondary mitochondrial disease (which are all non-ambulatory) are resting more and have a lower AUC during half an hour for the upper leg. Patients with biochemically confirmed mitochondrial dysfunction did not differ significantly from the patients genetically confirmed primary mitochondrial disease.
Not surprisingly, the leg activity of non-ambulatory patients was lower compared to ambulatory patients (Table 2). In fact, the activity of ambulatory patients did not deviate from healthy peers (p = 0.05–0.9), with the exception of the largest AUC in 30 min for the upper leg. The other peak intensity parameters did not reach significance, but showed substantially lower values compared to their peers. Non-ambulatory patients deviated from healthy controls – as expected – in all leg parameters and position parameters, but also in peak activity. The maximal intensity parameters for the arm were decreased to the largest extent and most significantly.
Table 2.
Average activity over the weekend for children with mitochondrial disease compared to age- and sex-matched controls (including subgroup analyses for ambulatory and non-ambulatory patients and patients with myopathy or encephalopathy. We excluded a boy who was not able to walk but had excellent abilities to move (on his buttock), but not to walk, from the ambulatory/non-ambulatory analyses since we could not define in which group he belonged)
| Patients (n = 17) | Healthy controls (n = 15) | Ambulatory patients (n = 9) | Non ambulatory patients (n = 7) | Myopathy (n = 7) | Enchephalomyopathy (n = 10) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Median | Range | Median | Range | p-value for difference between patients and healthy controls | Median | Range | p-value for difference between ambulatory patients and healthy controls | Median | Range | p-value for difference between non-ambulatory patients and healthy controls | p-value for difference between ambulatory and non-ambulatory patients | Median | Range | Median | Range | p-value for difference between patients with myopathy and patients with encephalomyopathy | |
| Wear time (h/day) | 10 | (8–13) | 12 | (6–15) | 0.004 | 12 | (9–14) | 0.04 | 11 | (9–12) | 0.2 | 0.5 | 11 | (8–13) | 10 | (8–12) | 0.3 |
| Average % rest | 86 | (44–99) | 60 | (2–89) | <0.001 | 73 | (44–90) | 0.2 | 98 | (86–99) | 0.003 | 0.006 | 77 | (63–99) | 89 | (44–98) | 0.6 |
| Average % standing | 4 | (0–23) | 18 | (7–48) | 0.004 | 16 | (4–23) | 0.6 | 1 | (0–3) | 0.001 | <0.001 | 10 | (0–23) | 3 | (0–22) | 0.8 |
| Average % dynamic activity | 13 | (1–50) | 18 | (5–33) | 0.02 | 14 | (3–34) | 0.2 | 5 | (1–50) | 0.3 | 0.09 | 13 | (1–50) | 9 | (5–35) | 0.4 |
| Average counts upper leg (1,000 counts/h) | 9.4 | (3.1–29.6) | 18.0 | (7.0–27.2 | 0.008 | 12.7 | (8.2–29.6) | 0.2 | 5.5 | (3.1–17.4) | 0.02 | 0.03 | 9.1 | (3.1–16.9) | 10.0 | (5.1–29.6) | 0.7 |
| Average counts upper arm (1,000 counts/h) | 15.7 | (11.3–36.2) | 19.0 | (10.5–30.2) | 0.3 | 18.9 | (14.2–32.2) | 0.9 | 14.3 | (11.3–36.2) | 0.5 | 0.1 | 16.5 | (11.3–25.2) | 15.5 | (13.2–36.2) | 0.6 |
| Average counts lower arm (1,000 counts/h) | 21.5 | (16.1–41.8) | 27.1 | (15.3–37.1) | 0.3 | 23.3 | (18.9–40.3) | 0.8 | 20.0 | (16.1–41.8) | 0.6 | 0.1 | 21.5 | (16.1–31.8) | 21.2 | (17.8–41.8) | 0.9 |
| Average counts wheelchair (1,000 counts/h) | 1.3 | (0.7–1.4) | NA | NA | NA | 0.8 | NA | 1.3 | (0.7–1.5) | NA | 0.6 | NA | 1.4 | 1.2 | (0.7–1.5) | 0.9 | |
| Maximal intensity upper leg (1,000 counts/min) | 1.0 | (0.5–2.6) | 1.9 | (0.9–2.6 | <0.001 | 1.3 | (0.7–2.6) | 0.09 | 0.9 | (0.5–1.0) | 0.006 | 0.03 | 0.9 | (0.5–1.7) | 1.0 | (0.9–2.6) | 0.6 |
| Maximal intensity upper arm (1,000 counts/min) | 1.0 | (0.8–1.9) | 1.6 | (0.9–2.1) | <0.001 | 1.1 | (0.8–1.9) | 0.08 | 0.9 | (0.8–1.2) | 0.008 | 0.06 | 1.0 | (0.9–1.4) | 1.0 | (0.8–1.9) | 0.8 |
| Maximal intensity lower arm (1,000 counts/min) | 1.2 | (0.9–2.2) | 1.9 | (1.0–2.5) | <0.001 | 1.4 | (1.0–2.2) | 0.09 | 1.1 | (0.9–1.4) | 0.009 | 0.09 | 1.2 | (1.0–1.8) | 1.2 | (0.9–2.2) | 0.9 |
| Largest AUC during 1/2 h upper leg (1,000 counts) | 10.4 | (6.4–37.3) | 26.8 | (11.4–66.2) | <0.001 | 20.2 | (8.9–37.2) | 0.02 | 9.9 | (6.4–17.3) | 0.006 | 0.03 | 9.5 | (6.4–37.3) | 13.9 | (7.4–30.7) | 0.4 |
| Largest AUC during 1/2 h upper arm (1,000 counts) | 15.8 | (1.1–27.3) | 25.3 | (11.7–368) | 0.003 | 17.9 | (14.0–27.3) | 0.07 | 14.6 | (11.1–25.1) | 0.03 | 0.09 | 15.5 | (11.1–27.3) | 17.0 | (11.9–25.1) | 0.5 |
| Largest AUC during 1/2 h lower arm (1,000 counts) | 21.2 | (16.0–34.8) | 30.8 | (16.7–43.4) | 0.004 | 25.6 | (18.5–34.8) | 0.05 | 18.2 | (16.0–29.3) | 0.2 | 0.04 | 20.4 | (16.0–34.8) | 22.9 | (16.7–29.9) | 0.5 |
A level of p = 0.05 was used for significance; significant values are indicated in bold
AUC area under the curve, min minutes
Patients with myopathy and encephalopathy were comparable with respect to all activity parameters (p = 0.3–0.9).
Recommendations
Based on the difference (both significance and the magnitude of the difference) between patients and healthy subjects, we made recommendations on which variables to use in future accelerometry studies in children with mitochondrial disease (Table 3). We recommend the use of maximal intensity and largest AUC in 30 min variables. For non-ambulatory patients, arm activity variables should be used instead of leg variables.
Table 3.
Recommendations for future accelerometry studies in children with mitochondrial disease
| Ambulatory patients | Non-ambulatory patients | |
|---|---|---|
| The percentage of the day spent resting or in dynamic activity | NR | NR |
| The level of activity of legs | NR | NR |
| The level of activity of arms | NR | NR |
| Maximal intensity of legs | H | NR |
| Maximal intensity of arms | R | R |
| Largest AUC in 30 min of legs | H | NR |
| Largest AUC in 30 min of arms | R | R |
AUC area under the curve, H highly recommended, NR not recommended, R recommended
Discussion
In this exploratory study, we aimed to select the domains of daily physical activity, measured by 3D accelerometry, that could be sensitive end points in future clinical trials. Although we experienced technical difficulties with the hardware in 18% of the subjects (4% of the measurements), we showed that measuring physical activity in a home-situation with 3D accelerometers was feasible and had good face validity in all seventeen children with mitochondrial disease. By comparing the children to an age- and sex-matched healthy peer who was measured within the same weekend, we selected domains of movement that deviated from normal in children with mitochondrial disorders (Table 3).
The percentages of rest, standing and dynamic activity were significantly different between patients and their healthy peers. However, since we observed no differences in these percentages between matched controls and non-ambulatory patients (who are sitting in a wheelchair), we don’t recommend the use domains of physical activity for future studies. Arm activity levels (amount of activity) were comparable between patients and healthy controls. Our data could not confirm that this was due to compensatory use of arms in non-ambulatory children or due to high levels of unpurposeful arm activity in children with movement disorders such as ataxia. We advice not to include arm activity level as a domain of physical activity in children with mitochondrial disorders. The peak activity and largest area under the curve for both arms and legs showed the largest magnitude of difference and was significantly different between patients and controls for most parameters, also when only the nine patients with genetically confirmed primary mitochondrial disease were included. For ambulatory patients, the largest magnitude of change is observed in the peak activity of the upper leg, although a significant difference was observed only for the largest AUC in half an hour for leg activity. Both variables have a large spread across both healthy controls and patients. For non-ambulatory patients, the peak activity and the largest AUC of the arms were decreased to the same extent, but this decrease was most significant for the peak activity. All peak intensity parameters correlated moderately to gross motor function.
For this study, we selected the MOX-accelerometer, a device with opportunities to design a tailored data-analysis. It is known that measuring for longer periods decreased variability substantially in patients with cerebral palsy (CP) (Mitchell et al. 2015). However, we deliberately chose to measure physical activity only during the days in which the child was performing physical activity to their own desire and was not challenged with mental exercise (i.e. the weekend versus school week), since the aim of this study was to select domains of physical activity that deviated from the normal population and not to reliably quantify the amount of daily movement. We used 4–5 accelerometers instead of one, to be able to quantify movement patterns of the upper- and lower arm, the chest and the upper leg. This allowed us to draw conclusions about the orientation and the intensity of movements of these body parts, but is obviously less desirable from a patient perspective (Kirby et al. 2012).
Previously, Martens et al. (2014) showed that children with mitochondrial disease have a lower activity level compared to healthy controls and less time spent in moderate to vigorous activities, using a commercially available physical activity device. The resting percentage in the study by Martens et al. was lower compared to what we found, even in ambulatory patients only. This could be due to selection bias: Martens et al. studied ambulatory children without severe cognitive impairment and none of them had a genetically confirmed mitochondrial disease. Adults with mitochondrial disease also show lower habitual physical activity compared to matched controls (Apabhai et al. 2011). Interestingly, also the number of breaks in sedentary activity was reduced and longer periods of rest were observed. The resting percentage found in our study was comparable to the resting percentage in ambulatory normally weighted children with Down’s-, William’s- and Prader-Willi syndrome (Nordstrom et al. 2013) and patients with CP, when both patient groups were stratified based on their walking abilities (Gorter et al. 2012). Five young ambulatory boys (4–6 years) with Duchenne Muscular Dystrophy (DMD) (Jeannet et al. 2011), who were significantly younger than our study subjects, spent much less time resting, but had comparable levels of dynamic activity when compared to our ambulatory patients. In contrast to most previous studies, we also included non-ambulatory patients in our study. Measuring daily activity in these patients is challenging, because accelerations elicited by the wheelchair are also measured by the other sensors. We found that the wheelchair accounted for about one fourth of the leg activity level in non-ambulatory children.
This study was performed on a relatively small and clinically heterogeneous group of patients but indicates abnormal domains of physical activity in patients with both high and limited abilities. Patients are compared to age- and sex-matched controls, who were measured in the same weekend to correct for weather conditions. Weaknesses include the substantial level of technical failures and the relatively short measurement time, which were caused by the use of the studied accelerometers and are not likely to play a major role in commercially available accelerometers. Other methodological issues inherent to measuring activity with accelerometers include: the lack of measurement of stable, sustained body positions and that the current data analysis is not able to differentiate between meaningful movements and aberrant movements, such as myoclonus or ataxia. Another weakness is that we included a heterogeneous population and that not all patients have a confirmed genetic mitochondrial disease: out of 17 patients, only 9 patients have a genetically confirmed “primary” mitochondrial disease. During analysis of this subgroup, we confirmed that also if only these patients were analysed, peak activity parameters had the highest magnitude and significance of difference. The activity parameters did not differ between patients with genetically confirmed primary mitochondrial disease and patients with biochemically confirmed mitochondrial dysfunction. Patients with genetically confirmed secondary mitochondrial disease did differ on three parameters, but these findings need to be interpreted carefully since all patients with genetically confirmed secondary mitochondrial disease were non-ambulatory. Since most patients were not able to reliably complete questionnaires regarding their level of fatigue, these were not included in this study.
This study provides insight into the domains of physical activity that are abnormal in children with mitochondrial disease. Since this approach is data-driven only, we are currently performing a more patient-centred approach in which the clinically relevant domains of movement are explored in close dialogue with patients and their families. Combining the results of both studies, we aim to select a simple, commercially available activity monitor, that provides insight in the clinically relevant aspects of daily physical activity of children with mitochondrial disease. This accelerometer should be validated in a larger and more homogeneous study population, with longer measurement period and standardization of family activities during the measurements, to provide representative activity patterns and reduce random variability. Validation should include at least the following aspects: acceptability, feasibility, test-retest reliability, including the influence of weather conditions and seasons, validity, especially in wheelchair bound patients, and responsivity.
In conclusion, accelerometry is a promising method to quantify the highly burdensome fatigue in children with mitochondrial disease in daily practice as well as natural history studies. The method is safe, feasible and well tolerated. Whether accelerometry is reliable and sensitive enough to detect changes during clinical trials needs to be studied in more detail.
Electronic Supplementary Material
Characteristics of patients and their age- and sex-matched controls. A level of p = 0.05 was used for significance; significant values are indicated in bold; BMI Body Mass Index (XLSX 13 kb)
Patient characteristics. Genetic, biochemical and clinical details for each patient. Psychomotor retardation is defined: IQ <50 = severe; IQ 50–70 = mild and IQ >70 = normal. 1 = genetically confirmed primary mitochondrial disease; 2 = genetically confirmed secondary mitochondrial disease; 3 = biochemically confirmed mitochondrial dysfunction. # siblings, A ankle, CI complex I, F female, GMFM gross motor function measure, L leukocytes, M male, Mu muscle, ND not done, PEDI pediatric evaluation of disability inventory, PMR psychomotor retardation, UEC urinary epithelial cells (XLSX 14 kb)
Correlations between the GMFM and the PEDI and activity parameters measured with the accelerometer. A level of p = 0.001 was used for significance; significant values are indicated in bold; AUC area under the curve, GMFM gross motor function measure, PEDI pediatric evaluation of disability inventory (XLSX 9 kb)
Percentage of rest during the weekend for patients versus matched controls. The dark bars represent the patients and the light bars represent their matched controls. Group 1 = genetically confirmed primary mitochondrial disease; group 2 = genetically confirmed secondary mitochondrial disease; group 3 = biochemically confirmed mitochondrial dysfunction (PNG 10 kb)
Take Home Message
Children with mitochondrial disorders have lower peak activity rather than a lower amount activity compared to healthy peers.
Details of the Contributions of Individual Authors
| Saskia Koene | Planning, supervising and reporting of study |
| Ilse Dirks | Execution and planning of study, critical review manuscript |
| Esmee van Mierlo | Execution of study, critical review manuscript |
| Pascal de Vries | Analysis of data, critical review manuscript |
| Anjo J. W. M. Janssen | Performance physiotherapeutic tests, critical review manuscript |
| Jan A. M. Smeitink | Supervision of study, critical review manuscript |
| Arjen Bergsma | Data analysis, critical review manuscript |
| Hans Essers | Data analysis, critical review manuscript |
| Kenneth Meijer | Critical review manuscript |
| Imelda J. M. de Groot | Supervision of planning of study, critical review manuscript |
Name of One Author Who Serves as Guarantor
Saskia Koene.
A Competing Interest Statement
Imelda J. M. de Groot is consultant for Biomarin, PTC, Treat-NMD. She has collaborations in scientific projects on the development of technical aids (arm and hand exoskeletons) with Focal Meditech, Hankamp, BAAT Medical products, Summit, Laevo, Xsens, Maastricht Instruments. She has no financial interest in these firms. Jan Smeitink is the founding CEO of Khondrion BV.
Details of Funding
We thank all patients and parents participating in this study. This project was sponsored by Stofwisselkracht, Zeldzame Ziekten Fonds and ZonMW (The Netherlands Organization for Health Research and Development). The author(s) confirm(s) independence from the sponsors; the content of the article has not been influenced by the sponsors.
Details of Ethics Approval/A Patient Consent Statement
This study was approved by the regional Medical Research Ethics Committee (MREC NL50560.091.14). In accordance with the Helsinki agreement, written informed consent was obtained from participant’s legal guardian and, where indicated, the participant.
Approval from the Institutional Committee for Care and Use of Laboratory Animals
Not indicated.
Footnotes
Ilse Dirks and Esmee van Mierlo contributed equally to this work.
Electronic supplementary material
The online version of this chapter (doi:10.1007/8904_2016_35) contains supplementary material, which is available to authorized users.
Contributor Information
Saskia Koene, Email: Saskia.koene@radboudumc.nl.
Collaborators: Matthias R. Baumgartner, Marc Patterson, Shamima Rahman, Verena Peters, Eva Morava, and Johannes Zschocke
References
- Abel MF, Damiano DL, Blanco JS, et al. Relationships among musculoskeletal impairments and functional health status in ambulatory cerebral palsy. J Pediatr Orthop. 2003;23(4):535–541. [PubMed] [Google Scholar]
- Apabhai S, Gorman GS, Sutton L, et al. Habitual physical activity in mitochondrial disease. PLoS One. 2011;6(7):e22294. doi: 10.1371/journal.pone.0022294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beenakker EA, Maurits NM, Fock JM, Brouwer OF, van der Hoeven JH. Functional ability and muscle force in healthy children and ambulant Duchenne muscular dystrophy patients. Eur J Paediatr Neurol. 2005;9(6):387–393. doi: 10.1016/j.ejpn.2005.06.004. [DOI] [PubMed] [Google Scholar]
- Bjornson KF. Physical activity monitoring in children and youths. Pediatr Phys Ther. 2005;17(1):37–45. doi: 10.1097/01.PEP.0000154107.30252.FE. [DOI] [PubMed] [Google Scholar]
- Cain KL, Sallis JF, Conway TL, Van Dyck D, Calhoon L. Using accelerometers in youth physical activity studies: a review of methods. J Phys Act Health. 2013;10(3):437–450. doi: 10.1123/jpah.10.3.437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capio CM, Sit CH, Abernethy B. Physical activity measurement using MTI (actigraph) among children with cerebral palsy. Arch Phys Med Rehabil. 2010;91(8):1283–1290. doi: 10.1016/j.apmr.2010.04.026. [DOI] [PubMed] [Google Scholar]
- Caspersen CJ, Powell KE, Christenson GM. Physical activity, exercise, and physical fitness: definitions and distinctions for health-related research. Public Health Rep. 1985;100(2):126–131. [PMC free article] [PubMed] [Google Scholar]
- Clanchy KM, Tweedy SM, Boyd RN, Trost SG. Validity of accelerometry in ambulatory children and adolescents with cerebral palsy. Eur J Appl Physiol. 2011;111(12):2951–2959. doi: 10.1007/s00421-011-1915-2. [DOI] [PubMed] [Google Scholar]
- Clanchy KM, Tweedy SM, Boyd R. Measurement of habitual physical activity performance in adolescents with cerebral palsy: a systematic review. Dev Med Child Neurol. 2011;53(6):499–505. doi: 10.1111/j.1469-8749.2010.03910.x. [DOI] [PubMed] [Google Scholar]
- Custers JW, Wassenberg-Severijnen JE, Van der Net J, Vermeer A, Hart HT, Helders PJ. Dutch adaptation and content validity of the pediatric evaluation of disability inventory (PEDI) Disabil Rehabil. 2002;24(5):250–258. doi: 10.1080/09638280110076036. [DOI] [PubMed] [Google Scholar]
- Gordijn M, Cremers EM, Kaspers GJ, Gemke RJ. Fatigue in children: reliability and validity of the Dutch PedsQL multidimensional fatigue scale. Q Life Res. 2011;20(7):1103–1108. doi: 10.1007/s11136-010-9836-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorter JW, Noorduyn SG, Obeid J, Timmons BW. Accelerometry: a feasible method to quantify physical activity in ambulatory and nonambulatory adolescents with cerebral palsy. Int J Pediatr. 2012;2012:329284. doi: 10.1155/2012/329284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeannet PY, Aminian K, Bloetzer C, Najafi B, Paraschiv-Ionescu A. Continuous monitoring and quantification of multiple parameters of daily physical activity in ambulatory Duchenne muscular dystrophy patients. Eur J Paediatr Neurol. 2011;15(1):40–47. doi: 10.1016/j.ejpn.2010.07.002. [DOI] [PubMed] [Google Scholar]
- Kirby J, Tibbins C, Callens C, et al. Young people’s views on accelerometer use in physical activity research: findings from a user involvement investigation. ISRN Obes. 2012;2012:948504. doi: 10.5402/2012/948504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koene S, Jansen M, Verhaak CM, De Vrueh RL, De Groot IJ, Smeitink JA. Towards the harmonization of outcome measures in children with mitochondrial disorders. Dev Med Child Neurol. 2013;55:698. doi: 10.1111/dmcn.12119. [DOI] [PubMed] [Google Scholar]
- Koene S, Wortmann SB, de Vries MC, et al. Developing outcome measures for pediatric mitochondrial disorders: which complaints and limitations are most burdensome to patients and their parents? Mitochondrion. 2013;13(1):15–24. doi: 10.1016/j.mito.2012.11.002. [DOI] [PubMed] [Google Scholar]
- LeMura LM, von Duvillard SP, Cohen SL, et al. Treadmill and cycle ergometry testing in 5- to 6-year-old children. Eur J Appl Physiol. 2001;85(5):472–478. doi: 10.1007/s004210100461. [DOI] [PubMed] [Google Scholar]
- Martens AM, Gorter H, Wassink RG, Rietman H. Physical activity of children with a mitochondrial disease compared to children who are healthy. Pediatr Phys Ther. 2014;26(1):19–26. doi: 10.1097/PEP.0000000000000016. [DOI] [PubMed] [Google Scholar]
- McDonald CM, Widman L, Abresch RT, Walsh SA, Walsh DD. Utility of a step activity monitor for the measurement of daily ambulatory activity in children. Arch Phys Med Rehabil. 2005;86(4):793–801. doi: 10.1016/j.apmr.2004.10.011. [DOI] [PubMed] [Google Scholar]
- Meijer K, Annegarn J, Lima Passos V, et al. Characteristics of daily arm activities in patients with COPD. Eur Respir J. 2014;43(6):1631–1641. doi: 10.1183/09031936.00082513. [DOI] [PubMed] [Google Scholar]
- Mitchell LE, Ziviani J, Boyd RN. Variability in measuring physical activity in children with cerebral palsy. Med Sci Sports Exercise. 2015;47(1):194–200. doi: 10.1249/MSS.0000000000000374. [DOI] [PubMed] [Google Scholar]
- Nordstrom M, Hansen BH, Paus B, Kolset SO. Accelerometer-determined physical activity and walking capacity in persons with down syndrome, Williams syndrome and Prader-Willi syndrome. Res Dev Disabil. 2013;34(12):4395–4403. doi: 10.1016/j.ridd.2013.09.021. [DOI] [PubMed] [Google Scholar]
- Parreira SL, Resende MB, Zanoteli E, Carvalho MS, Marie SK, Reed UC. Comparison of motor strength and function in patients with Duchenne muscular dystrophy with or without steroid therapy. Arq Neuropsiquiatr. 2010;68(5):683–688. doi: 10.1590/S0004-282X2010000500002. [DOI] [PubMed] [Google Scholar]
- Pfeffer G, Horvath R, Klopstock T, et al. New treatments for mitochondrial disease-no time to drop our standards. Nat Rev Neurol. 2013;9(8):474–481. doi: 10.1038/nrneurol.2013.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodenburg RJ. Biochemical diagnosis of mitochondrial disorders. J Inherited Metabol Dis. 2011;34(2):283–292. doi: 10.1007/s10545-010-9081-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanink CM, Vercoulen JH, Bleijenberg G, Fennis JF, Galama JM, van der Meer JW. Chronic fatigue syndrome: a clinical and laboratory study with a well matched control group. J Int Med. 1995;237(5):499–506. doi: 10.1111/j.1365-2796.1995.tb00876.x. [DOI] [PubMed] [Google Scholar]
- Vos-Vromans DC, Ketelaar M, Gorter JW. Responsiveness of evaluative measures for children with cerebral palsy: the gross motor function measure and the pediatric evaluation of disability inventory. Disabil Rehabil. 2005;27(20):1245–1252. doi: 10.1080/09638280500076178. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Characteristics of patients and their age- and sex-matched controls. A level of p = 0.05 was used for significance; significant values are indicated in bold; BMI Body Mass Index (XLSX 13 kb)
Patient characteristics. Genetic, biochemical and clinical details for each patient. Psychomotor retardation is defined: IQ <50 = severe; IQ 50–70 = mild and IQ >70 = normal. 1 = genetically confirmed primary mitochondrial disease; 2 = genetically confirmed secondary mitochondrial disease; 3 = biochemically confirmed mitochondrial dysfunction. # siblings, A ankle, CI complex I, F female, GMFM gross motor function measure, L leukocytes, M male, Mu muscle, ND not done, PEDI pediatric evaluation of disability inventory, PMR psychomotor retardation, UEC urinary epithelial cells (XLSX 14 kb)
Correlations between the GMFM and the PEDI and activity parameters measured with the accelerometer. A level of p = 0.001 was used for significance; significant values are indicated in bold; AUC area under the curve, GMFM gross motor function measure, PEDI pediatric evaluation of disability inventory (XLSX 9 kb)
Percentage of rest during the weekend for patients versus matched controls. The dark bars represent the patients and the light bars represent their matched controls. Group 1 = genetically confirmed primary mitochondrial disease; group 2 = genetically confirmed secondary mitochondrial disease; group 3 = biochemically confirmed mitochondrial dysfunction (PNG 10 kb)


