Abstract
Gamma-hydroxybutyrate (GHB) is a drug of abuse, an approved therapeutic for narcolepsy, an agent employed for facilitation of sexual assault, as well as a biomarker of succinic semialdehyde dehydrogenase deficiency (SSADHD). Our laboratory seeks to identify surrogate biomarkers in SSADHD that can shed light on the developmental course of this neurometabolic disease. Since GHB may be quantified in hair as a potential surrogate to identify victims of drug-related assault, we have opted to examine its level in SSADHD. We quantified GHB in hair derived from ten patients with SSADHD, and documented a significant negative age correlation. These findings are consistent with recent results in patient biological fluids, including plasma and red blood cells. These findings may provide additional insight into the developmental course of SSADHD (Jansen et al., J Inherit Metab Dis 39:795–800, 2016).
Keywords: GABA metabolism, Gamma-hydroxybutyrate (GHB), Hair analysis, Succinic semialdehyde dehydrogenase (SSADH), Succinic semialdehyde dehydrogenase deficiency (SSADHD), Tandem mass spectrometry
Introduction
Succinic semialdehyde dehydrogenase (SSADH) deficiency (SSADHD), a rare disorder of GABA metabolism, manifests with accumulation of both gamma-aminobutyrate (GABA) and gamma-hydroxybutyrate (GHB) in patient biological fluids (Malaspina et al. 2016). While the neurochemistry of GABA is well known (Hillmer et al. 2015), the pharmacology of GHB remains unclear. GHB, an endogenous intermediate in the central nervous system present at ~1% of parent GABA, is neuromodulatory in its effects on dopamine release and uptake (Maitre et al. 2016). Additionally, GHB is used therapeutically for the treatment of narcolepsy, and employed illicitly as a drug of abuse and agent in the facilitation of sexual assault (Malaspina et al. 2016). Because of its short terminal plasma half-life (~0.5 h) (Brenneisen et al. 2004), forensic methods have been developed to quantify GHB in non-physiological fluids, including hair (Jagerdeo et al. 2015).
The natural history of SSADHD remains undefined, and a systematic developmental analysis of patients with age is lacking. In an effort to explore metabolic developmental characteristics, we recently quantified GABA and GHB in plasma and red blood cell (RBC) lysates obtained from a cohort of 18 patients (age range 5–41 years; median 8 years) and found that both compounds negatively correlated with age (Jansen et al. 2016). In that study, plasma and RBC GHB levels reached a nadir and approximate steady-state by 10 years of age, whereas plasma GABA achieved an approximate steady state level at 30–40 years of age. These biomarker interactions shed light on additional GABA- and GHB-ergic neurotransmission imbalances potentially correlated with the onset of adolescent/adulthood neuropsychiatric morbidity and epilepsy. Here, we extend these studies by examining the levels of GHB in hair derived from a cohort of SSADHD patients, with the goal of investigating the potential of this method for long-term monitoring of GHB levels in SSADHD patients.
Patient Samples and Methodological Approach
Testing for drugs in hair is an established methodology complementing other methodologies in clinical and forensic toxicology (Cooper et al. 2012). Hair represents a durable sample, which is less affected by other contaminants (as in the case of urine and blood). Moreover, hair can provide an overview of drug exposure (e.g., narcotics, benzodiazepines) over extended periods depending upon the length of the sample analyzed. Hair samples contain keratinized cells composed of three distinct layers, including the medulla (core, or soft keratin), the cortex (composed of thick and hard keratin), and the cuticle (surface, composed also of hard keratin which is thinner than the cortex). Drugs of abuse (including GHB) are believed to disperse throughout all these regions, deposited from blood capillaries located at the root bulb (Cooper et al. 2012). Our methodology closely follows the guidelines of the Society of Hair Testing (SoHT) established for evaluation of drugs of abuse in hair. They include appropriate washing of collected samples with both aqueous and organic solvents, pulverization of samples prior to extraction, and analysis of an extract of each segment and washed fraction (Cooper et al. 2012). For GHB quantitation, the method of Wang et al. (2016) was used with multiple reaction monitoring (MRM) and quantification of the m/z 85 fragment from parent ion m/z 103, using 2H6-GHB as internal standard. Hair samples were pulverized into a fine powder in extraction media containing solvent and buffer (Wang et al. 2016) at 37°C for 1.5 h. The limit of quantification (LOQ) for GHB was 0.32 ng/mg hair, with linearity of the assay to 50 ng/mg. Extraction recoveries were 62–92%, and the accuracy 90–108%. Relative standard deviations (%) obtained from daily controls for GHB were 9.1–11.3%. Quantitation of the glucuronide of GHB followed the same methodology (Wang et al. 2016).
Hair specimens from patients with genetically confirmed SSADHD were obtained with informed consent (WSU IRB #14100; Gibson, PI). Sample collection kits were sent to all participants following consent. Patient demographics included 10 patients, age range 3–36 years (median age, 13 years; 9 males), representing approximately 5% of published patients (Malaspina et al. 2016). Race as well as hair color can influence the concentration of GHB in hair (Goullé et al. 2003). Seven patients resided in the USA, presumed to be of N. European descent. The three remaining patients were from Germany, Greece, and Uruguay, respectively. All had brown hair, with the exception of one patient with blond hair. Hair was clipped from the back upper area of the skull (vertex posterior region), where hair growth is considered most regular. Hair shafts of 1 cm length were gathered by the individual collecting the specimen (with gloves; approximately 60 hairs), and clipped as close to the scalp as possible. Three samples were collected, placed in individual foil, and the foil crimped. Samples were shipped and stored at room temperature until analysis. Hair samples were processed into either 0.5 or 1 cm segments and washed with isopropanol and water several times before analysis.
Control samples were obtained from 10 controls (8 Caucasian, 2 Chinese), and hair color ranging from blond to black with ages from 9 to 44 years (median age 29 y.o.; 9 females). The GHB range was <0.32–1.00 ng/mg hair in controls, with 0.32 ng/mg representing LOQ. Other investigators have reported higher values, including Goullé et al. (2003), 0.32–1.86 ng/mg, n = 61; Bertol et al. (2015), 0–5.09 ng/mg, n = 30; and Shi et al. (2016), 0.28–4.91 ng/mg, n = 66. Statistical analysis of GHB concentrations in hair employed the Spearman rank order correlation for non-parametric data, with a post-hoc Bonferroni analysis employing a standard two-way t-test. Significance was set at the 95th centile. Data analysis employed the GraphPad 6.0 program (San Diego, CA).
Results
GHB levels in hair samples of patients are shown in Fig. 1, and a representative ion chromatogram of GHB analysis in Fig. 2. The left side of Fig. 1 depicts all data for patients with all hair segments (either in 0.5 or 1 cm segments). The four graphs of Fig. 1 on the right depict the same data stratified for cm 1–4 of hair segments as a function of patient age. The concentrations of GHB in hair reached a level within the control range (<0.32–1.00 ng/mg) at approximately age 12–13 years, with elevated levels observed in patients below this age (3–7 years). When stratified for age with the first cm of hair, there was a highly significant negative correlation as a function of age. This correlation was maintained in cm 2, 3 and 4 of hair samples. It was not as strong but still significant. For cm 5, 6 and 7, correlations failed to achieve significance but the sample number was low due to short hair for several patients. Additionally, the levels of GHB in the most distant samples measured from the hair root (cm 5–7) might reflect wash out due to personal hygiene. Only younger patients showed hair GHB concentrations significantly above the control range; conversely, older patients demonstrated hair GHB levels within the control range of up to 1.0 ng/mg in all hair segments. There was no significant GHB vs age correlation in the control group (Fig. 3).
Fig. 1.
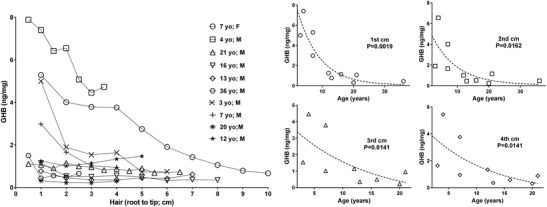
(Left) GHB concentrations in all hair segments measured from ten patients with SSADHD. Age and gender of patients are shown in the legend. (Right) Stratification of GHB content in hair segments in cm 1, 2, 3, and 4 for all patients from whom samples were available. Each data point for a patient hair sample represents a unique measurement of GHB content within that single 0.5–1.0 cm hair segment derived from the entire length of the hair sample (depicted as analysis from root to tip). Statistical analyses employed the Spearman rank order correlation, with significance set at the 95th centile
Fig. 2.
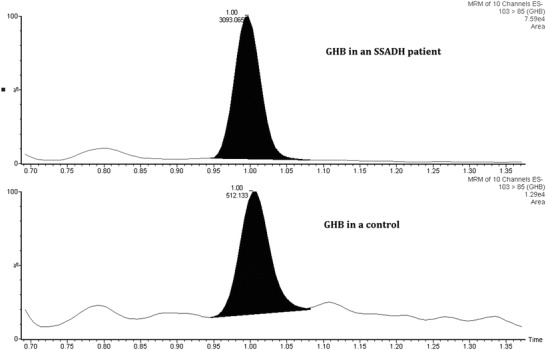
Multiple reaction monitoring (MRM) of GHB in a representative hair sample from a patient with SSADHD and a control. The transition from m/z 103 > 85 was monitored with 2H6-GHB as internal standard. This obviated interference from other potential contaminants, including other butyric acid compounds, which also elute at different retention times than GHB (Wang et al. 2016). The GHB level in the patient’s hair sample was approximately 5.9-fold increased in comparison to control based upon peak area
Fig. 3.
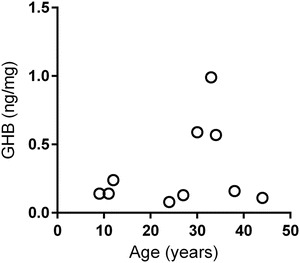
Total hair GHB content for controls. The concentrations were calculated by standard addition method for each individual (age-dependent correlation was non-significant)
An explanation for the longitudinal decrease of GHB with length of hair is not readily available, although this implies that transport of GHB from blood to hair is not reversible. Several other factors could contribute to this phenomena, including diet and drug interactions, but direct studies to address this have not been presented. The analysis of the glucuronide of GHB in hair was also measured for the first seven patients, since the glucuronide of GHB represents its main metabolite (Ainslie et al. 2016). For all segments in these patients, the concentrations of GHB-glucuronide were below the LOQ or within the levels observed in controls (not shown) (Wang et al. 2016).
Discussion
These are the first data on GHB concentrations in the hair of patients with SSADHD. These findings are consistent with our recent study of GHB in plasma and red-blood cell lysates derived from patients with SSADHD. They showed similar age-dependent negative correlations that reached a steady-state at approximately 10 years of age. In the current study, patients in the median age-range of the cohort, ~12–13 years, showed hair GHB values within the control range (<1 ng/mg hair). Whether hair represents a better surrogate of GHB content in the brain than blood or urine remains to be determined (Staeheli et al. 2016). To answer this question, a longitudinal analysis of brain GHB in patients (either employing cerebrospinal fluid, or methods to quantify GHB in vivo using edited magnetic resonance spectroscopy) will be needed. Nonetheless, surrogate studies to date (hair, plasma, red blood cell GHB content) all point to an age-dependent lowering of GHB in brain in SSADHD (Jansen et al. 2016). It is important to point out that no negative correlation of GHB vs. age was observed in unaffected controls. Future studies will thus be needed to confirm the diagnostic value of GHB concentration in hair from SSADHD patients, as well as to explain the significance of elevated hair GHB concentration in younger SSADHD patients.
Direct comparisons of the level of GHB and GABA in cerebrospinal fluid (CSF) of SSADHD patients with hair GHB content are somewhat challenging based upon the matrices involved. CSF GHB in four patients with SSADHD were 116–1,110 nmol/ml (control range, 0–2.6, n = 10) and the values for total GABA in these same patients were 13.6–22.4 nmol/ml (control range, 4.7–11.8, n = 10) (Gibson et al. 1995). The corresponding highest GHB concentrations measured in the hair of the 4 youngest patients with SSADHD in our study (Fig. 1) was approximately 3–8 ng/mg (0.03–0.08 nmol/mg). Nonetheless, blood or CSF levels of GHB cannot be directly correlated to hair concentration in the present study. To achieve this, we will need controlled studies of GHB intake and GHB hair measurement (Busardò et al. 2016).
As mentioned previously, the content of GHB in hair can be dependent upon race and hair color. All patients in our cohort were Caucasian and the hair color for 9 of 10 patients was brown, but for one 3 years old patient the hair color was blond. This may provide an explanation for the observation that the GHB hair content of this 3-year-old patient was less elevated than we predicted (Fig. 1) (Goullé et al. 2003). The phenotype of SSADHD routinely includes neuropsychiatric morbidity from adolescence to adulthood, including attention deficit-hyperactivity, oppositional defiant and obsessive compulsive disorders (Malaspina et al. 2016), representing significant disease morbidity. It is tempting to speculate that the nadir of hair GHB levels we observed (~12–13 years of age) may correlate with altered GABA levels and the onset of neuropsychiatric morbidity in SSADHD (Parviz et al. 2014). Disruption of GABA-ergic neurotransmission has been confirmed in SSADHD (Pearl et al. 2009; Reis et al. 2012; Buzzi et al. 2006), using both mouse models and patients. High levels of GHB and GABA during ontogeny almost certainly offsets the balance of GABA-ergic/glutamatergic neurotransmission (Jansen et al. 2008; Vogel et al. 2016; Talaei et al. 2016). High levels of GHB may impact this balance both through GHB-ergic and GABAB-ergic and GABAA-ergic effects (Absalom et al. 2012).
Decreasing GHB levels with age will likely reset the homeostatic balance between inhibitory and excitatory neurotransmission, a process we plan to address in a more comprehensive longitudinal evaluation of SSADHD. A major goal in such a study will be to identify biomarkers that reflect chronic exposure to high GHB levels, and correlate with the clinical evolution of the disease. Our data suggest that hair GHB concentration which declines with age like plasma and RBC GHB levels, and is not subject to fast metabolic clearance, may be a suitable biomarker in a SSADHD natural history study. The data also suggest that hair GHB measurement will be useful as a SSADHD diagnostic tool. However, the present findings need to be confirmed in studies with SSADHD patients against strictly age-matched controls.
Acknowledgements
The authors gratefully acknowledge the patients and families who contributed hair samples for this study. The ongoing support of the SSADH association (www.ssadh.net) and Speragen, Inc., for longitudinal analyses of patient biological samples, is gratefully acknowledged.
Author Contributions
JSS, WX, SPD: acquisition and analysis of data
PLP, JBR, KRV, GRA: conception and design of study; drafting of manuscript
KMG: conception and design of study; data analysis; manuscript drafting
Conflicts of Interest
The authors cumulatively declare they have no direct or perceived conflicts of interest.
Guarantor
K. M. Gibson.
Details of Funding
Described in acknowledgements.
Ethics Approval
Approved by WSU IRB (Institutional Review Board for Human Studies).
Contributor Information
K. M. Gibson, Email: mike.gibson@wsu.edu
Collaborators: Matthias R. Baumgartner, Marc Patterson, Shamima Rahman, Verena Peters, Eva Morava, and Johannes Zschocke
References
- Absalom N, Eghorn LF, Villumsen IS, Karim N, Bay T, Olsen JV, Knudsen GM, Bräuner-Osborne H, Frølund B, Clausen RP, Chebib M, Wellendorph P. α4βδ GABA(A) receptors are high-affinity targets for γ-hydroxybutyric acid (GHB) Proc Natl Acad Sci U S A. 2012;109:13404–13409. doi: 10.1073/pnas.1204376109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ainslie GR, Gibson KM, Vogel KR. A pharmacokinetic evaluation and metabolite identification of the GHB receptor antagonist NCS-382 in mouse informs novel therapeutic strategies for the treatment of GHB intoxication. Pharmacol Res Perspect. 2016;4(6):e00265. doi: 10.1002/prp2.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertol E, Mari F, Vaiano F, Romano G, Zaami S, Baglìo G, Busardò FP. Determination of GHB in human hair by HPLC-MS/MS: development and validation of a method and application to a study group and three possible single exposure cases. Drug Test Anal. 2015;7:376–384. doi: 10.1002/dta.1679. [DOI] [PubMed] [Google Scholar]
- Brenneisen R, Elsohly MA, Murphy TP, Passarelli J, Russmann S, Salamone SJ, Watson DE. Pharmacokinetics and excretion of gamma-hydroxybutyrate (GHB) in healthy subjects. J Anal Toxicol. 2004;28:625–630. doi: 10.1093/jat/28.8.625. [DOI] [PubMed] [Google Scholar]
- Busardò FP, Vaiano F, Mannocchi G, Bertol E, Zaami S, Marinelli E (2016) Twelve months monitoring of hair GHB decay following a single dose administration in a case of facilitated sexual assault. Drug Test Anal. doi:10.1002/dta.2100 [Epub ahead of print] [DOI] [PubMed]
- Buzzi A, Wu Y, Frantseva MV, Perez Velazquez JL, Cortez MA, Liu CC, Shen LQ, Gibson KM, Snead OC. Succinic semialdehyde dehydrogenase deficiency: GABAB receptor-mediated function. Brain Res. 2006;1090:15–22. doi: 10.1016/j.brainres.2006.02.131. [DOI] [PubMed] [Google Scholar]
- Cooper GAA, Kronstrand R, Kintz P. Society of Hair Testing (SoHT) guidelines for drug testing in hair. Forensic Sci Int. 2012;218:20–24. doi: 10.1016/j.forsciint.2011.10.024. [DOI] [PubMed] [Google Scholar]
- Gibson KM, Jakobs C, Ogier H, Hagenfeldt L, Eeg-Olofsson KE, Eeg-Olofsson O, Aksu F, Weber H-P, Rossier E, Vollmer B, Lehnert W. Vigabatrin therapy in six patients with succinic semialdehyde dehydrogenase deficiency. J Inherit Metab Dis. 1995;18:143–146. doi: 10.1007/BF00711750. [DOI] [PubMed] [Google Scholar]
- Goullé JP, Chèze M, Pépin G. Determination of endogenous levels of GHB in human hair. Are there possibilities for the identification of GHB administration through hair analysis in cases of drug-facilitated sexual assault? J Anal Toxicol. 2003;27:574–580. doi: 10.1093/jat/27.8.574. [DOI] [PubMed] [Google Scholar]
- Hillmer AT, Mason GF, Fucito LM, O'Malley SS, Cosgrove KP. How imaging glutamate, γ-aminobutyric acid, and dopamine can inform the clinical treatment of alcohol dependence and withdrawal. Alcohol Clin Exp Res. 2015;39:2268–2282. doi: 10.1111/acer.12893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jagerdeo E, Montgomery MA, LeBeau MA. An improved method for the analysis of GHB in human hair by liquid chromatography tandem mass spectrometry. J Anal Toxicol. 2015;39:83–88. doi: 10.1093/jat/bku130. [DOI] [PubMed] [Google Scholar]
- Jansen EE, Struys E, Jakobs C, Hager E, Snead OC, Gibson KM. Neurotransmitter alterations in embryonic succinate semialdehyde dehydrogenase (SSADH) deficiency suggest a heightened excitatory state during development. BMC Dev Biol. 2008;8:112. doi: 10.1186/1471-213X-8-112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansen EE, Vogel KR, Salomons GS, Pearl PL, Roullet J-B, Gibson KM. Correlation of blood biomarkers with age informs pathomechanisms in succinic semialdehyde dehydrogenase deficiency (SSADHD), a disorder of GABA metabolism. J Inherit Metab Dis. 2016;39:795–800. doi: 10.1007/s10545-016-9980-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maitre M, Klein C, Mensah-Nyagan AG. Mechanisms for the specific properties of γ-hydroxybutyrate in brain. Med Res Rev. 2016;36:363–388. doi: 10.1002/med.21382. [DOI] [PubMed] [Google Scholar]
- Malaspina P, Roullet JB, Pearl PL, Ainslie GR, Vogel KR, Gibson KM. Succinic semialdehyde dehydrogenase deficiency (SSADHD): pathophysiological complexity and multifactorial trait associations in a rare monogenic disorder of GABA metabolism. Neurochem Int. 2016;99:72–84. doi: 10.1016/j.neuint.2016.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parviz M, Vogel K, Gibson KM, Pearl PL. Disorders of GABA metabolism: SSADH and GABA-transaminase deficiencies. J Pediatr Epilepsy. 2014;3(4):217–227. doi: 10.3233/PEP-14097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearl PL, Gibson KM, Quezado Z, Dustin I, Taylor J, Trzcinski S, Schreiber J, Forester K, Reeves-Tyer P, Liew C, Shamim S, Herscovitch P, Carson R, Butman J, Jakobs C, Theodore W. Decreased GABA-A binding on FMZ-PET in succinic semialdehyde dehydrogenase deficiency. Neurology. 2009;73:423–429. doi: 10.1212/WNL.0b013e3181b163a5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reis J, Cohen LG, Pearl PL, Fritsch B, Jung NH, Dustin I, Theodore WH. GABAB-ergic motor cortex dysfunction in SSADH deficiency. Neurology. 2012;79:47–54. doi: 10.1212/WNL.0b013e31825dcf71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Y, Cui X, Shen M, Xiang P. Quantitative analysis of the endogenous GHB level in the hair of the Chinese population using GC/MS/MS. J Forensic Leg Med. 2016;39:10–15. doi: 10.1016/j.jflm.2016.01.002. [DOI] [PubMed] [Google Scholar]
- Staeheli SN, Baumgartner MR, Gauthier S, Gascho D, Jarmer J, Kraemer T, Steuer AE. Time-dependent postmortem redistribution of butyrfentanyl and its metabolites in blood and alternative matrices in a case of butyrfentanyl intoxication. Forensic Sci Int. 2016;266:170–177. doi: 10.1016/j.forsciint.2016.05.034. [DOI] [PubMed] [Google Scholar]
- Talaei SA, Azami A, Salami M. Postnatal development and sensory experience synergistically underlie the excitatory/inhibitory features of hippocampal neural circuits: glutamatergic and GABAergic neurotransmission. Neuroscience. 2016;318:230–243. doi: 10.1016/j.neuroscience.2016.01.024. [DOI] [PubMed] [Google Scholar]
- Vogel KR, Ainslie GR, Gibson KM. mTOR inhibitors rescue premature lethality and attenuate dysregulation of GABAergic/glutamatergic transcription in murine succinate semialdehyde dehydrogenase deficiency (SSADHD), a disorder of GABA metabolism. J Inherit Metab Dis. 2016;39:877–886. doi: 10.1007/s10545-016-9959-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Linnet K, Johansen SS. Development of a UPLC–MS/MS method for determining γ-hydroxybutyric acid (GHB) and GHB glucuronide concentrations in hair and application to forensic cases. Forensic Toxicol. 2016;34:51–60. doi: 10.1007/s11419-015-0285-6. [DOI] [Google Scholar]


