Abstract
Mucopolysaccharidosis type I (MPS I), a rare autosomal recessive disease, is caused by a deficiency of the lysosomal enzyme alfa-l-iduronidase. Impaired enzyme activity promotes glycosaminoglycans accumulation in several tissues and organs, leading to complex multisystemic complications. Several studies using animal models indicated different intracellular pathways involving MPS I physiopathology; however, the exact mechanisms underlying this syndrome are still not understood. Previous results from our group showed alterations in ionic homeostasis and cell viability of splenocytes and macrophages in Idua−/− mice. In the present study, we found altered intracellular ionic homeostasis in a different cell type (fibroblasts) from the same murine model. Idua−/− fibroblasts from 3-month-old mice presented higher cytoplasmatic and endoplasmic reticulum Ca2+ concentration, lower levels of mitochondrial Ca2+ and mitochondrial membrane potential and higher cytoplasmatic pH when compared to Idua+/+ animals. Also, Idua−/− fibroblasts were more resistant to the apoptotic induction with staurosporine, indicating a possible resistance to apoptotic induction in those cells. In addition, despite the intracellular ionic imbalance, no significant alterations were found in apoptosis and autophagy in Idua−/− fibroblasts, which implies that the ionic alterations did not activate those pathways. The investigation of mechanisms underlying the cellular physiopathology of lysosomal diseases is crucial for a better understanding about the progression of these diseases. Since splenocytes, macrophages, and fibroblasts have different embryonic origins and distinct structural and functional features, potentially altered signaling pathways found in a cell-specific manner in an alfa-l-iduronidase-deficient environment provide additional understanding of the clinical multisystemic presentation of this disease and provide new basis for improved therapeutic approaches.
Keywords: Apoptosis, Autophagy, Calcium, Fibroblasts, Mucopolysaccharidosis type I
Introduction
Mucopolysaccharidosis type I (MPS I) is a progressive lysosomal storage disease caused by deficient α-l-iduronidase, responsible for GAG (heparan and dermatan sulfate) catabolism. These non-degraded substrates accumulates in several organs and tissues, leading to multisystemic complications (Neufeld and Meunzer 2001). The exact mechanisms underlying MPS physiopathology are still not well understood; however, several studies using cellular and animal models indicated alterations in different intracellular signaling cascades, not related to the enzyme deficiency (and subsequent GAG accumulation) itself (Clarke 2008). Dysfunctions in intracellular pathways such as Ca2+ signaling, apoptosis/autophagy, bone turnover, and inflammation were also observed in different cell types derived from MPS animals (Kiselyov et al. 2007; Baldo et al. 2012; Lieberman et al. 2012). An impairment of the autophagic pathway was observed in mouse embryonic fibroblasts from MPS IIIA mice, leading to accumulation of dysfunctional organelles and toxic substrates and consequently cell death (Settembre et al. 2008). Mitochondrial defect and altered autophagy were related to neuronal death in MPS IIIC brains (Pshezhetsky 2016). Elevated levels of gangliosides (GM2 and GM3) and cholesterol were also found in neurons from MPS IIIA animals, indicating a secondary accumulation of storage material in MPS brains leading to a neuronal dysfunction (McGlynn et al. 2004). Decreased autophagy could also lead to an imbalance of reactive oxygen species production. Changes in redox state balancing have been described in MPS models. In a study using MPS VI rats, it was described a higher expression of cytochrome b5588, involved in oxidative burst of phagocytes (Simonaro et al. 2008). Our group have also found evidences of oxidative stress in blood cells from MPS I patients, where increased toxic products from lipid peroxidation, as malondialdehyde, led to lysosomal destabilization and consequently permeabilization (Pereira et al. 2008). Evidences of multi-organellar Ca2+ storage, intracellular pH alterations, and lysosomal permeability with higher cysteine proteases activity and apoptotic rate in murine Idua−/− splenocytes were also shown (Pereira et al. 2010). In a recent paper, we also demonstrated that changes in Ca2+ signaling itself were not sufficient to induce apoptosis in Idua−/− macrophages (in contrast to splenocytes); however, these cells appeared to be more sensitive to apoptotic induction and presented impaired phagocytosis ability (Viana et al. 2016). Thus, the present work shows altered homeostasis (ionic imbalance, apoptosis/autophagy induction, and GAG storage) of another cell type (fibroblasts) from 3-month-old Idua−/− mice.
Methods
Mice
C57BL/6 (Idua+/+) and α-l-iduronidase-deficient mice (Idua−/−), both 3-month-old, were used. MPS I mice (Ohmi et al. 2003) were kindly donated by Dr. Elizabeth Neufeld (UCLA, USA) and Dr. Nance B. Nardi (UFRGS, Brazil). The colony was established at Universidade Federal de São Paulo (UNIFESP) after heterozygous (Idua+/−) mating breeding and maintained on a light-dark 12:12 cycle under controlled temperature conditions (20 ± 2°C), with free access to food and water. Euthanasia was performed using cervical dislocation. All animal procedures were in accordance to the guidelines for care and use of animals and were approved by the Animal Care Ethics Committee of UNIFESP (#0594/10). After weaning, mice were identified according to their genotypes, by polymerase chain reaction, using specific oligonucleotides that amplify a fragment of Idua gene. After genotyping, 3-month old Idua+/+ and Idua−/− animals were selected for all experiments.
Peritoneal Fibroblast Culture
After euthanasia, the peritoneal membranes from both Idua+/+ and Idua−/− were removed and sliced into several small pieces of approximately 10 mm × 10 mm square sections. Under a laminar flow cabinet, pieces were then placed in a 35-mm Petri dish with R10 (RPMI-1640 medium supplemented with 10% of fetal bovine serum) to allow cell migration from tissue to the plate. After 5–10 days, cells were harvested, centrifuged at 200 × g for 6 min at 4°C, and seeded at a density of 106 cells/mL.
Ca2+ Signaling and Mitochondrial Membrane Potential
Ca2+ measurements were assessed using fluorescence microscopy (DM6000 microscope; Leica Microsystems Inc., Wetzlar, Hesse, Germany). The following fluorophores were used for cytoplasmic Ca2+ (Ca2+ cit), mitochondrial Ca2+ (Ca2+ mit), and mitochondrial membrane potential (ψmit) quantifications: Fluo-4AM (4 μM), Rhod-2AM (1 μM), and TMRE (250 nM), respectively. All fluorophores were purchased from Life Technologies (Carlsbad, CA, USA). Fluorescence was acquired with the following excitation/emission filter cubes, respectively: I3 (BP 470/40 nm and BP 525/50 nm) for Fluo-4AM and N2.1 (BP 515–560 nm and LP 590 nm) for Rhod-2AM and TMRE.
Intacellular pH
Lysosomal pH was quantified using Acridine Orange (AO; Molecular Probes, Invitrogen, USA). AO is a metachromatic fluorophore, which is accumulated in acidic organelles, especially in lysosomes. When excited by a blue light (BP 470/40 nm), AO emits green fluorescence (BP 505–530) related to cytosolic pH, or red fluorescence (LP 560 nm), related to lysosomic pH. Cells were plated in Petri dishes (35 mm; Mat Tek Corp, USA) at a density of 5 × 106. After adhesion, cells were incubated with AO (0.5 μM, 15 min), washed twice with HBSS, and visualized under fluorescence microscopy. Fluorescence intensities were then quantified and expressed as relative fluorescence units (RFU) for cytoplasmic pH (alkaline) and lysosomal pH (acidic), as well the ratio alkaline/acidic.
Cellular Viability Assay
For cell viability evaluation, peritoneal fibroblasts from Idua+/+ and Idua−/− (2 × 105 cells/mL) were treated with 5 μM staurosporine (STS; Sigma–Aldrich, USA) for 24 h. After this period, cells were harvested and incubated for additional 15 min with Annexin-V-FITC (Life Technologies, USA) and Propidium Iodide (PI; Life Technologies, USA), indicators of apoptosis and necrosis, respectively. Assessment of cell viability and cell death (apoptosis and necrosis) was performed comparing values between both Idua+/+ and Idua−/− groups and between stimulated and non-stimulated cells (basal). The fluorescence readings were acquired in a Tali® ImageBased Cytometer (Life Technologies, USA).
Western Blotting: Apoptosis and Autophagy
Fibroblast protein extracts were obtained using NP-40 buffer supplemented with 10% of protease inhibitor cocktail (Sigma–Aldrich, USA). After quantification, proteins were then denatured in sample buffer (2% SDS, 62.5 mM Tris–HCl pH 6.8, 10% glycerol, 0.04 mg/mL bromophenol blue and 350 mM beta-mercaptoethanol), heated at 95°C for 5 min, and loaded (50 μg) into 15% (LC3-II, Bcl-2, Bax and GAPDH) or 10% (Beclin-1 and p62) polyacrylamide gels. Electrophoresis were done at constant voltage of 100 V. Proteins were then transferred to the nitrocellulose membranes at constant current (230 mA) for 90–120 min. After transfer, the nitrocellulose membranes were blocked in blocking solution (5% non-fat milk) for 1 h and incubated with primary antibodies overnight. All primary antibodies (produced in rabbit) were purchased from Novus Biologicals (Novus Biologicals Inc., USA), Sigma (Sigma–Aldrich, USA), or CST (Cell Signaling Technology, USA), with specific reactivity to mice. After primary antibody incubation, membranes were incubated with Alexa Fluor® 680-conjugated anti-rabbit IgG (Life Technologies, USA) for 45 min. The normalization of the results was performed with the GAPDH protein (glyceraldehyde 3-phosphate dehydrogenase) and the visualization of the results and band intensities quantified using the Odyssey Infrared Imaging System (Li-Cor Biosciences, USA).
Caspases 3, 8, and 9 Activities
The analysis of caspases activities was assessed using Image-iT™ LIVE Green Poly Caspases Detection Kit (Life Technologies, USA), with a fluorescent caspase inhibitor (FLICA) and a fluorescent nuclear marker (Hoeschst 33342). Approximately 5 × 105 cells/well were plated in a 96-wells black plate with a light bottom (Nunc, Germany). After adhesion, cells were treated with STS (5 μM; 4 h) or R10 (non-stimulated control); washed and incubated with FLICA and Hoeschst for 1 h. Cells were washed twice with a specific kit buffer and fluorescent emissions were read using SpectraMax M2 – MDS (Molecular Devices, USA). Green fluorescence indicated caspases activities (ex/em: 488/520 nm) and blue fluorescence indicated nuclear staining (ex/em: 350/461 nm). Results were expressed as a ratio of green and blue fluorescences to normalize the emission according to the cell density.
Statistics
All data were expressed as mean ± SEM. Comparisons were performed with unpaired Student’s t test using Statistica 8.0 software (Stat Soft Inc., USA). The level of significance was set at P < 0.05.
Results
Ca2+ Signaling and Mitochondrial Membrane Potential
Measurements of Ca2+ homeostasis showed that basal cytosolic Ca2+ was significantly decreased in Idua−/− mice compared to Idua+/+ (Fig. 1a, d). No statistical difference was observed in basal mitochondrial Ca2+ (Fig. 1b, e). However, an increase in mitochondrial potential was found in Idua−/− group compared to control (Fig. 1c). Inhibition of Ca2+-ATPase pump from endoplasmatic reticulum (ER) by thapsigargin (THG) produced a lower Ca2+ release in Idua−/− group (Fig. 1d). Similarly, mitochondrial Ca2+ and mitochondrial potential were also decreased after THG stimulus (Fig. 1e, f).
Fig. 1.
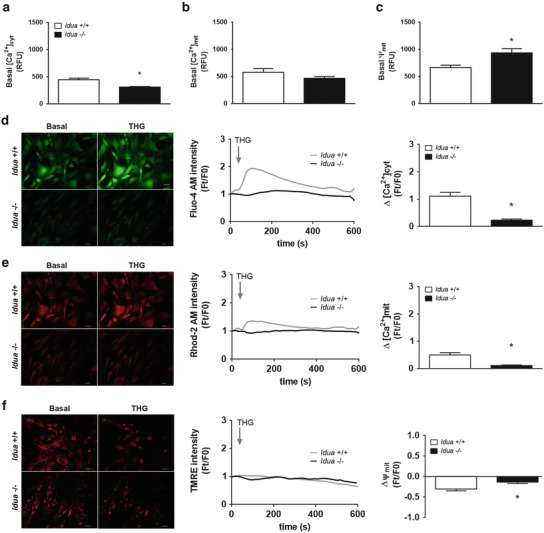
Alterations in intracellular Ca2+ and mitochondrial homeostasis in Idua −/− fibroblasts. Peritoneal fibroblasts from Idua +/+ and Idua −/− mice were incubated with Fluo-4AM (4 μM) for cytoplasmatic Ca2+, Rhod-2AM (1 μM) for mitochondrial Ca2+, and TMRE (250 nM) for mitochondrial membrane potential measurements. Fluorescence microscopy was used to capture images, which were pseudocolored green (for cytoplasmatic Ca2+) and red (mitochondrial Ca2+) or mitochondrial membrane potential). As shown in (a), Idua −/− fibroblasts showed lower basal cytoplasmatic Ca2+ and higher mitochondrial membrane potential (c), despite no alterations in basal mitochondrial Ca2+ (b). To evaluate possible alterations in intracellular Ca2+ mobilization, cells were also incubated with THG (10 mM), a specific ER Ca2+/ATPase inhibitor (d–f). The fluorescence intensity as a function of time corresponds to the images shown in the middle and the ratio between fluorescence readings before and after THG addition (Ft/F0) indicates the relative concentration of Ca2+ at ER (right). A reduced relative concentration ER Ca2+ was found in Idua −/− fibroblasts after THG addition (d). Lower mitochondrial Ca2+ variations were also found in these cells (e), indicating a reduced ability of Ca2+ buffering from this organelle, despite normal mitochondrial membrane potential (f). Ca2+ cit: cytoplasmatic Ca2+; Ca2+ mit: mitochondrial Ca2+; ψmit: mitochondrial membrane potential. Data are expressed as the mean ± S.E.M. of three independent experiments. P < 0.05 (Student’s t test). n = 4–6
Lysosomal Homeostasis
Acridine orange has been known for many years as a convenient metachromatic dye to stain acidic vesicles, with fluorescence wavelength emission strongly dependent on concentration. The mechanism of AO accumulation in those vesicles is related to the protonation of AO molecules in a low pH environment, creating an electric charge that consequently hinders the ability of the dye molecules to cross the vesicular membrane (e.g., lysosomes) and escape back into the surrounding cytoplasm (Traganos and Darzynkiewicz 1994; Dobrucki et al. 2007). After incubation with AO, Idua−/− fibroblasts showed a higher cytosolic pH (Fig. 2a) and the ratio of lysosomal and cytosolic pH decreased in the same group, compared to the control (Fig. 2b).
Fig. 2.
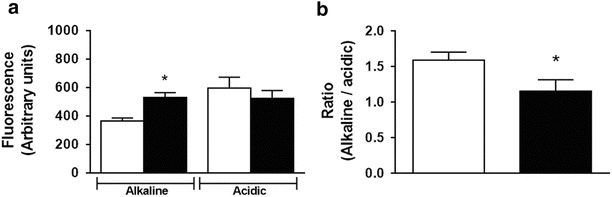
Evidence of cytoplasmic alkalinization in Idua −/− fibroblasts. Idua −/− fibroblasts (5 × 105) were seeded in glass-bottom 35-mm Petri dishes and incubated with AO (0.5 μM) for 15 min and visualized under fluorescence microscopy. Idua −/− fibroblasts showed elevated cytoplasmic pH (alkaline) but normal lysosomal pH (acidic), when compared to Idua +/+ cells (a). In addition, alkaline/acidic ratio was lower in Idua −/−, indicating a disruption of intracellular H+ homeostasis (b). Data are expressed as the mean ± S.E.M. of three independent experiments. P < 0.05 (Student’s t test). n = 4–6
Apoptosis and Cellular Viability
There were no differences in the relative expression of apoptotic proteins (Bax and Bcl-2) and autophagic proteins (p62, beclin and LC3 II) between Idua+/+ and Idua−/− groups (Fig. 3a–d and Fig. 3e–h, respectively). Also, basal cellular viability appeared similar between both groups (Fig. 3i). However, after STS treatment, fibroblasts from Idua−/− group appeared more resistant to cell death, since we observed a higher percentage of viable fibroblasts and a lower number of apoptotic cells in Idua−/− mice (Fig. 3j). We also examined the activity of caspases 3, 6, and 9, but no statistical difference was observed between groups (Fig. 3k).
Fig. 3.
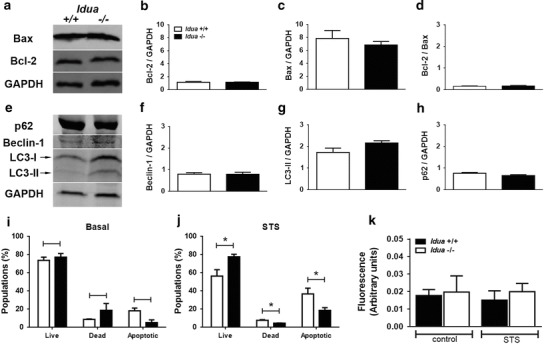
Idua −/− fibroblasts are more resistant to apoptotic induction. Total protein extracts of Idua +/+ and Idua −/− fibroblasts (30–50 mg) were loaded into 10–15% SDS-polyacrylamide gels for evaluation of basal apoptotic (Bcl-2 and Bax) and autophagic (p62, beclin-1 and LC3-II) markers. No differences were observed in Bcl-2 and Bax relative expression (a–d), as well as in autophagy markers such p62, beclin-1, and LC3-II (e–h). Moreover, no changes in basal viability of peritoneal Idua −/− fibroblasts were observed (i). However, incubation with STS (5 μM) for 4 h resulted in an increase of viable (live) and a decrease of dead and apoptotic cells (j). In addition, no differences were observed in caspase activity of control (no STS) and STS-treated cells (k). Data are expressed as the mean ± S.E.M. of three independent experiments. P < 0.05 (Student’s t test). n = 4–6
Discussion
Ionic intracellular homeostasis, autophagy, and apoptosis are some of the most affected mechanisms reported in studies related to animal models of lysosomal storage diseases (Kiselyov et al. 2010; Settembre et al. 2013). It is a consensus that MPS I is a progressive and multisystemic disease; however, it is still unclear whether the same mechanisms are similarly altered in different cell types. We decided to describe some physiological cellular parameters in fibroblasts of Idua−/− mice to implement the characterization of this important animal model and to compare previous data observed in splenocytes (Pereira et al. 2010) and macrophages (Viana et al. 2016). Although they share the same microenvironment, peritoneal macrophages and fibroblasts exert distinct functions in vivo. Macrophages act in immunological response against pathogenic agents, while fibroblasts maintain extracellular matrix and participate of tissue repairing and remodeling.
Ca2+ homeostasis is essential to cellular functions such as hormonal secretion, neurotransmitter release, muscle contraction, induction of cell death by apoptosis, necrosis, and autophagy (Harr and Distelhorst 2010). In fibroblasts, we observed a decrease in ER Ca2+, as we have previously observed in macrophages of Idua−/− mice (Viana et al. 2016). Otherwise, our group observed a higher stock of ER Ca2+ in splenocytes of 6-month-old Idua−/− mice (Pereira et al. 2010). This suggests a failure of ER Ca2+/ATPase, since Ca2+ maintenance depends on a coordinated action among calcium Ca2+ channels and Ca2+/ATPase activity. Failure of Ca2+ homeostasis may also functionally affect lysosomes and mitochondria, such as the maintenance of cellular viability upon the cell death pathways control (Kiselyov and Muallem 2008). Pereira et al. (2010) encountered a higher level of cysteine proteases in the cytosol and an increased number of apoptotic splenocytes of Idua−/−. Furthermore, we had also previously described diminished ER Ca2+ in Idua−/− macrophages and an increase in mitochondrial Ca2+ basal levels (Viana et al. 2016).
Besides, after THG treatment, the Ca2+ mitochondrial uptake was lower, probably by low Ca2+ stocks in the ER, which reinforces the hypothesis of Ca2+/ATPase impairment in this model. Although ER Ca2+ levels were lower in fibroblasts of Idua−/−, we have not found any difference in basal mitochondrial Ca2+. However, the basal mitochondrial potential was increased in the same group. This result may be related to a protective cellular mechanism, as it has been described in cancer cells (Fulda et al. 2010; Ubah and Wallace 2014). On the other hand, an exaggerated increase of mitochondrial Ca2+ may also outcome in a metabolic overload, deficiency of ATP synthesis, excess of reactive oxygen species production, decrease in membrane potential, and consequently loss of mitochondrial functions and cell death (Patron et al. 2013). Ca2+ fluctuations may interfere in lysosomal homeostasis, which interfere in a variety of mechanisms.
Non-degraded substrates inside lysosomes change the membrane permeability and interfere in vesicle trafficking, altering the balance among cell proliferation and cell death (Lagadic-Gossmann et al. 2004). Activation of apoptosis has been described in different cell types from MPS animal models (Simonaro et al. 2008; Pereira et al. 2010). However, despite higher cytoplasmic pH and altered alkaline/acidic ratio found in cytoplasm of Idua−/− fibroblasts, no differences in cell viability were observed, as observed by reduced caspase activity and number of Idua−/− apoptotic fibroblasts after STS. Also, we have found no significant difference in Bcl-2 relative expression, neither in Bax expression in Idua−/− fibroblasts, indicating no mitochondrial damage.
Although some authors have indicated signs of autophagic deregulation in different tissues from MPS models (Ballabio 2009; Tessitore et al. 2009), we have not detected significant differences in relative expression of autophagic proteins in fibroblasts of Idua−/− mice. This data indicated that lysosomal dysfunction found in fibroblasts from MPS I mice could not impair endosomal vesicular traffic and cellular recycling, which is crucial to maintenance of cell viability.
However, it is important to stress that this lack of differences in autophagy markers in those cells is probably due to the absence of paracrine modulation between adjacent cells/tissues and possible differences in GAG deposition profile in several biological microenvironments in vivo. In fact, some studies on evaluation of intracellular signaling cascades can be only performed in isolated cultured cells from animal models, so general extrapolation of these results to humans should be carefully addressed.
In summary, the present study indicated significant differences of ionic homeostasis of ER, mitochondria, and lysosomes in Idua−/− fibroblasts. Despite the intracellular ionic imbalance in Idua−/− cells, no significant alterations were found in the relative expression of apoptosis and autophagy proteins in both wild type and Idua−/− fibroblasts, implying that such alterations did not activate these pathways. These evidences indicate that MPS I cellular physiopathology can be highly heterogeneous in a cell-specific manner and brings new perspectives to understand how multisystemic complications are possible related to changes in cellular physiology, tissue/cell type, storage substrate, and disease progression.
Acknowledgments
This work was supported by Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP) research grant # 2011/18050-9 (Vânia D’Almeida). The authors would also like to thank CAPES, CNPq, and AFIP for additional financial and infrastructural support, Dr. Helena Nader for providing access to the microscopy facility at INFAR, UNIFESP, and Dr. Marcelo Lima for his critical reading of this manuscript. Vânia D’Almeida was recipient of a fellowship from CNPq. Gustavo Viana was a recipient of a FAPESP Ph.D. scholarship (# 2010/10458-6).
Synopsis
Evidence of cell-specificity in murine MPS I physiopathology.
Compliance with Ethics Guideline
Conflict of Interest
Gustavo Viana, Cinthia Nascimento, Edgar Paredes Gamero e Vânia D’Almeida declare that they have no conflict of interest.
Animal Rights
All institutional and national guidelines for the care and use of laboratory animals were followed.
Details of the Contributions of Individual Authors
Gustavo Viana performed the experiments. Gustavo Viana, Cinthia Nascimento, Edgar Paredes Gamero, and Vânia D’Almeida analyzed data. Gustavo Viana, Cinthia Nascimento, and Vânia D’Almeida wrote the manuscript.
Contributor Information
Gustavo Monteiro Viana, Email: gvianabiomed@gmail.com.
Collaborators: Matthias R. Baumgartner, Marc Patterson, Shamima Rahman, Verena Peters, Eva Morava, and Johannes Zschocke
References
- Baldo G, Mayer FQ, Martinelli B, et al. Evidence of a progressive motor dysfunction in mucopolysaccharidosis type I mice. Behav Brain Res. 2012;233(1):169–175. doi: 10.1016/j.bbr.2012.04.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballabio A. Disease pathogenesis explained by basic science: lysosomal storage diseases as autophagocytic disorders. Int J Clin Pharmacol Ther. 2009;47(Suppl 1):S34–S38. doi: 10.5414/cpp47034. [DOI] [PubMed] [Google Scholar]
- Clarke LA (2008) The mucopolysaccharidoses: a success of molecular medicine. Expert Rev Mol Med 10(1): e1 [DOI] [PubMed]
- Dobrucki JW, Feret D, Noatynska A. Scattering of exciting light by live cells in fluorescence confocal imaging: phototoxic effects and relevance for FRAP studies. Biophys J. 2007;93(5):1778–1786. doi: 10.1529/biophysj.106.096636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fulda S, Galluzzi L, Kroemer G. Targeting mitochondria for cancer therapy. Nat Rev Drug Discov. 2010;9(6):447–464. doi: 10.1038/nrd3137. [DOI] [PubMed] [Google Scholar]
- Harr MW, Distelhorst CW. Apoptosis and autophagy: decoding calcium signals that mediate life or death. Cold Spring Harb Perspect Biol. 2010;2(10):a005579. doi: 10.1101/cshperspect.a005579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiselyov K, Jennigs JJ, Jr, Rbaibi Y, Chu CT. Autophagy, mitochondria and cell death in lysosomal storage diseases. Autophagy. 2007;3(3):259–262. doi: 10.4161/auto.3906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiselyov K, Muallem S. Mitochondrial Ca2+ homeostasis in lysosomal storage diseases. Cell Calcium. 2008;44(1):103–111. doi: 10.1016/j.ceca.2007.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiselyov K, Yamaguchi S, Lyons CW, Muallem S. Aberrant Ca2+ handling in lysosomal storage disorders. Cell Calcium. 2010;47(2):103–111. doi: 10.1016/j.ceca.2009.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagadic-Gossmann D, Huc L, Lecureur V. Alterations of intracellular pH homeostasis in apoptosis: origins and roles. Cell Death Differ. 2004;11(9):953–961. doi: 10.1038/sj.cdd.4401466. [DOI] [PubMed] [Google Scholar]
- Lieberman AP, Puertollano R, Raben N, Slaugenhaupt S, Walkley SU, Ballabio A. Autophagy in lysosomal storage disorders. Autophagy. 2012;8(5):719–730. doi: 10.4161/auto.19469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGlynn R, Dobrenis K, Walkley SU. Differential subcellular localization of cholesterol, gangliosides, and glycosaminoglycans in murine models of mucopolysaccharide storage disorders. J Comp Neurol. 2004;480(4):415–426. doi: 10.1002/cne.20355. [DOI] [PubMed] [Google Scholar]
- Neufeld EF, Meunzer J (2001) The mucopolysaccharidoses. In: Scriver CR, Beaudet AL, Sly WS, Valle D (eds) Metabolic and molecular basis of inherited disease, vol 3, pp. 3421–3452
- Ohmi K, Greenberg DS, Rajavel KS, Ryazantsev S, Li HH, Neufeld EF. Activated microglia in cortex of mouse models of mucopolysaccharidoses I and IIIB. Proc Natl Acad Sci U S A. 2003;100(4):1902–1907. doi: 10.1073/pnas.252784899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patron M, Raffaello A, Granatiero V, et al. The mitochondrial calcium uniporter (MCU): molecular identity and physiological roles. J Biol Chem. 2013;288(15):10750–10758. doi: 10.1074/jbc.R112.420752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira VG, Gazarini ML, Rodrigues LC, et al. Evidence of lysosomal membrane permeabilization in mucopolysaccharidosis type I: rupture of calcium and proton homeostasis. J Cell Physiol. 2010;223(2):335–342. doi: 10.1002/jcp.22039. [DOI] [PubMed] [Google Scholar]
- Pereira VG, Martins AM, Micheletti C, D'Almeida V. Mutational and oxidative stress analysis in patients with mucopolysaccharidosis type I undergoing enzyme replacement therapy. Clin Chim Acta. 2008;387(1–2):75–79. doi: 10.1016/j.cca.2007.09.008. [DOI] [PubMed] [Google Scholar]
- Pshezhetsky AV. Lysosomal storage of heparan sulfate causes mitochondrial defects, altered autophagy, and neuronal death in the mouse model of mucopolysaccharidosis III type C. Autophagy. 2016;12(6):1059–1060. doi: 10.1080/15548627.2015.1046671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Settembre C, Fraldi A, Jahreiss L, et al. A block of autophagy in lysosomal storage disorders. Hum Mol Genet. 2008;17(1):119–129. doi: 10.1093/hmg/ddm289. [DOI] [PubMed] [Google Scholar]
- Settembre C, Fraldi A, Medina DL, Ballabio A. Signals from the lysosome: a control centre for cellular clearance and energy metabolism. Nat Rev Mol Cell Biol. 2013;14(5):283–296. doi: 10.1038/nrm3565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simonaro CM, D'Angelo M, He X, et al. Mechanism of glycosaminoglycan-mediated bone and joint disease: implications for the mucopolysaccharidoses and other connective tissue diseases. Am J Pathol. 2008;172(1):112–122. doi: 10.2353/ajpath.2008.070564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tessitore A, Pirozzi M, Auricchio A. Abnormal autophagy, ubiquitination, inflammation and apoptosis are dependent upon lysosomal storage and are useful biomarkers of mucopolysaccharidosis VI. Pathogenetics. 2009;2(1):4. doi: 10.1186/1755-8417-2-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traganos F, Darzynkiewicz Z. Lysosomal proton pump activity: supravital cell staining with acridine orange differentiates leukocyte subpopulations. Methods Cell Biol. 1994;41:185–194. doi: 10.1016/S0091-679X(08)61717-3. [DOI] [PubMed] [Google Scholar]
- Ubah OC, Wallace HM. Cancer therapy: targeting mitochondria and other sub-cellular organelles. Curr Pharm Des. 2014;20(2):201–222. doi: 10.2174/13816128113199990031. [DOI] [PubMed] [Google Scholar]
- Viana GM, Buri MV, Paredes-Gamero EJ, Martins AM, D'Almeida V. Impaired hematopoiesis and disrupted monocyte/macrophage homeostasis in mucopolysaccharidosis type I mice. J Cell Physiol. 2016;231(3):698–707. doi: 10.1002/jcp.25120. [DOI] [PubMed] [Google Scholar]


