The deep superior colliculus is involved in steering the saccade toward the current location of a moving target. During interceptive saccades, the active population consists of a continuum of cells ranging from neurons issuing commands related to past locations of the target to neurons issuing commands related to its current location. The motor burst of collicular neurons does not contain commands related to the future location of a moving target.
Keywords: brain stem, foveation, interception, motion, saccade
Abstract
Following the suggestion that a command encoding current target location feeds the oculomotor system during interceptive saccades, we tested the involvement of the deep superior colliculus (dSC). Extracellular activity of 52 saccade-related neurons was recorded in three monkeys while they generated saccades to targets that were static or moving along the preferred axis, away from (outward) or toward (inward) a fixated target with a constant speed (20°/s). Vertical and horizontal motions were tested when possible. Movement field (MF) parameters (boundaries, preferred vector, and firing rate) were estimated after spline fitting of the relation between the average firing rate during the motor burst and saccade amplitude. During radial target motions, the inner MF boundary shifted in the motion direction for some, but not all, neurons. Likewise, for some neurons, the lower boundaries were shifted upward during upward motions and the upper boundaries downward during downward motions. No consistent change was observed during horizontal motions. For some neurons, the preferred vectors were also shifted in the motion direction for outward, upward, and “toward the midline” target motions. The shifts of boundary and preferred vector were not correlated. The burst firing rate was consistently reduced during interceptive saccades. Our study demonstrates an involvement of dSC neurons in steering the interceptive saccade. When observed, the shifts of boundary in the direction of target motion correspond to commands related to past target locations. The absence of shift in the opposite direction implies that dSC activity does not issue predictive commands related to future target location.
NEW & NOTEWORTHY The deep superior colliculus is involved in steering the saccade toward the current location of a moving target. During interceptive saccades, the active population consists of a continuum of cells ranging from neurons issuing commands related to past locations of the target to neurons issuing commands related to its current location. The motor burst of collicular neurons does not contain commands related to the future location of a moving target.
the primate oculomotor system for saccade generation has been used as a model to understand the neuronal processes underlying the ability to localize an object in the external world and to produce an accurate movement toward its location (Goffart 2017). In most studies, the stimulus is static, leaving unexplored the processes responsible for the generation of saccades toward the changing location of a moving object (Fig. 1, A and B). Yet, quite remarkably, these interceptive saccades are almost as accurate as saccades toward a static target (Cassanello et al. 2008; Fleuriet et al. 2011; Guan et al. 2005; Keller and Johnsen 1990). Among the numerous brain regions that are involved, the deep superior colliculus (dSC) and the caudal fastigial nucleus (cFN) are considered to play synergistic and complementary roles. Their involvement is suggested by the emission of bursts of action potentials by some of their neurons during interceptive and catch-up saccades toward a moving target (Fuchs et al. 1994; Keller et al. 1996). Moreover, their anatomical situation between on the one hand the cerebral (Cassanello et al. 2008; Erlikhman and Caplovitz 2017; Konen and Kastner 2008; Maioli et al. 1992; Rosano et al. 2002) and cerebellar (Robinson and Fuchs 2001; Suzuki et al. 1981; Suzuki and Keller 1988) cortices where neurons responsive to the motion of a target are found and on the other hand the saccade-related premotor neurons in the reticular formation (Gandhi and Katnani 2011; Moschovakis et al. 1996; Scudder et al. 2002; Sparks 2002) corroborates their involvement.
Fig. 1.
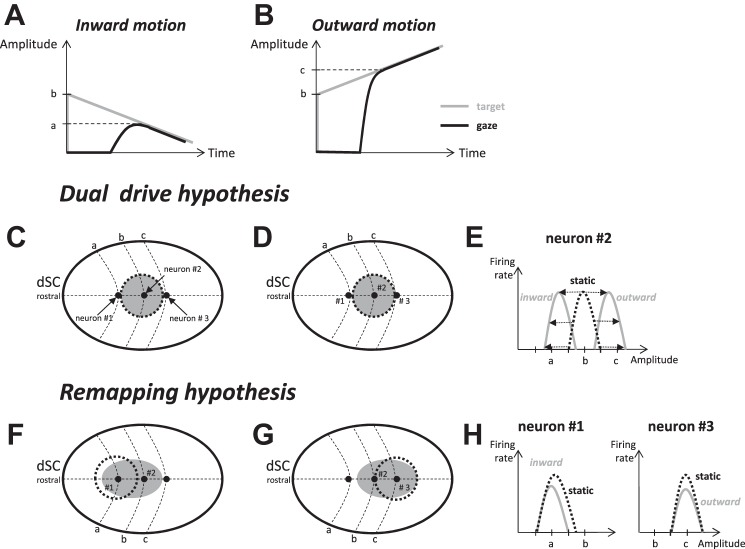
Schematic representation of the “dual drive” and “remapping” hypotheses and their predictions for the movement field (MF) of dSC neurons. Let us consider prototypical interceptive saccades directed toward a target moving toward the fixation target (A; inward motion) or along the same axis but in the opposite direction (B; outward motion). According to the dual drive hypothesis, population of activity in the dSC encodes the location where the target appears initially. Thus, the dSC activity is identical regardless of whether the saccade is aimed at a static target (dotted circle in C and D) or at a target moving inward (C) or outward (D). Neuron 2 (preferring amplitude b) situated at the center of the population should fire during both interceptive saccades. However, its MF is expected to be different between the 2 types of saccades (E). Compared with the MF observed with static targets (dashed black curve), the entire profile (preferred amplitude and boundaries) should shift toward smaller values of saccade amplitude during inward target motion and toward larger values during outward motion (gray solid curves). By contrast, neurons 1 (preferring amplitude a) and 3 (preferring amplitude c) are situated outside the active population and thus should not fire during these interceptive saccades. According to the remapping hypothesis, the activity can change after the target appearance; it would recruit neuron 1 during inward target motion (F) and neuron 3 during outward motion (G). The analysis of their MF should reveal more complex changes in MF, with some overlap between saccades toward a static vs. moving target (H).
According to the “dual drive” hypothesis, interceptive saccades are driven by a combination of commands issued by the dSC and cFN (Optican 2009). The locus of dSC activity encodes the location where the target first appears (Fig. 1, C and D), whereas the cFN component encodes the command related to the target motion after the collicular “snapshot” (see also Optican and Pretegiani 2017). This hypothesis rests upon the observation that the “centers” of the movement field (MF) of dSC neurons (i.e., the amplitude and direction of saccades associated with the most vigorous burst) shift to larger amplitudes during saccades made toward a target moving away from the central visual field (Keller et al. 1996). However, the magnitude of the shift spans over a notable range, since some neurons exhibit no change (see Fig. 3A in Keller et al. 1996). This scattering could instead indicate that the population of collicular neurons that burst during interceptive saccades consists of a continuum of cells ranging from cells issuing commands related to past locations of the target (cells with a shift) to cells issuing commands related to its current location (cells with no shift). Thus, as an alternative to the dual drive hypothesis, the “remapping” hypothesis (Fig. 1, F and G) proposes that the population of active neurons does not correspond to a snapshot of the past but spreads across the dSC (Fleuriet et al. 2011). Crucially, the supplementary command envisioned by the dual drive hypothesis would be incorporated within the dSC itself, making the signals originating in the cFN different from merely compensating for the target motion after the snapshot. The saccade-related burst of the dSC and the cFN would then steer the saccade in parallel, making their combined output (possibly with other signals, too) at the origin of the expected “here-and-now” command that has been proposed to feed the saccade premotor system during interceptive saccades (Fleuriet and Goffart 2012). The remapping of activity in the deep layers of SC could be made in interaction with the parabigeminal nucleus (Cui and Malpeli 2003; Ma et al. 2013) under the influence of input signals from its superficial layers (Isa and Hall 2009; Moors and Vendrik 1979; Schiller and Koerner 1971), the frontal eye fields (Cassanello et al. 2008; Ferrera and Barborica 2010; Hanes and Wurtz 2001; Lynch 1987), and the lateral intraparietal area (Bremmer et al. 2016; Paré and Wurtz 2001).
One goal of this study was to evaluate the dual drive and remapping hypotheses by comparing the MFs of dSC neurons between saccades toward static vs. moving targets. According to the dual drive hypothesis, the population of active neurons encodes the location where the target appears initially (Fig. 1, C and D). Thus, it would be identical regardless of the target motion after its appearance. In comparison to the MF recorded during saccades to a static target, the preferred vector and boundaries of MF should be identically shifted in the direction of target motion during the interceptive saccades (see neuron 2 in Fig. 1E). According to the remapping hypothesis, the active population does not remain static after the target onset but diffuses across the dSC. During inward target motions, the activity would spread toward the neuron labeled 1 (Fig. 1F), and toward neuron 3 during outward motions (Fig. 1G). In this scenario, changes in MF can be more complex; two possibilities are highlighted in Fig. 1H.
Another goal of our study was to examine whether the population of dSC neurons that burst during interceptive saccades includes commands that are related to future locations of the target along its motion path, i.e., locations that are going to be reached. Such a possibility would be indicated by shifts of the boundaries of the MF in the direction opposite to the target motion, an option that cannot be deduced from the recordings made by Keller et al. (1996) since their study focused on the MF preferred vector, a parameter that does not tell us what the activity is during “nonpreferred” amplitudes. In contrast, the MF boundaries are very informative because they indicate whether a cell discharged or not, and thus they tell us something about the extent of the population of active neurons. On the basis of the available data (Keller et al. 1996), it cannot be excluded that dSC neurons emit action potentials during interceptive saccades and not during saccades to a static target with matched amplitudes. Therefore, we complemented the electrophysiological characterization initiated by Keller and colleagues (1996) by comparing the MF of saccade-related SC neurons between saccades to a static target and saccades toward a similar target moving with a constant speed along various straight trajectories in the visual field.
Our results show a continuum of neurons in the dSC, ranging from cells that exhibit a shift in the boundary (or in the preferred vector) of their MF to cells that do not exhibit any change. When shifts were observed, they were always in the same direction as the target motion, never in the opposite direction. This absence of boundary shift in the opposite direction indicates that there was no recruitment of neurons to issue a command related to a future target location. When MF boundary shifts are observed, they likely correspond to residual activity due to the fact that the locus of active neurons across the dSC does not change as fast as the target in the visual field. The observation of cells with no shift is consistent with their involvement in steering the saccade toward the current location of a moving target, as if it were static.
MATERIALS AND METHODS
Subjects and surgical procedures.
All surgical and experimental protocols were approved by the University of Pittsburgh Animal Care and Use Committee and performed in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals. Three adult rhesus monkeys (Macaca mulatta; male: BB and BL, female: WI) underwent aseptic surgeries to secure a small head-restraint device to the skull, cement a stainless steel chamber over a craniotomy, and attach a Teflon-coated stainless steel wire (search coil) on the sclera of one eye. The chamber was placed stereotaxically on the skull, slanted posteriorly at an angle of 38° in the sagittal plane. This approach allowed access to both SCs and permitted electrode penetrations roughly perpendicular to their surface. Antibiotics and analgesics were administered postoperatively as detailed in an approved protocol.
Behavioral tasks and experimental apparatus.
After full recovery, the subjects were trained to sit in a primate chair with their head restrained and a sipper tube placed near the mouth for reward delivery. They were subsequently trained to perform standard oculomotor tasks involving stationary targets. The monkeys were not previously trained to pursue moving targets, which were introduced only during the recording sessions. Visual stimuli, behavioral control, and data acquisition were implemented by a custom-built program that uses LabVIEW conventions on a real-time operating system supported by National Instruments (Austin, TX) (Bryant and Gandhi 2005). Each animal sat inside a frame containing two alternating magnetic fields that induced voltages in the search coil, thereby permitting measurement of horizontal and vertical eye orientations (Robinson 1963). Visual targets were red dots subtending ~0.5° of visual angle that were displayed on a 55-in. (140 cm), 120-Hz-resolution LED monitor.
Every trial began with the illumination of an initial target (T0) that the subjects were required to fixate for a variable duration (300–700 ms, 100-ms increments). Trials were aborted if the gaze direction deviated beyond a computer-defined window (3° radius) surrounding T0. If fixation was maintained, then T0 was extinguished and another target (T1) was simultaneously presented in the visual periphery. During static trials, the subjects were rewarded for orienting their gaze within a window that surrounded T1 with a radius of 3–6° for a minimum of 350 ms. During motion trials, target T1 moved at a constant speed of 20°/s immediately after it appeared on the screen. The reward window associated with T1 was elliptical, with a long axis that extended from the starting position of T1 to at least 5° beyond its final position. The subjects were required to be within this window for at least 500 ms before receiving a reward. The starting position and the direction of target motion depended upon the MF properties of the recorded cell as determined during static trials (see Single-unit recording and movement fields).
Single-unit recording and movement fields.
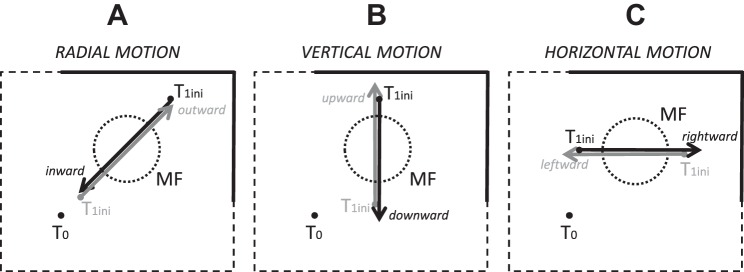
Tungsten microelectrodes (Microprobe) were used to record extracellular activity from the intermediate and deep layers of SC, at depths greater than ~1 mm below its dorsal surface. The dSC was identified online by the presence of distinctive bursts of activity associated with flashes of room lights and saccades as well as identifiable saccade-related cells during static trials. After we isolated a single saccade-related neuron, we estimated the boundaries of its MF by pseudorandomly presenting targets and observing peak firing rates displayed online by the acquisition software. Once the optimal vector was approximated, a series of static target locations was chosen along 1) an imaginary line that passed through the preferred amplitude (“center”) of the MF and the initial target T0, 2) an imaginary line that passed through the “center” and parallel to the vertical meridian, or 3) an imaginary line that passed through the “center” and parallel to the horizontal meridian. Approximately 75–100 static trials were collected before static and motion trials were pseudorandomly intermixed. The starting positions of moving targets, which we denote T1ini, were pseudorandomly selected among locations situated along the same imaginary lines used for the targets during static trials. Target motion could be radial (Fig. 2A, inward or outward relative to T0), vertical (Fig. 2B, upward or downward relative to T1ini, motion along an axis parallel and different from the vertical meridian), or horizontal (Fig. 2C, rightward or leftward motion along an axis parallel and different from the horizontal meridian). Recordings of action potentials during saccades toward a target moving along one of these axes were performed in block mode until the cell was lost. Therefore each neuron could not be recorded during all the different types of target motion. Moreover, no specific order was followed except that the radial motion was more privileged than other motions in order to preserve the continuity of our work with the previous study of Keller et al. (1996). Introducing variability in the location of T1ini during the motion trials, as well as the natural variability in the subjects’ reaction times, allowed the collection of neural data during interceptive saccades that fell both within and outside of the boundaries of the MF as defined during static trials.
Fig. 2.
Different target motion paths relative to a canonical movement field. The starting positions of moving targets (T1ini) were pseudorandomly selected among locations situated along the same imaginary lines used for the targets during static trials. Target motion could be radial (A, inward or outward relative to fixation target T0), vertical (B, upward or downward relative to T1ini, motion along an axis parallel to and different from the vertical meridian) or horizontal (C, rightward or leftward motion along an axis parallel to and different from the horizontal meridian).
Data set and analysis.
The horizontal and vertical eye positions for each trial were digitized and stored with a resolution of 1 ms and then analyzed off-line with custom software and MATLAB. The onset and offset of saccades were identified with a velocity criterion of 15°/s. Saccade metrics (amplitude, peak velocity, latency, etc.) reported here were obtained by measuring the first saccade (primary saccade) made after the presentation of T1 (equivalently, offset of T0). The primary saccade needed to occur between 100 ms and 500 ms after the offset of T0 to be considered for further analysis.
The present study concerns the discharge properties of 52 neurons (11, 16, and 25 neurons recorded in monkeys BB, BL, and WI, respectively) that fired a burst of action potentials during saccades. These are visuomotor and motor neurons found in the intermediate and deep SC layers. We did not attempt to differentiate between the two classes because both project to the saccade burst generator in the reticular formation (Raybourn and Keller 1977; Rodgers et al. 2006). Moreover, even putative motor neurons have the capacity to exhibit a visual response under certain conditions (Jagadisan and Gandhi 2016). We did not segregate the neurons according to the animal in which they were recorded because of the small size of our sample of neurons. Indeed, no significant difference was found when the shifts in boundary were compared between monkeys (nonparametric Mann-Whitney test, P > 0.05). Response fields were obtained by plotting firing rate (calculated as the number of spikes per second during a period beginning 20 ms before saccade onset and continuing until 10 ms before saccade end) as a function of horizontal, vertical, or radial saccade amplitude during either the static or motion trials. The MF boundaries and the vector for which the neuron fired the most (preferred amplitude) were estimated from a smoothing spline fit of the data with the curve-fitting toolbox in MATLAB. For each neuron, the same spline parameter was used for fitting the data of both tasks. The boundary was defined as the saccade amplitude from which the neuron starts firing with a rate >30 spikes/s. When the saccade-related burst was preceded by a prelude activity, the threshold was adjusted to the minimal value that characterizes the burst onset. For some neurons, the amplitude tuning was such that the firing rate did not exhibit a well-defined maximum; its curve exhibited either a plateau or a slope indicating that the peak would be attained with larger amplitudes. Therefore, to avoid erroneous values, the preferred amplitude was not measured in 6 of 39 neurons recorded with a radially moving target (5 neurons in monkey WI, 1 in monkey BL). Moreover, for some neurons, the proximal boundary of the MF could not be estimated because the monkey did not make the interceptive saccades with the amplitude that we “desired,” despite our efforts to vary the starting position of the moving target. This case was encountered for two neurons during rightward motions, two neurons during leftward motions, two neurons during inward motions, three neurons during outward motions, six neurons during upward motions, and nine neurons during downward motions. The Wilcoxon test (P < 0.05) was used to test for statistically significant differences in MF properties across neurons between the saccades toward a static and a moving target.
RESULTS
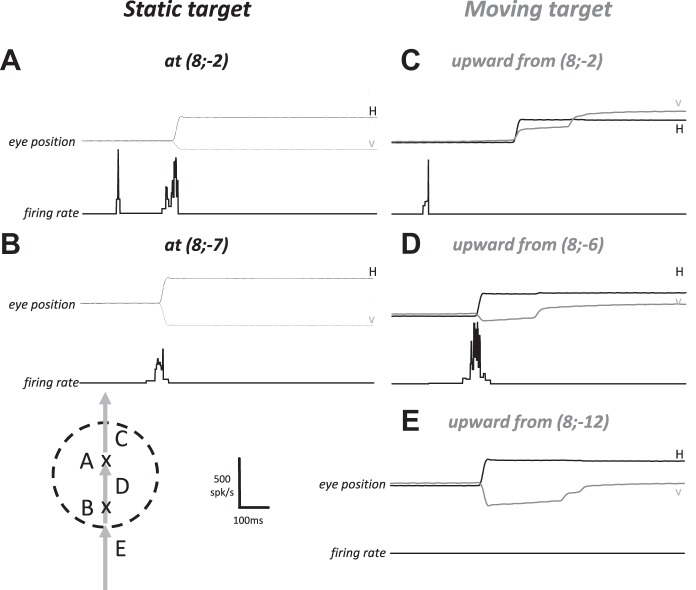
Figure 3A illustrates the firing rate of a typical visuomotor SC neuron during a static target trial. The first phasic response occurred ~100 ms after the onset of the visual target and was followed by a second, more vigorous burst timed with the saccade toward its location. The neuron also produced a weaker burst during saccades whose amplitude and direction slightly deviated from the neuron’s preferred vector (Fig. 3B); the visual response was absent for this particular location. In response to a target moving upward at the same horizontal eccentricity, the neuron’s discharge was different. When the target motion started from the location that elicited vigorous visual and perisaccadic responses during the static condition, the visual response was not followed by the saccade-related burst (Fig. 3C). Thus, the response of this neuron could signal the presence of the target within its response field, but it did not participate in the population activity that drives this particular interceptive saccade. The cell was active during saccades whose vectors matched the vectors that elicited the most vigorous perisaccadic bursts with a static target (compare Fig. 3A to Fig. 3D). Another observation is the absence of firing when the monkey made an interceptive saccade whose vector was associated with a perisaccadic burst if the target had been static (compare Fig. 3B to Fig. 3E). During this particular condition, the neuron was silent even though the saccade vector belonged to the MF measured with static targets (hereafter referred to as “static MF”) and even though the target was going to enter this MF.
Fig. 3.
Instantaneous firing rate of a dSC visuomotor neuron after target onset and during single trials. A and B: visual and saccade-related activity following the appearance of a static target at different locations (Cartesian coordinates) of the right visual field. C–E: firing rate of the same neuron after the target appears and moves upward at the same horizontal eccentricity. In A and C, the target appears at a location corresponding to the preferred amplitude of the neuron’s movement field (MF). In D, the saccade is aimed at the same location as in A: the visual response is absent because the moving target appears outside the neuron’s response field. In E, the saccade is aimed at the same location as in B: the neuron does not fire when the target moves. Schema at bottom left shows the boundary of a putative MF (dashed line) and 3 saccade vectors. Crosses labeled A and B schematically represent starting position of saccades illustrated in A and B, respectively. Labels C, D, and E illustrate the saccade vectors shown in C–E.
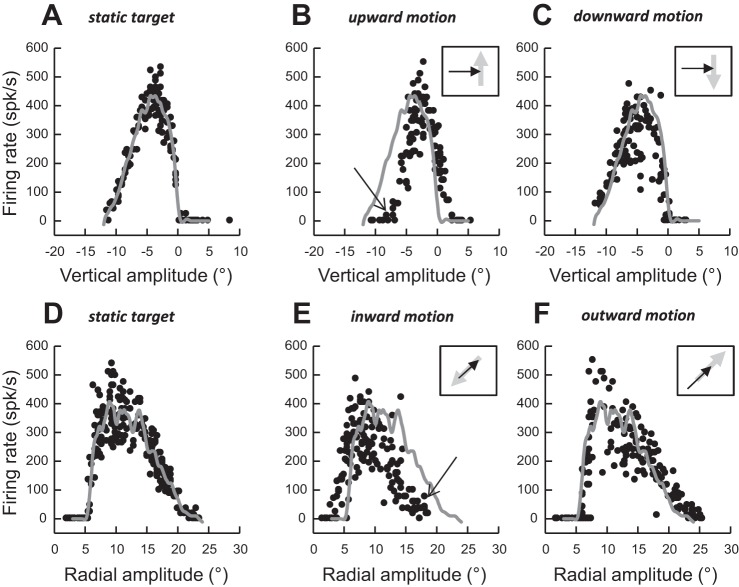
Figure 4 plots “slices” through the MF of the same cell during six target conditions: static (Fig. 4, A and D) and moving upward (Fig. 4B), downward (Fig. 4C), inward (Fig. 4E), and outward (Fig. 4F). In Fig. 4, A–C, the slices were generated by presenting targets along a vertical axis situated at a horizontal eccentricity of 8° to the right. During these target conditions, saccades had horizontal amplitudes ranging from 7.2° to 9.2°. With static targets, the neuron fired maximally during rightward saccades with a small (−4.4°) downward component (Fig. 4A); the discharge of this neuron was weaker when the saccade deviated from this preferred vertical amplitude. Estimated by a spline fitting procedure, the lower and upper boundaries of the vertical amplitude tuning curve were −11.2° and 0.1°, respectively. Compared with the static MF, the peak and the boundaries of the tuning (Fig. 4B) were shifted upward (toward positive values) during saccades made to a target moving upward (peak: −2.3°, shift Δ = 2.1°; lower boundary = −7.3°, Δ = 3.9°; upper boundary = 2.4°, Δ = 2.3°). When the target appeared below the lower edge of the static MF and moved upward toward the inside of the MF, the neuron did not fire unless the interceptive saccade involved a vertical component larger than −7.3° (see arrow in Fig. 4B). Thus, instead of emitting spikes that would promote the foveation of a target that was going to enter its MF, the neuron remained silent. Likewise, when the vertical amplitude of the interceptive saccade exceeded the amplitude corresponding to the upper boundary of the amplitude tuning observed with a static target (0.1°), instead of pausing and facilitating the generation of saccades with a larger upward component, this neuron emitted spikes, biasing the population of active neurons with a command encoding an oblique downward vector. While differences of amplitude tuning between the static and moving targets were clearly visible during saccades directed to a target moving upward, changes were barely visible in the saccade-related burst of this neuron when the target moved downward (Fig. 4C). Thus, the effects of a moving target on the MF properties of this particular neuron were consistent with the dual drive hypothesis when the saccades were made to a target moving upward and with the remapping hypothesis when they were made to a target moving downward. In Fig. 4, D–F, we describe the burst during saccades made along the radial axis of its MF. During saccades to static targets, the neuron fired during saccades of radial amplitudes ranging from 5.5° (inner boundary) to 20.7° (outer boundary), with the most vigorous bursts occurring for 8.9° saccades (Fig. 4D). During saccades to a target moving from the peripheral to the central visual field (inward motions), the amplitude tuning was shifted toward smaller amplitude values (Fig. 4E). When the target started its motion from outside the MF and moved inward, the neuron did not fire unless the monkey made a 17° saccade (see arrow in Fig. 4E). Thus, instead of emitting spikes that would promote the reduction of saccade amplitudes, the neuron remained silent. Moreover, although no firing was observed during small saccades toward static targets with eccentricity <5°, the neuron discharged during small saccades made to an inward target motion. A small shift of the MF was also observed in the direction of the target motion during outward motions (Fig. 4F): the outer boundary shifted toward larger amplitudes (Δ = 2.1°), whereas the inner boundary barely changed (Δ = 0.4°).
Fig. 4.
Movement field (MF) of the same neuron as in Fig. 3 during saccades toward targets located on axis parallel to the vertical meridian (top) or along the radial axis of its MF (bottom). A and D: static target. B: target moving upward. C: target moving downward. E: target moving inward (toward the fixation target). F: target moving outward (away from the fixation target). Arrows in B and E show the shift in the boundary of the MF. Gray traces show the spline fit from static target trials. Insets in B, C, E, and F schematize the moving target (gray arrow) and 1 possible interceptive saccade (black arrow) in a head-centered reference frame.
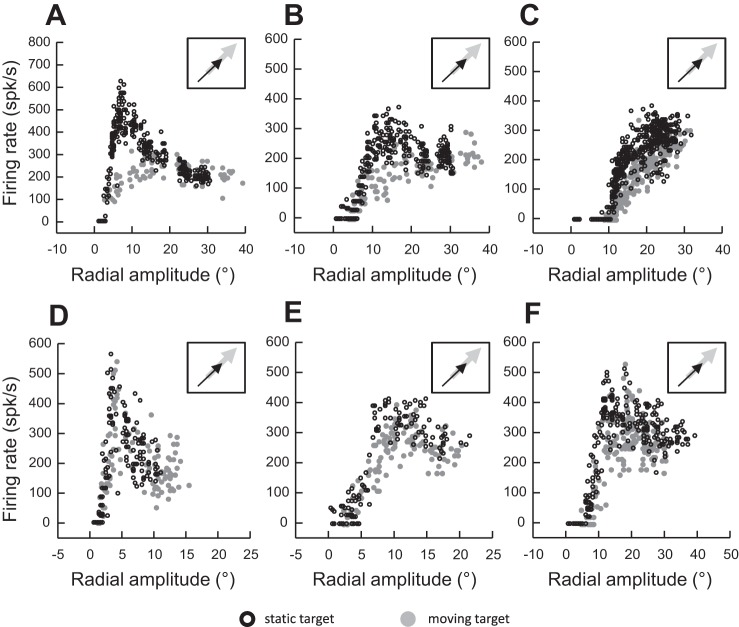
Many of the cells that we recorded exhibited open MFs, so only the proximal boundary could be identified. Figure 5 shows four examples of such neurons where the amplitude tuning curves exhibited a shift in boundary (consistent with the dual drive hypothesis), whereas Fig. 6 shows examples of neurons where the shift was absent or barely visible (consistent with the remapping hypothesis). Figure 5 shows the amplitude tunings during saccades made to a static target or to a target moving along an axis orthogonal to the vertical meridian (Fig. 5A: rightward motion), a radial axis (Fig. 5B: outward motion), or an axis perpendicular to the horizontal meridian (Fig. 5C: downward motion; Fig. 5D: upward motion). For each of these neurons, the boundary of the MF is shifted in the same direction as the target motion. By contrast, Fig. 6 shows examples of neurons that exhibited no shift or a barely visible shift in the MF boundary during interceptive saccades (as in Fig. 4F). Some of them exhibited a lower firing rate during saccades made to the “center” of the MF (Fig. 6, A–C and F). However, this reduced firing rate was not observed during small (Fig. 6, A and D) or large (Fig. 6, C and F) saccades.
Fig. 5.
Movement fields of 4 other neurons exhibiting a shift during saccades toward a moving target in comparison to saccades toward a static target. A: target moves to the right. B: target moves outward along the radial axis. C: target moves downward. D: target moves upward. Insets schematize the moving target (gray arrow) and 1 possible interceptive saccade (black arrow) in a head-centered reference frame.
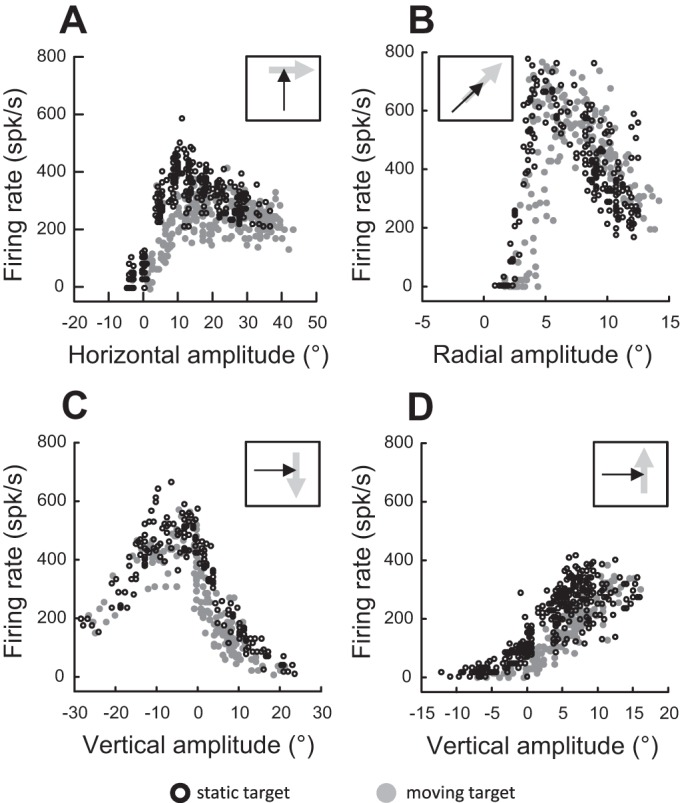
Fig. 6.
Examples of 6 other neurons (A–F) where the shift of either the preferred amplitude or the inner boundary of the movement field was barely visible or almost absent. Gray, firing rate during interceptive saccades; black, firing rate during saccades toward a static target. Insets schematize the moving target (gray arrow) and 1 possible interceptive saccade (black arrow) in a head-centered reference frame.
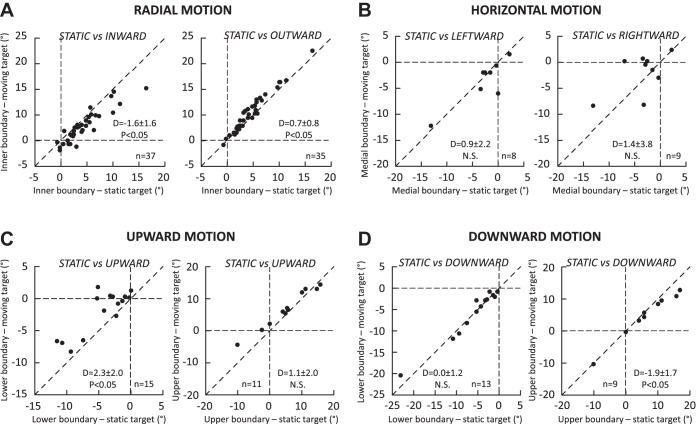
Figure 7 compares, for all neurons, the boundaries of static MF to those of MF measured during saccades made toward a target that moved radially (Fig. 7A), horizontally (Fig. 7B), or vertically (Fig. 7C: upward, Fig. 7D: downward) across their MF. In comparison to the static target conditions, the inner boundary shifted toward small amplitude values when the saccades were made to a target that moved inward, i.e., toward the central visual field (Fig. 7A, left; average difference = −1.6 ± 1.6°, nonparametric Wilcoxon test, P < 0.05). During outward motion (Fig. 7A, right), a small but significant shift toward larger amplitude values, in the same direction as the target motion, was also observed (0.7 ± 0.8°, P < 0.05). When the target moved horizontally across the MF (Fig. 7B), no significant difference in the medial boundary was observed during leftward (0.9 ± 2.2°, P value = 0.25) or rightward (1.4 ± 3.8°, P value = 0.29) motion. The absence of significant difference is likely due to the small sample of neurons recorded during this motion condition of target motion (3 neurons in monkey BL, 7 in monkey WI, all in the left dSC). In contrast, when the target moved upward (Fig. 7C), a shift in the same direction as the target motion was observed for the lower boundary (Fig. 7C, left; 2.3 ± 2.0°, P < 0.05). For the upper boundary (Fig. 7C, right), the difference failed to reach our threshold of statistical significance (1.1 ± 2.0°, P value = 0.07). During downward target motion (Fig. 7D), a significant shift was observed for the upper boundary (−1.9 ± 1.7°, P < 0.05; Fig. 7D, right) but not for the lower boundary (0.0 ± 1.2°, P value = 0.81; Fig. 7D, left). In summary, average shifts in the MF boundaries were observed but not in every condition. Crucially, whenever a significant difference was found between the static and dynamic MFs, the shift was always in the same direction as the target motion.
Fig. 7.
Comparison of the MF boundaries between saccades toward a static target (x-axis) and saccades toward a target (y-axis) moving along the radial axis (A), a horizontal axis (B), and a vertical axis passing through the preferred amplitude (C and D). The moving target moves upward in C and downward in D. Each dot corresponds to the measurement provided by spline fitting the amplitude tuning curves for each neuron when this was possible (see Data set and analysis). In each graph, the mean and SD of differences (D values) and the statistical significance of their comparison with the Wilcoxon test are documented. P values obtained for not statistically significant differences (N.S., P > 0.05) can be found in the text.
While Keller et al. (1996) did not describe the MF boundaries, they reported a shift in MF preferred vectors during saccades made toward a stimulus moving outward; other directions of target motion were not tested. Figure 8 complements and extends their study by comparing the preferred amplitude values during radial (Fig. 8A), vertical (Fig. 8B), and horizontal (Fig. 8C) target motions. The preferred amplitudes significantly changed during saccades aimed at a target moving outward (Fig. 8A, right; average difference = 2.6 ± 3.5°, P < 0.05). No consistent shift was observed during saccades aimed at a target moving inward (−0.4 ± 3.0°, P value = 0.25). During vertical motions (Fig. 8B), a shift was observed when the target moved upward (3.1 ± 3.3°, P < 0.05; Fig. 8B, right) but not when it moved downward (−0.5 ± 2.7°, P value = 0.81; Fig. 8B, left). During horizontal target motion (Fig. 8C), a significant change was observed during leftward motion (−3.5 ± 4.0°, P < 0.05) but not during rightward motion (2.0 ± 3.9°, P value = 0.29). In summary, shifts in the preferred amplitude were observed but not in every condition. Whenever a significant difference of preferred amplitude was found in the tuning between the static and moving targets, the shift was always in the same direction as the target motion.
Fig. 8.
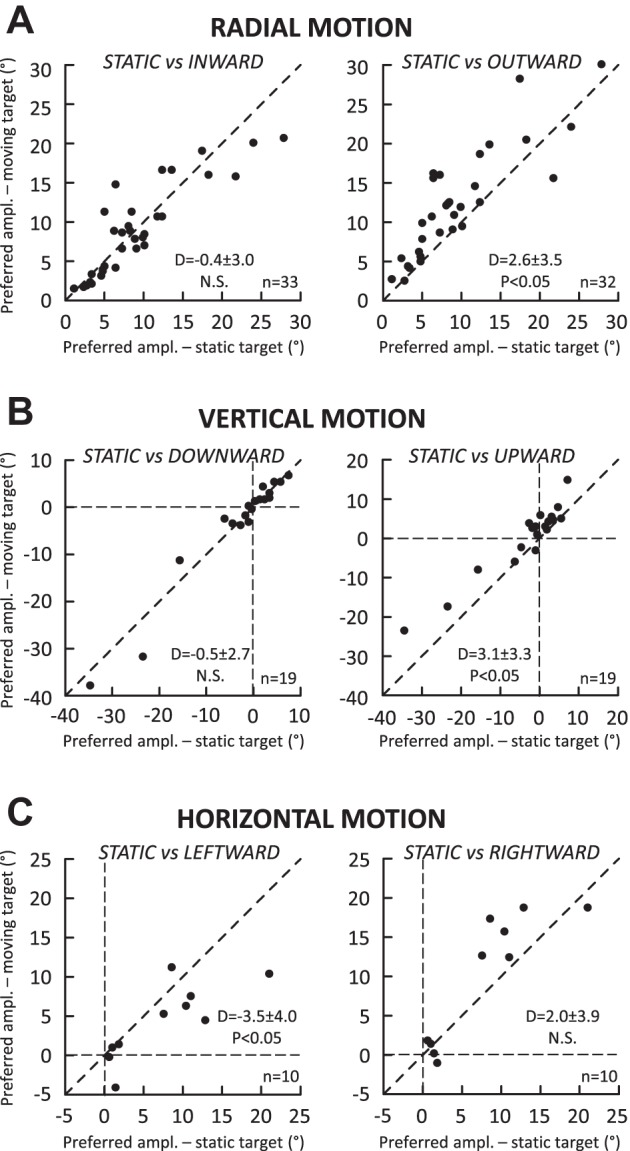
Comparison of the MF preferred amplitude between saccades toward a static target (x-axis) and saccades toward a target (y-axis) moving along the radial axis (A), the vertical axis (B), and the horizontal axis passing through the preferred amplitude (C). Each dot corresponds to the measurement provided by spline fitting the amplitude tuning curves for each neuron when this was possible (see Data set and analysis). In each graph, the mean and SD of differences (D values) and the statistical significance of their comparison with the Wilcoxon test are documented. P values obtained for not statistically significant differences (N.S., P > 0.05) can be found in the text.
During saccades toward a target moving outward, significant shifts were observed in the inner boundary (0.7 ± 0.8°; Fig. 7A) and preferred amplitude (2.6 ± 3.5°; Fig. 8A) of amplitude tunings. However, these changes were not correlated (Spearman correlation coefficient R = 0.05, P > 0.05). There was also no dependence between the shifts of the inner boundary and preferred amplitude of the tunings recorded with the saccades toward a target moving inward (R = 0.32, P > 0.05). Figure 9 plots the relation between these MF parameters during saccades toward a target moving inward (Fig. 9A) and outward (Fig. 9B). No correlation was found between the shifts of the inner boundary measured for the saccades made to targets moving inward and outward (R = 0.09; Fig. 9C). A significant correlation was found between the shifts of preferred amplitude (R = 0.58, P < 0.05). However, this correlation should be interpreted very carefully. The top right quadrant of Fig. 9D (positive values of shifts) corresponds to cells (11 of 31) for which the preferred amplitude shifted in the direction opposite to target motion during inward target motions whereas the shift was in the same direction as the target motion during outward motions. The lower left quadrant of Fig. 9D (negative values of shifts) corresponds to cells (6 of 31) for which the preferred amplitude shifted in the same direction as the target motion during inward target motions but in the opposite direction during outward motions. Thus, for the remaining cells (14/31), the shift of preferred amplitude was in the same direction as the target motion, regardless of whether the target was moving inward or downward. In other words, the shift of preferred amplitude is a poor indicator of the target motion direction.
Fig. 9.
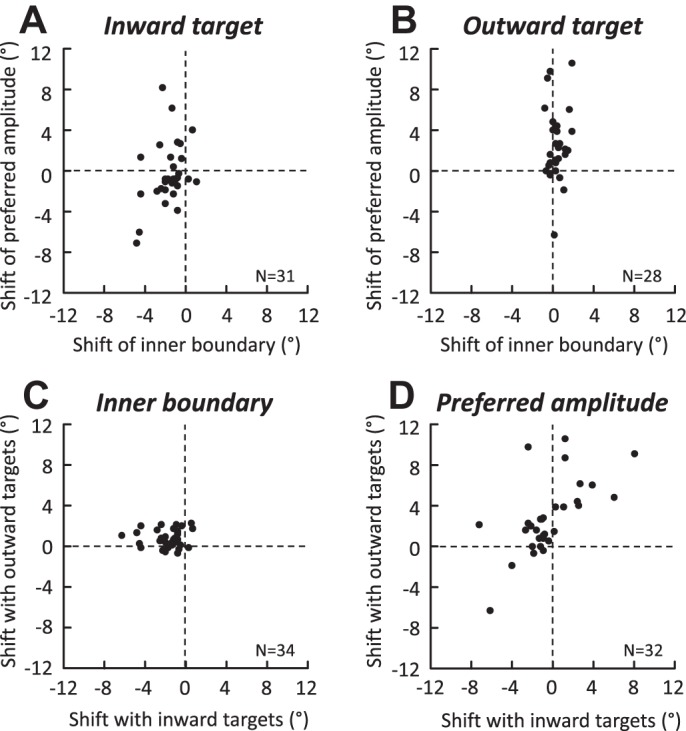
Comparison of the shifts of inner boundary and preferred amplitude during saccades toward a target moving inward (A) and outward (B). The shifts of inner boundary (C) and preferred amplitude (D) observed with the inward and outward targets are also shown. Each dot corresponds to the difference between the measurements shown in Figs. 7A and 8A (value during moving target condition − value during static target condition).
Finally, when the average firing rates were compared between saccades toward a static and a moving target, significant reductions were consistently observed during radial motions (Fig. 10A; −90 ± 91 and −89 ± 90 spikes/s for inward and outward targets, corresponding to 23% reductions), during vertical motions (Fig. 10B; −54 ± 92 and −52 ± 100 spikes/s for downward and upward motions; 15% reductions), and during horizontal motions (Fig. 10C; −123 ± 87 and −141 ± 98 spikes/s for leftward and rightward motions; 32% and 36% reductions). Contrary to the suggestion made by Berthoz et al. (1986), the firing rate of collicular cells during saccades made toward a moving target is not related to their velocity. Figure 11 shows two examples of cells where the largest difference in MF was found between inward and outward target motions. For the first neuron, when one considers the saccades of amplitudes <5°, the firing rate was higher during inward motions than during outward motions, whereas for saccades of amplitudes >5°, the firing rate was lower during inward motions than during outward motions (Fig. 11A, left). Yet the relation between the amplitude and the peak velocity of saccades does not show any difference between the two groups (Fig. 11A, right). For the other neuron, the firing rate was always lower during saccades made toward a target moving outward than toward a target moving inward (Fig. 11B, left) and, again, no difference in velocity was observed between the two saccade types (Fig. 11B, right). Our results contrast with the qualitative impression illustrated in the work of Keller et al. (1996) (see their Fig. 1). Perhaps the “shoulder” or double peaks in the velocity waveform were due to accompanying gaze-evoked blinks (Gandhi 2012). The attenuation reported here could be related to the uncertainty about the exact location of the saccade goal (Basso and Wurtz 1998).
Fig. 10.
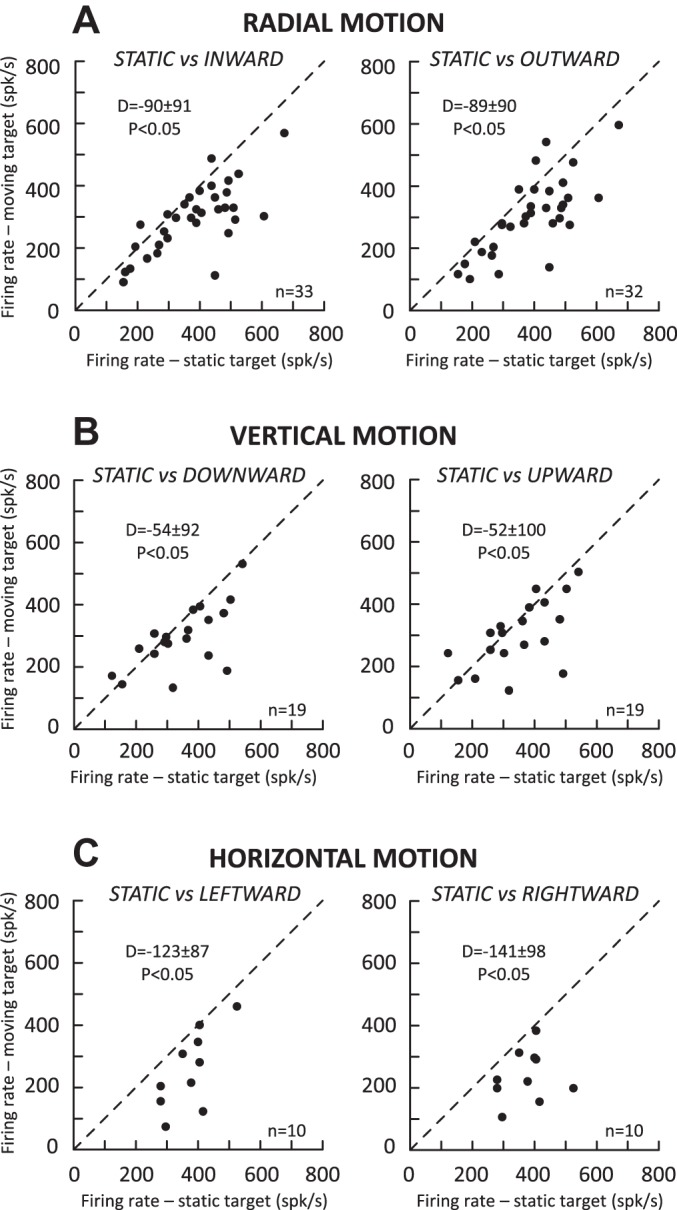
Comparison of the average firing rate (at MF preferred amplitude) of the motor burst between saccades toward a static target (x-axis) and saccades toward a target (y-axis) moving along the radial axis (A), the vertical axis (B), and the horizontal axis passing through the preferred amplitude (C). Each dot corresponds to the measurement provided by spline fitting the amplitude tuning curves for each neuron when this was possible (see Data set and analysis). In each graph, the mean and SD of differences (D values) and the statistical significance of their comparison with the Wilcoxon test are documented.
Fig. 11.
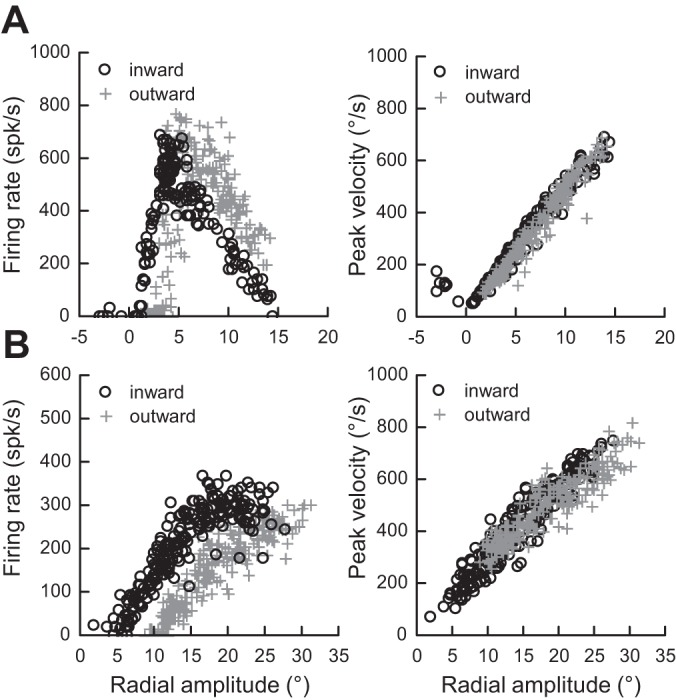
The firing rate of dSC cells is not related to the velocity of interceptive saccades. Two examples of cells are shown where the largest difference in MF was found between inward and outward target motions. For the neuron shown in A, the firing rate was higher during saccades of amplitude <5° when they were made to a target moving inward than to a target moving outward but lower for amplitudes >5° (left). The relation between the amplitude and the peak velocity of saccades does not show any difference between the 2 groups of saccades (right). For the neuron shown in B, the firing rate was lower for outward moving targets than for inward moving targets (left). Again, the relation between the amplitude and the peak velocity of saccades does not show any difference between the 2 groups of saccades (right).
DISCUSSION
In this work, we studied the MF of saccade-related neurons in the dSC while monkeys made saccades toward a static or moving visual target. For some neurons, significant shifts were found in the preferred vector of the MF, in their boundaries, and in the firing rate. The changes indicate that for a given saccade the population of bursting neurons is not identical between the two types of saccade. However, the shifts were not always observed and their size varied across the cells. When they were present, they were always in the direction of target motion, never in the opposite direction. The absence of shift of boundaries in the direction opposite to the target motion implies that the SC activity does not contain action potentials corresponding to commands related to upcoming locations of the moving target; no evidence was found for a predictive coding. A reduction in the discharge was also observed during interceptive saccades. Unrelated to any change in saccade velocity, this lower firing rate could be due to the uncertainty about the exact location of the saccade goal when the neuron’s response field is traveled by a moving object rather than when it was excited by a static stimulus.
No predictive coding in SC for generation of interceptive saccades.
The idea has diffused that the dSC would identify the position and speed of an object and, in a predictive and anticipatory manner, trigger the movement required to orient the gaze toward its future location (Berthoz 2012; Optican and Pretegiani 2017). More precisely, the target motion signals would be “used to predict the future target position so as to assure a spatial lead of the gaze at the saccade end, instead of attempting a precise capture of the target” (Klam et al. 2001). The present study presents a physiological argument refuting this conjecture and is congruent with previous results showing 1) the maintenance of stable pursuit during partial inactivation of the rostral SC (Hafed et al. 2008), 2) the relatively accurate capture of a moving target by interceptive saccades even when they are perturbed (Fleuriet and Goffart 2012), and 3) the landing of interceptive saccades on locations that do not correspond to the future target location (Quinet and Goffart 2015a). During the emission of the saccade-related burst, the active population does not include cells whose firing codes for saccades toward future locations of the moving target. Our study shows that during inward motions, when the target moved from a location outside the MF toward its inside, none of our neurons emitted action potentials that would promote the reduction of saccade amplitude; the outer boundary of their MF did not shift toward larger values of saccade amplitude (e.g., Fig. 4E). Likewise, during outward motions, when the target moved from a location inside the MF toward a location outside, instead of pausing and facilitating the amplitude increase the neurons continued to fire, biasing the vector encoded by the population of active neurons toward past locations of the target and not to its forthcoming locations (e.g., Fig. 4F). In summary, contrary to what would be expected if the dSC neurons fired in a predictive manner, the boundaries did not shift in the direction opposite to the target motion. The neurons did not fire during saccades toward a target that was going to enter their response field. Moreover, their firing persisted when the target, after crossing the response field, moved away from it.
It may be argued that our testing conditions did not favor the possibility of emitting predictive responses because our subjects were not trained to pursue the target or because the direction of target motion and the trials with static and moving targets were pseudorandomly interleaved. Under restricted conditions, anticipatory saccades could have been observed if the target always moved from the same starting location, in the same direction, and after a constant fixation delay. Such saccades might even be triggered before the target appears, associated with bursting activities in the dSC, more likely if a gap were introduced between the offset of the fixation target and the onset of the moving target. However, the generation of such premature saccades does not necessarily involve a shift of the MF of dSC neurons in the direction opposite to the target motion. If the dSC activity steers the interceptive saccades like saccades toward a static target, i.e., toward the location where the target is estimated to be here and now (Fleuriet and Goffart 2012), then the MFs should overlap between saccades toward static and moving targets.
Dual drive and remapping hypotheses.
Consistent with the study of Keller et al. (1996), we found that, on average, the preferred amplitude of MF shifted in the direction of the target motion during outward motions (Fig. 7A, right). But the shift was small and not consistently observed across all neurons (see examples in Fig. 4C and Fig. 6), comparable to observations made in the frontal eye fields (Cassanello et al. 2008). Should we consider that the generation of saccades involves two subgroups in the SC, with one subgroup composed of neurons that exhibit a shift and another of neurons that do not? This option would require that we consider subgroups of neurons also for the generation of saccades toward a target moving inward, and likewise for upward and downward target motions. Indeed, the preferred vector of our example neuron was shifted during inward (Fig. 4E) and upward (Fig. 4B) motions but not during outward (Fig. 4F) or downward (Fig. 4C) motions. Current knowledge of the dSC physiology does not support such a segregation (Gandhi and Katnani 2011; Hall and Moschovakis 2003; May 2006). The only known segregation takes place in the pontomedullary and mesencephalic reticular formations, at the level of the premotor neurons that are involved in the generation of the horizontal and vertical components of saccades (Barton et al. 2003; Moschovakis et al. 1996) or in the generation of eye and head components of gaze shifts (Gandhi and Katnani 2011). Therefore, instead of segregation, we propose a continuum of commands within the dSC, ranging from commands related to the past location of the target to commands related to its present location. By present location, we mean the location that is targeted by saccades toward a stationary stimulus.
Neurophysiological studies indicate that the generation of saccades is under the influence of activity originating in the dSC and the cFN. According to the dual drive hypothesis, the MF changes observed during interceptive saccades result from the fact that the saccade-related premotor neurons in the reticular formation are summing commands from these two structures. Because the locus of activity in the dSC is supposed to encode the location where the target appears initially, the MF (preferred vector and boundaries) is expected to be shifted in the direction of the target motion. The lack of a correlation between the shifts of preferred amplitude and the shifts of boundaries (Fig. 9, A and B) is not consistent with this hypothesis. Yet, several experimental results indicate independent influences of cFN and dSC on the reticular formation, i.e., that the fastigial-induced changes of premotor activity do not influence the activity of neurons in the dSC (see discussion of Quinet and Goffart 2015b). The strongest evidence comes from microstimulation studies. During electrical stimulation of the dSC, a movement of the head is almost always observed in addition to the eye saccade (Freedman et al. 1996; Walton et al. 2007). When the stimulation is applied in the fastigial nucleus, the head barely moves (Quinet and Goffart 2009). However, other observations indicate that the cFN influence on the premotor neurons is modulatory rather than additive (Goffart et al. 2004; Quinet and Goffart 2007). Let us consider a target appearing at some eccentric location along the vertical meridian and moving horizontally away from it (as in the protocols used by Fleuriet et al. 2011 and Quinet and Goffart 2015a). If the cFN provides a command that compensates for the motion of the target after its appearance, we should expect that this supplementary command is constant (or zero) when the target is static. This inference is not supported by the amplitude-dependent horizontal deviation (ipsipulsion) of vertical saccades when the cFN is unilaterally inactivated with muscimol (Goffart et al. 2004; Iwamoto and Yoshida 2002; Quinet and Goffart 2007). The observation that unilateral cFN inactivation does not affect the vertical component of saccades toward static (Goffart et al. 2004; Quinet and Goffart 2007; Robinson et al. 1993) or moving (Bourrelly et al. 2017) targets also indicates that the cFN is not sufficient for complementing the dSC activity during oblique interceptive saccades. Additional structures must be involved, for controlling not only their vertical component but also their coupling with a movement of the head. Indeed, all neurophysiological approaches indicate that the cFN activity essentially influences the generation of the eye component of gaze shifts (Fuchs et al. 2010; Quinet and Goffart 2007, 2009). Finally, the dual drive hypothesis considers that the dSC encodes the location of the target appearance, overlooking the possibility of subsequent changes in the distribution of active neurons. However, this view is supported by neither our observations of cells whose MF does not differ between saccades toward a static and a moving target nor the demonstration that the population of active neurons can change during saccades made toward a target that jumps toward a new location (McPeek et al. 2003; Port and Wurtz 2003).
The shift of the MF boundaries indicates that the locus of activity in the dSC is different between identical saccades made toward a static and a moving target. The fact that on average the shift is in the same direction as the target motion indicates that the population of active neurons includes commands for generating a saccade toward a past location of the target. The larger shifts of preferred vectors observed by Keller et al. (1996) are consistent with this view, since in their work the target moved two to three times faster than in our study. Moreover, the examination of the shift for each individual neuron shows a continuum of neurons ranging from cells that exhibited a shift to cells with no change or a very small shift. Therefore, instead of considering that all dSC neurons provide a discrete snapshot command and that another drive is added downstream, we propose that the shifts illustrate the fact that the population of active neurons does not change in the dSC as fast as the target does in the visual field. It would actually consist of a continuum of neurons issuing commands, ranging from commands related to antecedent target locations to commands related to its current location. Thus, the saccade-related burst would continuously steer the saccade until its end, in accordance with the demonstration that a cessation of dSC activity is rapidly followed by an arrest of the saccade (Freedman et al. 1996; Stanford et al. 1996). More generally, this population burst feeds the oculomotor system with a continuous command that specifies the target location, be it static or moving (Goffart et al. 2017), in the peripheral (Katnani and Gandhi 2012; Lee et al. 1988; McPeek et al. 2003; Noto and Gnadt 2009; Sparks et al. 1990; Watanabe et al. 2005) or central (Goffart et al. 2012; Hafed et al. 2008) visual field. Downstream adjustments for improving the accuracy of the foveation are still possible, from the cFN but from other regions also. Indeed, experimental studies indicate that the cFN is essentially involved in the control of the horizontal component of fixational saccades (Guerrasio et al. 2010) and regular saccades made with the head fixed (Goffart et al. 2004; Quinet and Goffart 2015b) or with the head free to move (Quinet and Goffart 2007, 2009) (see also the anatomical study of Sato and Noda 1991). Downstream from the dSC, modulatory adjustments are still required for spatially and temporally coordinating [i.e., space-timing (Pellionisz and Llinás 1982) or spatially synchronizing (Bourrelly et al. 2016; Goffart et al. 2017)] the orientation of gaze with the motion of a visual target, even when a rotation of the head or a body movement must accompany the eye movement.
GRANTS
This work was supported by National Eye Institute Grants EY-022854 and EY-02831 to N. Gandhi. L. Goffart was supported by the Centre National de la Recherche Scientifique and the European Research Council under the European Union’s Seventh Framework Program (FP7/2007-2013/ERC Grant Agreement No. AG324070 to Dr. Patrick Cavanagh).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
L.G. and N.J.G. conceived and designed research; L.G., A.L.C., and N.J.G. performed experiments; L.G. and N.J.G. analyzed data; L.G. and N.J.G. interpreted results of experiments; L.G. prepared figures; L.G., A.L.C., and N.J.G. drafted manuscript; L.G., A.L.C., and N.J.G. edited and revised manuscript; L.G., A.L.C., and N.J.G. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Dr. Uday K. Jagadisan for helpful assistance during the experiments.
REFERENCES
- Barton EJ, Nelson JS, Gandhi NJ, Sparks DL. Effects of partial lidocaine inactivation of the paramedian pontine reticular formation on saccades of macaques. J Neurophysiol 90: 372–386, 2003. doi: 10.1152/jn.01041.2002. [DOI] [PubMed] [Google Scholar]
- Basso MA, Wurtz RH. Modulation of neuronal activity in superior colliculus by changes in target probability. J Neurosci 18: 7519–7534, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berthoz A. Simplexity: Simplifying Principles for a Complex World, translated by Weiss G. New Haven, CT: Yale Univ. Press, 2012. doi: 10.12987/yale/9780300169348.001.0001. [DOI] [Google Scholar]
- Berthoz A, Grantyn A, Droulez J. Some collicular efferent neurons code saccadic eye velocity. Neurosci Lett 72: 289–294, 1986. doi: 10.1016/0304-3940(86)90528-8. [DOI] [PubMed] [Google Scholar]
- Bourrelly C, Quinet J, Cavanagh P, Goffart L. Learning the trajectory of a moving visual target and evolution of its tracking in the monkey. J Neurophysiol 116: 2739–2751, 2016. doi: 10.1152/jn.00519.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourrelly C, Quinet J, Cavanagh P, Goffart L. Cerebellar control of the ability to track a moving target: role of the fastigial oculomotor region (Abstract). Neuroscience Meeting Planner 2017: 59.01, 2017. [Google Scholar]
- Bremmer F, Kaminiarz A, Klingenhoefer S, Churan J. Decoding target distance and saccade amplitude from population activity in the macaque lateral intraparietal area (LIP). Front Integr Neurosci 10: 30, 2016. doi: 10.3389/fnint.2016.00030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryant CL, Gandhi NJ. Real-time data acquisition and control system for the measurement of motor and neural data. J Neurosci Methods 142: 193–200, 2005. doi: 10.1016/j.jneumeth.2004.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassanello CR, Nihalani AT, Ferrera VP. Neuronal responses to moving targets in monkey frontal eye fields. J Neurophysiol 100: 1544–1556, 2008. doi: 10.1152/jn.01401.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui H, Malpeli JG. Activity in the parabigeminal nucleus during eye movements directed at moving and stationary targets. J Neurophysiol 89: 3128–3142, 2003. doi: 10.1152/jn.01067.2002. [DOI] [PubMed] [Google Scholar]
- Erlikhman G, Caplovitz GP. Decoding information about dynamically occluded objects in visual cortex. Neuroimage 146: 778–788, 2017. doi: 10.1016/j.neuroimage.2016.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrera VP, Barborica A. Internally generated error signals in monkey frontal eye field during an inferred motion task. J Neurosci 30: 11612–11623, 2010. doi: 10.1523/JNEUROSCI.2977-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleuriet J, Goffart L. Saccadic interception of a moving visual target after a spatiotemporal perturbation. J Neurosci 32: 452–461, 2012. doi: 10.1523/JNEUROSCI.3896-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleuriet J, Hugues S, Perrinet L, Goffart L. Saccadic foveation of a moving visual target in the rhesus monkey. J Neurophysiol 105: 883–895, 2011. doi: 10.1152/jn.00622.2010. [DOI] [PubMed] [Google Scholar]
- Freedman EG, Stanford TR, Sparks DL. Combined eye-head gaze shifts produced by electrical stimulation of the superior colliculus in rhesus monkeys. J Neurophysiol 76: 927–952, 1996. [DOI] [PubMed] [Google Scholar]
- Fuchs AF, Brettler S, Ling L. Head-free gaze shifts provide further insights into the role of the medial cerebellum in the control of primate saccadic eye movements. J Neurophysiol 103: 2158–2173, 2010. doi: 10.1152/jn.91361.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs AF, Robinson FR, Straube A. Participation of the caudal fastigial nucleus in smooth-pursuit eye movements. I. Neuronal activity. J Neurophysiol 72: 2714–2728, 1994. [DOI] [PubMed] [Google Scholar]
- Gandhi NJ. Interactions between gaze-evoked blinks and gaze shifts in monkeys. Exp Brain Res 216: 321–339, 2012. doi: 10.1007/s00221-011-2937-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gandhi NJ, Katnani HA. Motor functions of the superior colliculus. Annu Rev Neurosci 34: 205–231, 2011. doi: 10.1146/annurev-neuro-061010-113728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goffart L. Saccadic eye movements: basic neural processes. In: Reference Module in Neuroscience and Biobehavioral Psychology. Amsterdam: Elsevier, 2017. doi: 10.1016/B978-0-12-809324-5.02576-1. [DOI] [Google Scholar]
- Goffart L, Bourrelly C, Quinet J. Synchronizing the tracking eye movements with the motion of a visual target: basic neural processes. Prog Brain Res, 2017. doi: 10.1016/bs.pbr.2017.07.009. [DOI] [PubMed] [Google Scholar]
- Goffart L, Chen LL, Sparks DL. Deficits in saccades and fixation during muscimol inactivation of the caudal fastigial nucleus in the rhesus monkey. J Neurophysiol 92: 3351–3367, 2004. doi: 10.1152/jn.01199.2003. [DOI] [PubMed] [Google Scholar]
- Goffart L, Hafed ZM, Krauzlis RJ. Visual fixation as equilibrium: evidence from superior colliculus inactivation. J Neurosci 32: 10627–10636, 2012. doi: 10.1523/JNEUROSCI.0696-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan Y, Eggert T, Bayer O, Büttner U. Saccades to stationary and moving targets differ in the monkey. Exp Brain Res 161: 220–232, 2005. doi: 10.1007/s00221-004-2070-3. [DOI] [PubMed] [Google Scholar]
- Guerrasio L, Quinet J, Büttner U, Goffart L. Fastigial oculomotor region and the control of foveation during fixation. J Neurophysiol 103: 1988–2001, 2010. doi: 10.1152/jn.00771.2009. [DOI] [PubMed] [Google Scholar]
- Hafed ZM, Goffart L, Krauzlis RJ. Superior colliculus inactivation causes stable offsets in eye position during tracking. J Neurosci 28: 8124–8137, 2008. doi: 10.1523/JNEUROSCI.1317-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall WC, Moschovakis AK, editors. The Superior Colliculus: New Approaches for Studying Sensorimotor Integration. Boca Raton, FL: CRC, 2003. doi: 10.1201/9780203501504. [DOI] [Google Scholar]
- Hanes DP, Wurtz RH. Interaction of the frontal eye field and superior colliculus for saccade generation. J Neurophysiol 85: 804–815, 2001. [DOI] [PubMed] [Google Scholar]
- Isa T, Hall WC. Exploring the superior colliculus in vitro. J Neurophysiol 102: 2581–2593, 2009. doi: 10.1152/jn.00498.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwamoto Y, Yoshida K. Saccadic dysmetria following inactivation of the primate fastigial oculomotor region. Neurosci Lett 325: 211–215, 2002. doi: 10.1016/S0304-3940(02)00268-9. [DOI] [PubMed] [Google Scholar]
- Jagadisan UK, Gandhi NJ. Disruption of fixation reveals latent sensorimotor processes in the superior colliculus. J Neurosci 36: 6129–6140, 2016. doi: 10.1523/JNEUROSCI.3685-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katnani HA, Gandhi NJ. The relative impact of microstimulation parameters on movement generation. J Neurophysiol 108: 528–538, 2012. doi: 10.1152/jn.00257.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller E, Johnsen SD. Velocity prediction in corrective saccades during smooth-pursuit eye movements in monkey. Exp Brain Res 80: 525–531, 1990. doi: 10.1007/BF00227993. [DOI] [PubMed] [Google Scholar]
- Keller EL, Gandhi NJ, Weir PT. Discharge of superior collicular neurons during saccades made to moving targets. J Neurophysiol 76: 3573–3577, 1996. [DOI] [PubMed] [Google Scholar]
- Klam F, Petit J, Grantyn A, Berthoz A. Predictive elements in ocular interception and tracking of a moving target by untrained cats. Exp Brain Res 139: 233–247, 2001. doi: 10.1007/s002210100759. [DOI] [PubMed] [Google Scholar]
- Konen CS, Kastner S. Representation of eye movements and stimulus motion in topographically organized areas of human posterior parietal cortex. J Neurosci 28: 8361–8375, 2008. doi: 10.1523/JNEUROSCI.1930-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee C, Rohrer WH, Sparks DL. Population coding of saccadic eye movements by neurons in the superior colliculus. Nature 332: 357–360, 1988. doi: 10.1038/332357a0. [DOI] [PubMed] [Google Scholar]
- Lynch JC. Frontal eye field lesions in monkeys disrupt visual pursuit. Exp Brain Res 68: 437–441, 1987. doi: 10.1007/BF00248811. [DOI] [PubMed] [Google Scholar]
- Ma R, Cui H, Lee SH, Anastasio TJ, Malpeli JG. Predictive encoding of moving target trajectory by neurons in the parabigeminal nucleus. J Neurophysiol 109: 2029–2043, 2013. doi: 10.1152/jn.01032.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maioli MG, Domeniconi R, Squatrito S, Riva Sanseverino E. Projections from cortical visual areas of the superior temporal sulcus to the superior colliculus, in macaque monkeys. Arch Ital Biol 130: 157–166, 1992. [PubMed] [Google Scholar]
- May PJ. The mammalian superior colliculus: laminar structure and connections. Prog Brain Res 151: 321–378, 2006. doi: 10.1016/S0079-6123(05)51011-2. [DOI] [PubMed] [Google Scholar]
- McPeek RM, Han JH, Keller EL. Competition between saccade goals in the superior colliculus produces saccade curvature. J Neurophysiol 89: 2577–2590, 2003. doi: 10.1152/jn.00657.2002. [DOI] [PubMed] [Google Scholar]
- Moors J, Vendrik AJ. Responses of single units in the monkey superior colliculus to moving stimuli. Exp Brain Res 35: 349–369, 1979. [DOI] [PubMed] [Google Scholar]
- Moschovakis AK, Scudder CA, Highstein SM. The microscopic anatomy and physiology of the mammalian saccadic system. Prog Neurobiol 50: 133–254, 1996. doi: 10.1016/S0301-0082(96)00034-2. [DOI] [PubMed] [Google Scholar]
- Noto CT, Gnadt JW. Saccade trajectories evoked by sequential and colliding stimulation of the monkey superior colliculus. Brain Res 1295: 99–118, 2009. doi: 10.1016/j.brainres.2009.07.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Optican LM. Oculomotor system: models. In: Encyclopedia of Neuroscience, edited by Squire LR. Oxford, UK: Academic, 2009, p. 25–34. doi: 10.1016/B978-008045046-9.01095-0. [DOI] [Google Scholar]
- Optican LM, Pretegiani E. What stops a saccade? Philos Trans R Soc Lond B Biol Sci 372: 20160194, 2017. doi: 10.1098/rstb.2016.0194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paré M, Wurtz RH. Progression in neuronal processing for saccadic eye movements from parietal cortex area lip to superior colliculus. J Neurophysiol 85: 2545–2562, 2001. [DOI] [PubMed] [Google Scholar]
- Pellionisz A, Llinás R. Space-time representation in the brain. The cerebellum as a predictive space-time metric tensor. Neuroscience 7: 2949–2970, 1982. doi: 10.1016/0306-4522(82)90224-X. [DOI] [PubMed] [Google Scholar]
- Port NL, Wurtz RH. Sequential activity of simultaneously recorded neurons in the superior colliculus during curved saccades. J Neurophysiol 90: 1887–1903, 2003. doi: 10.1152/jn.01151.2002. [DOI] [PubMed] [Google Scholar]
- Quinet J, Goffart L. Head-unrestrained gaze shifts after muscimol injection in the caudal fastigial nucleus of the monkey. J Neurophysiol 98: 3269–3283, 2007. doi: 10.1152/jn.00741.2007. [DOI] [PubMed] [Google Scholar]
- Quinet J, Goffart L. Electrical microstimulation of the fastigial oculomotor region in the head-unrestrained monkey. J Neurophysiol 102: 320–336, 2009. doi: 10.1152/jn.90716.2008. [DOI] [PubMed] [Google Scholar]
- Quinet J, Goffart L. Does the brain extrapolate the position of a transient moving target? J Neurosci 35: 11780–11790, 2015a. doi: 10.1523/JNEUROSCI.1212-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinet J, Goffart L. Cerebellar control of saccade dynamics: contribution of the fastigial oculomotor region. J Neurophysiol 113: 3323–3336, 2015b. doi: 10.1152/jn.01021.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raybourn MS, Keller EL. Colliculoreticular organization in primate oculomotor system. J Neurophysiol 40: 861–878, 1977. [DOI] [PubMed] [Google Scholar]
- Robinson DA. A method of measuring eye movement using a scleral search coil in a magnetic field. IEEE Trans Biomed Eng 10: 137–145, 1963. [DOI] [PubMed] [Google Scholar]
- Robinson FR, Fuchs AF. The role of the cerebellum in voluntary eye movements. Annu Rev Neurosci 24: 981–1004, 2001. doi: 10.1146/annurev.neuro.24.1.981. [DOI] [PubMed] [Google Scholar]
- Robinson FR, Straube A, Fuchs AF. Role of the caudal fastigial nucleus in saccade generation. II. Effect of muscimol inactivation. J Neurophysiol 70: 1741–1758, 1993. [DOI] [PubMed] [Google Scholar]
- Rodgers CK, Munoz DP, Scott SH, Paré M. Discharge properties of monkey tectoreticular neurons. J Neurophysiol 95: 3502–3511, 2006. doi: 10.1152/jn.00908.2005. [DOI] [PubMed] [Google Scholar]
- Rosano C, Krisky CM, Welling JS, Eddy WF, Luna B, Thulborn KR, Sweeney JA. Pursuit and saccadic eye movement subregions in human frontal eye field: a high-resolution fMRI investigation. Cereb Cortex 12: 107–115, 2002. doi: 10.1093/cercor/12.2.107. [DOI] [PubMed] [Google Scholar]
- Sato H, Noda H. Divergent axon collaterals from fastigial oculomotor region to mesodiencephalic junction and paramedian pontine reticular formation in macaques. Neurosci Res 11: 41–54, 1991. doi: 10.1016/0168-0102(91)90065-7. [DOI] [PubMed] [Google Scholar]
- Schiller PH, Koerner F. Discharge characteristics of single units in superior colliculus of the alert rhesus monkey. J Neurophysiol 34: 920–936, 1971. [DOI] [PubMed] [Google Scholar]
- Scudder CA, Kaneko CS, Fuchs AF. The brainstem burst generator for saccadic eye movements: a modern synthesis. Exp Brain Res 142: 439–462, 2002. doi: 10.1007/s00221-001-0912-9. [DOI] [PubMed] [Google Scholar]
- Sparks DL. The brainstem control of saccadic eye movements. Nat Rev Neurosci 3: 952–964, 2002. doi: 10.1038/nrn986. [DOI] [PubMed] [Google Scholar]
- Sparks DL, Lee C, Rohrer WH. Population coding of the direction, amplitude, and velocity of saccadic eye movements by neurons in the superior colliculus. Cold Spring Harb Symp Quant Biol 55: 805–811, 1990. doi: 10.1101/SQB.1990.055.01.075. [DOI] [PubMed] [Google Scholar]
- Stanford TR, Freedman EG, Sparks DL. Site and parameters of microstimulation: evidence for independent effects on the properties of saccades evoked from the primate superior colliculus. J Neurophysiol 76: 3360–3381, 1996. [DOI] [PubMed] [Google Scholar]
- Suzuki DA, Keller EL. The role of the posterior vermis of monkey cerebellum in smooth-pursuit eye movement control. II. Target velocity-related Purkinje cell activity. J Neurophysiol 59: 19–40, 1988. [DOI] [PubMed] [Google Scholar]
- Suzuki DA, Noda H, Kase M. Visual and pursuit eye movement-related activity in posterior vermis of monkey cerebellum. J Neurophysiol 46: 1120–1139, 1981. [DOI] [PubMed] [Google Scholar]
- Walton MM, Bechara B, Gandhi NJ. Role of the primate superior colliculus in the control of head movements. J Neurophysiol 98: 2022–2037, 2007. doi: 10.1152/jn.00258.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe M, Kobayashi Y, Inoue Y, Isa T. Effects of local nicotinic activation of the superior colliculus on saccades in monkeys. J Neurophysiol 93: 519–534, 2005. doi: 10.1152/jn.00558.2004. [DOI] [PubMed] [Google Scholar]