Glutamate and GABA signaling in the dorsal vagal complex is elevated after several days of chronic hyperglycemia in a mouse model of type 1 diabetes. We report persistently enhanced GABAA receptor-mediated responses to the somnolescent zolpidem in preganglionic vagal motor neurons. These results imply a broader impact of chronic hyperglycemia on central vagal function than previously appreciated and reinforce the hypothesis that diabetes effects in the brain can impact regulation of metabolic homeostasis.
Keywords: GABA, hyperglycemia, patch clamp, vagus, zolpidem
Abstract
Chronic experimentally induced hyperglycemia augments subunit-specific γ-aminobutyric acid A (GABAA) receptor-mediated inhibition of parasympathetic preganglionic motor neurons in the dorsal motor nucleus of the vagus (DMV). However, the contribution of α1 or γ GABAA receptor subunits, which are ubiquitously expressed on central nervous system neurons, to this elevation in inhibitory tone have not been determined. This study investigated the effect of chronic hyperglycemia/hypoinsulinemia on α1- and γ-subunit-specific GABAA receptor-mediated inhibition using electrophysiological recordings in vitro and quantitative RT-PCR. DMV neurons from streptozotocin-treated mice demonstrated enhancement of both phasic and tonic inhibitory currents in response to application of the α1-subunit-selective GABAA receptor-positive allosteric modulator zolpidem. Responses to low concentrations of the GABAA receptor antagonist gabazine suggested an additional increased contribution of γ-subunit-containing receptors to tonic currents in DMV neurons. Consistent with the functional elevation in α1- and γ-subunit-dependent activity, transcription of both the α1- and γ2-subunits was increased in the dorsal vagal complex of streptozotocin-treated mice. Overall, these findings suggest an increased sensitivity to both zolpidem and gabazine after several days of hyperglycemia/hypoinsulinemia, which could contribute to altered parasympathetic output from DMV neurons in diabetes.
NEW & NOTEWORTHY Glutamate and GABA signaling in the dorsal vagal complex is elevated after several days of chronic hyperglycemia in a mouse model of type 1 diabetes. We report persistently enhanced GABAA receptor-mediated responses to the somnolescent zolpidem in preganglionic vagal motor neurons. These results imply a broader impact of chronic hyperglycemia on central vagal function than previously appreciated and reinforce the hypothesis that diabetes effects in the brain can impact regulation of metabolic homeostasis.
diabetes compromises several indexes of parasympathetic function, including gastric function (Rayner et al. 2001; Saltzman and McCallum 1983), insulin secretion (Ahrén 2000; Mussa and Verberne 2008; Yamatani et al. 1998), and regulation of hepatic gluconeogenesis (Pocai et al. 2005). The preganglionic parasympathetic motor neurons innervating most of the subdiaphragmatic viscera are located in the brain stem dorsal motor nucleus of the vagus (DMV). Together with second-order viscerosensory neurons in the nucleus tractus solitarii (NTS), the DMV regulates visceral function via the vagus nerve. Inhibitory GABAergic neurotransmission is arguably the most prominent regulator of ongoing vagal motor neuron activity (Babic et al. 2011; Travagli et al. 2006), and GABAA receptor-mediated activity in the vagal complex regulates vagally mediated visceral functions relevant to energy homeostasis (Feng et al. 1990; Mussa and Verberne 2008; Washabau et al. 1995). Therefore, the fidelity of GABAergic neurotransmission to the DMV is critical in the maintenance of energy homeostasis.
GABAA receptor-mediated signaling has two distinct signaling modalities, phasic (i.e., synaptic) and tonic (i.e., peri- or extrasynaptic), and both modalities contribute significantly to vagal motor neuron activity (Bouairi et al. 2006; Gao and Smith 2010a, 2010b). DMV neurons themselves contain KATP channels (Balfour et al. 2006; Trapp and Ballanyi 1995; Williams et al. 2007), but a relatively small proportion of DMV neurons are directly responsive to glucose (Balfour et al. 2006). In contrast, acutely elevated glucose concentration consistently hyperpolarizes the membrane of DMV neurons in a manner consistent with increased GABA release and subsequent activation of tonic GABA currents in the DMV (Ferreira et al. 2001), and GABAergic NTS neurons are glucose sensitive (Boychuk et al. 2015a). In addition, glutamatergic modulation of GABAergic phasic currents in DMV neurons is elevated after hyperglycemia/hypoinsulinemia is experimentally induced (Boychuk and Smith 2016), which could contribute to chronically elevated GABA concentrations in vivo. Moreover, DMV neurons from hyperglycemic mice demonstrate a sustained elevation of tonic current signaling mediated by GABAA receptors containing δ-subunits, and this elevation involves posttranslational modification of receptor function (Boychuk et al. 2015b). The typical synaptic GABAA receptor isoforms in the brain, however, contain two α-subunits, two β-subunits, and a γ-subunit (Fritschy and Brünig 2003); when GABA concentration is elevated, GABAA receptors containing an α1- and/or γ-subunit can also contribute to the tonic inhibitory GABA current in a subset of DMV neurons (Gao and Smith 2010a, 2010b).
Whereas increased functional expression of δ-subunit-containing receptors has been reported after several days of systemic hyperglycemia (Boychuk et al. 2015b), diabetes-associated plasticity of GABAA receptors containing α1/γ2-subunits has not been investigated adequately in the DMV. Given that GABA concentration in the dorsal vagal complex is likely to be elevated after chronic hyperglycemia (Boychuk and Smith 2016), this is a significant oversight. Therefore, the present study tested whether GABAergic signaling arising from postsynaptic receptors containing either the α1- or γ-subunit was significantly altered in DMV neurons after chronic hyperglycemia/hypoinsulinemia in a mouse model of type 1 diabetes. We hypothesized that both phasic and tonic currents mediated by these traditionally synaptic GABAA receptor subunits would be significantly elevated. We also sought to determine if zolpidem, a widely used somnolescent, differentially altered GABA-mediated cellular responses in this model. This is particularly relevant in light of recent clinical evidence that zolpidem use increases patient risk for diabetes (Lin et al. 2015).
METHODS
All experiments were performed on young adult male CD-1 mice (Harlan, Indianapolis, IN) housed in the University of Kentucky Division of Laboratory Animal Resources housing facilities. Animals were under a normal 14:10-h light-dark cycle with food and water available ad libitum. The University of Kentucky Animal Care and Use Committee approved all animal procedures.
Diabetes Induction
To induce type 1 diabetes with frank hyperglycemia, mice were fasted for 5–6 h before receiving a randomized intraperitoneal injection of either streptozotocin (STZ; 200 mg/kg), to kill insulin-secreting pancreatic beta cells, or the saline vehicle (0.1 ml). After injection (day 0), mice were returned to their home cages for 4–9 days until the day of experimentation (see Fig. 1 for timeline); mice typically became hyperglycemic (blood glucose ≥300 mg/dl) 2–3 days after STZ injection. Blood glucose levels were monitored daily by tail lance (Nova Max, Waltham, MA), and animals were used for experiments after ≥3 consecutive days of sustained blood glucose levels.
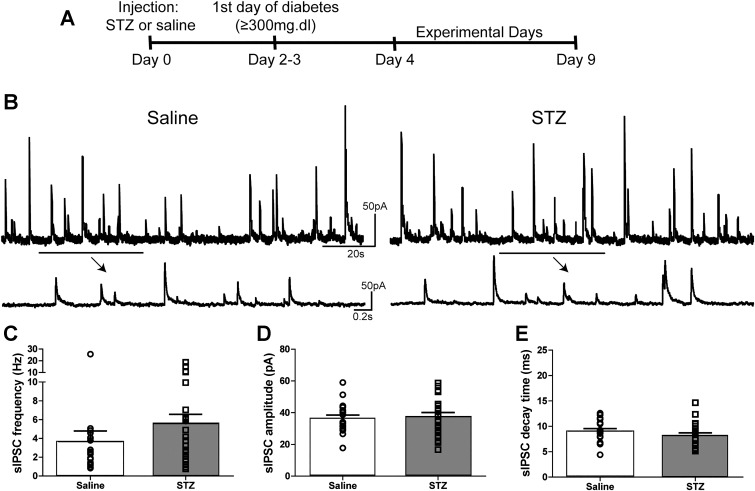
Fig. 1.
sIPSC parameters in DMV neurons from mice with STZ-induced diabetes. A: experimental timeline. B: representative traces showing sIPSCs in DMV neurons from saline (left)- and STZ-treated mice (right) in the presence of elevated GABA concentration ([GABA]; either 3 μM GABA or 1 mM NIP). Arrows indicate temporally expanded sections of the trace above. C: mean sIPSC frequency comparison between neurons from saline (n = 20)- and STZ-treated mice (n = 21) in the presence of elevated [GABA], with individual neuronal frequencies overlaid. D: mean sIPSC amplitude comparison between neurons from saline- and STZ-treated mice in the presence of elevated [GABA] with individual neuronal amplitudes overlaid. E: mean sIPSC decay time constant in neurons from saline- and STZ-treated mice in the presence of elevated [GABA] with individual neuronal amplitudes overlaid. No statistical differences were observed for any of these measures (P > 0.05); all solutions contained kynurenic acid (1 mM).
Electrophysiology
On the day of experimentation, mice were anesthetized by isoflurane inhalation to effect (i.e., lack of tail-pinch response) and decapitated while anesthetized. The brain stem was rapidly removed and submerged in ice-cold (0–4°C), oxygenated (95% O2-5%CO2) artificial cerebrospinal fluid (aCSF) with the following composition (in mM): 124 NaCl, 3 KCl, 26 NaHCO3, 1.4 NaH2PO4, 11 glucose, 1.3 CaCl2, 1.3 MgCl, and 1 kynurenic acid (KYN). The osmolarity and pH of all solutions was 290–305 mosmol/kg H2Oand 7.3–7.4, respectively. The brain stem was mounted on an ice-cold stage, and slices (300 µm) were cut in the coronal plane using a vibratome (Technical Products International, St Louis, MO). Slices containing the dorsal vagal complex ±300 µm rostral and caudal to area postrema were transferred to a holding chamber and incubated in warmed (30–33°C), oxygenated aCSF for 1 h before being transferred to a recording chamber mounted on the fixed stage of an upright microscope (BX51WI; Olympus, Melville, NY), where they were continually superfused with warmed, oxygenated aCSF.
Whole cell patch-clamp recordings were performed under visual control using infrared illumination and differential interference contrast optics (IR-DIC). For recordings, glass recording pipettes (2–5 MΩ; King Precision Glass, Claremont, CA) were filled with a solution containing the following (in mM): 130 Cs+-gluconate, 1 NaCl, 5 EGTA, 10 HEPES, 1 MgCl2, 1 CaCl2, 3 CsOH, and 2–3 Mg-ATP, pH 7.3–7.4 adjusted with 5 M CsOH. Cs+ was used as the primary cation to block K+ currents and minimize any influence of K+-dependent, postsynaptic GABAB receptors in recorded neurons (Hestrin et al. 1990; Sah 1995) and also allowed consistent voltage-clamp at depolarized membrane potentials. Inhibitory GABAA receptor-mediated currents (both tonic and phasic, I-tonic and I-phasic, respectively) were examined at a holding potential of 0 mV. Recordings were discarded if series resistance was >25 MΩ or changed by >20% throughout the course of the experiment; mean series resistance was 12.18 ± 1.00 MΩ in the saline-treated group (n = 20) and 12.43 ± 1.08 MΩ in the STZ-treated group (n = 21). Electrophysiological signals were acquired at 20 kHz and recorded using an Axoclamp 700B amplifier (Molecular Devices, Union City, CA), low-pass filtered at 2 kHz, and stored to a computer using a Digidata 1440A digitizer and pClamp 10.2 software (Molecular Devices).
All drugs were dissolved in aCSF containing 1 mM KYN to block ionotropic glutamate receptors and bath applied until a steady state was reached. Drugs used included bicuculline methiodide (BIC; 30 µM) to block GABAA receptors, gabazine (GBZ; 1 µM) to block putative γ-subunit-containing GABAA receptors, and zolpidem (ZOL; 1 µM) to potentiate α1-subunit-dependent currents. As described previously (Gao and Smith 2010a, 2010b), ambient GABA concentration was elevated by bath application of either GABA (3 µM) or the GABA transporter inhibitor nipecotic acid (NIP; 1 mM) for 10 min to determine the effects of gabazine and zolpidem on GABAA receptor-mediated tonic currents. GABA, GBZ, and NIP and were received from Sigma-Aldrich (St. Louis, MO). BIC and ZOL were received from R&D Systems (Minneapolis, MN).
Quantitative RT-PCR
mRNA expression of GABAA receptor subunits (α1 and γ2) was assessed in separate subsets of mice. In these animals, three to four slices were generated in a manner similar to the procedure used for electrophysiological recordings. From each slice, a 1-mm-diameter punch (Miltex, York, PA) was taken containing most of the dorsal vagal complex, with minimal tissue sampled from other surrounding structures. Punches from one animal were pooled into one sample. Each sample was immediately homogenized, placed in 0.5 ml of Trizol (Sigma), and centrifuged according to the manufacturer’s instructions. RNA samples were then used to create cDNA. Spectrophotometry (NanoDrop Technologies, Wilmington, DE) was used to determine cDNA concentration and purity. All quantitative RT-PCR were run in triplicate in 96-well optical grade plates using a 7500 Fast Real-Time PCR System (Applied Biosystems, Foster City, CA). The abundance of all cDNA was determined using RT-PCR Master Mix (Applied Biosystems). Total volume for each run was 20 µl containing 50 ng of cDNA. The reaction times and temperatures were 50°C for 2 min, 95°C for 10 min, followed by 50 cycles of 95°C for 15 s and 60°C for 1 min. Primer and TaqMan probe sets were purchased from Applied Biosystems. The sequences for each were generated from the listed references within GenBank: α1 (TaqMan probe Mm00439046), γ2 (TaqMan probe Mm00433489), and β-actin (TaqMan probe Mm00607939). All reactions used forward and reverse primer concentrations of 100 nM. Probe concentrations for all reactions were 50 nM. No-template and no-RT controls were run for each plate. Fold change in receptor subunit expression was calculated by the formula 2–ΔΔCt, using β-actin as an internal reference (Livak and Schmittgen 2001). Relative mRNA abundance is presented as fold increase; the change in threshold cycle (ΔCt) was used to determine statistical significance with an unpaired, two-tailed Student’s t-test (Wood and Giroux 2003).
Data Analysis
To determine drug effects on both phasic (i.e., inhibitory postsynaptic currents, IPSCs) and tonic inhibitory currents, 2 min of continuous steady-state activity were analyzed offline using Clampex 10.2 (Molecular Devices) and MiniAnalysis 6.0.3 (Synaptosoft, Decatur, CA). All synaptic events during the period analyzed were used to assess spontaneous IPSC (sIPSC) frequency (minimum 100 events), but only unitary events (i.e., single peak) were used for sIPSC amplitude and decay time constant measurements. Decay time was fitted using a single exponential fit in MiniAnalysis.
Tonic currents were measured as ZOL induced (the difference in holding current between control aCSF and ZOL) and also as total current (the holding current difference between peak response to ZOL application and steady state after 5–10 min in the presence of BIC). Changes in tonic current amplitude were considered significant if they were greater than twice the average root mean square value; significant tonic current amplitude was therefore ≥5.8 pA (Boychuk et al. 2015b). Tonic currents were corrected for membrane area by normalizing to whole cell capacitance and are therefore presented as current density (pA/pF). For all electrophysiological experiments, only one cell was used per slice. Data are means ± SE. Statistical measurements were performed using GraphPad Prism 5 (GraphPad Software, La Jolla, CA). The Shapiro-Wilkes normality test was used on all data sets to determine the appropriate use of parametric or nonparametric statistical analysis. Parametric, two-tailed unpaired Student’s t-tests were used to determine statistical differences between saline- and STZ-treated animals for both electrophysiological and molecular biological analyses. Where noted, the nonparametric Mann-Whitney U-test was used to assess differences in nonnormally distributed data. A correlational analysis with a linear regression was also used to assess relationships between several parameters. Probability values ≤0.05 were considered significant for all statistical analysis.
RESULTS
Characteristics of STZ-Induced Diabetes
There was no difference in the age of animals used (38 ± 2 days for saline-treated vs. 39 ± 3 days for STZ-treated animals; P ≥ 0.05) or number of days after injection (8 ± 1 days for saline-treated vs. 7 ± 1 days for STZ-treated animals; P ≥ 0.05). The average blood glucose concentration of saline-treated CD-1 mice was 165 ± 3 mg/dl (n = 19). Mice were considered diabetic after their blood glucose concentration measured >300 mg/dl for at least 3 consecutive days following STZ treatment. The average blood glucose of STZ-treated mice on the day of experimentation was 501 ± 16 mg/dl (n = 21). Animals were maintained in a hyperglycemic state for 3–7 days, with an average diabetic duration of 5 ± 0.3 days. By the day of experimentation, STZ-treated mice weighed significantly less (26.3 ± 1.2 g) than saline-treated mice (33.4 ± 1.1 g; P = 0.00005), which is similar to previous reports (Boychuk et al. 2015b; Rerup and Tarding 1969).
I-phasic in Elevated GABA Concentrations
Previous studies demonstrated that the induction of α1/γ-subunit-containing GABAA receptor-mediated I-tonic requires elevated GABA concentration in the DMV (Gao and Smith 2010a, 2010b). Therefore, the present study used application of either NIP (1 mM) or GABA (3 µM) to experimentally increase extracellular GABA concentration in the slice. Under these conditions, sIPSC parameters were measured to determine differences in I-phasic parameters in DMV neurons from saline- and STZ-treated mice (Fig. 1). There were no significant difference in sIPSC parameters between NIP and exogenously applied GABA. When GABA concentration was elevated, mean sIPSC frequency in DMV neurons from STZ-treated mice (5.6 ± 1.0 Hz; n = 23 neurons) was not significantly different from that in neurons from saline-treated animals (3.7 ± 1.2 Hz; n = 21 neurons; Mann-Whitney U-test; P = 0.06). Mean amplitude (36.4 ± 2.1 pA in saline vs. 37.5 ± 2.6 pA in STZ; P = 0.75) and decay time constant (9.1 ± 0.5 ms, saline-treated vs. 8.2 ± 0.5 ms, STZ-treated; P = 0.23) were not different between saline- and STZ-treated animals. There were no statistical differences in I-phasic parameters between DMV neurons from saline- and STZ-treated mice when GABA concentration was elevated.
Zolpidem-Sensitive GABA Currents
Phasic current.
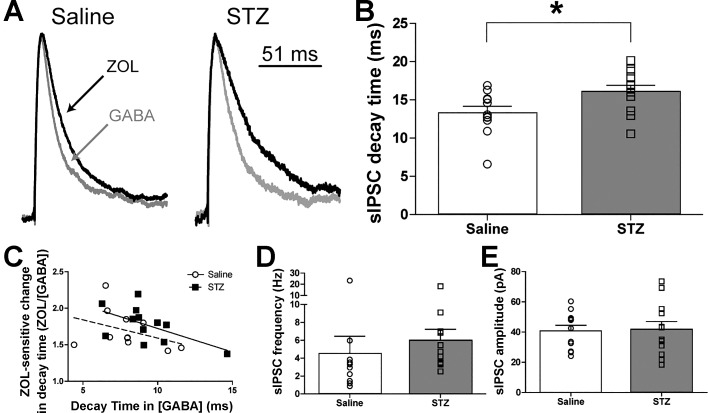
In the DMV, GABAA receptors containing α1-subunits contribute to both phasic and tonic currents when GABA concentration is elevated (Gao and Smith 2010a, 2010b). Zolpidem (ZOL; 1 µM), a positive allosteric modulator of GABAA receptors containing α1-subunits, was used to assess the contribution of putative α1-subunit-containing receptors to both phasic and tonic currents in DMV neurons from control and STZ-treated mice. In the continued presence of elevated GABA concentration, the sIPSC decay time constant was significantly longer in DMV neurons from STZ-treated (16.1 ± 0.8 ms; n = 12) vs. saline-treated mice (13.3 ± 0.9 ms; n = 11; P = 0.03; Fig. 2B) after ZOL application. There was no relationship between the level of hyperglycemia (slope = 0.003; 95%CI: −0.03 to 0.03; R2 = 0.004; P = 0.85) or number of days diabetic (slope = −0.6; 95%CI: −2.6 to 1.4; R2 = 0.05; P = 0.5) and the length of decay time in the STZ-treated animals. The ratio of the decay time constant during ZOL application to that measured before drug application (Fig. 2C) was correlated with the decay time constant before ZOL application to determine if the effect of ZOL on decay time could be predicted by the predrug decay time constant. No significant relationship existed in DMV neurons from saline-treated animals (slope = −0.05; 95%CI: −0.1 to 0.04; r = −0.4; R2 = 0.14; P = 0.26). DMV neurons from STZ-treated animals, however, demonstrated a significant negative relationship (slope = 0.06; 95%CI: −0.1 to −0.0001; r = −0.6; R2 = 0.33; P = 0.04). These data suggest that longer baseline decay times may predict lower sensitivity of sIPSC decay time constant to ZOL. However, the goodness of fit for both groups was low (<0.5), suggesting high levels of variability in these parameters, and more direct testing of this relationship should be investigated. The mean frequency of sIPSCs in DMV neurons from STZ-treated diabetic mice (6.0 ± 1.2 Hz; n = 12) was significantly different in the presence of ZOL compared with the saline-treated group (4.5 ± 2.0 Hz; n = 11; Mann-Whitney U-test; P = 0.04; Fig. 2D). Mean sIPSC amplitude was also not different between groups (40.7 ± 3.8 pA, saline-treated vs. 42.0 ± 5.2 pA, STZ-treated; P = 0.86; Fig. 2E) in the presence of ZOL. Taken together, these results suggest that ZOL-sensitive receptor subunits contribute significantly more to decay time constant of phasic sIPSCs in DMV neurons from chronically hyperglycemic mice compared with controls. This elevation in ZOL sensitivity may be more likely to occur in neurons that tend to express subunit(s) of the GABAA receptors that impart faster decay times (e.g., higher α1-to-α4 ratios; Kapur et al. 1999), but full characterization of the relationship between cell-specific subunit composition and ZOL sensitivity of phasic and tonic currents in diabetic mice requires additional investigation.
Fig. 2.
Increased zolpidem (ZOL)-induced augmentation of sIPSC decay time in DMV neurons from STZ-treated mice. A: averaged trace of sIPSCs in DMV neurons from saline- and STZ-treated mice in the presence of ZOL (1 μM; black lines). Averaged traces were normalized for amplitude to demonstrate the ZOL-induced lengthened decay time constant. There was no difference in decay time constant between the groups before the addition of ZOL (see Fig. 1). B: mean sIPSC decay time constant differences between neurons from saline (n = 11)- and STZ-treated mice (n = 12) in the presence of ZOL, with individual neuronal responses overlaid. *P < 0.05 (unpaired 2-tailed t-test). C: correlation between the ZOL sensitivity of decay time constant as a function of the decay time constant in elevated [GABA]. Only DMV neurons from STZ-treated mice demonstrated a significant relationship (slope = −0.06; 95%CI: −0.1 to −0.0001; r = −0.6; R2 = 0.33; P < 0.05), suggesting that the effect of hyperglycemia on DMV neuron ZOL sensitivity preferentially occurs in neurons with faster baseline decay times. D: effect of ZOL on mean sIPSC frequency in neurons from saline- and STZ-treated mice, with frequencies from individual neurons overlaid. E: effect of ZOL on mean sIPSC amplitude in neurons from saline- and STZ-treated mice, with individual neuronal sIPSC amplitudes overlaid. aCSF for all experiments included 1 M kynurenic acid and 3 μM GABA.
Tonic current.
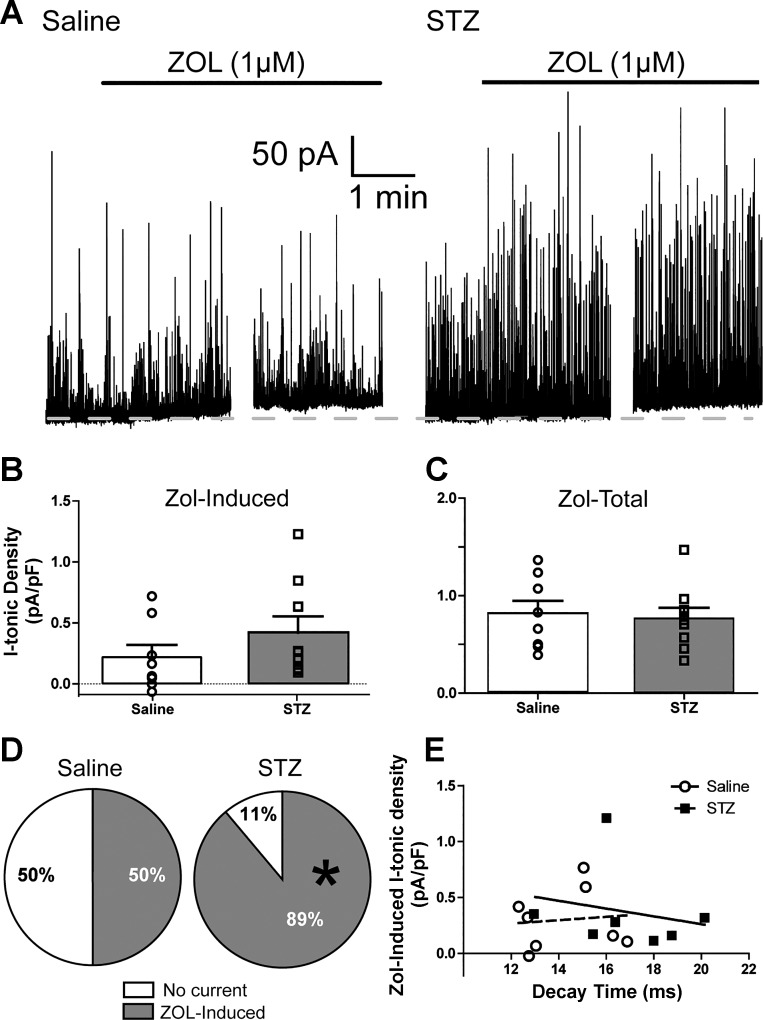
GABAA receptors containing the α1-subunit can also contribute to the tonic current in DMV neurons (Gao and Smith 2010b), and this current exerts potent control over DMV neuron excitability (Gao and Smith 2010a). Therefore, the ZOL sensitivity of the tonic current was investigated after hyperglycemia/hypoinsulinemia was experimentally induced. Similar to a previous report in normal mice, ZOL augmented tonic currents in DMV neurons from both saline- and STZ-treated mice. The mean ZOL-induced tonic current was not significantly different between neurons from saline-treated (0.22 ± 0.10 pA/pF; n = 8) and STZ-treated mice (0.42 ± 0.13 pA/pF; n = 9; Mann-Whitney U-test; P = 0.14; Fig. 3B). Total tonic current after ZOL application was assessed by measuring the maximum ZOL-induced current before and after addition of BIC (i.e., IZOL − IBIC). The total tonic current density was not significantly different in neurons from diabetic (0.77 ± 0.11 pA/pF; n = 8) vs. saline-treated animals (0.82 ± 0.13 pA/pF; n = 9; P = 0.76; Fig. 3B). The STZ-treated group, however, demonstrated a significantly higher proportion of neurons (8/9) with ZOL-inducible currents than did saline-treated mice (4/8; Fig. 3C; Mann-Whitney U-test; P < 0.05). To ensure that the proportion of responsive neurons did not skew the variability of the responses and eliminate potential differences, data from only those neurons with significant ZOL-induced currents were analyzed separately. In this sample of neurons, there still was no difference in ZOL-induced current density (0.42 ± 0.13 pA/pF; n = 4 in saline vs. 0.46 ± 0.14 pA/pF; n = 8 in diabetic). Thus the ZOL-sensitive augmentation of tonic current in DMV cells from the STZ-treated group was not significantly different from that in cells expressing ZOL sensitivity from saline-treated mice, but the proportion of DMV neurons expressing this current was significantly increased after several days of hyperglycemia/hypoinsulinemia.
Fig. 3.
Increased ZOL-sensitive tonic current in DMV neurons from STZ-treated mice. A: representative traces of ZOL-induced (1 mM) tonic currents in saline (left)- and STZ-treated mice (right). Dashed lines represent baseline holding current. aCSF contained GABA (3 μM) and kynurenic acid (1 mM). B: group statistics for ZOL-induced I-tonic current density for all neurons in saline (n = 8)- and STZ-treated mice (n = 9) with overlaid individual neuronal responses. C: group statistics for ZOL-induced total tonic current density for both groups, with overlaid individual neuronal responses. D: pie chart illustrating the significant increase in the proportion of cells expressing significant ZOL-induced responses. *P < 0.05 (Mann-Whitney U-test). E: correlation between ZOL-induced I-tonic current density as a function of sIPSC decay time constant. Because short sIPSC decay times did not predict the amplitude of ZOL-induced tonic current density (P > 0.05), hyperglycemia/hypoinsulinemia-mediated plasticity of ZOL-sensitive inhibitory synaptic and tonic currents in the DMV are likely to affect receptors at the synapse and those at peri-/extrasynaptic locations independently.
Because both phasic and tonic currents were altered, a correlational analysis was used to determine if sIPSC decay time constant following ZOL application predicted the amplitude of ZOL-induced tonic current (Fig. 3E), a relationship that may exist if tonic currents are generated by synaptic receptors (Farrant and Nusser 2005). In the saline-treated group, no relationship was determined for sIPSC decay time constant vs. the ZOL-induced tonic current density (r = 0.1; R2 = 0.01; P = 0.82), suggesting that ZOL-sensitive receptors at the synapses of DMV neurons function independently from the receptor pool mediating the tonic current. Similarly, DMV neurons from STZ-treated mice demonstrated no relationship between ZOL-sensitive decay time and tonic current density (r = −0.2; R2 = 0.05; P = 0.64), suggesting that hyperglycemia/hypoinsulinemia-induced plasticity of ZOL-sensitive inhibitory phasic and tonic currents in the DMV affects these receptors independently.
Gabazine-Sensitive I-tonic
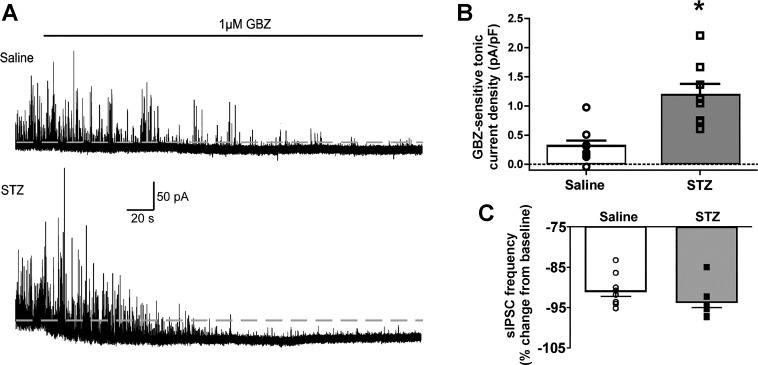
Previous reports indicate that GABA receptors containing γ-subunits can also contribute to tonic currents in the DMV when extracellular GABA concentration is elevated (Gao and Smith 2010a). A low concentration of GBZ (1 µM) was used to assess the contribution of putative γ-subunit-containing receptors to the tonic current after STZ treatment (Fig. 4). Unless GABA concentration is exogenously elevated, γ-subunit containing receptors do not actively contribute to the tonic current (Gao and Smith 2010a), probably because low levels of ambient GABA bind the low-affinity, γ-subunit containing receptors incompletely (Haas and Macdonald 1999). Under baseline recording conditions, application of GBZ did not reveal a tonic current (≥5.8 pA) in neurons from STZ-treated diabetic (−0.04 ± 0.04 pA/pF; n = 7) or saline-treated control mice (−0.04 ± 0.05 pA/pF; n = 4). As demonstrated previously (Gao and Smith 2010a), applying NIP (1mM) to block GABA transport and elevate extracellular GABA concentration unmasked a GBZ-sensitive tonic GABA current in neurons from both saline-treated and STZ-treated mice. In the continued presence of NIP, the GBZ-sensitive tonic currents were significantly greater in neurons from STZ-treated mice (1.2 ± 0.20 pA/pF; n = 8; Fig. 4B) compared with saline-treated mice (0.31 ± 0.10 pA/pF; n = 9; P = 0.0007). There was no relationship between the level of hyperglycemia (slope = −0.003; 95%CI: −0.02 to 0.009; R2 = 0.07; P = 0.5) or number of days diabetic (slope = −0.1; 95%CI: −0.6 to 0.3; R2 = 0.06; P = 0.6) and the GBZ-sensitive tonic currents in STZ-treated animals. There was no difference in the proportion of neurons expressing this current (8/9 in saline-treated and 9/9 in STZ-treated mice). Frequency, amplitude and decay time changes in sIPSCs were not examined, because GBZ-application decreased the frequency of events by >90% (to <0.2 Hz), which did not allow for a sufficient number of analyzable events (Fig. 4C).
Fig. 4.
Increased gabazine (GBZ)-sensitive tonic current in DMV cells from STZ-treated mice. A: representative traces of GBZ-sensitive (1 µM) tonic currents in neurons from saline (top)- and STZ-treated mice (bottom). Dashed lines represent baseline holding current. B: a significant mean increase in GBZ-sensitive tonic current density was detected in neurons from STZ-treated mice (n = 9) relative to controls (n = 8). *P < 0.05 (unpaired 2-tailed t-test. Overlaid individual neuronal responses are also shown. C: sIPSC frequency in DMV neurons was reduced by >90% in the presence of GBZ. Recording solutions contained kynurenic acid (1 mM) and nipecotic acid (1 mM).
Quantitative RT-PCR
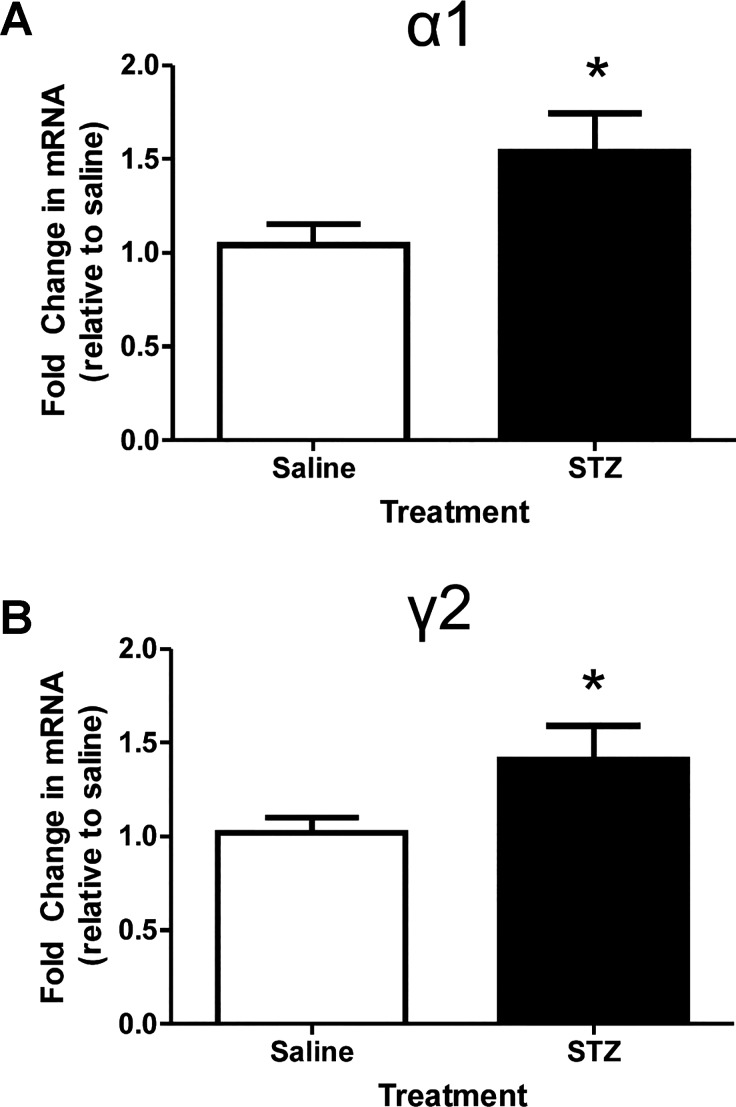
Quantitative RT-PCR was employed to determine if the increase in tonic inhibition reflected an increase in the transcription of traditionally synaptic GABAA receptor subunits, γ2 and its predominant partner, α1. Fold change in mRNA expression is illustrated in Fig. 5. Messenger RNA expression increased for both α1- and γ2-subunits after STZ treatment (n = 7 mice) compared with saline treatment (n = 8 mice). The fold increases were relatively small but significant (α1: 1.54 ± 0.20-fold increase; P = 0.03; γ2: 1.41 ± 0.18-fold increase; P = 0.03).
Fig. 5.
Increased relative mRNA abundance of the α1 and γ2 GABAA receptor subunits in the vagal complex. A: mean and SE for the fold change of the GABAA receptor α1-subunit for both groups. *P < 0.05 (unpaired 2-tailed t-test). B: mean and SE for the fold change of the GABAA receptor γ2-subunit for both groups (n = 7–8 mice per group). *P < 0.05 (unpaired 2-tailed t-test).
DISCUSSION
The present study identified that both phasic and tonic modalities of subunit-specific GABAA receptor-mediated inhibition are significantly elevated after STZ-induced diabetes. These changes are sustained, even after glucose concentrations are normalized in vitro, suggesting plasticity of GABA responses that outlast prolonged periods of hyperglycemia. Similar to previous reports, sIPSC frequency was not significantly elevated in DMV neurons from STZ-treated mice when ionotropic glutamate receptors were blocked and GABA concentration was exogenously elevated. The present study also extends previous reports of functionally elevated δ-subunit-containing GABAA receptor-mediated activity (Boychuk et al. 2015b) to include functional and molecular augmentation of currents mediated by receptors containing α1- and γ-subunits. Specifically, although sIPSC decay time constant was not different between the two groups under control conditions, the augmentation of sIPSC decay time constant following bath application of ZOL was significantly larger in DMV neurons from STZ-treated mice, suggesting increased contribution of receptors containing the α1-subunit. Although this was not associated with increased ZOL-induced tonic currents, significantly more DMV neurons from the STZ-treated group expressed a ZOL-induced tonic current compared with their saline-treated counterparts.
DMV neurons from STZ-treated mice also expressed significantly elevated GBZ-sensitive tonic currents, but a similar proportion of neurons expressed this current, consistent with the conclusion that GBZ and ZOL act at different receptor subunits. Parallel experiments demonstrated that the transcription of GABAA receptor α1- and γ-subunit mRNA in the vagal complex was elevated after experimentally induced diabetes. Taken together, these results suggest that diabetes-associated GABAergic plasticity in DMV neurons results in a functional upregulation of the GABAA receptor α1- and γ-subunits at both synaptic and peri-/extrasynaptic locations, paralleled by transcriptional upregulation of these subunits. This differs from diabetes-related plasticity of δ-subunits, which was not accompanied by a change in mRNA expression and was likely mediated by posttranslational receptor modification (Boychuk et al. 2015b).
Although STZ-induced hyperglycemia/hypoinsulinemia results in elevated glutamatergic facilitation of GABA release (Boychuk and Smith 2016), the present study confirms previous reports that GABAergic sIPSC frequency was similar when ionotropic glutamate receptors are blocked (Boychuk et al. 2015b). It also extends this work by demonstrating that sIPSC frequency, amplitude, and decay time constant remained similar between the two groups after GABA concentration was experimentally elevated in the perfusate, and the values for all sIPSC measures reported in the presence of elevated GABA concentration are comparable to those reported in the presence of normal ambient GABA concentration (Boychuk et al. 2015b; Boychuk and Smith 2016). However, hyperglycemia/hypoinsulinemia resulted in a ZOL-induced augmentation of mean sIPSC decay time in DMV neurons from diabetic mice, suggesting an overall increased effect of ZOL on phasic current. The ZOL sensitivity of decay time also correlated negatively with shorter decay times in DMV neurons from diabetic mice, making it possible that this type of functional plasticity occurs more often in neurons that express subunit(s) of the GABAA receptors that impart faster decay times (e.g., higher α1-to-α4 ratios) (Kapur et al. 1999).
Similar to previous reports (Gao and Smith 2010b), about half of the DMV neurons from normoglycemic control mice demonstrated a ZOL-sensitive tonic inhibitory current. Although the amplitude of the ZOL-sensitive inhibitory tonic current was not different between groups, the proportion of neurons expressing a significant ZOL-induced tonic current increased to nearly all DMV neurons (8/9) after STZ-induced hyperglycemia. Notably, our inability to detect differences using analyses restricted only to those cells expressing the ZOL-induced tonic current could have been influenced by the relatively low percentage of neurons expressing the current in control mice or by differing variability of responses between groups. Correspondingly, transcriptional expression of the α1-subunit was also elevated in the dorsal vagal complex after STZ-induced hyperglycemia. Although the intracellular triggers leading to increased α1 expression remain to be elucidated, these results suggest that, unlike δ-subunit plasticity after STZ-induced hyperglycemia, which likely involves posttranslational mechanisms, the functional increase in ZOL-induced tonic current expression is paralleled by increased α1-subunit transcriptional expression.
Although ZOL has the highest affinity for the α1-subunit, it does have moderate effects on receptors containing the α2- and α3-subunits (Barnard et al. 1998; Möhler et al. 2002; Rudolph et al. 2001). Therefore, it is possible that some of these effects are not specific to the α1-subunit. However, the α1-subunit is highly expressed throughout the brain stem, including the DMV (Broussard et al. 1997; Pirker et al. 2000), and α2- and α3-subunits are expressed mainly during early development (Okada et al. 2000; Vicini et al. 2001). Nonetheless, the transcriptional upregulation of the α1-subunit is consistent with the hypothesis that the increased ZOL sensitivity is due to a functional increase in α1-subunit-containing receptors.
Several days of STZ-induced hyperglycemia also resulted in a significant increase in GBZ-sensitive tonic GABA currents. The present data confirm previous reports demonstrating that a low concentration of GBZ abolished sIPSCs, with no effect on tonic inhibitory current (Gao and Smith 2010a), and this was also observed in DMV neurons from diabetic mice. However, in the presence of elevated GABA concentration, DMV neurons from mice treated with STZ demonstrated a significantly larger GBZ-sensitive tonic current. By inference, this concentration of GBZ likely targets γ-subunits preferentially (Bai et al. 2001; Park et al. 2006; Semyanov et al. 2003; Yeung et al. 2003). Consistent with this functional outcome, transcriptional expression of the γ2-subunit was elevated. Therefore, the increase in the functional expression of GBZ-sensitive tonic current likely reflects the increased transcriptional expression of the γ2-subunit. Factors that might induce the increased transcription of GABA receptor subunits include plasticity events that occur subsequent to decreased insulin levels or to the chronically increased glucose, GABA (Boychuk et al. 2015b), or glutamate (Boychuk and Smith 2016; Zsombok et al. 2011) concentrations in the DMV of STZ-treated mice. Although direct effects of STZ in the DMV are unlikely several days postinjection, because the drug is rapidly cleared from serum (Karunanayake et al. 1974; Schein and Loftus 1968) and the STZ treatment does not induce cell death in the vagal complex (Bach et al. 2015), other effects secondary to STZ treatment (e.g., sustained inflammatory responses; STZ- or hyperglycemia-induced damage to vagal afferents or enteric neurons) might also contribute to the changes observed. The mechanisms leading to the increased functional and molecular expression of α1- and γ2-subunits are not known.
Regardless of mechanism, it is apparent that experimentally induced, sustained hyperglycemia results in significant neuronal plasticity within the central neuron population that is principally responsible for the regulation of visceral parasympathetic activity. This plasticity is multifaceted and results in altered function involving both presynaptic (i.e., facilitated neurotransmitter release) and postsynaptic signaling (i.e., receptor reorganization, increased transcription). The increased GABAA receptor function in DMV cells from hyperglycemic/hypoinsulinemic mice implies that the inhibitory influence on vagal motor output of GABA mimetics, including zolpidem or other benzodiazepine-like drugs, may be enhanced during or after bouts of hyperglycemia. Given the importance of parasympathetic activity and its prominent role in digestion and metabolic regulation (Zsombok and Smith 2009), extensive future work is warranted to understand how pathophysiological regulation of glucose metabolism changes neurotransmission to hindbrain parasympathetic regulatory regions.
GRANTS
This work was supported by National Institute of Diabetes and Digestive and Kidney Diseases Grant R01DK056132 (to B. N. Smith).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
C.R.B., K.C.S., and B.N.S. conceived and designed research; C.R.B. and K.C.S. performed experiments; C.R.B., K.C.S., and B.N.S. analyzed data; C.R.B., K.C.S., and B.N.S. interpreted results of experiments; C.R.B. and K.C.S. prepared figures; C.R.B. and B.N.S. drafted manuscript; C.R.B., K.C.S., and B.N.S. edited and revised manuscript; C.R.B., K.C.S., and B.N.S. approved final version of manuscript.
REFERENCES
- Ahrén B. Autonomic regulation of islet hormone secretion—implications for health and disease. Diabetologia 43: 393–410, 2000. doi: 10.1007/s001250051322. [DOI] [PubMed] [Google Scholar]
- Babic T, Browning KN, Travagli RA. Differential organization of excitatory and inhibitory synapses within the rat dorsal vagal complex. Am J Physiol Gastrointest Liver Physiol 300: G21–G32, 2011. doi: 10.1152/ajpgi.00363.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bach EC, Halmos KC, Smith BN. Enhanced NMDA receptor-mediated modulation of excitatory neurotransmission in the dorsal vagal complex of streptozotocin-treated, chronically hyperglycemic mice. PLoS One 10: e0121022, 2015. doi: 10.1371/journal.pone.0121022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai D, Zhu G, Pennefather P, Jackson MF, MacDonald JF, Orser BA. Distinct functional and pharmacological properties of tonic and quantal inhibitory postsynaptic currents mediated by γ-aminobutyric acidA receptors in hippocampal neurons. Mol Pharmacol 59: 814–824, 2001. doi: 10.1124/mol.59.4.814. [DOI] [PubMed] [Google Scholar]
- Balfour RH, Hansen AM, Trapp S. Neuronal responses to transient hypoglycaemia in the dorsal vagal complex of the rat brainstem. J Physiol 570: 469–484, 2006. doi: 10.1113/jphysiol.2005.098822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnard EA, Skolnick P, Olsen RW, Mohler H, Sieghart W, Biggio G, Braestrup C, Bateson AN, Langer SZ. International Union of Pharmacology. XV. Subtypes of γ-aminobutyric acidA receptors: classification on the basis of subunit structure and receptor function. Pharmacol Rev 50: 291–313, 1998. [PubMed] [Google Scholar]
- Bouairi E, Kamendi H, Wang X, Gorini C, Mendelowitz D. Multiple types of GABAA receptors mediate inhibition in brain stem parasympathetic cardiac neurons in the nucleus ambiguus. J Neurophysiol 96: 3266–3272, 2006. doi: 10.1152/jn.00590.2006. [DOI] [PubMed] [Google Scholar]
- Boychuk CR, Gyarmati P, Xu H, Smith BN. Glucose sensing by GABAergic neurons in the mouse nucleus tractus solitarii. J Neurophysiol 114: 999–1007, 2015a. doi: 10.1152/jn.00310.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boychuk CR, Halmos KC, Smith BN. Diabetes induces GABA receptor plasticity in murine vagal motor neurons. J Neurophysiol 114: 698–706, 2015b. doi: 10.1152/jn.00209.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boychuk CR, Smith BN. Glutamatergic drive facilitates synaptic inhibition of dorsal vagal motor neurons after experimentally induced diabetes in mice. J Neurophysiol 116: 1498–1506, 2016. doi: 10.1152/jn.00325.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broussard DL, Li H, Altschuler SM. Colocalization of GABAA and NMDA receptors within the dorsal motor nucleus of the vagus nerve (DMV) of the rat. Brain Res 763: 123–126, 1997. doi: 10.1016/S0006-8993(97)00344-2. [DOI] [PubMed] [Google Scholar]
- Farrant M, Nusser Z. Variations on an inhibitory theme: phasic and tonic activation of GABAA receptors. Nat Rev Neurosci 6: 215–229, 2005. doi: 10.1038/nrn1625. [DOI] [PubMed] [Google Scholar]
- Feng HS, Lynn RB, Han J, Brooks FP. Gastric effects of TRH analogue and bicuculline injected into dorsal motor vagal nucleus in cats. Am J Physiol 259: G321–G326, 1990. [DOI] [PubMed] [Google Scholar]
- Ferreira M Jr, Browning KN, Sahibzada N, Verbalis JG, Gillis RA, Travagli RA. Glucose effects on gastric motility and tone evoked from the rat dorsal vagal complex. J Physiol 536: 141–152, 2001. doi: 10.1111/j.1469-7793.2001.t01-1-00141.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritschy JM, Brünig I. Formation and plasticity of GABAergic synapses: physiological mechanisms and pathophysiological implications. Pharmacol Ther 98: 299–323, 2003. doi: 10.1016/S0163-7258(03)00037-8. [DOI] [PubMed] [Google Scholar]
- Gao H, Smith BN. Tonic GABAA receptor-mediated inhibition in the rat dorsal motor nucleus of the vagus. J Neurophysiol 103: 904–914, 2010a. doi: 10.1152/jn.00511.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao H, Smith BN. Zolpidem modulation of phasic and tonic GABA currents in the rat dorsal motor nucleus of the vagus. Neuropharmacology 58: 1220–1227, 2010b. doi: 10.1016/j.neuropharm.2010.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haas KF, Macdonald RL. GABAA receptor subunit γ2 and δ subtypes confer unique kinetic properties on recombinant GABAA receptor currents in mouse fibroblasts. J Physiol 514: 27–45, 1999. doi: 10.1111/j.1469-7793.1999.027af.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hestrin S, Nicoll RA, Perkel DJ, Sah P. Analysis of excitatory synaptic action in pyramidal cells using whole-cell recording from rat hippocampal slices. J Physiol 422: 203–225, 1990. doi: 10.1113/jphysiol.1990.sp017980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapur J, Haas KF, Macdonald RL. Physiological properties of GABAA receptors from acutely dissociated rat dentate granule cells. J Neurophysiol 81: 2464–2471, 1999. [DOI] [PubMed] [Google Scholar]
- Karunanayake EH, Hearse DJ, Mellows G. The synthesis of [14C] streptozotocin and its distribution and excretion in the rat. Biochem J 142: 673–683, 1974. doi: 10.1042/bj1420673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin CL, Yeh MC, Harnod T, Lin CL, Kao CH. Risk of type 2 diabetes in patients with nonapnea sleep disorders in using different types of hypnotics: a population-based retrospective cohort study. Medicine (Baltimore) 94: e1621, 2015. doi: 10.1097/MD.0000000000001621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔC(T) method. Methods 25: 402–408, 2001. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Möhler H, Fritschy JM, Rudolph U. A new benzodiazepine pharmacology. J Pharmacol Exp Ther 300: 2–8, 2002. doi: 10.1124/jpet.300.1.2. [DOI] [PubMed] [Google Scholar]
- Mussa BM, Verberne AJ. Activation of the dorsal vagal nucleus increases pancreatic exocrine secretion in the rat. Neurosci Lett 433: 71–76, 2008. doi: 10.1016/j.neulet.2007.12.048. [DOI] [PubMed] [Google Scholar]
- Okada M, Onodera K, Van Renterghem C, Sieghart W, Takahashi T. Functional correlation of GABAA receptor α subunits expression with the properties of IPSCs in the developing thalamus. J Neurosci 20: 2202–2208, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park JB, Skalska S, Stern JE. Characterization of a novel tonic γ-aminobutyric acidA receptor-mediated inhibition in magnocellular neurosecretory neurons and its modulation by glia. Endocrinology 147: 3746–3760, 2006. doi: 10.1210/en.2006-0218. [DOI] [PubMed] [Google Scholar]
- Pirker S, Schwarzer C, Wieselthaler A, Sieghart W, Sperk G. GABAA receptors: immunocytochemical distribution of 13 subunits in the adult rat brain. Neuroscience 101: 815–850, 2000. doi: 10.1016/S0306-4522(00)00442-5. [DOI] [PubMed] [Google Scholar]
- Pocai A, Obici S, Schwartz GJ, Rossetti L. A brain-liver circuit regulates glucose homeostasis. Cell Metab 1: 53–61, 2005. doi: 10.1016/j.cmet.2004.11.001. [DOI] [PubMed] [Google Scholar]
- Rayner CK, Samsom M, Jones KL, Horowitz M. Relationships of upper gastrointestinal motor and sensory function with glycemic control. Diabetes Care 24: 371–381, 2001. doi: 10.2337/diacare.24.2.371. [DOI] [PubMed] [Google Scholar]
- Rerup C, Tarding F. Streptozotocin- and alloxan-diabetes in mice. Eur J Pharmacol 7: 89–96, 1969. doi: 10.1016/0014-2999(69)90169-1. [DOI] [PubMed] [Google Scholar]
- Rudolph U, Crestani F, Möhler H. GABAA receptor subtypes: dissecting their pharmacological functions. Trends Pharmacol Sci 22: 188–194, 2001. doi: 10.1016/S0165-6147(00)01646-1. [DOI] [PubMed] [Google Scholar]
- Sah P. Different calcium channels are coupled to potassium channels with distinct physiological roles in vagal neurons. Proc Biol Sci 260: 105–111, 1995. doi: 10.1098/rspb.1995.0066. [DOI] [PubMed] [Google Scholar]
- Saltzman MB, McCallum RW. Diabetes and the stomach. Yale J Biol Med 56: 179–187, 1983. [PMC free article] [PubMed] [Google Scholar]
- Schein PS, Loftus S. Streptozotocin: depression of mouse liver pyridine nucleotides. Cancer Res 28: 1501–1506, 1968. [PubMed] [Google Scholar]
- Semyanov A, Walker MC, Kullmann DM. GABA uptake regulates cortical excitability via cell type-specific tonic inhibition. Nat Neurosci 6: 484–490, 2003. doi: 10.1038/nn1043. [DOI] [PubMed] [Google Scholar]
- Trapp S, Ballanyi K. KATP channel mediation of anoxia-induced outward current in rat dorsal vagal neurons in vitro. J Physiol 487: 37–50, 1995. doi: 10.1113/jphysiol.1995.sp020859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Travagli RA, Hermann GE, Browning KN, Rogers RC. Brainstem circuits regulating gastric function. Annu Rev Physiol 68: 279–305, 2006. doi: 10.1146/annurev.physiol.68.040504.094635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vicini S, Ferguson C, Prybylowski K, Kralic J, Morrow AL, Homanics GE. GABAA receptor α1 subunit deletion prevents developmental changes of inhibitory synaptic currents in cerebellar neurons. J Neurosci 21: 3009–3016, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Washabau RJ, Fudge M, Price WJ, Barone FC. GABA receptors in the dorsal motor nucleus of the vagus influence feline lower esophageal sphincter and gastric function. Brain Res Bull 38: 587–594, 1995. doi: 10.1016/0361-9230(95)02038-7. [DOI] [PubMed] [Google Scholar]
- Williams KW, Zsombok A, Smith BN. Rapid inhibition of neurons in the dorsal motor nucleus of the vagus by leptin. Endocrinology 148: 1868–1881, 2007. doi: 10.1210/en.2006-1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood CE, Giroux D. Central nervous system prostaglandin endoperoxide synthase-1 and -2 responses to oestradiol and cerebral hypoperfusion in late-gestation fetal sheep. J Physiol 549: 573–581, 2003. doi: 10.1113/jphysiol.2002.038398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamatani K, Ohnuma H, Niijima A, Igarashi M, Sugiyama K, Daimon M, Manaka H, Tominaga M, Sasaki H. Impaired vagus nerve-mediated control of insulin secretion in Wistar fatty rats. Metabolism 47: 1167–1173, 1998. doi: 10.1016/S0026-0495(98)90318-3. [DOI] [PubMed] [Google Scholar]
- Yeung JY, Canning KJ, Zhu G, Pennefather P, MacDonald JF, Orser BA. Tonically activated GABAA receptors in hippocampal neurons are high-affinity, low-conductance sensors for extracellular GABA. Mol Pharmacol 63: 2–8, 2003. doi: 10.1124/mol.63.1.2. [DOI] [PubMed] [Google Scholar]
- Zsombok A, Bhaskaran MD, Gao H, Derbenev AV, Smith BN. Functional plasticity of central TRPV1 receptors in brainstem dorsal vagal complex circuits of streptozotocin-treated hyperglycemic mice. J Neurosci 31: 14024–14031, 2011. doi: 10.1523/JNEUROSCI.2081-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zsombok A, Smith BN. Plasticity of central autonomic neural circuits in diabetes. Biochim Biophys Acta 1792: 423–431, 2009. doi: 10.1016/j.bbadis.2008.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]