Highlights
-
•
The chlorite-based drug WF10 improved healing of foot wounds in diabetic patients.
-
•
Infusion with WF10 markedly reduced HbA1c values in patients with diabetic foot syndrome.
-
•
After administration of WF10 HbA1c values remained low over at least 8 to 12 weeks.
-
•
The chlorite component of WF10 is known to inactivate efficiently cytotoxic hemoglobin forms.
-
•
This treatment prevented below knee amputation in patients with diabetic foot syndrome.
Keywords: Foot ulcers, Healing, WF10, HbA1c, Diabetes
Graphical Abstract
Abstract
Aims
The intravenous application of the chlorite-based drug solution WF10 is known to improve wound healing in patients with diabetic foot syndrome. In this retrospective study, we addressed the question, which effects are caused by this drug in patients with diabetic foot ulcers on the hemoglobin A1c value.
Methods
Patients received five consecutive daily infusions of WF10. Three patients received a second cycle of WF10, and one patient a third cycle.
Results
On a group of twelve patients with diabetic foot syndrome, WF10 gradually reduced the HbA1c values from a high-risk range (9.1 ± 1.6% (76 ± 13 mmol/mol)) into a low-risk range in all patients but one. These values remain low over at least 8 to 12 weeks after the administration of WF10. This drug improved also considerably wound healing processes in eleven patients.
Conclusions
The chlorite component of WF10 is known to inactivate efficiently free cytotoxic hemoglobin forms that might accumulate in peripheral blood after hemolysis and induces the removal of pre-damaged red blood cells from circulation. By these mechanisms WF10 diminished toxic effects of hemolysis, improved microcirculation and glucose consumption in affected tissues, and prevented, thus, below knee amputation.
Introduction
Diabetic foot syndrome (DFS) is a severe pathology of diabetes mellitus type 1 and 2 and is associated with a 12-month mortality of 16.7%, which doubles after major amputation [1]. The risk for this and other common complications of diabetes like retinopathy, nephropathy, cardiovascular disease and others increases with enhanced values for hemoglobin A1c (HbA1c) [2], an important parameter for the long-term harm of hyperglycemia. Elevated HbA1c values are known to decrease the wound healing rate in neuropathic foot wounds and in those with peripheral artery disease [3].
The enhanced, insulin-independent uptake of glucose by erythrocytes favors the glycation of their proteins, impairs the deformability of these cells, and contributes to an osmotic stress by conversion of glucose into sorbitol via aldose reductase [4], [5], [6]. Hence, the impermeable sorbitol decreases the mechanic fragility of red blood cells and favors hemolysis [7]. There is a clear relationship between hemolysis degree and fasting plasma glucose concentrations as well as the HbA1c value in patients suffering from diabetes mellitus type 2 [8]. Otherwise, hemolysis diminishes the bioavailability of nitric monoxide (NO) and decreases, thus, blood circulation in affected tissues [9]. There is also a general association between enhanced glucose concentrations and decreased NO availability in diabetes [10], [11]. In these patients, hemolysis is also linked to an enhanced thrombotic risk [12], [13]. In their blood, the non-enzymatic modification of fibrinogen leads to the formation of complexes between fibrin fibers and red blood cells and to a changed morphology of these cells [14].
The chlorite-based drug solution WF10, which is given to patients in form of an intravenous infusion, has successfully been applied to improve the clinical outcome in patients with diabetic foot ulcer syndrome [15], [16]. This adjunct therapy to standard diabetes treatment significantly accelerated the wound healing process and reduced the rate of foot amputation. However, effects of this therapy on glucose status and especially on the long-term behavior of the HbA1c value remain unknown.
To deepen our knowledge about the beneficial action of WF10 in diabetic patients with foot syndrome, we addressed here the question whether the administration of WF10 improves besides the clinical outcome also important parameters of glycemic control. On twelve DFS patients, WF10 caused a normalization of enhanced HbA1c values. Follow up of a comparable group of diabetic patients with DFS, which were subjected to conventional wound care therapy, revealed no such reduction in HbA1c values [17].
Patients and methods
Patient's characteristics at baseline
This retrospective study was undertaken from March 2015 to December 2015 on diabetic patients with foot ulcers at a wound care clinic, Surgery Department of the General Police Hospital, Bangkok, Thailand. These patients received WF10 treatment in an attempt to treat diabetic foot ulcer and prevent below knee amputation (BK). The data of demographic, coexisting medical conditions, wound staging, and the glycemic control data before and after treatment were reviewed.
This study was approved by the ethical committee of the General Police Hospital, Bangkok, Thailand.
Assessment of wounds
Patient's ulcer were assessed and classified into different grades and stages according to Wagner's Grading System [18]. Grade 0 corresponds to intact skin, grade 1 to superficial diabetic foot ulcer, grade 2 to extensive ulcer involving ligament, tendon, joint capsule, or fascia without abscess or osteomyelitis. The higher wound grades correspond to deep ulcer with abscess or osteomyelitis (grade 3), gangrene to portion of forefoot (grade 4) or extensive gangrene of foot (grade 5).
Ulcers were graded as infected or not on the basis of the presence or absence of purulent discharge together with other local signs (warmth, erythema, lymphangitis, lymphadenopathy, edema and pain) and confirmed with swab wound culture. Diagnosis of osteomyelitis was made on the basis of radiological findings.
Determination of parameters of glucose metabolism
Hemoglobin A1c testing was performed by Integra 400 (Roche Diagnostics, Laval, QC, Canada). Fasting blood glucose level was performed daily by Dextrostix (Dtx) with a Glucocheck meter. Blood chemistry tests were performed by the Siemens Advia 1800 analyzer. Complete blood count was performed by Beckman Coulter hematology analyzer.
Treatment protocol with WF10
Five consecutive infusions of WF10 (referred to as ‘cycle’) at a dose of 0.3 ml/kg body weight, diluted in 500 ml physiological saline, were administered to DFS patients in an attempt to prevent BK and to induce healing of the chronic ulcer. As the primary focus was on wound healing, in three patients (NP, PS, SA), a second cycle of WF10, and in one patient (SR) after two cycles at 0.3 ml/kg even a third cycle at 0.5 ml/kg body weight were deemed clinically beneficial and consequently administered.
The usually administered WF10 dose of 0.3 ml/kg was lower than the recommended dose of 0.5 ml/kg. Probably in cases of NP, PS, and SA a selected dose of 0.5 ml/kg body weight for the first cycle may have lessened the need for another treatment cycle.
Results
Patient's characteristics during treatment with WF10
This retrospective study was undertaken from March 2015 to December 2015 on twelve diabetic patients with severe foot ulcers with gangrened toes and suspected osteomyelitis. These patients received WF10 treatment in an attempt to prevent below knee amputation (BK) at the General Police Hospital, Bangkok, Thailand. Baseline characteristics of these patients at hospitalization are summarized in Table 1.
Table 1.
Baseline characteristics of twelve diabetic patients with severe infected foot syndrome at hospitalization before WF10 treatment
| Baseline characteristics | Range | Average ± SD | NT | PP | AS | KP | NP | UA | PS | CW | SR | SA | NY | WW |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sex, male (%) | M | M | M | F | M | M | M | F | M | M | M | M | ||
| Age (years) | 40–71 | 55.1 ± 11.1 | 54 | 70 | 40 | 62 | 62 | 59 | 58 | 41 | 71 | 59 | 43 | 42 |
| Duration of diabetes (years) | 2–30 | 13.1 ± 8.4 | 10 | 20 | 8 | 20 | 18 | 30 | 6 | 10 | 20 | 2 | 10 | 3 |
| Nutritional status | ||||||||||||||
| Body weight (kg) | 50–116 | 72.6 ± 16.0 | 65 | 72 | 80 | 50 | 80 | 65 | 70 | 65 | 63.5 | 77 | 116 | 68 |
| Height (cm) | 152–182 | 169.7 ± 8.9 | 165 | 176 | 175 | 152 | 175 | 165 | 163 | 160 | 168 | 182 | 177 | 178 |
| Body mass index (kg/m2) | 21.5–37.0 | 25.1 ± 4.1 | 23.9 | 23.2 | 26.1 | 21.6 | 26.1 | 23.9 | 26.3 | 25.4 | 22.5 | 23.2 | 37.0 | 21.5 |
| Serum albumin (g/dL) | 2.8–4.6 | 3.8 ± 0.5 | 4.1 | 3.7 | 3.3 | 4.6 | 3.8 | 2.8 | 3.7 | 4.4 | 4.4 | 3.3 | 4.1 | 3.7 |
| Comorbidities (n) | ||||||||||||||
| Hypertension | 9 | Y | Y | Y | Y | – | Y | Y | Y | Y | – | Y | – | |
| Nephropathy | 1 | – | – | – | – | Y | – | – | – | – | – | – | – | |
| Retinopathy | 1 | – | – | – | – | Y | – | – | – | – | – | – | – | |
| Cardiovascular disease | 5 | Y | Y | Y | Y | Y | – | – | – | – | – | – | – | |
| Peripheral arterial occlusion disease | 2 | – | – | Y | Y | – | – | – | – | – | – | – | – | |
| Dyslipidemia | 3 | – | Y | – | – | – | – | – | Y | – | – | Y | – | |
| Diabetic foot ulcer | ||||||||||||||
| Ulcer duration (weeks) | 6.7 ± 8.4 | 1 | 3 | 24 | 8 | 2 | 24 | 1 | 1 | 4 | 6 | 2 | 4 | |
| Previous minor amputation (n) | 3 | – | Y | Y | Y | – | – | – | – | – | – | – | – | |
| Previous major amputation (n) | 0 | – | – | – | – | – | – | – | – | – | – | – | – | |
| Suggested below knee amputation | 8 | Y | – | Y | Y | Y | Y | – | – | – | Y | Y | Y | |
| Wagner wound score | 4 | 3 | 4 | 4 | 3 | 3 | 3 | 1 | 3 | 3 | 3 | 3 | ||
| Suspected osteomyelitis (n) | 11 | Y | Y | Y | Y | Y | Y | Y | N | Y | Y | Y | Y | |
| Diagnosed ulcer infection (n) | 11 | Y | Y | Y | Y | Y | Y | Y | N | Y | Y | Y | Y | |
| Swab culture positive (n) | 11 | Y | Y | Y | Y | Y | Y | Y | N | Y | Y | Y | Y | |
| Laboratory findings | ||||||||||||||
| Hemoglobin A1c (%) | 6.9–11.5 | 9.1 ± 1.6 | 9.7 | 9.2 | 11.5 | 8.9 | 7.7 | 6.9 | 11.1 | 9.8 | 7.7 | 8.4 | 11.0 | 7.1 |
| White blood cell count (106/L) | 6.6–34.4 | 13.9 ± 7.4 | 34.4 | 13.6 | 10.6 | 13.8 | 6.6 | 9.6 | 19.9 | 9.7 | 7.7 | 11.7 | 15.4 | 13.3 |
| Hematocrit (%) | 26.2–42.9 | 32.9 ± 4.5 | 30.1 | 33.3 | 26.2 | 30.8 | 33.9 | 33.3 | 33.0 | 32.0 | 30.2 | 29.5 | 42.9 | 39.4 |
| Hemoglobin (g/dL) | 8.1–14.4 | 10.8 ± 1.7 | 9.8 | 11.1 | 8.1 | 9.7 | 11.2 | 10.9 | 11.2 | 10.7 | 9.5 | 9.7 | 14.4 | 12.9 |
| Platelet (106/L) | 205–759 | 433 ± 155 | 409 | 382 | 424 | 759 | 280 | 598 | 432 | 245 | 556 | 448 | 456 | 205 |
| Fasting blood sugar (mg/dL) | 107–332 | 202.8 ± 62.5 | 187 | 210 | 288 | 229 | 124 | 160 | 196 | 332 | 107 | 220 | 198 | 183 |
| Blood urea nitrogen (mg/dL) | 10–31 | 18.4 ± 7.4 | 19 | 24 | 23 | 31 | 16 | 10 | 12 | 29 | 12 | 22 | 13 | 10 |
| Serum creatinine (mg/dL) | 0.54–1.78 | 1.00 ± 0.38 | 1.11 | 1.30 | 0.90 | 0.75 | 1.57 | 1.11 | 0.71 | 1.78 | 0.79 | 0.86 | 0.62 | 0.54 |
| Estimated GFR (eGFR) | 43.3–252 | 98.9 ± 60.5 | 69.9 | 53.8 | 123.5 | 61.4 | 55.2 | 65.9 | 112.3 | 43.3 | 78.0 | 101.0 | 252.0 | 171.0 |
| Blood Tx (unit) before WF10 Rx | 2 | – | – | – | 2 | – | – | – | – | 3 | – | – | ||
| Previous anti-DM medication | ||||||||||||||
| Oral anti-diabetic | N | N | Y | Y | Y | Y | Y | Y | Y | Y | Y | N | ||
| Previous insulin treatment (n) | Y | Y | N | N | N | N | N | Y | N | N | N | Y | ||
| Total dose insulin (U/day) | 10 | 50 | – | – | – | – | – | 35 | – | – | – | – |
M, male; F, female; Y, yes; N, no.
For eight patients, BK had been recommended by physicians from transferring hospitals. All patients had been diabetics for an average of 16 years and were associated with hypertension. Six patients had cardiovascular disease and three dyslipidemia. Two patients received insulin treatment and ten received only oral anti-diabetic medications prior DFS treatment. Based on this standard of care patients exhibited mean fasting glucose values of 202.8 mg/dl (107 mg/dl–332 mg/dl) and mean HbA1c levels of 9.1% (6.9%–11.5%) at baseline. Ten patients had been anemic (hematocrit less than 35%). Two patients exhibited peripheral occlusive disease symptoms. The glomerular filtration rate (GFR) for four patients were at or below 60 ml/min. Platelets of eight patients had been elevated.
Upon treatment with WF10, clinically all patients but one (SA) have shown either complete healing of their wounds or at least improvements by two points on the Wagner Score. Clinical parameters of the twelve diabetics, time to complete healing, medications against diabetes mellitus, and blood transfusions are summarized in Table 2.
Table 2.
Specific patient's characteristics concerning diabetic foot ulcers, anti-diabetic medications and results of WF10 treatment
| Characteristics | Range | Average ± SD | NT | PP | AS | KP | NP | UA | PS | CW | SR | SA | NY | WW |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| DFS treatment outcome | ||||||||||||||
| Wagner score at week 0 | 1–4 | 4 | 3 | 4 | 4 | 3 | 3 | 3 | 1 | 3 | 3 | 3 | 3 | |
| Wagner score at week 12 | 0–2 | NA | 0 | 2 | 2 | 0 | 0 | 1 | 0 | 1 | NA | 0 | 0 | |
| Time to heal (week) | 1–28 | 11 ± 8 | – | 14 | – | 28 | 1 | 4 | – | 6 | 14 | – | 10 | 10 |
| Hemoglobin A1c (%) | ||||||||||||||
| At week 0 before WF10 | 9.7 | 9.2 | 11.5 | 8.9 | 7.7 | 6.9 | 11.1 | 9.8 | 7.7 | 8.4 | 11.0 | 7.1 | ||
| At week 4 | 5.5 | 7.8 | 4.8 | 6.2 | 6.5 | 5.1 | 6.8 | 7.4 | 8.4 | 5.4 | 9.5 | 6.7 | ||
| At week 8 | 5.3 | 5.4 | 6.4 | 5.2 | 5.1 | 5.5 | 7.0 | 8.7 | 6.9 | 6.1 | ||||
| At week 12 | 8.4 | 5.9 | 6.4 | 6.2 | 6.1 | 6.1 | 8.4 | 7.3 | 6.5 | |||||
| At week 16 | 6.2 | 7.4 | 8.2 | 5.9 | 6.4 | 10.1 | 6.9 | |||||||
| Blood Tx (unit) before WF10 Rx | 2 | – | – | – | 2 | – | – | – | – | 3 | – | – | ||
| Blood Tx (unit) during WF10 Rx | 8 | – | 4 | 4 | - | 2 | – | – | – | – | – | – | ||
| Previous anti-DM medication | ||||||||||||||
| Oral anti-diabetic | N | N | Y | Y | Y | Y | Y | Y | Y | Y | Y | N | ||
| Previous insulin treatment (n) | Y | Y | N | N | N | N | N | Y | N | N | N | Y | ||
| Total dose insulin (U/day) | 10 | 50 | – | – | – | – | – | 35 | – | – | – | – | ||
| Anti-DM medication during treatment | ||||||||||||||
| Oral anti-diabetic | N | N | Y | Y | Y | Y | Y | Y | Y | Y | Y | N | ||
| Insulin treatment (n) | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | ||
| Total dose insulin (U/day) | 10 | 50 | 30 | 15 | 10 | 6 | 10 | 35 | 4 | 6 | 3 | 62 |
Y, yes; N, no; NA, not analyzed.
Clinically a visible improved blood circulation in affected tissues could be seen, granulation developed like a manicured lawn. Kidney function remained stable for at least six months. Elevated platelets became normal.
Alterations in glucose status upon WF10 treatment
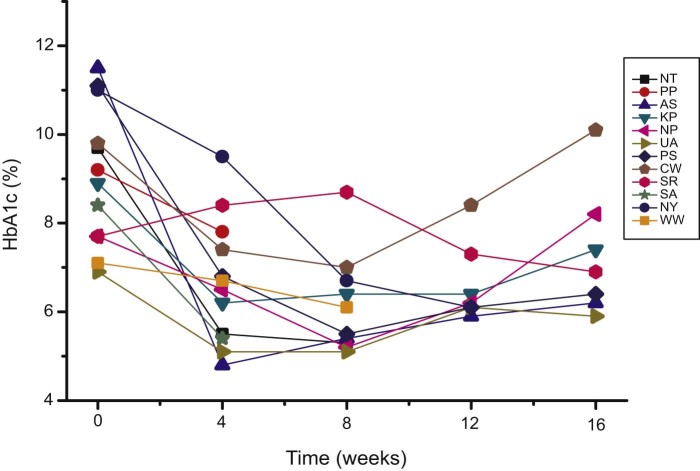
In all patients but one (SR), the HbA1c level gradually declined from high risk levels (9.1 ± 1.6%) to low risk levels of 6. 7 ± 1.4% at week 4, and 6.2 ± 1.1% at week 8 after five consecutive daily infusions of WF10 (Table 3). The mean value of 6.8 ± 1.0% at week 12 was also considerably lower than the baseline one, but slightly higher than the value at week 8. A mean HbA1c value of 7.3 ± 1.5% (n = 7) was determined at week 16. Statistical analysis applying the unpaired Student's t-test revealed a significant decrease of HbAc1 data in comparison to the baseline values with p < 0.001 (week 4, week 8), p < 0.01 (week 12), and p < 0.05 (week 16).
Table 3.
Time-dependent alterations in glucose parameters and hematocrit of twelve diabetics with severe foot syndrome after treatment with WF10
| Parameter | Baseline | Week 4 | Week 8 | Week 12 | Week 16 |
|---|---|---|---|---|---|
| Number of patients considered | 12 | 12 | 10 | 9 | 7 |
| HbA1c (%) | 9.1 ± 1.6 | 6.7 ± 1.4 | 6.2 ± 1.1 | 6.8 ± 1.0 | 7.3 ± 1.5 |
| HbA1c (mmol/mol) | 76 ± 13 | 50 ± 10 | 44 ± 8 | 51 ± 8 | 56 ± 12 |
| Fasting blood glucose (mg/dL) | 208 ± 64 | 170 ± 42 | 149 ± 22 | 207 ± 61 | |
| Hematocrit (%) | 32.9 ± 4.5 | 30.1 ± 3.8 | 33.3 ± 8.1 | 31.1 ± 6.7 |
Individual data for alterations of HbA1c values are given in Fig. 1. HbA1c declined during treatment from high risk into low risk range for at least eight and up to twelve weeks. Patient SR did not respond during the first two cycles of WF10, but after receiving the recommended dose of 0.5 ml/kg in the third cycle HbA1c declined to 7.3% at week 12 and further to 6.9% at week 16 (Table 3).
Figure 1.
WF10 consistently and drastically reduces HbA1c percentage into normal range. Individual HbA1c values of twelve diabetic DFS patients are given in dependence on the time after the first cycle of WF10 administration. One cycle (5 consecutive infusions on day 1, 2, 3, 4, 5) of WF10 at a dose of 0.3–0.5 ml/kg body mass was administered to twelve patients, two cycles to three patients (NP, PS, SA), and three cycles to one patient (SR).
Fasting glucose control improved after WF10 treatment (Table 3). Additionally the amount of oral hypoglycemic drugs and insulin required before and during treatment were gradually reduced and fasting glucose remained at a level of around 150 mg/dl for at least eight weeks.
Hematocrit and anemia control
WF10 induced an accelerated splenic clearance of dysfunctional erythrocytes bearing high levels of HbA1c. Patients with the highest HbA1c levels experienced the highest reduction. Expectedly, a transient drop in hematocrit values during the infusion period has been observed accompanied by a compensating erythropoiesis. As found earlier in healthy individuals and in virus infected patients, no sign of hemolysis was observed in this cohort of patients either [19]. In experimental investigations, fast generation of red blood precursor cells and erythropoietin generation have been shown after the first WF10 infusion suggesting a strong, compensating erythropoiesis (data not shown). Over a period of 12 weeks the mean hematocrit remained stable (Table 3) without further blood transfusions indicating a termination of hemolytic anemia by inactivating cytotoxic free hemoglobin metabolites.
One patient (UA) presented with severe anemia before treatment has shown repeatedly waves of ups (after infusions of two units RBC to 28% hematocrit) and downs (nearly exactly six weeks later), but the initial level of 23.5% hematocrit measured at baseline was never undercut and increased steadily after WF10 treatment to 25.6% (December 3, 2015) without further RBC transfusions.
Discussion
In diabetic patients with foot syndrome, the adjuvant therapy with the chlorite-based drug solution WF10 improves not only the clinical outcome [15], [16], but also as shown in the present study, this therapy improves significantly blood parameters. Intriguingly, there was a drastic and consistent decline in the HbA1c level into a well-defined low risk range. Thus, WF10 is remarkably directed toward basic processes associated with hyperglycemia-mediated pathologies. Remarkably, WF10 administration improved healing of foot wounds in eleven of the twelve patients. In eight patients, a complete wound healing was observed. In no case, a below knee amputation was necessary in this patient group.
Hyperglycemia disturbs the redox status and osmotic stability of red blood cells [4], [5], [6], [7], [20], favors hemolysis, diminishes the bioavailability of nitric monoxide, and impairs blood flow parameters [8], [10], [11], [21]. Release of hemoglobin from stressed erythrocytes has long-lasting consequences and is associated with many other disease scenarios [22], [23]. After hemolysis methemoglobin and protoporphyrin IX (also called hemin) are derived from hemoglobin. As the capacity of the protective plasma proteins haptoglobin and hemopexin for scavenging and inactivation of these oxidized heme forms is limited [24], a massive hemolysis is a dangerous condition to our organism. The hydrophobic hemin, a very toxic component, incorporates easily into hydrophobic areas of lipoproteins and cell membranes [25], [26]. In red blood cells, it causes hemolytic events [27]. Thus, we can hypothesize that in diabetic patients with severe pathologies such as foot syndrome, not only enhanced hyperglycemia, but also the presence of toxic hemolysis products contributes to the clinical picture.
Chlorite, the active principle of WF10, is known to interact with heme proteins [28], [29], [30], [31]. It inactivates hemoglobin and oxidized hemoglobin forms [31] that might accumulate in peripheral blood after hemolysis. Otherwise, WF10 causes a methemoglobin formation in intact red blood cells [32], [33], [34], [35]. As methemoglobin formation is counter-regulated by cellular redox processes [36] WF10 effects will be favored in cells with a reduced redox state. Erythrocytes with slightly enhanced methemoglobin levels will be removed from circulation by spleen and liver macrophages [37]. Red blood cells from diabetic individuals have a screwed morphology. They are more elongated and twist around fibrin fibers [14]. Whether this property contributes to an enhanced removal of pre-damaged red blood cells upon treatment with WF10 should be clarified in ongoing experiments.
From this background, it is not surprising that in some patients, a strong decline in hematocrit was observed after WF10 administration. The higher HbA1c was at baseline the stronger was the transient decline in hematocrit. Four of twelve already anemic patients received compensatory blood transfusion after WF10 administration.
The most intriguing result of this study is the long-lasting strong decrease in hemoglobin A1c. This parameter serves as an important predictor for the long-term harm of hyperglycemia [2]. In patients with chronic heart failure, increasing HbA1c value is a risk factor for further cardiovascular complications [38], [39]. Enhanced HbA1c level is also associated with a higher risk for neuropathy, retinopathy, and nephropathy [40], [41]. And finally, HbA1c is a predictor of healing rate in diabetic wounds [3]. Thus, the decrease in this parameter is of paramount importance in the therapy of diabetes complications.
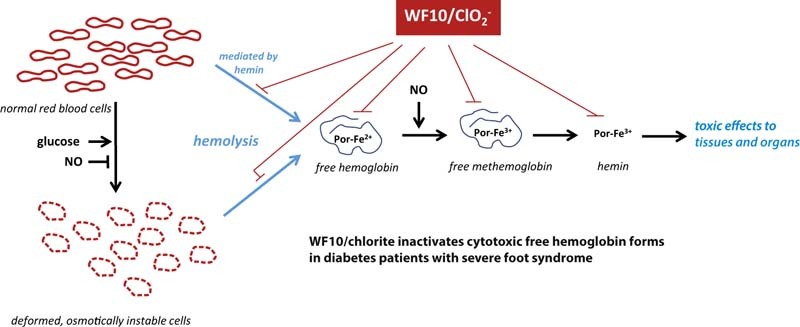
Apparently, the following sequence of events should be responsible for the observed strong decrease of HbA1c. As WF10 inactivates serum heme components and induces the removal of pre-damaged erythrocytes (Fig. 2), these two main sources for hemolytic events are eliminated or at least are strongly reduced. This improves the bioavailability of nitric monoxide and blood circulation, because nitric monoxide cannot further be utilized by hemolytic hemoglobin [9], [42], [43]. Enhanced NO levels also depress the activity of aldose reductase in red blood cells [44], [45]. Finally, the enhanced blood supply to tissues increases the consumption of glucose and reduces thus the hyperglycemic state.
Figure 2.
Proposed mode of action of the chlorite-based drug solution WF10 in hyperglycemic patients. Red blood cells of these patients are prone to hemolysis. Hemoglobin might be released from deformed, osmotically instable cells. A second hemolytic mechanism is mediated by hemin, a highly toxic hemolysis product, which can accumulate after exhaustion of the natural protecting serum proteins haptoglobin and hemopexin. WF10 prevents hemolysis events by inactivation of hemoglobin and oxidized hemoglobin forms and induces a removal of pre-damaged red blood cells.
It is assumed that glycated hemoglobin forms like HbA1c are unevenly distributed in the red blood cell population. Higher values are expected in elder cells and especially already damaged cells. WF10 induces the predominant removal of those cells from circulation and the formation of novel red blood cells with low level of glycated proteins. Thus, this decrease in HbA1c should occur within only few days after WF10 administration. Remarkably, this low level of HbA1c remains stable over at least eight weeks after therapy indicating a significant improvement of physiologic blood flow parameters and stabilization of glucose utilization. It is still unknown when hemolysis and osmotic stress flares up again. Currently a stable HbA1c level in the low risk range of five months in one patient has been measured. Thereafter, HbA1c started to rise again, but very slow.
Our data should also be important for other pathologic complications associated with hyperglycemia developing over years like chronic kidney disease, retinopathy, coronary arterial disease, and peripheral arterial disease, referred to as metabolic syndrome or syndrome X [46], [47], [48], [49]. Also, in these cases, the generation of toxic metabolites due to a smoldering hemolysis will contribute to disease progression. It is therefore a promising approach to focus on red blood cells as a therapeutic target having a relative short lifetime with the objective to interrupt the vicious cycle of hemolysis and to get rid of those cytotoxic hemoglobin metabolites.
Conclusion
The preliminary findings in this cohort of hyperglycemic patients presented with severe ulcers with gangrened toes and osteomyelitis and suggested for below knee amputation (BK) have shown besides good clinical outcome an improved glycemic control and a dramatic and consistent reduction in HbA1c, a predictor for death and micro-vascular complications in diabetics, after five infusions of WF10. Transient anemia during infusion could be managed by compensatory blood transfusions. Hemolysis was controlled by WF10-mediated inactivation of cytotoxic free hemoglobin products and removal of dysfunctional erythrocytes prone to a smoldering hemolysis. This therapy improved significantly blood circulation in affected tissues and blood parameters signaling a better control and consumption of glucose. Further studies are warranted to verify this approach.
Author contributions
Paiboon Maraprygsavan treated the patients, Jarasporn Mongkolsuk collected the data, Juergen Arnhold provided the scientific data and background, Friedrich-Wilhelm Kuehne initiated this research. All authors were involved in writing this manuscript. The final manuscript was approved by all authors.
Conflict of interest
Juergen Arnhold has pure scientific interests. Friedrich-Wilhelm Kuehne is the inventor of the drug WF10. Both Friedrich-Wilhelm Kuehne and Jarapsorn Mongkolsuk are researchers at OXO Chemie (Thailand) Co., Ltd., Bangkok, Thailand. Friedrich-Wilhelm Kuehne and Paiboon Maraprygsavan applied a patent about the use of WF10 to treat hemorrhagic diseases.
Acknowledgement
We acknowledge support from the German Research Foundation (DFG) and University of Leipzig within the program of Open Access Publishing.
References
- 1.Braun L.R., Fisk W.A., Lev-Tov H., Kirsner R.S., Isseroff R.R. Diabetic foot ulcer: an evidence-based treatment update. Am J Clin Dermatol. 2014;15:267–281. doi: 10.1007/s40257-014-0081-9. [DOI] [PubMed] [Google Scholar]
- 2.Gallagher E.J., Le Roith D., Bloomgarden Z. Review of hemoglobin A1c in the management of diabetes. J Diabetes. 2009;1:9–17. doi: 10.1111/j.1753-0407.2009.00009.x. [DOI] [PubMed] [Google Scholar]
- 3.Christman A.L., Selvin E., Margolis D.J., Lazarus G.S., Garza L.A. Hemoglobin A1c is a predictor of healing rate in diabetic wounds. J Invest Dermatol. 2011;131:2121–2127. doi: 10.1038/jid.2011.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gugliucci A. Glycation as the glucose link to diabetic complications. J Am Osteopath Assoc. 2000;100:621–634. [PubMed] [Google Scholar]
- 5.Brownlee M. The pathophysiology of diabetic complications. A unifying mechanism. Diabetes. 2005;54:1615–1625. doi: 10.2337/diabetes.54.6.1615. [DOI] [PubMed] [Google Scholar]
- 6.Shin S., Ku Y.-H., Ho J.-X., Kim Y.-K., Suh J.-S., Singh M. Progressive impairment of erythrocyte deformability as indicator of microangiopathy in type 2 diabetes melittus. Clin Hemorheol Microcirc. 2007;36:253–261. [PubMed] [Google Scholar]
- 7.Kung C.-M., Tseng Z.-L., Wang H.-L. Erythrocyte fragility with level of glycosylated hemoglobin in type 2 diabetic patients. Clin Hemorheol Microcirc. 2009;43:345–351. doi: 10.3233/CH-2009-1245. [DOI] [PubMed] [Google Scholar]
- 8.Lippi G., Mercadanti M., Aloe R., Targher G. Erythrocyte mechanical fragility is increased in patients with type 2 diabetes. Eur J Intern Med. 2012;23:150–153. doi: 10.1016/j.ejim.2011.11.004. [DOI] [PubMed] [Google Scholar]
- 9.Rother R.P., Bell L., Hillmen P., Gladwin M.T. The clinical sequelae of intravascular hemolysis and extracellular plasma hemoglobin. A novel mechanism of human disease. J Am Med Assoc. 2005;293:1653–1662. doi: 10.1001/jama.293.13.1653. [DOI] [PubMed] [Google Scholar]
- 10.Pieper G.M. Review of alterations in endothelial nitric oxide production in diabetes. Hypertension. 1998;31:1047–1060. doi: 10.1161/01.hyp.31.5.1047. [DOI] [PubMed] [Google Scholar]
- 11.Hink U., Li H., Mollnau H., Oelze M., Matheis E., Hartmann M. Mechanisms underlying endothelial dysfunction in diabetes mellitus. Circ Res. 2001;88:E14–22. doi: 10.1161/01.res.88.2.e14. [DOI] [PubMed] [Google Scholar]
- 12.Hess K., Grant P.J. Inflammation and thrombosis in diabetes. Thromb Haemost. 2011;105(Suppl. 1):S43–54. doi: 10.1160/THS10-11-0739. [DOI] [PubMed] [Google Scholar]
- 13.Lipinski B., Pretorius E. Novel pathway of iron-induced blood coagulation: implications for diabetes mellitus and its complications. Pol Arch Med Wewn. 2012;122:115–122. [PubMed] [Google Scholar]
- 14.Buys A., Van Rooy M.-J., Soma P., Van Papendrop D., Lipinski B., Pretorius E. Changes in red blood cell membrane structure in type 2 diabetes: a scanning electron and atomic force microscopy study. Cardiovasc Diabetol. 2013;12:25. doi: 10.1186/1475-2840-12-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yingsakmongkol N., Maraprygsavan P., Sukosit P. Effect of WF10 (Immunokine) on diabetic foot ulcer therapy: a double-blind, randomized, placebo-controlled trail. J Foot Ankle Surg. 2011;50:635–640. doi: 10.1053/j.jfas.2011.05.006. [DOI] [PubMed] [Google Scholar]
- 16.Yingsakmongkol N. Clinical outcome of WF10 adjunct to standard treatment of diabetic foot ulcers. J Wound Care. 2013;22:1–6. doi: 10.12968/jowc.2013.22.3.130. [DOI] [PubMed] [Google Scholar]
- 17.Markuson M., Hanson D., Anderson J., Langemo D., Hunter S., Thompson P. The relationship between hemoglobin A(1c) values and healing time for lower extremity ulcers in individuals with diabetes. Adv Skin Wound Care. 2009;22:365–372. doi: 10.1097/01.ASW.0000358639.45784.cd. [DOI] [PubMed] [Google Scholar]
- 18.Wagner F.W., Jr The diabetic foot. Orthopedics. 1987;10:163–172. doi: 10.3928/0147-7447-19870101-28. [DOI] [PubMed] [Google Scholar]
- 19.Raffanti S.P., Schaffner W., Federspiel C.F., Blackwell R.B., Ching O.A., Kühne F.-W. Randomized, double-blind, placebo-controlled trial of the immune modulator WF10 in patients with advanced AIDS. Infection. 1998;26:202–207. doi: 10.1007/BF02962364. [DOI] [PubMed] [Google Scholar]
- 20.Bravi M.C., Pietrangeli P., Laurentia O., Basili S., Cassone-Faldetta M., Ferri C. Polyol pathway activation and glutathione redox status in non-insulin-dependent diabetic patients. Metabolism. 1997;46:1194–1198. doi: 10.1016/s0026-0495(97)90216-x. [DOI] [PubMed] [Google Scholar]
- 21.Bahktiari N., Hosseinkhani S., Larijani B., Mohajeri-Tehrani M.R., Fallah A. Red blood cell ATP/ADP & nitric oxide: the best vasodilators in diabetic patients. J Diabetes Metab Disord. 2012;11:9. doi: 10.1186/2251-6581-11-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Adamzik M., Hamburger T., Petrat F., Peters J., de Groot H., Hartmann M. Free hemoglobin concentration in severe sepsis: methods of measurement and prediction of outcome. Crit Care. 2012;16:R125. doi: 10.1186/cc11425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schaer D.M., Buehler P.W., Alayah A.I., Belcher J.D., Vercellotti G.M. Hemolysis and hemoglobin revisited: exploring hemoglobin and hemin scavengers as a novel class of therapeutic proteins. Blood. 2013;121:1276–1284. doi: 10.1182/blood-2012-11-451229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chiabrando D., Vinchi F., Fiorito V., Tolosano E. Haptoglobin and hemopexin in heme detoxification and iron recycling. In: Veas F., editor. Acute phase proteins – regulation and functions of acute phase proteins. Intech; Rijeka, Croatia: 2011. pp. 261–288. [Google Scholar]
- 25.Jeney V., Balla J., Yachie A., Varga Z., Vercellotti G.M., Eaton J.W. Pro-oxidant and cytotoxic effects of circulating heme. Blood. 2002;100:879–887. doi: 10.1182/blood.v100.3.879. [DOI] [PubMed] [Google Scholar]
- 26.Balla J., Vercellotti G.M., Jeney V., Yachie A., Varga Z., Eaton J.W. Heme, heme oxygenase and ferritin in vascular endothelial cell injury. Mol Nutr Food Res. 2005;49:1030–1043. doi: 10.1002/mnfr.200500076. [DOI] [PubMed] [Google Scholar]
- 27.Kumar S., Bandyopadhyay U. Free heme toxicity and its detoxification systems in human. Toxicol Lett. 2005;157:175–188. doi: 10.1016/j.toxlet.2005.03.004. [DOI] [PubMed] [Google Scholar]
- 28.Schempp H., Reim M., Dornisch K., Elstner E. Chlorite-hemoprotein interaction as key role for the pharmacological activity of the chlorite-based drug WF10. Drug Res. 2001;51:3–11. doi: 10.1055/s-0031-1300079. [DOI] [PubMed] [Google Scholar]
- 29.Jakopitsch C., Spalteholz H., Furtmüller P.G., Arnhold J., Obinger C. Mechanism of reaction of horseradish peroxidase with chlorite and chlorine dioxide. J Inorg Biochem. 2008;102:108–128. doi: 10.1016/j.jinorgbio.2007.09.002. [DOI] [PubMed] [Google Scholar]
- 30.Jakopitsch C., Pirker K.F., Flemmig J., Hofbauer S., Schlorke D., Furtmüller P.G. Mechanism of reaction of chlorite with mammalian heme peroxidases. J Inorg Biochem. 2014;135:10–19. doi: 10.1016/j.jinorgbio.2014.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pichert A., Arnhold J. Interaction of the chlorite-based drug WF10 with hemoglobin, methemoglobin and ferryl hemoglobin. Arch Biochem Biophys. 2015;585:82–89. doi: 10.1016/j.abb.2015.09.009. [DOI] [PubMed] [Google Scholar]
- 32.Bercz J.P., Jones L., Garner L., Murray D., Ludwig D.A., Boston J. Subchronic toxicity of chlorine dioxide and related compounds in drinking water in the nonhuman primate. Environ Health Perspect. 1982;46:47–55. doi: 10.1289/ehp.824647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.French C.L., Yaun S.-S., Baldwin A., Leonard D.A., Zhao X.Q., Calabrese E.J. Potency ranking of methemoglobin-forming agents. J Appl Toxicol. 1995;15:167–174. doi: 10.1002/jat.2550150306. [DOI] [PubMed] [Google Scholar]
- 34.Habermann E., Müller B. Oxiferin und Natriumchlorit – Ein Vergleich. Klin Wochenschr. 1989;67:20–25. doi: 10.1007/BF01736530. [DOI] [PubMed] [Google Scholar]
- 35.Langlois C.J., Calabrese E.J. The interactive effect of chlorine, copper and nitrite on methemoglobin formation in red blood cells of Dorset sheep. Hum Exp Toxicol. 1992;11:223–228. doi: 10.1177/096032719201100311. [DOI] [PubMed] [Google Scholar]
- 36.Rodkey F.L., O'Neal J.D. Effects of carboxyhemoglobin on the determination of methemoglobin in blood. Biochem Med. 1974;9:261–270. doi: 10.1016/0006-2944(74)90061-1. [DOI] [PubMed] [Google Scholar]
- 37.Arashiki N., Kimata N., Manno S., Mohandas N., Takakuwa Y. Membrane peroxidation and methemoglobin formation are both necessary for band 3 protein clustering: mechanistic insights into human erythrocyte senescence. Biochemistry. 2013;52:5760–5769. doi: 10.1021/bi400405p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gerstein H.C., Swedberg K., Carlsson J., McMurray J.J.V., Michelson E.L., Olofsson B. The hemoglobin A1c level as a progressive risk factor for cardiovascular death, hospitalization for heart failure, or death in patients with chronic heart failure. Arch Intern Med. 2005;168:1699–1704. doi: 10.1001/archinte.168.15.1699. [DOI] [PubMed] [Google Scholar]
- 39.Wang H., Calhoun D., Shara N.M., de Simone G., Lee E.T., Umans J.G. Hemoglobin A1c, fasting glucose, and cardiovascular risk in a population with high prevalence of diabetes. Diabetes Care. 2011;34:1952–1958. doi: 10.2337/dc11-0329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.McCarter R.J., Gomez R., Hempe J.M., Chalew S.A. Biological variation in HbA1c predicts risk of retinopathy and nephropathy in type 1 diabetes. Diabetes Care. 2004;27:1259–1264. doi: 10.2337/diacare.27.6.1259. [DOI] [PubMed] [Google Scholar]
- 41.El-Salem K., Ammari F., Khader Y., Dhaimat O. Elevated glycosylated hemoglobin is associated with subclinical neuropathy in neurologically asymptomatic diabetic patients: a prospective study. J Clin Neurophysiol. 2009;26:50–53. doi: 10.1097/WNP.0b013e31819862ee. [DOI] [PubMed] [Google Scholar]
- 42.Reiter C.D., Wang X., Tanus-Santos J.E., Hogg N., Cannon R.O., Schechter A.N. Cell-free hemoglobin limits nitric oxide bioavailability in sickle-cell disease. Nat Med. 2002;8:1383–1389. doi: 10.1038/nm1202-799. [DOI] [PubMed] [Google Scholar]
- 43.Olson J.S., Foley E.W., Rogge C., Tsai A.-L., Doyle M.P., Lemon D.D. NO scavenging and the hypertensive effect of hemoglobin-based blood substitutes. Free Radic Biol Med. 2004;36:685–697. doi: 10.1016/j.freeradbiomed.2003.11.030. [DOI] [PubMed] [Google Scholar]
- 44.Chandra D., Jackson E.B., Ramana K.V., Kelley R., Srivastava S.K., Bhatnagar A. Nitric oxide prevents aldose reductase activation and sorbitol accumulation during diabetes. Diabetes. 2002;51:3095–3101. doi: 10.2337/diabetes.51.10.3095. [DOI] [PubMed] [Google Scholar]
- 45.Srivastava S.K., Ramana K.V., Chandra D., Srivastava S., Bhatnagar A. Regulation of aldose reductase and the polyol pathway activity by nitric oxide. Chem Biol Interact. 2003;143–144:333–340. doi: 10.1016/s0009-2797(02)00214-4. [DOI] [PubMed] [Google Scholar]
- 46.Oishi N., Kubo E., Takamura Y., Maekawa K., Tanimoto T., Akagi Y. Correlation between erythrocyte aldose reductase level and human diabetic retinopathy. Br J Ophthalmol. 2002;86:1363–1366. doi: 10.1136/bjo.86.12.1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dominiczak M.H. Obesity, glucose intolerance and diabetes and their links to cardiovascular disease. Implication for laboratory medicine. Clin Chem Lab Med. 2003;41:1266–1278. doi: 10.1515/CCLM.2003.194. [DOI] [PubMed] [Google Scholar]
- 48.Gross J.L., Canini L.H., de Azevedo M.J., Caramori M.L., Silveiro S.P., Zelmanovitz T. Diabetic nephropathy: diagnosis, prevention, and treatment. Diabetes Care. 2005;28:164–176. doi: 10.2337/diacare.28.1.164. [DOI] [PubMed] [Google Scholar]
- 49.Ramasamy R., Goldberg I.J. Aldose reductase and cardiovascular diseases, creating human-like diabetic complications in an experimental model. Circ Res. 2010;106:1449–1458. doi: 10.1161/CIRCRESAHA.109.213447. [DOI] [PMC free article] [PubMed] [Google Scholar]