Highlights
-
•
Blood glucose and fat oxidation were simultaneously measured in Asian males.
-
•
The whole body calorimetre measured fat oxidation over 10 hours.
-
•
Low GI meals increased fat oxidation in subjects who were in a sedentary state.
-
•
Low GI meals minimized large blood glucose fluctuations throughout the day.
Keywords: Glycaemic index, Diet, Continuous glucose monitoring, Energy flux, Fat oxidation, Asian
Abstract
Objective
Low glycaemic index (GI) foods are known to minimize large fluctuations in blood glucose levels and have been suggested to increase fat oxidation. The objective of this study was to simultaneously investigate glucose excursion and substrate oxidation in a whole body calorimetre when Chinese male subjects were provided a low or high GI meal.
Materials/Methods
In a randomized, controlled crossover non blind design, 12 healthy Chinese male adults (BMI 21.8 ± 1.3 kgm−2) attended two sessions consisting of either four low or high glycaemic meals (LGI vs HGI). Breakfast, lunch and snack were consumed in a whole body calorimetre while dinner was consumed at home. Daily changes in glycaemic response (GR) and postprandial GR responses were measured using a continuous glucose monitoring system. The GR was further calculated to obtain the incremental area under the curve (iAUC) for glucose concentrations. Glycaemic variability was calculated as mean amplitude of glycaemic excursion (MAGE). Substrate oxidation was calculated by measuring respiratory quotient and urine nitrogen excretion.
Results
After LGI meals in the whole body calorimetre, iAUC for glucose (P = 0.008) was lower compared to the HGI session. The HGI treatment produced a significantly greater MAGE than the LGI treatment over the 24 hour period (P < 0.001). Additionally, higher fat oxidation and lower carbohydrate oxidation were observed following breakfast and lunch when comparing LGI to HGI (P < 0.05)
Conclusions
Consumption of LGI meals was capable of attenuating 24-hour blood glucose profiles and decreasing postprandial glucose excursions in healthy Asian males. Additionally, LGI mixed meals were able to promote fat oxidation over carbohydrate oxidation when compared to HGI mixed meals. The consumption of low GI meals may be a strategic approach in improving overall glycaemia and increasing fat oxidation in Asians consuming a high carbohydrate diet.
Introduction
Asia has the unenviable reputation as being the epicentre for type 2 diabetes. The Asian phenotype has been shown to be more susceptible to diabetes than Caucasians [1], [2]. More significantly, the transition from prediabetes to diabetes is more dramatic and severe in Asians. Overweight and obesity are driving the global diabetes epidemic [3]. This is an important public health concern as these individuals are at greater risk of developing type 2 diabetes and impaired glucose tolerance. Larger fluctuations in blood glucose during postprandial periods trigger more oxidative stress and are also considered as a risk factor in the onset for type 2 diabetes and impaired glucose tolerance [4].
There is good evidence to suggest that the consumption of low glycaemic index (GI) foods minimizes large fluctuations in blood glucose levels [5], [6]. This is especially significant in insulin resistant or diabetic people [7]. There is also increasing interest in the use of low GI foods in the management of obesity [8], [9]. Substrate oxidation is reflected by the respiratory quotient (RQ). A high fasting RQ corresponds to low fat oxidation and has been associated with higher prospective weight gain [10], [11] and fat storage [11]. Low GI (LGI) foods have been hypothesized to affect weight control by promoting satiety and fat oxidation at the expense of carbohydrate oxidation [12]. Low GI-carbohydrate (CHO) meals have been shown to be beneficial in reducing postprandial blood glucose and increasing fat oxidation during subsequent exercise in males [13] and females [14]. The results from previous studies indicated that the consumption of LGI foods maintains plasma glucose concentrations whilst favouring an increase in fat oxidation [13], [15], [16]. There is a significant shift in substrate utilization from CHO to fat when an LGI meal was ingested before exercise compared with a high GI (HGI) meal [16], [17]. These studies have assessed the effect of GI of a meal consumed before or after exercise on metabolic and biochemical parameters. No studies have been initiated to determine the effect of LGI and HGI foods on postprandial blood glucose fluctuations and energy metabolism measured simultaneously over a 10 hour period. The novelty of the study is that it demonstrates how a relatively small intervention to the daily diet could affect 24 h glucose levels and energy flux in Asians. Simultaneous measurements using the continuous glucose monitoring systems (CGMS™) and the whole body calorimetre (WBC) were carried out. The CGMS™ provided a detailed glucose profile of a person. Indirect calorimetry using the WBC provided data to enable us to compute substrate oxidation and how this is modulated when subjects are fed an LGI or an HGI diet.
Research design and methods
Fifteen healthy Chinese adults were recruited using a variety of methods which included flyers, online advertisements and personal communication. Volunteers who fulfilled all the inclusion criteria (male, age: 21–40 years; body mass index 17–25 kg/m2; no metabolic diseases; not on prescription medication; not allergic/intolerant to any of the test foods; does not intentionally restrict food intake; fasting blood glucose < 6 mmol/L) were accepted into the study. Baseline anthropometric and biochemistry data of the study participants are shown in Table 1. Physical activity was quantified using the questionnaire by Baecke et al. [18]. Eating behaviour was quantified using a Dutch eating behaviour questionnaire by Van Strien et al. [19].
Table 1.
Baseline characteristics of study participants (n = 12)a
| Characteristic | Statistic |
|---|---|
| Age (years) | 26.2 ± 3.8 |
| Height (cm) | 171.9 ± 6.2 |
| Weight (kg) | 64.6 ± 6.8 |
| BMI (kg/m2) | 21.8 ± 1.3 |
| Waist circumference (cm) | 74.5 ± 4.8 |
| Fasting blood glucose (mmol/L) | 4.4 ± 0.3 |
| HbA1c (%)b | 5.4 ± 0.2 |
| Body fat (%) | 14.1 ± 2.8 |
| Systolic blood pressure (mmHg) | 121.4 ± 7.6 |
| Diastolic blood pressure (mmHg) | 73.5 ± 8.7 |
Data presented as mean ± SD.
HbA1c normal range 2.5–14.0%.
The study was conducted at the Clinical Nutrition Research Centre (CNRC), Singapore. Ethical approval of all procedures involving human subjects was obtained from the National Healthcare Group Domain Specific Review Board (NHG DSRB). Research procedures and trial protocols were followed in accordance to the good clinical practice (GCP) guidelines and with the ethical standards in concordance to the Declaration of Helsinki, 1983. Written informed consent was obtained from all eligible participants before commencement. A crossover design of 11 participants would suffice in detecting a 15% change in area under the 24 h glucose curve, using a power of 85% and at a significance level of 0.05 [20], [21].
Study design
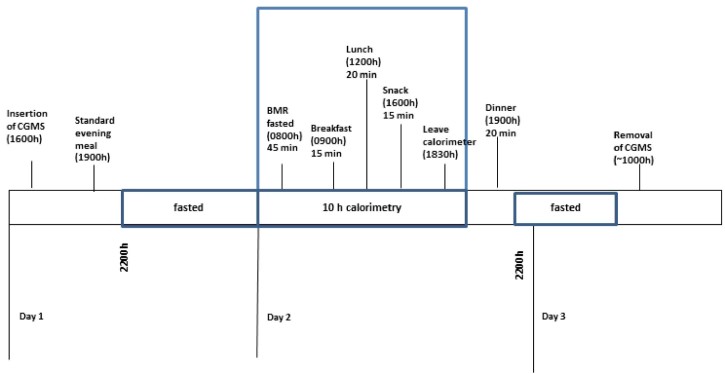
The study had a randomized, controlled crossover non-blind design. Volunteers attended two test sessions separated by a wash-out period of at least five days. Each session spanned over three consecutive days, of which one complete 24 h period of glucose measure was captured with a continuous glucose monitoring system (CGMS) device on day 2. A food diary was given on day 1 to capture their own breakfast and lunch consumption, and a session log sheet to assess their physical activity for the rest of the day. Subjects were advised to refrain from any strenuous physical activity during the study period. The food diary and session log sheet were used to ensure subjects followed the same routine on day 1 for the next session. A standardized dinner was also provided on day 1. On day 2, participants stayed in the whole body calorimetre (WBC) room from 0800 to 1800 (10 hr) where they were asked to lie in a supine position on the bed for the first 45 min to measure their basal metabolic rate (BMR). Participants were then given either a low glycaemic index (LGI) or high glycaemic index (HGI) breakfast, lunch and snack to consume. Postprandial diet-induced thermogenesis (DIT) was measured for breakfast and lunch for 120 min followed by 100 min postprandial DIT for snack. Thereafter, an LGI or an HGI dinner was provided. Participants were free to study, surf the net, watch television, listen to radio, use the telephone or lie on the bed. However, they were not allowed to sleep during their time in the WBC. They were also encouraged to keep to one activity after consuming the meal and to minimize movement. A schematic study flow is presented as shown in Fig. 1.
Figure 1.
Study protocol. From evening of day 1, subjects consumed a standardized dinner. Overnight fast from 2200 h to 0600 h. For day 2, subjects entered the calorimetre in a fasted state at 0800 h and underwent a basal metabolism test (BMR). Breakfast, lunch and snack test meals were provided in the calorimetre. Subjects remained sedentary during the 10 hours in the calorimetre. Test dinner provided after leaving calorimetre. The CGMS was removed on day 3.
Test meals
A standardized dinner was provided on day 1 consisting of a ready-to-eat teriyaki chicken with rice, one drink and one mango-flavoured jelly pudding. The entire meal reflected a typical local rice-based meal accompanied with a drink and dessert (energy: 879 kcal; protein: 44.3 g; fat: 18.3 g; carbohydrate:132.7 g).
The high and low GI test meals provided were as follows:
-
1
Low GI breakfast: bran cereal (Kellogg's, Thailand), GI: 42; high GI breakfast: Honey Stars (Nestlé, Malaysia), GI: 87
-
2
Low GI lunch: parboiled basmati rice (Diabetic Specialities Pte Ltd, Singapore), GI: 55, chicken stock (Knorr chicken stock, Malaysia), fresh spinach, GI: 15; high GI lunch: glutinous rice (New Moon, Tek Seng Rice Mill Co. Ltd, Thailand), GI: 92, chicken stock (Knorr chicken stock, Malaysia), fresh carrots, GI: 49, margarine (Flora, Unilever, Australia)
-
3
Low GI snack: multigrain bread (Bakels Pte Ltd, Singapore), GI: 44, strawberry spread (Fifty 50; USA), GI: 6; high GI snack: white bread (Gardenia brand, Singapore) GI: 79, strawberry jam (Bonne Maman, France), GI: 51
-
4
Low GI dinner: parboiled basmati rice (Diabetic Specialities Pte Ltd, Singapore), GI: 55, chicken stock (Knorr chicken stock, Malaysia), fresh spinach, teriyaki chicken (Charoen Pokphand Intertrade, Singapore), Chunky Organic Hazelnut Oat Krunch (Munchy's, Malaysia), GI: 49; high GI dinner: glutinous rice (New Moon, Tek Seng Rice Mill Co. Ltd, Thailand), GI: 92, chicken stock (Knorr chicken stock, Malaysia), fresh carrots, GI: 49, margarine (Flora, Unilever, Australia), teriyaki chicken (Charoen Pokphand Intertrade, Singapore), rice cracker (Bin-Bin, Singapore), GI: 83
All meals were served with a cup of chamomile tea (300 mL). The energy values, macronutrient composition and available carbohydrates of the test meals are provided in Table 2. The GI of individual foods was obtained using GI values from recognized tables [4] and from manufacturers' information. The meal GI was calculated using the mixed meal formula [22]. Subjects were requested to consume both breakfast and snack within 15 min and lunch within 20 min.
Table 2.
Macronutrient composition and menu for the test meals provided in the study
| LGI diet | HGI diet | |
|---|---|---|
| Macronutrient composition (kcal) | ||
| Energy | 1827 | 1848 |
| Carbohydrate | 1339(72%)a | 1377(74%)a |
| Protein | 283(15%) | 217(12%) |
| Fat | 241(13%) | 254(14%) |
| Total calories for each meal (kcal) | ||
| Breakfast | 302 | 328 |
| Lunch | 417 | 439 |
| Snack | 368 | 338 |
| Dinnerb | 740 | 743 |
| Meal GI | ||
| Breakfast | 42 | 87 |
| Lunch | 52 | 90 |
| Snack | 36 | 73 |
| Dinner | 49 | 83 |
| Available CHO (g) | ||
| Breakfast | 63 | 63 |
| Lunch | 91 | 97 |
| Snack | 60 | 58 |
| Dinner | 119 | 119 |
Percent (%) kilocalories from carbohydrate, protein and fat in parentheses.
Dinner meal consumed at home. Breakfast, lunch and snack consumed in the whole body calorimetre.
Glucose measurement
iPro™2 continuous glucose monitoring (CGM) system
The iPro™2 continuous glucose monitoring (CGM) system (iPro™2 Professional CGM-Medtronic MiniMed, Northbridge, CA, USA) was used in this study. The insertion was performed on day 1 at 1400 h and the sensor was removed on day 3 of the study at 1000 h. Data were collated and processed using an online software (Medtronic Diabetes CareLink iPro; https://carelink.minimed.eu). The data reported in this paper represent interstitial glucose readings recorded every 5 minutes for up to 42 hours. At each test session, the CGMS sensor was calibrated against finger-stick blood glucose measurements four times a day before every meal and before sleeping using the OneTouch Ultra®2 blood glucose metre (LifeScan, Inc., Milpitas, CA, USA).
Energy expenditure and substrate oxidation assessment
Whole body calorimetry (WBC)
The WBC was a 13.5 m3 room furnished with a single-bed, a foldable chair, a bureau with built-in sink, deep-freeze toilet (Special Products, Mulder), a colour television, an alarm clock, a radio, a telephone, a laptop, WIFI connection and an automated intercom for communication between the researcher and the participant. In addition, the WBC was built to mimic a normal room with two windows for visual contact between the researcher and participant. Respiratory quotient (RQ), resting energy expenditure (REE) and substrate oxidation were measured on day 2 in the WBC. The used WBC was a modification on the system described by Schoffelen et al. [23].
Substrate oxidation
Substrate oxidation was calculated from urinary nitrogen excretion, oxygen consumption and carbon dioxide production [24]. Urine samples were collected in the WBC over 10 h in a 3 litre 24-hr urine collection container (Urisafe®, Canada). The total volume of urine over 10 h was measured and a randomized urine sample was stored for nitrogen analysis. Nitrogen content (%) was measured using the copper catalyst Kjeldahl method (AOAC Official Method 984.13). Protein oxidation (g/min) was calculated by multiplying 10 h urinary nitrogen (g) by 6.25 and converted to per minute values. CHO oxidation and fat oxidation were calculated by using the following equations based on the volumes of O2 consumed and CO2 produced in oxidation of glucose, fat and protein as published by Frayn [25]: CHO oxidation (g/min) = −3.21 × O2 (L/min) + 4.55 × CO2 (L/min) − 2.87 × N (g/min) and fat oxidation (g/min) = 1.67 × O2 (L/min) − 1.67 × CO2 (L/min) – 1.92 × N (g/min).
Statistical analysis
All statistical analyses were performed using Statistical Package for the Social Sciences version 16 (SPSS Inc.). Data and figures were processed in a Microsoft Excel spreadsheet (Microsoft Corporation). Values were presented as mean ± SD unless otherwise stated. Prior to statistical analysis, the normality of the data was assured using the Shapiro–Wilk test.
The primary outcome of this study was to determine how the inclusion of LGI and HGI diets (breakfast, lunch, snack and dinner) impacts on 24 hour blood glucose fluctuations and energy regulation. The GR was calculated by first using the first two hour average of CGM interstitial glucose readings under the fasting state as baseline value. To determine the daily baseline value for each subject, the average of each day's 2 hours of CGM readings under the fasted state, before the breakfast meal, was taken. The average baseline value was then used to convert every 5 min reading of 22 subsequent hours of CGMS interstitial glucose data as the ‘change in glucose’. The other primary outcome measure was the total glucose response expressed as the incremental area under the curve (i.e. the GR iAUC) calculated using the trapezoidal rule [26], [27]. The ‘change in glucose’ values was important for further analyses such as the GR iAUC calculations, CGMS glucose curve construction and statistics. The secondary outcome measures were the total daily AUC and the glycaemic variability. Several indicators have been developed and used to ascertain glycaemic variability [28] and the risk of developing hypo- or hyperglycaemia [29]. The MAGE was used as an indicator in the present study to assess glucose fluctuations during the day [30]. MAGE was calculated using EasyGV software (available free at http://www.easygv.co.uk), with this software being extensively reviewed [31]. Substrate oxidation was calculated from respiratory quotient (RQ) and urine nitrogen excretion resulting in non-protein RQ (npRQ) and oxidation rate of carbohydrates, fat and protein. Baseline npRQ was calculated from the REE measurement under the fasting state. Subsequently, npRQ values during each meal were used to calculate the postprandial changes. Paired t-test was performed to test the differences in the GR and substrate oxidation between LGI and HGI treatments over 24 hours and during the period in the calorimetre. These comparisons were also performed for the GR iAUC, total daily AUC and the MAGE. Alpha (α) was set at 0.05 for statistical analyses.
Results
Baseline characteristics of subjects
The baseline characteristics of the subjects are given in Table 1. The anthropometric data were within normal ranges for this population. Twelve out of fifteen study participants completed the study, with complete data for both the LGI and HGI diets. The food diary records for breakfast, lunch and standardized dinner were analysed for subjects' dietary intake on day 1. There were no significant difference in dietary intake on day 1 between the two sessions, for total energy, carbohydrate, fat and protein (kcal) prior to testing on day 2 (P > 0.05). This was to ensure that dietary intake prior to testing did not bias the results.
Continuous glucose monitoring interstitial glucose data
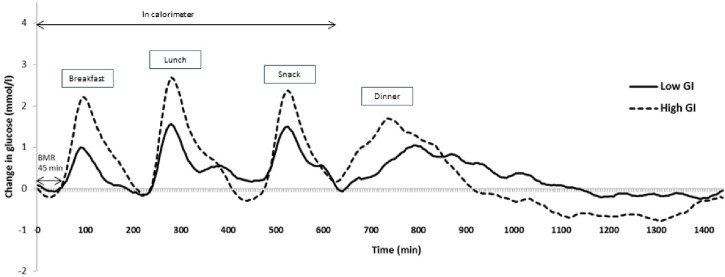
The glycaemic profiles for the LGI and HGI diets are graphically presented in Fig. 2. The calculated GR, GR iAUC and MAGE results are presented in Table 3. Generally the HGI intervention produced a sustained higher GR throughout the day (Fig. 2).
Figure 2.
Mean change in glucose concentrations from baseline of healthy Chinese male participants on a low GI or high GI meals for each day (n = 12). CGM, continuous glucose monitoring (overnight fast range from 960 min to 1440 min).
Table 3.
Mean (SD) glycaemic outcome variables of the 12 participants for whom a complete set of continuous glucose monitoring data were obtained
| Outcome measure | LGI | HGI | P value |
|---|---|---|---|
| Change in glucose (from baseline) | |||
| Breakfast (mmol/l)a | 0.46(0.2) | 1.27(0.5) | <0.001 |
| Lunch(mmol/l)a | 0.79(0.5) | 1.46(0.7) | 0.017 |
| Snack(mmol/l)a | 0.93(0.5) | 1.38(1.0) | 0.112 |
| Dinner(mmol/l)a | 0.81(0.5) | 1.58(1.29) | 0.055 |
| Overnight fast(mmol/l)a | 0.22(0.5) | -0.28(1.1) | 0.156 |
| Assessment of glycaemic variability | |||
| MAGE over 24 hours(mmol/l) | 1.75(0.5) | 3.05(1.0) | <0.001 |
| Daily positive AUC | |||
| Total daily AUC(mmol/L.min) (24 hours) | 692.14(395.7) | 1049.48(648.0) | 0.059 |
| Total AUC(mmol/L.min) (period of 10 hours in calorimetre) | 345.46(162.1) | 582.10(267.5) | 0.008 |
| Breakfast iAUC(mmol/L.min) | 60.50(23.2) | 158.52(60.2) | <0.001 |
| Lunch iAUC(mmol/L.min) | 105.14(51.8) | 184.39(82.6) | 0.014 |
| Snack iAUC(mmol/L.min) | 100.83(50.3) | 149.60(90.8) | 0.025 |
| Dinner iAUC(mmol/L.min) | 105.35(60.1) | 206.53(146.3) | 0.033 |
| Overnight iAUC(mmol/L.min) | 193.06(172.0) | 167.29(213.9) | 0.766 |
Values are expressed as mean with SD in parentheses; LGI: low glycaemic index; HGI: high glycaemic index.
MAGE, mean amplitude of glycaemic excursion.
Change from baseline glucose values.
In the LGI intervention, the incremental change in GR following breakfast and lunch was significantly lower (P < 0.001 and 0.017 respectively) compared to the HGI intervention. The incremental change in GR following the snack, dinner and overnight were not significant (P = 0.05). Although the snack, dinner and overnight glucose concentrations were not significantly different between the LGI and HGI treatments, there was a trend towards a lower GR than the latter. All the LGI meals (breakfast, lunch, snack and dinner) produced a significantly lower GR iAUC than the HGI meals (P < 0.05). The overnight GR iAUC failed to reach significance (P = 0.766). The daily total AUC was not significantly different for the HGI compared to the LGI intervention over the 24 h period (P = 0.059). The total AUC while in the calorimetre (10 hours) was shown to be significantly higher for the HGI treatment than the LGI treatment (P = 0.008). The glycaemic variability over the 24 hour period (MAGE) was assessed. The HGI treatment produced a significantly greater MAGE than the LGI treatment over the 24 hour period (P < 0.001).
Energy metabolism and substrate oxidation
There was no difference in energy expenditure over 10 hours in the whole body calorimetre between the two dietary conditions, LGI = 769 kcal and HGI = 776 kcal (P = 0.53). There was a significant correlation observed between energy expenditure during LGI and HGI (R2 = 0.83, P < 0.0001).
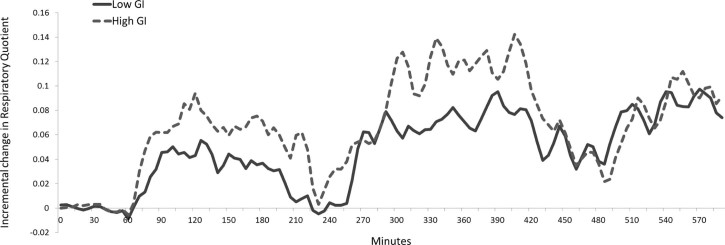
Fig. 3 shows the change in non-protein respiratory quotient (npRQ) values in the two dietary conditions over the 10 hours in the whole body calorimetre. The change in npRQ is expressed as the incremental RQ value with respect to the baseline value. There were no significant differences in npRQ between the two dietary conditions at baseline, during breakfast, during lunch and during snack. After breakfast and lunch, incremental npRQ was lower for LGI compared to HGI (P < 0.0001). This is reflected by increased carbohydrate (CHO) oxidation in grams per 5 minutes in HGI compared to LGI (P < 0.0001), and a smaller postprandial decrease in fat oxidation in grams per 5 minutes seen in LGI compared to HGI (P < 0.0001). There are no differences in npRQ, CHO or fat oxidation after the snack between the two conditions (Table 4).
Figure 3.
Average change in non-protein respiratory quotient (npRQ) from baseline of healthy Chinese male participants on a low GI or high GI meals for each day (n = 12).
Table 4.
Postprandial substrate oxidation parameters of the 12 participants
| Outcome measure | LGI | HGI | P value |
|---|---|---|---|
| Incremental npRQ breakfasta | 0.057(0.026) max:0.093 |
0.087(0.028) max:0.128 |
<0.0001 |
| Incremental npRQ luncha | 0.060(0.030) max:0.110 |
0.088(0.040) max:0.147 |
<0.0001 |
| Incremental npRQ snacka | 0.035(0.025) max:0.068 |
0.046(0.035) max:0.078 |
0.17 |
| Incremental CHO oxidation breakfasta (g/5 min) | 0.297(0.136) | 0.486(0.147) | <0.0001 |
| Incremental CHO oxidation luncha (g/5 min) | 0.277(0.164) | 0.467(0.259) | <0.0001 |
| Incremental CHO oxidation snacka (g/5 min) | 0.204(0.135) | 0.274(0.226) | 0.15 |
| Incremental fat oxidation breakfasta (g/5 min) | -0.208(0.084) | -0.282(0.073) | <0.0001 |
| Incremental fat oxidation luncha (g/5 min) | -0.205(0.087) | -0.265(0.101) | <0.0001 |
| Incremental fat oxidation snacka (g/5 min) | -0.113(0.075) | -0.139(0.096) | 0.26 |
Values are expressed as mean with SD in parentheses.
LGI, low glycaemic index; HGI, high glycaemic index; npRQ, no-protein respiratory quotient; CHO, carbohydrate.
Change from respectively breakfast, lunch and snack meal time values.
Table 5 shows the grams of oxidized macronutrients for LGI and HGI for breakfast, lunch, snack and all three meals combined. The combined meals showed lower CHO oxidation during LGI compared to HGI (P < 0.01). For the three meals combined, there was no significant difference in fat oxidation in LGI compared to HGI (P < 0.01). However, higher fat oxidation and lower carbohydrate oxidation were seen for breakfast and lunch separately after LGI compared to HGI (P < 0.05).
Table 5.
Postprandial oxidation of carbohydrates, fat and protein in grams for LGI and HGI meals
| LGI | HGI | |||||
|---|---|---|---|---|---|---|
| CHO (g) | Fat (g) | Protein (g) | CHO (g) | Fat (g) | Protein (g) | |
| Breakfast | 29.8(6.0) | 11.4(3.2) | 9.1(2.4) | 36.4(5.0)** | 9.7(1.3)* | 6.9(3.0)** |
| Lunch | 49.1(9.6) | 10.9(4.4) | 12.1(3.2) | 59.1(8.8)** | 8.6(2.7)* | 8.2(3.5)** |
| Snack | 27.1(3.3) | 4.6(1.6) | 5.8(1.6) | 26.5(3.5) | 5.2(1.3) | 5.4(2.3) |
| Total | 106.0(18.8) | 26.9(9.1) | 27.0(7.6) | 122.0(17.2)** | 23.5(4.1)(P = 0.07) | 20.5(9.1)** |
* p < 0.05, ** p < 0.01 when comparing LGI and HGI.
Values are expressed as mean with SD in parentheses.
LGI, low glycaemic index; HGI: high glycaemic index.
Discussion
The present study investigated the effects of low and high GI meals on glucose profile and substrate metabolism when the subjects were confined to the WBC throughout the postprandial periods. Predictably, lower glycaemic response was observed when LGI meals were consumed over a day compared to consuming HGI meals for the same time period. Significantly, lower carbohydrate oxidation and higher fat oxidation were observed with the LGI intervention compared to HGI during the 10 hours in the whole body calorimetre. This is in support of the relationship between glycaemic index and substrate oxidation hypotheses which suggests that high GI meals will increase serum insulin and consequently lead to lower fat oxidation [8], [12].
Previous studies have shown that ingesting LGI foods, compared to HGI foods, resulted in lower and more stable glucose levels [5], [22], [32]. Low GI foods produce a lower glycaemic response due to a slower rate of appearance of glucose in the systemic circulation. These findings are supported by our results which showed lower GR after LGI meals compared to HGI meals. Our study also showed that the glucose excursion after a meal was more prominent after breakfast than after lunch or dinner as insulin sensitivity is higher in the morning than in the afternoon [33]. This finding has also been reported previously in a study with healthy Chinese subjects using the CGM [34]. The MAGE was applied to assess glucose fluctuations, showing higher glycaemic variability over the entire 24 h compared to the LGI treatment. Glycaemic variability is of significant clinical concern due to its negative effects on oxidative stress [35] and insulin regulatory mechanisms. The daily total AUC for blood glucose was lower for the LGI intervention compared to the HGI intervention, although this did not reach a significant difference (P = 0.09). This could be explained by the hypoglycaemia during the night after the HGI intervention in contrast to the LGI intervention where the blood glucose was better maintained after a day with low GI meals [22]. Between 2 and 4 hours after an HGI meal, nutrient absorption from the gastrointestinal tract declines but the biological effects of the high insulin and low glucagon levels persist, causing blood glucose concentration to fall rapidly, often into the hypoglycaemic range [8]. The physiological significance of this hypoglycaemia is caused by a greater fall in glucose oxidation rate after consumption of a high compared to low GI carbohydrate [36].
In addition to lowered postprandial blood glucose after LGI meals, the simultaneous measurement of substrate oxidation showed higher fat oxidation and lower carbohydrate oxidation following the ingestion of the LGI breakfast and lunch. This resulted in more grams of fat and less grams of carbohydrates oxidized after the LGI breakfast and lunch compared to HGI. This is in line with existing data of studies that showed a significant shift in substrate utilization from carbohydrate to fat when an LGI meal was ingested before exercise compared to HGI [16], [17]. After LGI meals, there was higher protein oxidation compared to HGI which could be explained by the higher protein content of the LGI diet (14.9% vs 11.8%).
Stevenson and colleagues have shown that meals composed of low GI carbohydrates were able to reduce postprandial plasma glucose and increase fat oxidation during subsequent exercise [37]. In our study, we have shown that even in a sedentary state, after the consumption of a low GI mixed meal, blood glucose response was lower and stable, while simultaneously increasing fat oxidation. This is in agreement with previous studies that showed lower blood glucose and increased fat oxidation when LGI or HGI carbohydrates were used [36], [38]. However, some groups found that the GI of mixed meals was unable to modify fuel partitioning in sedentary obese women [39], [40]. Diaz and colleagues concluded that the lack of effect of serum insulin response on fat oxidation may be due to the short-time period in which serum insulin concentration was maintained at a quantitatively higher level when comparing HGI to LGI meals [39]. The main differences between these studies and our study were the use of obese subjects who are less susceptible to fat oxidation, and these subjects were overfed with a high carbohydrate load irrespective of whether the meals were LGI or HGI.
Several authors have consistently reported reduced fat deposition when fed with an LGI diet [8], [9], [32], [41]. The mechanistic explanation for this observation has eluded many researchers. We now report a possible mechanism for this observation. The concomitant measurement of blood glucose and RQ enables us to quantitate both glucose flux and fat tissue accretion. Our results demonstrate that the mechanism of reduced fat deposition when fed LGI is driven by increased fat oxidation. In contrast, it has been shown before that higher increase in insulin and inhibition of glucagon shortly after an HGI meal result in a notably higher insulin:glucagon ratio compared to LGI. This promotes the uptake of carbohydrates and fat by the liver and the muscles after HGI meals. This is supported by our results indicating lower fat oxidation after HGI meals. Over the breakfast and lunch periods, increased fat oxidation was 4 grams, equivalent to 36 kcal which when extrapolated to over 30 days leads to 120 grams (1080 kcal). The observation that even in a sedentary state, the consumption of LGI meals not only lowers blood glucose but also enhances fat oxidation is a key finding. This provides convincing evidence that even in the sedentary state, an LGI diet may play a key role in body weight regulation and weight maintenance.
A minor limitation of our study was that there were no serum insulin measurements which could further enhance the link between glycaemic response and fuel utilization. An additional limitation was that protein oxidation could not be measured in a time-specific way. It was therefore necessary to estimate the 24 hour protein oxidation based on the 10 hour cycle in the WBC. The rate of protein oxidation was assumed to be constant during the time in the WBC [42]. Another limitation was the inclusion of only male subjects which might bias the results to the sex of individuals. Despite these limitations, our study for the first time demonstrates how subjects fed with a low glycaemic index diet can benefit from increased fat oxidation mediated via a lowering of blood glucose.
Conclusions
The uniqueness of our study was the simultaneous measurement of blood glucose and respiratory quotient using a whole body calorimetre, when subjects were fed with low or high GI meals. This provided us with a unique dataset on glycaemic response in relation to fuel oxidation in normal weight Asians. This study demonstrates that low GI mixed meals are able to modulate glycaemic response while promoting fat oxidation over carbohydrate oxidation when compared to high GI meals. While the link between blood glucose levels and fat oxidation has been demonstrated in this study, further research is necessary to quantitate how insulin and other hormones may influence tissue oxidation in humans. The consistent observation that Asians living on a high glycaemic, high carbohydrate diet is susceptible to weight gain and obesity needs some explanation. It is likely that the nature of obesity and adipose tissue accretion seen in Asians may be triggered by the consumption of HGI diets and suggests that not only a high fat diet is a driver of obesity. Our observations provide substantial public health support for the encouragement of consuming LGI meals in Asians. Thus far, the role of low GI meals has focussed attention on its impact of glycaemia. Now, our study shows that it may also have an important impact on fat oxidation in Asians.
Conflict of interest
The authors declare they have no conflicts of interest.
Acknowledgments
This research is supported by the Singapore Institute for Clinical Sciences.
Footnotes
Clinical trial registration number: NCT02631083.
References
- 1.Venn B.J., Williams S.M., Mann J.I. Comparison of postprandial glycaemia in Asians and Caucasians. Diabet Med. 2010;27(10):1205–1208. doi: 10.1111/j.1464-5491.2010.03069.x. [DOI] [PubMed] [Google Scholar]
- 2.Dickinson S., Colagiuri S., Faramus E., Petocz P., Brand-Miller J. Postprandial hyperglycemia and insulin sensitivity differ among lean young adults of different ethnicities. J Nutr. 2002;132(9):2574–2579. doi: 10.1093/jn/132.9.2574. [DOI] [PubMed] [Google Scholar]
- 3.Hu F.B. Globalization of diabetes: the role of diet, lifestyle, and genes. Diabetes Care. 2011;34(6):1249–1257. doi: 10.2337/dc11-0442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Monnier L., Mas E., Ginet C., Michel F., Villon L., Cristol J.P. Activation of oxidative stress by acute glucose fluctuations compared with sustained chronic hyperglycemia in patients with type 2 diabetes. JAMA. 2006;295(14):1681–1687. doi: 10.1001/jama.295.14.1681. [DOI] [PubMed] [Google Scholar]
- 5.Kaur B., Ranawana V., Teh A.-L., Henry C.J.K. The impact of a low glycemic index (GI) breakfast and snack on daily blood glucose profiles and food intake in young Chinese adult males. J Clin Transl Endocrinol. 2015;2(3):92–98. doi: 10.1016/j.jcte.2015.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brand-Miller J., Hayne S., Petocz P., Colagiuri S. Low-glycemic index diets in the management of diabetes: a meta-analysis of randomized controlled trials. Diabetes Care. 2003;26(8):2261–2267. doi: 10.2337/diacare.26.8.2261. [DOI] [PubMed] [Google Scholar]
- 7.Thomas D.E., Elliott E.J. The use of low-glycaemic index diets in diabetes control. Br J Nutr. 2010;104(6):797–802. doi: 10.1017/S0007114510001534. [DOI] [PubMed] [Google Scholar]
- 8.Ludwig D.S. The glycemic index: physiological mechanisms relating to obesity, diabetes, and cardiovascular disease. JAMA. 2002;287(18):2414–2423. doi: 10.1001/jama.287.18.2414. [DOI] [PubMed] [Google Scholar]
- 9.Ludwig D.S. Dietary glycemic index and obesity. J Nutr. 2000;130(2):280S–283S. doi: 10.1093/jn/130.2.280S. [DOI] [PubMed] [Google Scholar]
- 10.Weyer C., Pratley R.E., Salbe A.D., Bogardus C., Ravussin E., Tataranni P.A. Energy expenditure, fat oxidation, and body weight regulation: a study of metabolic adaptation to long-term weight change. J Clin Endocrinol Metab. 2000;85(3):1087–1094. doi: 10.1210/jcem.85.3.6447. [DOI] [PubMed] [Google Scholar]
- 11.Pannacciulli N., Salbe A.D., Ortega E., Venti C.A., Bogardus C., Krakoff J. The 24-h carbohydrate oxidation rate in a human respiratory chamber predicts ad libitum food intake. Am J Clin Nutr. 2007;86(3):625–632. doi: 10.1093/ajcn/86.3.625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brand-Miller J.C., Holt S.H.A., Pawlak D.B., McMillan J. Glycemic index and obesity. Am J Clin Nutr. 2002;76(1):281S–285S. doi: 10.1093/ajcn/76/1.281S. [DOI] [PubMed] [Google Scholar]
- 13.Stevenson E., Williams C., Nute M. The influence of the glycaemic index of breakfast and lunch on substrate utilisation during the postprandial periods and subsequent exercise. Br J Nutr. 2005;93(06):885–893. doi: 10.1079/bjn20051430. [DOI] [PubMed] [Google Scholar]
- 14.Stevenson E.J., Williams C., Mash L.E., Phillips B., Nute M.L. Influence of high-carbohydrate mixed meals with different glycemic indexes on substrate utilization during subsequent exercise in women. Am J Clin Nutr. 2006;84(2):354–360. doi: 10.1093/ajcn/84.1.354. [DOI] [PubMed] [Google Scholar]
- 15.Stevenson E.J., Astbury N.M., Simpson E.J., Taylor M.A., Macdonald I.A. Fat oxidation during exercise and satiety during recovery are increased following a low-glycemic index breakfast in sedentary women. J Nutr. 2009;139(5):890–897. doi: 10.3945/jn.108.101956. [DOI] [PubMed] [Google Scholar]
- 16.Wee S.-L., Williams C., Gray S., Horabin J. Influence of high and low glycemic index meals on endurance running capacity. Med Sci Sports Exerc. 1999;31(3):393–399. doi: 10.1097/00005768-199903000-00007. [DOI] [PubMed] [Google Scholar]
- 17.DeMarco H.M., Sucher K.P., Cisar C.J., Butterfield G.E. Pre-exercise carbohydrate meals: application of glycemic index. Med Sci Sports Exerc. 1999;31(1):164–170. doi: 10.1097/00005768-199901000-00025. [DOI] [PubMed] [Google Scholar]
- 18.Baecke J.A., Burema J., Frijters J.E. A short questionnaire for the measurement of habitual physical activity in epidemiological studies. Am J Clin Nutr. 1982;36(5):936–942. doi: 10.1093/ajcn/36.5.936. [DOI] [PubMed] [Google Scholar]
- 19.Van Strien T., Frijters J.E.R., Bergers G., Defares P.B. The Dutch Eating Behavior Questionnaire (DEBQ) for assessment of restrained, emotional, and external eating behavior. Int J Eat Disord. 1986;5(2):295–315. [Google Scholar]
- 20.Henry C.J.K., Newens K.J., Lightowler H.J. Low-glycaemic index sweetener-based beverages reduce 24-h glucose profiles in healthy adults. JAMA. 2009;22(1):77–80. doi: 10.1111/j.1365-277X.2008.00930.x. [DOI] [PubMed] [Google Scholar]
- 21.Brynes A.E., Adamson J., Dornhorst A., Frost G.S. The beneficial effect of a diet with low glycaemic index on 24 h glucose profiles in healthy young people as assessed by continuous glucose monitoring. Br J Nutr. 2005;93(02):179–182. doi: 10.1079/bjn20041318. [DOI] [PubMed] [Google Scholar]
- 22.Liu A.G., Most M.M., Brashear M.M., Johnson W.D., Cefalu W.T., Greenway F.L. Reducing the glycemic index or carbohydrate content of mixed meals reduces postprandial glycemia and insulinemia over the entire day but does not affect satiety. Diabetes Care. 2012;35(8):1633–1637. doi: 10.2337/dc12-0329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schoffelen P.F., Westerterp K.R., Saris W.H., Ten Hoor F. A dual-respiration chamber system with automated calibration. J Appl Physiol. 1997;83(6):2064–2072. doi: 10.1152/jappl.1997.83.6.2064. [DOI] [PubMed] [Google Scholar]
- 24.Wulan S.N., Westerterp K.R., Plasqui G. Dietary and 24-h fat oxidation in Asians and whites who differ in body composition. Am J Clin Nutr. 2012;95(6):1335–1341. doi: 10.3945/ajcn.111.031369. [DOI] [PubMed] [Google Scholar]
- 25.Frayn K.N. Calculation of substrate oxidation rates in vivo from gaseous exchange. J Appl Physiol Respir Environ Exerc Physiol. 1983;55(2):628–634. doi: 10.1152/jappl.1983.55.2.628. [DOI] [PubMed] [Google Scholar]
- 26.Allison D.B., Paultre F., Maggio C., Mezzitis N., Pi-Sunyer F.X. The use of areas under curves in diabetes research. Diabetes Care. 1995;18(2):245–250. doi: 10.2337/diacare.18.2.245. [DOI] [PubMed] [Google Scholar]
- 27.Brouns F., Bjorck I., Frayn K.N., Gibbs A.L., Lang V., Slama G. Glycaemic index methodology. Nutr Res Rev. 2005;18(01):145–171. doi: 10.1079/NRR2005100. [DOI] [PubMed] [Google Scholar]
- 28.Guerci B. [Asymptomatic glycemic instability: how to measure it and which clinical applications?] Diabetes Metab. 2003;29(2 Pt 1):179–188. [PubMed] [Google Scholar]
- 29.Kovatchev B.P., Clarke W.L., Breton M., Brayman K., McCall A. Quantifying temporal glucose variability in diabetes via continuous glucose monitoring: mathematical methods and clinical application. Diabetes Technol Ther. 2005;7(6):849–862. doi: 10.1089/dia.2005.7.849. [DOI] [PubMed] [Google Scholar]
- 30.Service F.J., Molnar G.D., Rosevear J.W., Ackerman E., Gatewood L.C., Taylor W.F. Mean amplitude of glycemic excursions, a measure of diabetic instability. Diabetes. 1970;19(9):644–655. doi: 10.2337/diab.19.9.644. [DOI] [PubMed] [Google Scholar]
- 31.Rodbard D. Interpretation of continuous glucose monitoring data: glycemic variability and quality of glycemic control. Diabetes Technol Ther. 2009;11(S1):S-55–67. doi: 10.1089/dia.2008.0132. [DOI] [PubMed] [Google Scholar]
- 32.Ludwig D.S., Majzoub J.A., Al-Zahrani A., Dallal G.E., Blanco I., Roberts S.B. High glycemic index foods, overeating, and obesity. Pediatrics. 1999;103(3):e26. doi: 10.1542/peds.103.3.e26. [DOI] [PubMed] [Google Scholar]
- 33.Shih K., Ho L., Kou H., Liu P.C., Hsiao L.C., Li S.H. Diurnal variation of insulin sensitivity in NIDDM patients and normal subjects. J Formos Med Assoc. 1992;91(3):263–269. [PubMed] [Google Scholar]
- 34.Zhou J., Li H., Ran X., Yang W., Li Q., Peng Y. Reference values for continuous glucose monitoring in Chinese subjects. Diabetes Care. 2009;32(7):1188–1193. doi: 10.2337/dc09-0076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sun J., Xu Y., Sun S., Sun Y., Wang X. Intermittent high glucose enhances cell proliferation and VEGF expression in retinal endothelial cells: the role of mitochondrial reactive oxygen species. Mol Cell Biochem. 2010;343(1–2):27–35. doi: 10.1007/s11010-010-0495-5. [DOI] [PubMed] [Google Scholar]
- 36.Ritz P., Krempf M., Cloarec D., Champ M., Charbonnel B. Comparative continuous-indirect-calorimetry study of two carbohydrates with different glycemic indices. Am J Clin Nutr. 1991;54(5):855–859. doi: 10.1093/ajcn/54.5.855. [DOI] [PubMed] [Google Scholar]
- 37.Stevenson E., Williams C., Nute M., Swaile P., Tsui M. The effect of the glycemic index of an evening meal on the metabolic responses to a standard high glycemic index breakfast and subsequent exercise in men. Int J Sport Nutr Exerc Metab. 2005;15(3):308–322. doi: 10.1123/ijsnem.15.3.308. [DOI] [PubMed] [Google Scholar]
- 38.Blaak E.E., Saris W.H. Postprandial thermogenesis and substrate utilization after ingestion of different dietary carbohydrates. Metabolism. 1996;45(10):1235–1242. doi: 10.1016/s0026-0495(96)90241-3. [DOI] [PubMed] [Google Scholar]
- 39.Diaz E.O., Galgani J.E., Aguirre C.A., Atwater I.J., Burrows R. Effect of glycemic index on whole-body substrate oxidation in obese women. Int J Obes (Lond) 2005;29(1):108–114. doi: 10.1038/sj.ijo.0802592. [DOI] [PubMed] [Google Scholar]
- 40.McDevitt R.M., Poppitt S.D., Murgatroyd P.R., Prentice A.M. Macronutrient disposal during controlled overfeeding with glucose, fructose, sucrose, or fat in lean and obese women. Am J Clin Nutr. 2000;72(2):369–377. doi: 10.1093/ajcn/72.2.369. [DOI] [PubMed] [Google Scholar]
- 41.Ebbeling C.B., Ludwig D.S. Treatment of the obese patient. Springer; 2007. Glycemic index, obesity, and diabetes; pp. 281–298. [Google Scholar]
- 42.Sato M., Nakamura K., Ogata H., Miyashita A., Nagasaka S., Omi N. Acute effect of late evening meal on diurnal variation of blood glucose and energy metabolism. Obes Res Clin Pract. 2011;5(3):e220–8. doi: 10.1016/j.orcp.2011.02.001. [DOI] [PubMed] [Google Scholar]