Abstract
Background:
Rural Australians have poorer survival for most common cancers, due partially to later diagnosis. Internationally, several initiatives to improve cancer outcomes have focused on earlier presentation to healthcare and timely diagnosis. We aimed to measure the effect of community-based symptom awareness and general practice-based educational interventions on the time to diagnosis in rural patients presenting with breast, prostate, colorectal or lung cancer in Western Australia.
Methods:
2 × 2 factorial cluster randomised controlled trial. Community Intervention: cancer symptom awareness campaign tailored for rural Australians. GP intervention: resource card with symptom risk assessment charts and local cancer referral pathways implemented through multiple academic detailing visits. Trial Area A received the community symptom awareness and Trial Area B acted as the community campaign control region. Within both Trial Areas general practices were randomised to the GP intervention or control. Primary outcome: total diagnostic interval (TDI).
Results:
1358 people with incident breast, prostate, colorectal or lung cancer were recruited. There were no significant differences in the median or ln mean TDI at either intervention level (community intervention vs control: median TDI 107.5 vs 92 days; ln mean difference 0.08 95% CI −0.06–0.23 P=0.27; GP intervention vs control: median TDI 97 vs 96.5 days; ln mean difference 0.004 95% CI −0.18–0.19 P=0.99). There were no significant differences in the TDI when analysed by factorial design, tumour group or sub-intervals of the TDI.
Conclusions:
This is the largest trial to test the effect of community campaign or GP interventions on timeliness of cancer diagnosis. We found no effect of either intervention. This may reflect limited dose of the interventions, or the limited duration of follow-up. Alternatively, these interventions do not have a measurable effect on time to cancer diagnosis.
Keywords: primary care, cancer, early diagnosis, community awareness campaign, education, risk tool, cluster randomised trial
Rural Australians are more likely to die within 5 years of a cancer diagnosis than people from metropolitan areas (Underhill et al, 2006, Coory et al, 2013). While overall survival for most common cancers in Australia is improving, the rural–urban differential has been widening, with significant excess deaths due to lung, colorectal, breast and prostate cancer in regional Australia (AIHW, 2010). Similar disparities in cancer outcomes between rural and urban populations have been described internationally (Singh et al, 2011, Meilleur et al, 2013). As part of the International Cancer Benchmarking Partnership, a major focus on understanding variations in cancer outcomes has been later presentation to healthcare and later diagnosis (Butler et al, 2013)
Previous studies have shown that patients living in rural Australia are less likely to receive curative or reconstructive surgery, radiotherapy or anti-cancer drug treatment (.Coory and Baade, 2005, Hall et al, 2005, Mitchell et al, 2006, Baade et al, 2011b) Policy initiatives have focused, therefore, on reducing disparities in access to treatment (Department of Health and Ageing, 2010) but, while this is an important determinant of outcome, later presentation and stage at diagnosis have also been observed in cancer patients from rural compared with those from urban areas (Jong et al, 2005, Baade et al, 2011a). International research suggests that the time taken to appraise symptoms and seek help (the ‘patient interval’), management in primary care and access to diagnostic procedures are also key determinants of cancer outcomes (Allgar and Neal, 2005). Prolonged time to diagnosis is associated with poorer survival from several common cancers (Neal et al, 2015). Our previous exploratory mixed-methods research identified characteristics of rural Australians including optimism, stoicism and machismo which affected decisions to seek medical help (Emery et al, 2013a). Over-optimism towards health meant some participants were more likely to find alternative benign explanations for their symptoms. Optimism was associated with stoic responses to symptoms which meant that severe and continuous symptoms were self-managed. Related to such stoicism in men, was the need to be perceived as tough or macho and less willing to seek help. Many participants discussed these characteristics of optimism, stoicism and machismo as core features of what being ‘rural’ in Australia meant. These features, as well as poorer access to health care, contributed to later presentation of cancer (Emery et al, 2013a). In addition, there were significant overall differences between cancers in terms of time from presentation in general practice (GP) to referral, from GP referral to specialist appointment, and from specialist appointment to cancer diagnosis. These differences were due to the nature of presenting symptoms, limited access to diagnostic tests and a requirement for multiple visits to specialists (Emery et al, 2013b). However, there are no published studies which have compared symptom awareness, help seeking or diagnostic intervals between rural and urban people with cancer or in general community samples.
One of the approaches to reducing later presentation to healthcare has been community symptom awareness campaigns. These formed a major component of the UK National Awareness and Early Diagnosis Initiative as part of the policy to improve cancer outcomes (.Cancer Research UK, 2010) A systematic review of cancer symptom awareness campaigns published in 2009 found insufficient evidence to determine their effectiveness on presentation to healthcare (Austoker et al, 2009). Since then further studies examining the impact of the English ‘Be Clear on Cancer’ campaigns have begun to show potential effects on presentation and cancer diagnoses but these have all relied on before-and-after observational designs (Athey et al, 2011). There have been no trials in Australia of the efficacy of cancer symptom awareness campaigns, and relatively few studies examining community symptom awareness. The International Cancer Benchmarking Partnership compared symptom awareness and barriers to consultation between nations, including Australia, but did not compare rural and urban populations(Forbes et al, 2013).
A second approach has aimed at improving recognition of patients presenting to primary care whose symptoms may warrant early investigation for cancer. Until recently, there was little epidemiological evidence demonstrating how well symptoms predict risk of an underlying cancer from primary care populations (Emery et al, 2013a). Analysis of data in case-control studies using large UK general practice databases, notably the CAPER (CAncer Prediction in ExeteR) studies (Hamilton et al, 2005a, b, 2006, Hamilton, 2009) and QCancer research (Hippisley-Cox and Coupland, 2013a, 2013b), has led to significant advances in our understanding of the epidemiology of cancer symptoms in primary care. The CAPER studies quantified the risk of individual and paired symptoms, signs and primary care investigations for a number of cancers including colorectal, lung and prostate. These have been applied to create risk assessment tools (RATs) in paper versions (Hamilton et al, 2013) and are currently undergoing evaluation as computerised decision support tools embedded in the electronic medical records of general practices(Moore et al, 2016). Various interventions including audit and feedback, educational visits, guidelines and decision support have been tested in general practice to improve cancer diagnosis, but none of the 22 trials included in a systematic review of interventions to support cancer diagnosis in primary care examined effects on diagnostic delay (Mansell et al, 2011a).
The findings from our exploratory research in rural Western Australia (WA) (Emery et al, 2013a) (Emery et al, 2013b) contributed to the development of the interventions and design of the improving rural cancer outcomes (IRCO) Trial: a factorial cluster-randomised controlled trial of community-based and general practice-based interventions which aimed to measure the effect of these interventions on the time to diagnosis in rural patients presenting with prostate, breast, colorectal or lung cancer in WA.
Materials and methods
As the protocol for the IRCO trial has been published (http://bmjopen.bmj.com/content/4/9/e006156), (Emery et al, 2014) here we report the methods in brief. (Australian New Zealand Clinical Trial Registry ACTRN12610000872033).
Study design
This was a factorial cluster randomised controlled trial conducted in Western Australia with communities and general practices allocated to the interventions. Two Trial Areas were matched for population size, demographics including age and Aboriginality, and similar cancer incidence, based on the most recent available data when the trial was planned. Trial Area A comprised the Wheatbelt (155 256 km2; Australian Standard Geographical Classification RA3 (outer regional) and RA4 (remote)), Goldfields (770 488 km2; RA3, RA4 and RA5 (very remote)) and Great Southern (39 007 km2; RA3 and RA4) regions total population in 2010:140 554), and Trial Area B included the Peel/South West (29 646 km2;RA2 (inner regional) and RA3) and MidWest (470 000 km2; RA3, RA4 and RA5) regions (total population in 2010:233 063) (WA Country Health Service, 2011). In these areas, populations are widely distributed across many small and medium sized towns; smaller communities are served by general practices often based at least 50–100 kms away. Peel and the South West each have one large regional city (Mandurah and Bunbury respectively).
Primary ethics approval was obtained from The University of Western Australia’s Human Research Ethics Committee (RA/4/1/4527).
Randomisation and masking
Trial Area A was randomly allocated to receive the community symptom awareness campaign intervention and Trial Area B acted as the community campaign control region (Community level randomisation). Within Trial Area A (Community Intervention) and Trial Area B (Community Control) general practices were randomised to receive the education intervention or control, stratified by practice size (General Practice level randomisation). GPs who worked at more than one practice were identified, and these practices were treated as a single cluster for the purpose of randomisation to avoid contamination. We used a cluster version of Zelen’s method of post-randomised consent: intervention practices were invited to receive the educational intervention while control practices received no information about the trial (Adamson et al, 2006). This enabled non-intervention practices to act as true controls by minimising the Hawthorne effect in a situation where placebo and double-blind experimental conditions are impossible to achieve. Furthermore, it allowed a pragmatic delivery of the intervention and measure of its uptake in routine practice. Intervention practices which declined the invitation to receive the educational intervention were analysed on an intention-to-treat basis (i.e., patients from these practices were included in the GP Intervention group). Randomisation was performed by the trial statistician (MB). A database containing all practices categorised by workforce size was created and then random samples of practices were selected stratified for size of practice using ‘samplepps’ The research staff who collected outcome data and the trial statistician were blinded to group allocation.
Participants
While the whole community and general practices within the communities were the targets of the interventions, outcome measurement was at the level of the individual patient. From 1 March 2012, 4 months after the interventions commenced, all patients meeting the following criteria were invited to contribute their data for the trial:
adults aged over 18 years;
diagnosed with breast, lung, colorectal or prostate cancer between 1 January 2012 and the recruitment end date of 31 March 2014; and
resident of Campaign Intervention or Campaign Control regions at the time of cancer diagnosis.
Eligible participants were identified via:
The WA Cancer Registry (WACR), a population-based registry with mandatory notification since 1981. A letter and participant information sheet was mailed on behalf of the WACR directly to newly diagnosed cancer patients. After 3 weeks non-responders were followed up by the research team via phone or mail.
The Cancer Council Western Australia’s residential lodges, where a large proportion of rural cancer patients stayed during their treatment in metropolitan cancer centres. Lodge staff gave eligible patients a participant information sheet, followed up by contact from the research team.
Participants signed a consent form with agreement to access their medical records, and completed the SYMPTOM questionnaire. Participants were not specifically informed of the trial hypotheses; while those from Trial Area A may have been aware of the symptom awareness campaign, they did not know that the data they provided were used to measure the impact of the campaign.
Procedures
Both interventions were delivered from 1 November 2011 to 31 December 2013. The Community Intervention: the Find Cancer Early campaign The symptom awareness campaign materials were developed from the Cancer Research UK ‘Spot Cancer Early’ and the UK National Health Service ‘3-week cough’ campaigns. They were significantly modified to incorporate the findings of our previous exploratory research, including a focus on specific barriers to help-seeking such as stoicism and machismo, and to make them relevant to the rural Australian community following feedback from several community forums. (Emery et al, 2013a, 2013b) (Supplementary Materials for examples). Some of the campaign materials were explicitly targeted at individuals aged over 40 (e.g. the symptom checklist) given the low incidence of the four cancers below that age covered in the campaign. Five Cancer Council WA project officers, with a health promotion background, and a combined full time equivalent of 3.0, delivered the Find Cancer Early campaign across the three regions of WA in Trial Area A. They used a community engagement approach building local partnerships to deliver and disseminate the campaign messages with face-to-face presentations, displays and distribution of campaign materials. Paid advertising and promotional news media items in regional newspapers and local radio supplemented this dissemination strategy as these are known to have significant local reach and account for variable levels of literacy. Television was not used for news media or advertising to avoid contamination in the control area.
The GP Intervention
A GP education resource card was created which included the CAPER symptom RATs for colorectal (Hamilton et al, 2005b), lung (Hamilton et al, 2005a) and prostate (Hamilton et al, 2006) cancer. The resource card contained the clinical implications of these risk tools including guidelines on diagnostic assessment. (see Supplementary Material for example) The resource card also summarised the National Breast and Ovarian Cancer Centre guidelines for investigating new breast symptoms(National Breast and Ovarian Cancer Centre, 2006) and local referral guidelines and hospital contacts, including recommendations about access to cancer multidisciplinary teams (Department Of Health, WA, 2008). The GP resource card was implemented through a series of four academic detailing practice visits, supplemented by a series of case studies completed between visits designed to reinforce key messages (Grimshaw et al, 2004). Each practice visit discussed specific components of the resource card and facilitated discussion within the practice about recently diagnosed cancer patients. The educational visits were delivered by the Cancer Council WA project officers. The GP intervention was designed to reduce time to diagnosis by promoting earlier recognition and investigation of suspicious symptoms by GPs and clarifying cancer diagnostic pathways.
Outcomes
The primary outcome was the total diagnostic interval (TDI), defined as the time from first symptom to cancer diagnosis. We report our time intervals according to the principles outlined in the ‘Aarhus Statement’ on the conduct and reporting of research on cancer diagnosis,(Weller et al, 2012) and have used the Model of Pathways to Treatment as our theoretical framework (Walter et al, 2012, Scott et al, 2013). The date of first symptom was defined as ‘the time-point when first bodily change(s) and/or symptom(s) were noticed’ (up to two years before the date of diagnosis). For screen-detected cases we used the date of attendance for the screening test as the initial date in the patient pathway. Date of diagnosis was based on date of pathological diagnosis as reported to the WA Cancer Registry. Where data were available, we divided the TDI into: time from first symptom to first presentation to healthcare (Patient Interval); time from first healthcare presentation to first referral to specialist (First Healthcare Interval); and time from referral to specialist until date of diagnosis (Specialist Interval) (Weller et al, 2012). For patient-reported dates, where uncertainty existed, we applied mid-point rules to estimate the actual date (Allgar and Neal, 2005, Walter et al, 2015). Where necessary, a clinical consensus group of three reviewed the data, blinded to allocation, to confirm the date of first symptom and first presentation to healthcare.
Measurement of primary outcome
The following instruments were used to obtain information about symptoms and dates to calculate the TDI:
1. SYMPTOM questionnaire
This self-administered questionnaire was adapted from the C-SIM(Neal et al, 2014) measure and has been applied in the UK SYMPTOM study (Walter et al, 2015, 2016). It includes items specific to each tumour site to capture details of symptoms, their date of onset and time taken to seek help. For each tumour type, the participant is asked an open question about their first symptom, followed by a list of tumour-specific symptoms.
2. GP record audit tool
This tumour-specific proforma captures information on: the date, type and duration of presenting symptoms within the last 12 months, referral information including referral date, and date of first appointment with specialist.
Measurement of secondary outcomes
Process measures of intervention delivery
We collected data to measure the symptom awareness campaign dose including media exposure, distribution of resources and number of campaign events. We measured uptake of GP education including GP attendance at practice visits and completion of case studies. These data were used to estimate the cost to deliver each intervention.
As per our trial protocol we will report at a later date analyses from the WA linked datasets on the effects of interventions on healthcare utilisation and clinical outcomes. This will include data on cancer incidence, staging (or pseudo-staging where complete staging data are unavailable) and mortality.
Statistical analysis
Our original minimum sample size calculation before the trial commenced required 840 participants for 80% power and α=0.05 to detect a halving of the risk of long-delay from 30% to 15%. However, higher than expected accrual rates allowed us to revise our power estimates on the basis of a clinically more meaningful outcome, difference in the TDI. Accounting for the design effects from hierarchical correlations and an intra-class correlation coefficient of 0.09 based on similar trial designs,(Campbell et al, 2004) our final sample size of 1359 participants provided 80% power to detect a 10% difference in TDI between intervention groups for all four cancers combined, and a 20% difference in TDI for breast, colorectal and prostate cancer separately, based on the TDI distribution from our exploratory research (Emery et al, 2013a, 2013b). We therefore report differences in TDI for all our analyses.
The primary analysis compared the TDIs and its sub-components between trial groups. Given the skewed nature of time data, we applied a log transformation prior to conducting general linear modelling to compare intervals. Within the models we accounted for: exposure to the community and GP interventions and potential interactions relating to the factorial design, practice size and clustering effects at the general practice level using robust standard errors. Analyses were conducted as intention to treat only. We conducted sensitivity analyses excluding ‘vague’ first symptoms such as fatigue and ‘feeling different’ and screen-detected cases. Analyses were performed using Stata version 14.
Results
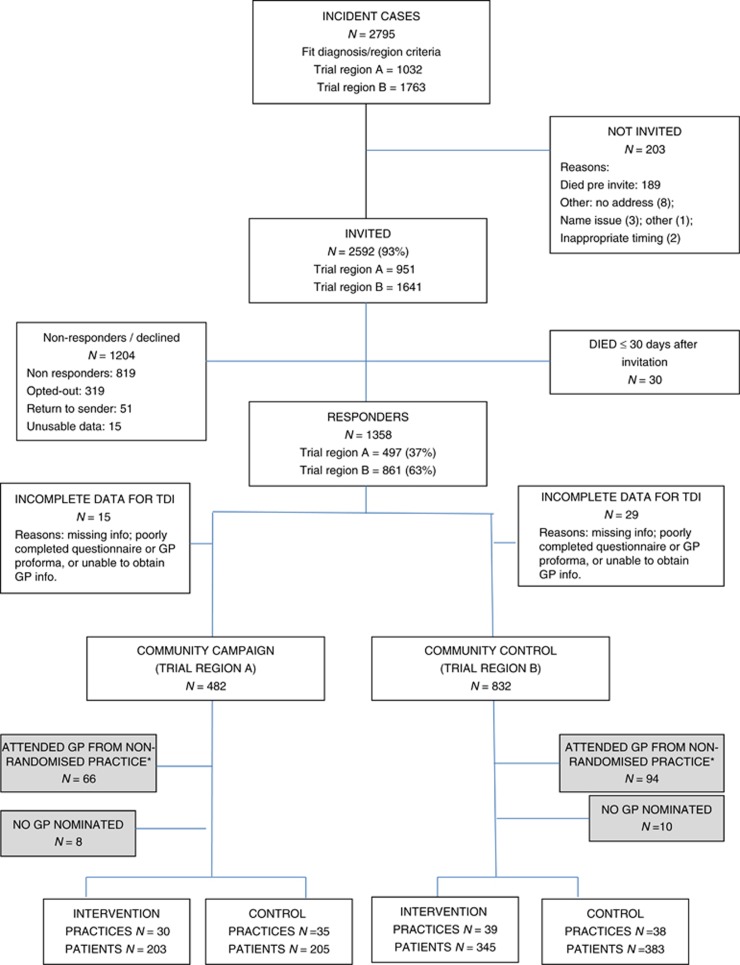
From 1 March 2012 to 31 March 2014 2592 potentially eligible participants were invited into the trial of whom 1358 (52.4%) consented (Figure 1). The vast majority of these were recruited via the WA Cancer Registry, only 71 (5.2%) through the CCWA residential lodges. We could calculate a TDI for 1314 (96.8%) participants at the level of the Community Intervention and for 1136 (83.7%) participants at the GP level. Table 1 presents the baseline characteristics of the participants at the Community level by tumour type showing balance in terms of age. Mean Socio-Economic Indexes for Areas (SEIFA) scores on the Index of Relative Socio-economic Advantage and Disadvantage (http://www.abs.gov.au/websitedbs/censushome.nsf/home/seifa) were similar (community intervention mean 977.8, mean rank 46.3; community control mean 980.6, mean rank 47.1) suggesting comparability in terms of a range of measures of income, qualifications and occupations. The larger population of the community control area meant an imbalance in total number of cases diagnosed compared to the community intervention.
Figure 1.
Trial flow chart. *Non-randomised practice refers to practices from the trial areas which were unknown at time of randomisation, and practices outside the trial regions.
Table 1. Trial participants’ baseline characteristics.
|
Breast |
Colorectal |
Lung |
Prostate |
|||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Age |
SEIFA |
Age |
SEIFA |
Age |
SEIFA |
Age |
SEIFA | |||||||||||||||||
| n | Mean | s.d. | Median | IQR | Mean | n | Mean | s.d. | Median | IQR | Mean | n | Mean | s.d. | Median | IQR | Mean | n | Mean | s.d. | Median | IQR | Mean | |
|
Community intervention | ||||||||||||||||||||||||
| Male | 56 | 67.2 | 10.7 | 68.5 | 13.5 | 973.9 | 30 | 67.1 | 9.3 | 66 | 13.3 | 983.3 | 200 | 66.0 | 8.8 | 67 | 13 | 977.9 | ||||||
| Female | 144 | 58.7 | 11.8 | 59 | 16.3 | 977.1 | 47 | 65.7 | 13.4 | 68 | 18.5 | 979.8 | 20 | 62.0 | 8.7 | 61.5 | 12.3 | 984.6 | ||||||
|
Community control | ||||||||||||||||||||||||
| Male | 79 | 68.0 | 9.2 | 70 | 11 | 980.7 | 40 | 69.0 | 8.0 | 70 | 9.3 | 971.6 | 365 | 66.9 | 8.0 | 67 | 11 | 980.5 | ||||||
| Female | 277 | 60.9 | 11.9 | 62 | 18 | 981.2 | 73 | 66.8 | 10.6 | 67 | 14 | 982.6 | 27 | 67.4 | 9.4 | 69 | 12 | 980.1 | ||||||
Abbreviation: IQR =interquartile range; SEIFA=Socio-Economic Indexes for Areas.
An estimated population of 121 600 aged over 40 years (the target population) resided in the community intervention area. During the Community Intervention there were: 130 388 symptom checklists, 4170 DVDs and 74 317 symptom postcards distributed; 312 community presentations attended by 6549 people; 89 articles in regional newspapers (reach: 573 380); 602 articles and/or symptom checklists published in local newspapers (reach: 283 520); 10 local radio interviews; 162 paid newspaper advertisements (reach 2 262 498) and 1490 30 s paid radio advertisements. The campaign was widely distributed across all regions maximising potential exposure to the whole target community. Sixty-nine general practices were invited to receive the GP Intervention. Fifty-six practices agreed to a single educational practice visit, 46 to two, 41 to three and 40 practices received all four visits.
The estimated cost of delivering the Community Intervention was $878 687 and in addition we received in-kind support to the level of $262 500. The estimated cost of delivering the GP Intervention was $179 965 with additional in-kind support of $125 250. Costs for teleconferencing, recruitment, and computing ($13 750) and in-kind office space ($105 000) were duplicated, so totals have been adjusted to reflect the interventions running independently of each other. Approximately 71% of campaign costs were accounted for by salaries, and 18% towards paid advertising. Approximately 75% of GP intervention costs were staff salaries. Costs reflect 2012/13 AUD.
Table 2 presents the TDI by group randomisation and by factorial level. The median TDIs by group randomisation were similar; after log transformation there were no statistically significant differences in the TDI at the Community or GP levels, or by factorial design: Community intervention vs control: median TDI 107.5 vs 92 days; ln mean difference 0.08 95% CI −0.06–0.23 P=0.27; GP intervention vs control: median TDI 97 vs 96.5 days; ln mean difference 0.004 95% CI −0.18–0.19 P=0.99. Table 3 presents the TDIs by group and factorial design and tumour type. The median TDIs by group randomisation were similar; after log transformation there were no statistically significant differences in the TDI at the Community or GP levels, or by factorial design for any tumour group (breast cancer: Community intervention vs control: median TDI 33 vs 32 days; ln mean difference 0.13 95% CI −0.13–0.39 P=0.32 ; GP intervention vs control: median TDI 34.5 vs 33 days; ln mean difference 0.12 95% CI −0.20–0.43 P=0.67; Colorectal cancer: community intervention vs control: median TDI 107 vs 133 days; ln mean difference −0.26 95% CI −0.63–0.11 P=0.16; GP intervention vs control: median TDI 124 vs 122 days; ln mean difference −0.03 95% CI −0.51–0.45 P=0.42; lung cancer: community intervention vs control: median TDI 114.5 vs 114 days; ln mean difference 0.06 95% CI −0.39–0.51 P=0.79; GP intervention vs control: 115 vs 125 days; ln mean difference 0.02 95% CI −0.56–0.60 P=0.45; prostate cancer: community intervention vs control: 107.5 vs 92 days; ln mean difference 0.16 95% CI −0.01–0.33 P=0.06 GP intervention vs control: 97 vs 96.5 days ln mean difference −0.05 95% CI −0.27–0.17 P=0.30).
Table 2. Total diagnostic interval by trial group and by factorial design.
| n | Meana Ln | Mean | Median | 25th | 75th | |
|---|---|---|---|---|---|---|
|
Trial group randomisation | ||||||
| CI | 482 | 4.48 | 168.5 | 107.5 | 36 | 239.5 |
| CC | 832 | 4.40 | 151.5 | 92 | 35 | 207.5 |
| GPI | 548 | 4.43 | 154.7 | 97 | 35 | 213 |
| GPC | 588 | 4.42 | 160.7 | 96.5 | 34.3 | 215 |
|
Factorial level of randomisation | ||||||
| Community and GP interventions | 203 | 4.40 | 157.6 | 100 | 36 | 228 |
| Community control and GP intervention | 345 | 4.42 | 153.0 | 92 | 34.5 | 204 |
| Community intervention and GP control | 205 | 4.46 | 176.4 | 103 | 33.5 | 241 |
| Community and GP control | 383 | 4.41 | 152.3 | 95 | 35 | 213 |
Abbreviations: CI=community intervention; CC=community control; GPC=general practice control; GPI=general practice intervention.
Natural log of mean. All other values in days.
Table 3. Total diagnostic interval by trial group, factorial design and tumour type.
|
Breast |
Colorectal |
Lung |
Prostate |
Total |
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Trial group | n | Mean Lna | Mean | Med’n | 25th | 75th | n | Mean Lna | Med’n | 25th | 75th | n | Mean Lna | Med’n | 25th | 75th | n | Mean Lna | Med’n | 25th | 75th | n | Mean Lna | Med’n | 25th | 75th |
| CI | 143 | 3.80 | 97.5 | 33 | 21 | 115 | 103 | 4.44 | 177.7 | 107 | 53 | 48 | 4.64 | 190.6 | 114.5 | 46.3 | 188 | 4.98 | 212.1 | 161.5 | 86 | 482 | 4.48 | 168.6 | 107.5 | 36 |
| CC | 275 | 3.67 | 82.5 | 34 | 18 | 97 | 146 | 4.70 | 195.3 | 133 | 53 | 65 | 4.58 | 157.4 | 114 | 53.5 | 346 | 4.82 | 186.8 | 123.5 | 66.8 | 832 | 4.40 | 151.5 | 92 | 35 |
| GPI | 176 | 3.77 | 83.0 | 34.5 | 19.3 | 100.8 | 118 | 4.54 | 185.3 | 124 | 43 | 55 | 4.66 | 180.7 | 115 | 55 | 199 | 4.88 | 192.9 | 139 | 74 | 548 | 4.43 | 154.7 | 97 | 35 |
| GPC | 196 | 3.69 | 91.8 | 33 | 18 | 91.5 | 94 | 4.57 | 185.3 | 122 | 57.3 | 45 | 4.64 | 170.4 | 125 | 49 | 253 | 4.93 | 203.1 | 148 | 76.5 | 588 | 4.42 | 160.7 | 96.5 | 34.25 |
| CI and GPI | 56 | 3.83 | 89.3 | 37 | 19.3 | 121 | 49 | 4.41 | 170.3 | 107 | 43 | 26 | 4.40 | 162.4 | 103.5 | 36.3 | 72 | 4.95 | 200.4 | 143 | 86.3 | 203 | 4.44 | 157.6 | 100 | 36 |
| CC and GPI | 120 | 3.74 | 80.1 | 34 | 19.3 | 97.8 | 69 | 4.64 | 195.9 | 128 | 42.5 | 29 | 4.89 | 197.1 | 119 | 71.5 | 127 | 4.83 | 188.6 | 129 | 69 | 345 | 4.42 | 153.0 | 92 | 34.5 |
| CI and GPC | 68 | 3.71 | 99.1 | 31 | 21.3 | 88.8 | 38 | 4.34 | 179.7 | 98 | 62 | 13 | 5.06 | 263.2 | 173 | 56.5 | 86 | 5.01 | 222.9 | 163.5 | 79.8 | 205 | 4.46 | 176.4 | 103 | 33.5 |
| CC and GPC | 128 | 3.63 | 87.9 | 33.5 | 17.3 | 92 | 56 | 4.73 | 189.2 | 134 | 55.8 | 32 | 4.47 | 132.7 | 110.5 | 42.8 | 167 | 4.88 | 193.0 | 132 | 73 | 383 | 4.41 | 152.3 | 95 | 35 |
Abbreviations: CI=community intervention; CC=community control; GPC=general practice control; GPI=general practice intervention.
Natural log of mean. All other values in days.
Table 4a, b, c presents the Patient, First Healthcare and Specialist Intervals by level of randomisation and tumour type. The median patient intervals between those in Community Intervention and Community Control regions were similar (44.5 vs 48.5 days), as were the First Healthcare Intervals between GP intervention and GP control groups (14 vs 15 days). After log transformation there were no statistically significant differences in the Patient, First Healthcare or Specialist Intervals by factorial design or tumour type (patient interval community intervention vs control ln mean difference −0.09 95% CI −0.36–0.18 P=0.51; First Healthcare Interval ln mean difference GP intervention vs control −0.27 95% CI −0.71–0.18 P=0.47; specialist interval ln mean difference Community intervention vs control 0.06 95% CI −0.18–0.29 P=0.65; GP intervention vs control 0.17 95% CI −0.12–0.47 P=0.49).
Table 4a. Patient interval by trial group, by factorial design and tumour type.
|
Breast |
Colorectal |
Lung |
Prostate |
Total |
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Trial group | n | Mean Lna | Mean | Med’n | 25th | 75th | n | Mean Lna | Mean | Med’n | 25th | 75th | n | Mean Lna | Mean | Med’n | 25th | 75th | n | Mean Lna | Mean | Med’n | 25th | 75th | n | Mean Lna | Mean | Med’n | 25th | 75th |
| CI | 104 | 2.92 | 73.8 | 18.5 | 4 | 77.8 | 86 | 3.64 | 118.1 | 50.5 | 14 | 180 | 36 | 3.70 | 111.5 | 54.5 | 17.8 | 155.5 | 80 | 3.97 | 136.0 | 86.5 | 18.8 | 183.8 | 306 | 3.49 | 106.9 | 44.5 | 10 | 160 |
| CC | 165 | 3.17 | 80.6 | 29 | 5.5 | 99 | 114 | 3.64 | 113.5 | 53 | 14 | 148.8 | 57 | 3.26 | 83.6 | 30 | 7 | 103 | 132 | 4.18 | 146.8 | 91 | 28.3 | 202.5 | 468 | 3.58 | 107.7 | 48.5 | 12 | 142.25 |
| GPI | 117 | 3.18 | 77.3 | 29 | 6.5 | 98 | 94 | 3.48 | 99.4 | 34 | 11 | 102 | 47 | 3.47 | 106.4 | 36 | 8 | 151 | 76 | 4.31 | 146.3 | 103.5 | 36 | 183.5 | 334 | 3.56 | 103.1 | 45 | 10.5 | 134 |
| GPC | 124 | 3.10 | 84.8 | 21 | 5 | 95 | 75 | 3.70 | 119.5 | 77.5 | 14 | 161 | 37 | 2.96 | 79.0 | 39.5 | 8.3 | 101.8 | 104 | 4.12 | 155.5 | 93.5 | 24 | 213.5 | 340 | 3.58 | 113.4 | 54 | 13 | 147 |
| CI and GPI | 40 | 3.04 | 85.5 | 21 | 3.3 | 106.8 | 38 | 3.71 | 108.1 | 54 | 15.5 | 209.3 | 22 | 3.59 | 107.5 | 54.5 | 16.8 | 152.5 | 31 | 4.05 | 122.0 | 78 | 21 | 177 | 131 | 3.568 | 104.4 | 54 | 12 | 160 |
| CC and GPI | 77 | 3.25 | 73.0 | 30 | 7.5 | 95.5 | 56 | 3.33 | 93.5 | 30 | 9 | 89 | 25 | 3.37 | 105.3 | 28 | 7.5 | 23.8 | 45 | 4.49 | 163.0 | 118 | 45 | 211.5 | 203 | 3.56 | 102.6 | 41 | 10 | 129 |
| CI and GPC | 51 | 2.82 | 67.5 | 14 | 4 | 73 | 33 | 3.33 | 112.3 | 43 | 10.5 | 92 | 8 | 3.76 | 107.7 | 61 | 23.8 | 100.5 | 40 | 4.04 | 152.6 | 106 | 15.8 | 197 | 132 | 3.37 | 106.9 | 40 | 7 | 116.5 |
| CC and GPC | 73 | 3.30 | 96.5 | 26 | 5.5 | 119.3 | 42 | 3.99 | 125.12 | 106 | 22.5 | 179 | 29 | 3.30 | 71.1 | 34.5 | 7 | 103.5 | 64 | 4.18 | 157.3 | 91.5 | 27.3 | 288.3 | 208 | 3.71 | 117.6 | 64 | 14 | 160 |
Abbreviations: CI=community intervention; CC=community control; GPC=general practice control; GPI=general practice intervention.
Natural log of mean. All other values in days.
Table 4b. First health encounter interval by trial group, by factorial design and tumour type.
|
Breast |
Colorectal |
Lung |
Prostate |
Total |
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Trial group | n | Mean Lna | Mean | Med’n | 25th | 75th | n | Mean Lna | Mean | Med’n | 25th | 75th | n | Mean Lna | Mean | Med’n | 25th | 75th | n | Mean Lna | Mean | Med’n | 25th | 75th | n | Mean Lna | Mean | Med’n | 25th | 75th |
| CI | 129 | 1.94 | 32.3 | 14 | 6 | 24.5 | 78 | 0.21 | 36.2 | 2.5 | 0 | 33.3 | 30 | 3.24 | 70.53 | 47.5 | 11.3 | 79 | 163 | 2.20 | 46.0 | 15 | 7 | 36 | 400 | 1.724 | 41.49 | 14 | 5 | 32.75 |
| CC | 258 | 1.95 | 25 | 14.5 | 6 | 28 | 131 | 0.56 | 44.9 | 10 | 0 | 40 | 47 | 2.32 | 58.13 | 16 | 6 | 50 | 329 | 2.15 | 46.6 | 14 | 6 | 41.5 | 765 | 1.822 | 39.72 | 14 | 5 | 35 |
| GPI | 160 | 1.73 | 20.4 | 13 | 6 | 25.8 | 100 | 0.41 | 50.4 | 10 | 0 | 51 | 40 | 2.33 | 68.15 | 16.5 | 6.3 | 68.8 | 182 | 2.29 | 47.1 | 14 | 6 | 36.25 | 482 | 1.719 | 40.66 | 14 | 4 | 33.5 |
| GPC | 186 | 2.14 | 31.9 | 15 | 7 | 27.5 | 84 | 0.45 | 36.9 | 8 | 0 | 33 | 32 | 2.93 | 53.63 | 26 | 7 | 63 | 239 | 2.27 | 44.9 | 17 | 7 | 42 | 541 | 1.984 | 39.7 | 15 | 6 | 35 |
| CI and GPI | 47 | 1.37 | 17.0 | 13 | 3 | 24 | 35 | 0.38 | 49.6 | 12 | 0 | 51 | 19 | 2.85 | 72.89 | 14 | 6 | 103 | 61 | 2.52 | 56.9 | 15 | 7 | 46 | 162 | 1.765 | 45.63 | 14 | 4.75 | 35 |
| CC and GPI | 113 | 1.88 | 21.8 | 13 | 6 | 28.5 | 65 | 0.42 | 50.8 | 10 | 0 | 63.8 | 21 | 1.87 | 63.86 | 17 | 5 | 41 | 121 | 2.18 | 42.2 | 14 | 5 | 31 | 320 | 1.696 | 38.14 | 13 | 4 | 32 |
| CI and GPC | 65 | 2.36 | 42.1 | 14 | 8 | 24.5 | 32 | 0.20 | 32.8 | 7 | 0 | 33.8 | 8 | 3.99 | 66.88 | 55 | 47.3 | 68.3 | 78 | 2.26 | 38.8 | 15 | 7 | 36 | 183 | 2.011 | 40.15 | 14 | 7 | 31 |
| CC and GPC | 121 | 2.03 | 26.4 | 15.5 | 7 | 28 | 52 | 0.61 | 39.4 | 8 | 0 | 31 | 24 | 2.57 | 50.54 | 10.5 | 5.3 | 59.8 | 161 | 2.28 | 47.7 | 18 | 6.25 | 47.75 | 358 | 1.970 | 39.46 | 16 | 6 | 35 |
Abbreviations: CI=community intervention; CC=community control; GPI=general practice intervention; GPC=general practice control.
Natural log of mean. All other values in days.
Table 4c. Specialist interval by trial group, by factorial design and tumour type.
|
Breast |
Colorectal |
Lung |
Prostate |
Total |
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Trial group | n | Mean Lna | Mean | Med’n | 25th | 75th | n | Mean Lna | Mean | Med’n | 25th | 75th | n | Mean Lna | Mean | Med’n | 25th | 75th | n | Mean Lna | Mean | Med’n | 25th | 75th | n | Mean Lna | Mean | Med’n | 25th | 75th |
| CI | 86 | 1.91 | 20.0 | 13 | 5.8 | 23 | 77 | 3.40 | 54.1 | 36 | 14 | 71 | 27 | 2.53 | 40.6 | 16 | 21 | 72 | 165 | 4.36 | 114.2 | 83 | 165 | 228 | 355 | 3.42 | 72.6 | 39 | 15 | 91 |
| CC | 146 | 1.70 | 22.3 | 11.5 | 4 | 23.3 | 133 | 3.48 | 71.6 | 39 | 21 | 72 | 47 | 2.80 | 28.3 | 16 | 8 | 36 | 330 | 4.14 | 94.4 | 63 | 330 | 63 | 656 | 3.37 | 69.0 | 41 | 16 | 78.8 |
| GPI | 95 | 2.04 | 23.9 | 13 | 6 | 29 | 100 | 3.61 | 76.4 | 41 | 23 | 93 | 38 | 2.64 | 25.3 | 14.5 | 6.75 | 26.5 | 183 | 4.29 | 106.8 | 73 | 38 | 140 | 416 | 3.36 | 73.1 | 39 | 16 | 93 |
| GPC | 113 | 1.49 | 17.3 | 10 | 5 | 21.8 | 83 | 3.36 | 60.3 | 36 | 15.5 | 58.5 | 31 | 2.84 | 44.2 | 19 | 11 | 36 | 240 | 4.17 | 98.3 | 68 | 38 | 119 | 467 | 3.29 | 68.4 | 40.5 | 14 | 77 |
| CI and GPI | 32 | 1.20 | 19.8 | 15.5 | 5 | 27.5 | 35 | 3.60 | 62.4 | 42 | 21 | 94 | 17 | 2.56 | 17.2 | 16 | 10 | 24 | 62 | 4.34 | 109.8 | 91 | 37.75 | 140.8 | 146 | 3.44 | 67.9 | 37.5 | 16 | 93.3 |
| CC and GPI | 63 | 2.06 | 25.9 | 13 | 6 | 29 | 65 | 3.62 | 83.9 | 40 | 23 | 92.5 | 21 | 2.70 | 31.9 | 14 | 6 | 40.5 | 121 | 4.27 | 105.2 | 71 | 38 | 140.5 | 270 | 3.48 | 75.9 | 41 | 15.5 | 92 |
| CI and GPC | 44 | 1.56 | 15.7 | 11 | 5.3 | 19.5 | 31 | 3.27 | 47.0 | 28 | 13 | 57 | 7 | 2.10 | 102 | 12 | 5 | 28 | 78 | 4.33 | 115.2 | 76.5 | 48 | 138.3 | 160 | 3.27 | 74.0 | 39 | 13.3 | 84 |
| CC and GPC | 69 | 1.44 | 18.3 | 10 | 4 | 22 | 52 | 3.40 | 68.3 | 41 | 17 | 60 | 24 | 3.06 | 27.4 | 19 | 11.75 | 44.25 | 162 | 4.10 | 90.2 | 62 | 35 | 111 | 307 | 3.30 | 65.4 | 41 | 16 | 73.8 |
Abbreviations: CI=community intervention; CC=community control; GPI=general practice intervention; GPC=general practice control.
Natural log of mean. All other values in days.
The protocol-driven sensitivity analyses excluding vague symptoms as the first symptom and screen-detected cases did not alter the main findings for the TDI or its sub-components. All interactions were statistically not significant so were not considered further.
Discussion
Summary findings
This is the first controlled trial worldwide to test the separate and combined effects of community symptom awareness campaign and GP educational interventions on time to cancer diagnosis. In this large rigorously conducted trial over a 2-year period of intervention, we found no evidence of an effect of either intervention on time to cancer diagnosis. Furthermore, we found no specific effect of the community intervention on the time participants took to present with symptoms or of the GP intervention on time from presentation to referral.
Strengths and limitations
There are several strengths to the design of this trial: the interventions were carefully developed from previous exploratory research conducted in rural Western Australia, and were tailored to meet specific identified barriers to timely cancer diagnosis. We rigorously applied the Aarhus guidelines to the measurement and reporting of studies of diagnostic intervals, underpinned by a theoretical model of Pathways to Treatment (Walter et al, 2012, Weller et al, 2012, Scott et al, 2013). Our measurement of the patient interval used a well-established instrument designed to collect information about duration of symptoms and dates of presentation to healthcare. There are inevitable limitations in the accuracy of such self-reported measures, such as recall bias and inaccurate recollection of precise dates, but there is no known better way of obtaining data on such intervals in cancer patients. Any measurement error is likely to be equally distributed between trial groups and so should not alter the trial comparisons.
We recruited over 50% of all incident cases who were eligible for the trial, a very good response rate for recruitment through a cancer registry. Although it is possible that we may have been subject to survival bias, in that those with more advanced disease were less likely to participate, this bias should not have differentially affected the trial groups in relation to the primary outcome. We chose to include screen-detected cases because the interventions could potentially have contributed to decisions to seek screening and GP referral pathways. Our sensitivity analysis excluding screen-detected cases did not alter our conclusions.
There are acknowledged weaknesses in our design: we had no urban comparator groups which could have provided additional useful comparative data on time to diagnosis. However, the community intervention was designed specifically for a rural Australian population making an urban comparison group of less direct relevance. We conducted the trial in Western Australia due to the isolated nature of many rural communities and because our previous exploratory research identifying barriers to help-seeking was based on patients from these regions. It would have been logistically even more challenging and expensive to compare rural regions in different States in Australia. Furthermore, conducting such a trial between Australian States would introduce significant confounding due to differences in healthcare systems. The selection of trial regions for the Community Intervention was matched as carefully as possible during the planning phase, within the geographical limitations of a large and sparsely populated state. However, there were only two community clusters in this trial, limiting our ability to account fully for clustering at this level in our analyses and raising the possibility of unbalanced confounders across the two community trial groups. Differential population growth in the community control regions during the trial meant a larger proportion of participants were in the control arm, but this did not impact our power to detect a small difference in the TDI.
In our Australian context, multiple cluster randomisation of small local communities was not possible, because of our reliance on regional radio and newspaper media outlets that covered large swaths of the Community Intervention area, but which did not overlap into the control area. Our design was thus necessitated by the imperative to avoid campaign contamination to the control area. We analysed the data on the basis of intention to treat. Not all general practices participated in the intervention and we do not know the precise extent to which individuals with cancer were exposed to the community campaign. Intention to treat analyses could potentially reduce our ability to identify an effect of the interventions. Despite these accepted limitations, we question if an improved experimental design using the same methods of community intervention could be achieved anywhere else in the world given the isolated nature of communities of rural Western Australia. Thus, our design represents the best level of evidence that is likely to be achieved in addressing the research question at hand.
Given these pragmatic challenges of conducting trials of community level interventions, how could potential imbalance between confounders explain our null result? If the community intervention was truly effective, baseline confounding would have been such that, in the absence of intervention, Trial Area A (community intervention) would have fared worse than the Trial Area B (community control). Alternatively, the intervention might have been harmful, causing greater delays, but baseline confounding was such that Trial Area A would have fared better than Trial Area B in the absence of intervention. We believe it is very unlikely that an intervention of this nature could actually cause greater delays in diagnosis. Thus the only credible alternative explanation for the null trial result is the first of these two possibilities.
Whilst we have limited data on the case-mix of those diagnosed with cancer during the trial, a wide range of socio-demographic, health care delivery measures and cancer incidence were compared between trial areas at baseline and found to have balanced distributions. Importantly, SEIFA scores, measuring several components of socio-economic disadvantage, were similar between trial participants from community intervention and control regions. Unfortunately we do not have pre-trial data on diagnostic intervals but, if there had been a material imbalance of independent determinants of diagnostic delay between the two trial areas, one would expect cancer mortality rates to have been worse in the Trial Area A before the trial. In fact this was not the case; age-standardised mortality rates for the four cancers were: Trial Area A male 56.3, female 43.9; Trial Area B male 57.1, female 46.3 per 100 000 person-years. If Trial Area A were destined to have a worse outcome than the control area, and this was mitigated by the intervention, one would expect to observe an unfavourable difference in delay times affecting the earliest cancers diagnosed during the trial, to then be balanced by a reversal of this for cancers diagnosed towards the end of the study as the community intervention took full effect. We examined this possibility but did not report it as it was not in the original protocol. This never occurred and the null result remained consistent across cancers diagnosed early and late in the intervention period. In summary, we believe that imbalance between confounders between the community intervention and control regions is not the explanation for our findings.
Context with existing literature
Why did we find no effect? It is possible that the dose of the interventions was simply too small to have a measureable influence on the primary outcome. The money spent on the Community Intervention was relatively modest compared to other national and international cancer awareness and prevention campaigns with a small staff committed over a large geographic area. The Community Intervention was based on principles of community engagement. To avoid contamination we did not use television and, although our process measures of reach showed significant awareness and initial cognitive impact of key components of the campaign,41 compared with other public health campaigns, our total expenditure on the Find Cancer Early campaign was relatively small. It was not feasible to obtain data either from a wider population of people presenting with symptoms associated with cancer or data on diagnostic intervals preceding the trial. Our community telephone survey mid-way through the campaign suggested differences between community and control regions in symptom awareness and intentions to seek help in favour of the intervention (Gray et al, 2015). Specifically, those in campaign regions were significantly more likely to recognise specific cancer symptoms (blood in stools and urine and an unusual lump); of those aware of the campaign, one quarter said they had visited their GP about a specific symptom (Gray et al, 2015). But these findings were not translated into differences in diagnostic intervals among those diagnosed with cancer during the follow-up period. Importantly, follow-up occurred during the two-year period and only for a further 3 months after intervention delivery. The intervention effects are likely to be cumulative over time and, given the lag between symptom onset and cancer diagnosis, our period of follow-up is likely to have been too short to identify small effects.
There is limited evidence about the benefits of symptom awareness campaigns despite their widespread use internationally (Austoker et al, 2009). Recent data from the English ‘Be Clear on Cancer Campaigns’ have suggested potentially useful short-term effects including increased presentations to general practice and earlier stage lung cancer, but these are based on comparisons with historical control data (Ironmonger et al, 2015). There are socio-demographic differences in symptom awareness and barriers to symptomatic presentation, with more deprived populations citing greater barriers (Niksic et al, 2015). Rural Australians may be a ‘hard to reach’ population who respond more stoically to symptoms, experience greater barriers to help-seeking, and are therefore less likely to respond to these types of campaign (Emery et al, 2013a).
Our GP Intervention was based on evidence about effective interventions to change health professional behaviour (O’Brien et al, 2007) and tailored to implement the CAPER cancer RATs and cancer diagnostic pathways. An observational study of implementing the same cancer RATs in English general practice showed an increase in referrals for suspected lung and colorectal cancer (Hamilton et al, 2013). That study included a computer mouse-mat and a paper flipchart to remind GPs about the RAT and may therefore have been a more constant reminder than our GP resource card. Furthermore, the English study was set in the context of a much larger and sustained national effort to raise awareness amongst GPs about early cancer diagnosis. Our model of implementation was based on a series of academic detailing visits to reinforce educational messages and seek GP reflection on their use. The Cochrane review of trials of academic detailing visits found small to moderate effects on changing professional behaviours but with large unexplained variation between trials (O’Brien et al, 2007). While 81% of all practices accepted at least one educational visit, only 58% received all four. Nonetheless, 71% of practices who participated in the intervention chose to receive all four visits suggesting high levels of acceptability. This is very good reach for a general practice intervention in such a large geographical area but may have been a factor in why we found no effect of the intervention.
Our interventions were aimed predominantly at reducing patient and GP delays along the diagnostic pathway. During this trial only one cancer diagnostic pathway was implemented in WA to reduce specialist delay, a one-stop prostate cancer clinic for rural patients (McCombie et al, 2015). A regional cancer centre was opened in mid-2011 in Bunbury (Community Control region) aimed at providing treatment for rural patients closer to home. The GP intervention included information about preferred specialist diagnostic pathways, including those linked to multidisciplinary teams, but these pathways were not designed specifically as ‘fast-track’ cancer referral pathways. Our interventions were therefore limited in the ability to reduce the specialist interval component of the TDI. The Specialist Interval can be a major contributor to the TDI, especially, for example, for uninsured patients with colorectal or prostate cancer who rely on public hospital services for diagnostic tests where they may be longer waiting times. There are no comparable published Australian data on TDIs for the cancers studied in this trial. The median durations in our trial cohort for lung and colorectal cancer were considerably longer than those reported in recent cohorts of these cancers from England(Walter et al, 2015, 2016) and are likely to represent clinically important delays (Neal et al, 2015).
This is the first such trial of community symptom awareness or GP interventions to use time to cancer diagnosis as a primary outcome (Austoker et al, 2009, Mansell et al, 2011b). Others have monitored a range of health service process measures such as GP visits and GP referrals, and some larger observational studies have measured cancer incidence. Given the focus of such interventions is to reduce diagnostic delay we believe that the TDI was the most appropriate primary outcome. Based on our pre-trial data, the trial was sufficiently powered to detect relatively small but potentially clinically important differences in the TDI. This paper reports on the primary outcome. By 2018 the WA linked data will be available for the trial period, at which point we will report any effects on cancer incidence, stage at diagnosis, healthcare utilisation and survival.
Conclusions and recommendations
In the United Kingdom, Denmark and other international jurisdictions, there have been significant investments in community cancer symptom awareness campaigns and GP interventions, including those aimed at implementing cancer RATs, with a focus on reducing cancer diagnostic delay (Hiom, 2015). While much emphasis has been made on a growing body of observational data about possible benefits, until now there have been no large-scale pragmatic trials reported which have measured their effect on time to diagnosis. Our trial in large geographically isolated regions of Australia, with known later cancer presentation and higher stage at diagnosis, failed to find an effect of either community or GP interventions on time to cancer diagnosis. This is unlikely to be due to contamination between groups or imbalance in confounders, but may reflect insufficient campaign dose or insufficient length of follow-up. Future trials of public awareness campaigns should consider approaches to measuring longer term effects and consider designs that allow for use of television and other media. Nonetheless, our results may in fact show that, at least in this setting, community campaigns and GP education initiatives may not be effective and alternative strategies, possibly focused on fast track specialist pathways and improved access to diagnostic tests, may be more important in reducing diagnostic delay and improving cancer outcomes (Moller et al, 2015).
Acknowledgments
We wish to thank the following for their contributions to this trial: Find Cancer Early Project Officers (Lisa Barr, Pam Foulkes-Taylor, Libby Foster, Karen Hansen, Amanda Harding, Kerryn Keating, Tracey Price, Jo Woodall), Cancer Council WA staff and associated staff, Cancer Council WA Lodges, participants, GPs and hospital specialists, Cancer Research UK, AH Crawford Treatment Society, Department of Health WA, WA Cancer and Palliative Care Network, WA Country Health Service, National Health and Medical Research Council, Cancer and Palliative Care Research and Evaluation Unit, Val Lishman Health Research Foundation, WA Cancer Registry Staff, WA Data Linkage Branch, Pat Booth, Rhonda Coleman, Hooi Ee, Dickon Hayne, Laura Keith, Andrew Kirke, Mike Mears, Leanne Monterosso, Violet Platt, Iain Steve Pratt, David Preen, Babu Simon, Craig Sinclair, Hayley Staples, Simon Towler, Clare Willix. The project was a partnership with Cancer Council Western Australia, the WA Cancer and Palliative Care Network, and the Department of Health Western Australia. JDE is funded by an NHMRC Practitioner Fellowship. FMW is funded by an NIHR Clinician Scientist award.
Disclaimer
The NHMRC had no role in the design of this study, the primary interpretation of data or decision to submit results. CCWA contributed to the design and provided substantial practical assistance in the delivery of the intervention; two authors (TS and EC) are CCWA staff. The funders had no role in data analysis or interpretation, or decision to publish the results.
Data sharing
Anonymised patient level data are available at on request from the corresponding author. Consent was not obtained for data sharing but the presented data are anonymised and risk of identification is low.
Author contributions
JDE, CDJH, CS, KA, AN, FMW, MB conceptualised and designed the study. All authors assisted with the development of the protocol, study design and refinement of study materials. All authors contributed to implementation of the protocol and acquisition of data. MB was responsible for the randomisation and statistical analyses. All authors have been involved in drafting and critical evaluation of the manuscript. All authors have read and approved the final version.
Footnotes
Supplementary Information accompanies this paper on British Journal of Cancer website (http://www.nature.com/bjc)
The authors declare no conflict of interest.
Supplementary Material
References
- Adamson J, Cockayne S, Puffer S, Torgerson DJ (2006) Review of randomised trials using the post-randomised consent (Zelen's) design. Contemp Clin Trials 27: 305–319. [DOI] [PubMed] [Google Scholar]
- AIHW (2010) Cancer in Australia 2010: an overview. Australian Institute of Health and Welfare: Canberra, Australia.
- Allgar VL, Neal RD (2005) Delays in the diagnosis of six cancers: analysis of data from the National Survey of NHS Patients: Cancer. Br J Cancer 92: 1959–1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Athey VL, Suckling RJ, Tod AM, Walters SJ, Rogers TK (2011) Early diagnosis of lung cancer: evaluation of a community-based social marketing intervention. Thorax 67(5): 412–417. [DOI] [PubMed] [Google Scholar]
- Austoker J, Bankhead C, Forbes LJ, Atkins L, Martin F, Robb K, Wardle J, Ramirez AJ (2009) Interventions to promote cancer awareness and early presentation: systematic review. Br J Cancer 101(Suppl 2): S31–S39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baade PD, Dasgupta P, Aitken J, Turrell G (2011. a) Geographic remoteness and risk of advanced colorectal cancer at diagnosis in Queensland: a multilevel study. Br J Cancer 105: 1039–1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baade PD, Youlden DR, Coory MD, Gardiner RA, Chambers SK (2011. b) Urban-rural differences in prostate cancer outcomes in Australia: what has changed? Med J Aust 194: 293–296. [DOI] [PubMed] [Google Scholar]
- Butler J, Foot C, Bomb M, Hiom S, Coleman M, Bryant H, Vedsted P, Hanson J, Richards M (2013) The international cancer benchmarking partnership: an international collaboration to inform cancer policy in Australia, Canada, Denmark, Norway, Sweden and the United Kingdom. Health Policy 112: 148–155. [DOI] [PubMed] [Google Scholar]
- Campbell MK, Thomson S, Ramsay CR, Maclennan GS, Grimshaw JM (2004) Sample size calculator for cluster randomized trials. Comput Biol Med 34: 113–125. [DOI] [PubMed] [Google Scholar]
- Cancer Research UK (2010) National Awareness and Early Diagnosis Initiative. Cancer Research UK: UK. [Google Scholar]
- Coory MD, Baade PD (2005) Urban-rural differences in prostate cancer mortality, radical prostatectomy and prostate-specific antigen testing in Australia. Med J Aust 182: 112–115. [DOI] [PubMed] [Google Scholar]
- Coory MD, Ho T, Jordan SJ (2013) Australia is continuing to make progress against cancer, but the regional and remote disadvantage remains. Med J Aust 199: 605–608. [DOI] [PubMed] [Google Scholar]
- Department of Health, WA.. Model of Care for Cancer. Cancer & Palliative Care Network, Department of Health: Perth, WA, Australia, 2008. [Google Scholar]
- Department of Health and Ageing (2010) Delivering Regional Cancer Centres. Canberra, Australia.
- Emery JD, Gray V, Walter FM, Cheetham S, Croager EJ, Slevin T, Saunders C, Threlfall T, Auret K, Nowak AK, Geelhoed E, Bulsara M, Holman CD (2014) The Improving Rural Cancer Outcomes (IRCO) Trial: a factorial cluster-randomised controlled trial of a complex intervention to reduce time to diagnosis in rural patients with cancer in Western Australia: a study protocol. BMJ Open 4: e006156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emery JD, Walter FM, Gray V, Sinclair C, Howting D, Bulsara M, Bulsara C, Webster A, Auret K, Saunders C, Nowak A, Holman CD (2013. a) Diagnosing cancer in the bush: a mixed-methods study of symptom appraisal and help-seeking behaviour in people with cancer from rural Western Australia. Fam Pract 30: 294–301. [DOI] [PubMed] [Google Scholar]
- Emery JD, Walter FM, Gray V, Sinclair C, Howting D, Bulsara M, Bulsara C, Webster A, Auret K, Saunders C, Nowak A, Holman D (2013. b) Diagnosing cancer in the bush: a mixed methods study of GP and specialist diagnostic intervals in rural Western Australia. Fam Pract 30(5): 541–550. [DOI] [PubMed] [Google Scholar]
- Forbes LJ, Simon AE, Warburton F, Boniface D, Brain KE, Dessaix A, Donnelly C, Haynes K, Hvidberg L, Lagerlund M, Lockwood G, Tishelman C, Vedsted P, Vigmostad MN, Ramirez AJ, Wardle J International Cancer Benchmarking Partnership Module 2 Working, G (2013) Differences in cancer awareness and beliefs between Australia, Canada, Denmark, Norway, Sweden and the UK (the International Cancer Benchmarking Partnership): do they contribute to differences in cancer survival? Br J Cancer 108: 292–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray V, Croager E, Emery J, Slevin T, Pratt I, Holman D Officers, F. C. E. P. (2015) Development and Evaluation of the Find Cancer Early community education campaign in regional Western Australia to reduce help-seeking interval. Eur J Cancer Care 24: 16–16. [Google Scholar]
- Grimshaw JM, Thomas RE, Maclennan G, Fraser C, Ramsay CR, Vale L, Whitty P, Eccles MP, Matowe L, Shirran L, Wensing M, Dijkstra R, Donaldson C (2004) Effectiveness and efficiency of guideline dissemination and implementation strategies. Health Technol Assess8: iii-iv, 1–72. [DOI] [PubMed]
- Hall SE, Holman CD, Wisniewski ZS, Semmens J (2005) Prostate cancer: socio-economic, geographical and private-health insurance effects on care and survival. BJU Int 95: 51–58. [DOI] [PubMed] [Google Scholar]
- Hamilton W (2009) The CAPER studies: five case-control studies aimed at identifying and quantifying the risk of cancer in symptomatic primary care patients. Br J Cancer 101: S80–S86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton W, Green T, Martins T, Elliott K, Rubin G, Macleod U (2013) Evaluation of risk assessment tools for suspected cancer in general practice: a cohort study. Br J Gen Pract 63: e30–e36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton W, Peters TJ, Round A, Sharp D (2005. a) What are the clinical features of lung cancer before the diagnosis is made? A population based case-control study. Thorax 60: 1059–1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton W, Round A, Sharp D, Peters TJ (2005. b) Clinical features of colorectal cancer before diagnosis: a population-based case-control study. Br J Cancer 93: 399–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton W, Sharp DJ, Peters TJ, Round AP (2006) Clinical features of prostate cancer before diagnosis: a population-based, case-control study. Br J Gen Pract 56: 756–762. [PMC free article] [PubMed] [Google Scholar]
- Hiom SC (2015) Diagnosing cancer earlier: reviewing the evidence for improving cancer survival. Br J Cancer 112(Suppl 1): S1–S5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hippisley-Cox J, Coupland C (2013. a) Symptoms and risk factors to identify men with suspected cancer in primary care: derivation and validation of an algorithm. Br J Gen Pract 63: e1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hippisley-Cox J, Coupland C (2013. b) Symptoms and risk factors to identify women with suspected cancer in primary care: derivation and validation of an algorithm. Br J Gen Pract 63: e11–e21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ironmonger L, Ohuma E, Ormiston-Smith N, Gildea C, Thomson CS, Peake MD (2015) An evaluation of the impact of large-scale interventions to raise public awareness of a lung cancer symptom. Br J Cancer 112: 207–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jong KE, Vale PJ, Armstrong BK (2005) Rural inequalities in cancer care and outcome. Med J Aust 182: 13–14. [DOI] [PubMed] [Google Scholar]
- Mansell G, Shapley M, Jordan JL, Jordan K (2011. a) Interventions to reduce primary care delay in cancer referral: a systematic review. Br J Gen Pract 61: e821–e835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansell G, Shapley M, Jordan JL, Jordan K (2011. b) Interventions to reduce primary care delay in cancer referral: a systematic review. Br J Gen Pract 61: e821–e835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCombie SP, Hawks C, Emery JD, Hayne D (2015) A 'One Stop' prostate clinic for rural and remote men: a report on the first 200 patients. BJU Int 116(Suppl 3): 11–17. [DOI] [PubMed] [Google Scholar]
- Meilleur A, Subramanian SV, Plascak JJ, Fisher JL, Paskett ED, Lamont EB (2013) Rural residence and cancer outcomes in the United States: issues and challenges. Cancer Epidemiol Biomarkers Prev 22: 1657–1667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell KJ, Fritschi L, Reid A, Mcevoy SP, Ingram DM, Jamrozik K, Clayforth C, Byrne MJ (2006) Rural-urban differences in the presentation, management and survival of breast cancer in Western Australia. Breast 15: 769–776. [DOI] [PubMed] [Google Scholar]
- Moller H, Gildea C, Meechan D, Rubin G, Round T, Vedsted P (2015) Use of the English urgent referral pathway for suspected cancer and mortality in patients with cancer: cohort study. BMJ 351: h5102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore HJ, Nixon C, Tariq A, Emery J, Hamilton W, Hoare Z, Kershenbaum A, Neal RD, Ukoumunne OC, Usher-Smith J, Walter FM, WHYTE S, RUBIN G (2016) Evaluating a computer aid for assessing stomach symptoms (ECASS): study protocol for a randomised controlled trial. Trials 17: 184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Breast And Ovarian Cancer Centre (2006) The Investigation of a New Breast Symptom: A Guide for General Practitioners. National Breast and Ovarian Cancer Centre: Australia. [Google Scholar]
- Neal RD, Nafees S, Pasterfield D, Hood K, Hendry M, Gollins S, Makin M, Stuart N, Turner J, Carter B, Wilkinson C, Williams N, Robling M (2014) Patient-reported measurement of time to diagnosis in cancer: development of the Cancer Symptom Interval Measure (C-SIM) and randomised controlled trial of method of delivery. BMC Health Serv Res 14: 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neal RD, Tharmanathan P, France B, Din NU, Cotton S, Fallon-Ferguson J, Hamilton W, Hendry A, Hendry M, Lewis R, Macleod U, Mitchell ED, Pickett M, Rai T, Shaw K, Stuart N, Torring ML, Wilkinson C, Williams B, Williams N, Emery J (2015) Is increased time to diagnosis and treatment in symptomatic cancer associated with poorer outcomes? Systematic review. Br J Cancer 112(Suppl): S92–S107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niksic M, Rachet B, Warburton FG, Wardle J, Ramirez AJ, Forbes LJ (2015) Cancer symptom awareness and barriers to symptomatic presentation in England—are we clear on cancer? Br J Cancer 113: 533–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Brien MA, Rogers S, Jamtvedt G, Oxman AD, Odgaard-Jensen J, Kristoffersen DT, Forsetlund L, Bainbridge D, Freemantle N, Davis DA, Haynes RB, Harvey EL (2007) Educational outreach visits: effects on professional practice and health care outcomes. Cochrane Database Syst Rev CD000409. [DOI] [PMC free article] [PubMed]
- Scott SE, Walter FM, Webster A, Sutton S, Emery J (2013) The model of pathways to treatment: conceptualization and integration with existing theory. Br J Health Psychol 18: 45–65. [DOI] [PubMed] [Google Scholar]
- Singh GK, Williams SD, Siahpush M, Mulhollen A (2011) Socioeconomic, rural-urban, and racial inequalities in US cancer mortality: part i-all cancers and lung cancer and part ii-colorectal, prostate, breast, and cervical cancers. J Cancer Epidemiol 2011: 107497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Underhill CR, Goldstein D, Grogan PB (2006) Inequity in rural cancer survival in Australia is not an insurmountable problem. Med J Aust 185: 479–480. [DOI] [PubMed] [Google Scholar]
- WA Country Health Service (2011) Regional Profiles. WA Country Health Service, Department of Health: Perth, WA, Australia. Available at : http://www.wacountry.health.wa.gov.au/index.php?id=506.
- Walter F, Webster A, Scott S, Emery J (2012) The andersen model of total patient delay: a systematic review of its application in cancer diagnosis. J Health Serv Res Policy 17: 110–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter FM, Emery JD, Mendonca S, Hall N, Morris HC, Mills K, Dobson C, Bankhead C, Johnson M, Abel GA, Rutter MD, Hamilton W, Rubin GP (2016) Symptoms and patient factors associated with longer time to diagnosis for colorectal cancer: results from a prospective cohort study. Br J Cancer 115: 533–541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter FM, Rubin G, Bankhead C, Morris HC, Hall N, Mills K, DOBSON C, Rintoul RC, Hamilton W, Emery J (2015) Symptoms and other factors associated with time to diagnosis and stage of lung cancer: a prospective cohort study. Br J Cancer 112: S6–S13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weller D, Vedsted P, Rubin G, Walter FM, Emery J, Scott S, Campbell C, Andersen RS, Hamilton W, Olesen F, Rose P, Nafees S, Van Rijswijk E, Hiom S, Muth C, Beyer M, Neal RD (2012) The Aarhus statement: improving design and reporting of studies on early cancer diagnosis. Br J Cancer 106: 1262–1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.