Highlights
-
•
We followed up a cohort of 3008 Type 2 DM patients with diabetic kidney disease.
-
•
We estimated the annual mortality rate by CKD stages.
-
•
Predictors of mortality were age, male gender, CKD stages and albuminuria.
-
•
Certain comorbid conditions and use of antiplatelet agents were predictors too.
Keywords: Epidemiology, Prognosis, Diabetic kidney disease, Mortality
Abstract
Background
The prognosis of diabetic kidney disease is poor because epidemiological data have shown that all-cause mortality increases with declining renal function. This study aims to estimate the annual mortality rate of diabetic kidney disease stratified by chronic kidney disease (CKD) stages and to identify the predictors of mortality.
Methods
Patients with Stage 3–5 CKD (estimated glomerular filtration rate [eGFR] less than 60 mL/min per 1.73 m2) with diabetic kidney disease from the National Healthcare Group CKD Registry from 1 January 2007 to 31 December 2007 were included in this study. The patients were followed up till 30 November 2013. Cox's proportional hazards regression modelling was used to assess the factors associated with all-cause mortality.
Results
Over a median follow up period of 6.0 years, 985 out of 3008 patients (32.8%) died. Of those who died, 363 (36.9%) died from cardiovascular causes. The annual mortality rate was 64.1 per 1000 individuals (95% confidence interval [CI] 60.2–68.3) and the mortality rate increased with severity of CKD [Stage 3A (37.0), Stage 3B (57.5), Stage 4 (98.3) and Stage 5 (198.5)]. Predictors of mortality were age, male gender, CKD stages, albuminuria, comorbid conditions such as peripheral vascular disease, neuropathy, retinopathy and the use of antiplatelet agents.
Conclusion
Our study estimated the annual all-cause mortality rate for Singaporean patients with diabetic kidney disease by CKD stages and identified predictors of all-cause mortality. This study has affirmed the poor prognosis of these patients and an urgency to intervene early so as to retard the progression to later stages of CKD.
Background
Chronic kidney disease (CKD) is defined as either functional or structural kidney damage or an estimated glomerular filtration rate (eGFR) <60 mL/min per 1.73 m2 for at least 3 months [1]. CKD has 5 stages (stage 1 to stage 5) with stage 3 being subdivided into stages 3A and 3B based on the eGFR.
Worldwide, the prevalence of CKD is estimated to be 7.2% in persons aged 30 years and above, with the prevalence varying from 23.4% to 35.8% in persons aged 64 years and above [2]. According to the 2010 Global Burden of Disease study CKD was ranked 18th in the list of causes of global deaths (annual death rate of 15.7 per 100,000) [3].
The prognosis of patients with CKD is poor [4] because lower eGFR and albuminuria are associated with incident cardiovascular disease and all-cause mortality [5], [6]. A systematic review showed that the unadjusted relative risk for all-cause mortality in CKD patients compared with non-CKD patients ranged from 0.94 to 5.0 and was significantly higher (relative risk more than 1.0) in 93% of the cohorts included [7]. The corresponding unadjusted relative risk for cardiovascular mortality ranged from 1.4 to 3.7 [7]. In Singapore, a recent study showed that the risks of both cardiovascular deaths and all-cause mortality increase with decreasing estimated GFR and increasing albuminuria [5]. The association between estimated GFR <60 mL/min per 1.73 m2 and all-cause mortality was even stronger among those with diabetes [5]. Furthermore, Singapore has the fifth highest incidence of end-stage renal failure in the world and the highest incidence of diabetic nephropathy causing end-stage renal failure compared to other countries [8]. A recent study has also found that the prevalence of diabetic nephropathy in a primary health cluster in Singapore is as high as 52.5% [9].
Therefore, it is important to be cognizant of the mortality rate in patients with chronic kidney disease for the purpose of health service provision planning. This study aims to quantify the mortality rate among diabetic CKD patients and to determine the predictors associated with all-cause mortality for these patients. The stage stratified mortality rates will provide a good estimate of the population heath while the predictors of mortality will help prognosticate patients for early and appropriate interventions to mitigate their risks of renal failure progression thereby reducing subsequent morbidity and mortality.
Methods
The National Healthcare Group (NHG) provides public healthcare services through an integrated network of primary healthcare polyclinics, acute care and tertiary hospitals, national specialty centres and business divisions [10]. Patients in the NHG CKD Registry were identified to have CKD if they were at least 16 years old and fulfilled any one of the following conditions:
-
1.
Coded with CKD diagnosis [International classification of disease codes, ninth edition (585, 585.1, 585.2, 585,3, 585.4, 585.5, 585.6, 585.9)];
-
2.
Two eGFR <60 mL/min/1.73 m2 90 days apart;
-
3.
Two urine albumin creatinine ratio (ACR) ≥2.5 mg/mmol (male) for males or ≥3.5 mg/mmol for females, or >30 mg/g taken 90 days apart
-
4.
Two urine protein creatinine ratio (PCR) ≥20 mg/mmol or >0.2 mg/mg 90 days apart;
-
5.
Two urine protein ≥0.2 g/day 90 days apart.
All patients who fulfilled the above criteria will be automatically included in the CKD registry. In addition, the CKD Registry contains administrative, clinical and pharmacy information of these patients, which would be extracted for the purpose of this study.
This is a retrospective cohort study of Type 2 diabetes patients with CKD stage 3A and above (estimated glomerular filtration rate <60 mL/min per 1.73 m2) from the Registry from 1 January 2007 to 31 December 2007. Patients with CKD stages 1–2 or unknown CKD stage status were excluded from the study.
To ensure comprehensive capture of all diabetes patients into the registry, the following rules, ranked in descending order, were used:
-
(i)
Rule 1, patients from existing standalone diabetes registries;
-
(ii)
Rule 2, patients with diagnosis code of §250 (§250.0–§250.9) under the International Classification of Diseases, 9th Revision, Clinical Modification (ICD9CM), coded as either the primary or secondary diagnosis;
-
(iii)
Rule 3, patients on anti-diabetes medication; and
-
(iv)
Rule 4, patients with 2-hour blood sugar level of ≥11.1 mmol/L on oral glucose tolerance test (OGTT), or a random blood sugar level of ≥11.1 mmol/L on 2 occasions within 2 years, or fasting plasma glucose ≥7.0 on 2 occasions within 2 years, or random blood sugar level of ≥11.1 mmol/L and fasting plasma glucose ≥7.0 within 2 years [11].
Variables extracted from the CKD Registry for the study included demographic data (age, gender and ethnicity), diabetes onset age, duration of diabetes, comorbidities (hypertension, dyslipidaemia, ischaemic heart disease, cerebrovascular disease, retinopathy, peripheral vascular disease and neuropathy), use of medications [angiotensin converting enzyme inhibitor (ACEi) and/or angiotensin receptor blocker (ARB), statins, oral hypoglycaemic agents and/or insulin and antiplatelet agents] and laboratory results (glycated haemoglobulin [HBA1c], serum creatinine, eGFR and albuminuria). Serum creatinine was measured using an Isotope Dilution Mass Spectrometry (IDMS) traceable standard and eGFR was estimated using the abbreviated Modification of Diet in Renal Disease (MDRD) equation. Microalbuminuria was defined as urine ACR 2.5–30 mg/mmol for males, urine ACR 3.5–30 mg/mmol for females, or urine PCR 20–50 mg/mmol or total urinary protein 0.2–0.5 g/day. Macroalbuminuria was defined as urine ACR >30 mg/mmol or urine PCR >50 mg/mmol or total urinary protein >0.5 g/day.
Mortality data were obtained from the relevant local death registry. Patients were followed up until 30 November 2013 where the outcomes of interest were deaths from all causes. In Singapore, the law requires all deaths occurring in Singapore to be registered within 24 hours of occurrence and a certificate of cause of death issued by doctors or authorized medical practitioners is required. Deaths were considered to be from cardiovascular causes if they were due to ischaemic heart diseases (ICD10: I20–I25), cerebrovascular diseases (ICD 10:I60–69), hypertensive diseases (ICD10: I10–I15) and other heart diseases (ICD10:I00–I09, I26–I51).
Statistical analysis
Characteristics of the study population are described for categorical variables by n (%) and for continuous variable as the mean ± SD. The unadjusted overall time to death was described using the Kaplan–Meier survival curve. Five year survival estimates (by CKD stage) were obtained via life tables. Univariate Cox's proportional hazards regression was used to assess associations, measured as hazard ratios (HR), between variables and all-cause deaths, followed by multivariate Cox's proportional hazards regression. The level of significance was set at p ≤ 0.20 for consideration to be used in multivariate regression using backward elimination of non-significant variables with p = 0.05 for the final model. All analyses were conducted using STATA (StataCorp, College Station, TX, USA) statistical software, version 12.0. The study was approved by the NHG's Domain-specific Ethics Review Board which is an independent committee constituting of medical, scientific and non-scientific members.
Results
Description
There were a total of 3008 Type 2 diabetes patients in 2007 who met the study criteria, i.e. stages 3A and above, and were followed up until 30 November 2013 (Table 1). A total of 19 patients were excluded from the survival analysis as they died at the start of the study. The mean age of the study cohort was 70.0 (standard deviation: 10.4) years. Majority (72.5%) of patients belonged to CKD stages 3A and 3B and had hypertension (95.8%) and dyslipidaemia (97.6%). At least 84.6% of patients were on ACEi, ARB or both.
Table 1.
Baseline characteristics of the study population
| n = 3008 | |
|---|---|
| Age in years, mean (SD) | 70.0(10.4) |
| Diabetes onset age in years, mean(SD) | 63.3(12.4) |
| Gender | |
| Male, no.(%) | 1533(50.3) |
| Ethnic group | |
| Chinese, no.(%) | 2115(70.3) |
| Malay, no.(%) | 556(18.5) |
| Indian, no.(%) | 222(7.4) |
| Others and unknown, no.(%) | 115(3.8) |
| Duration of diabetes in years, mean(SD) | 6.7(6.7) |
| HBA1c in %, mean(SD) | 7.7(1.6) |
| Creatinine, mean(SD) | 170.2(119.5) |
| Glomerular filtration rate in mL/min per 1.73 m2, mean(SD) | 38.2(14.0) |
| CKD stage | |
| 3A, no.(%) | 1091(36.3) |
| 3B, no.(%) | 1088(36.2) |
| 4, no.(%) | 603(20.1) |
| 5, no.(%) | 226(7.5) |
| Albuminuria | |
| None, no.(%) | 375(12.5) |
| Microalbuminuria, no.(%) | 1094(36.4) |
| Macroalbuminuria, no.(%) | 1025(34.1) |
| Unknown, no.(%) | 514(17.1) |
| Comorbid conditions | |
| Dyslipidaemia, no.(%) | 2935(97.6) |
| Hypertension, no.(%) | 2883(95.8) |
| Ischaemic heart disease, no.(%) | 684(22.7) |
| Cerebrovascular disease, no.(%) | 352(11.7) |
| Retinopathy, no.(%) | 350(11.6) |
| Peripheral vascular disease, no.(%) | 180(6.0) |
| Neuropathy, no.(%) | 127(4.2) |
| Medications | |
| ACEi/ARB | |
| None, no.(%) | 463(15.4) |
| ACEi, no.(%) | 1558(51.8) |
| ARB, no.(%) | 632(21.0) |
| Both, no.(%) | 355(11.8) |
| Diabetes treatment | |
| Diet, no.(%) | 468(15.6) |
| Oral hypoglycaemic agents only, no.(%) | 1762(58.6) |
| Insulin only, no.(%) | 219(7.3) |
| Both oral hypoglycaemic agents and insulin, no.(%) | 559(18.6) |
| Statin, no.(%) | 2429(80.8) |
| Antiplatelet agent, no.(%) | 1235(41.1) |
| Follow up period in years | |
| Mean(SD) | 5.0(1.8) |
| Median(Range) | 6.0(0–6.9) |
Mortality rate
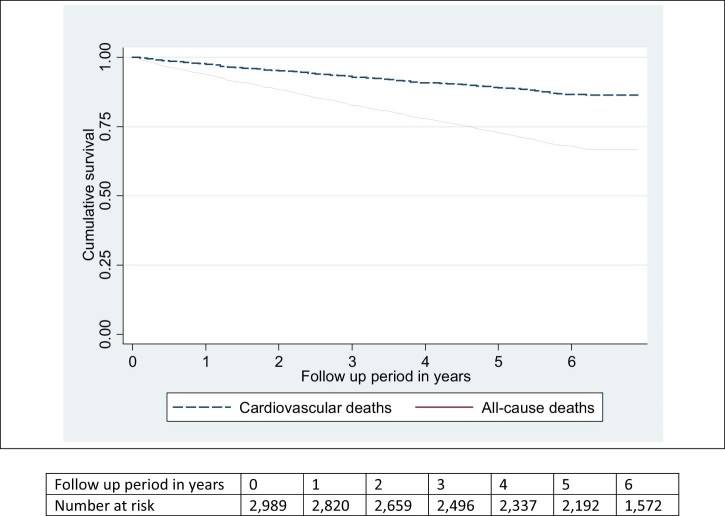
The median follow-up period was 6.0 years (range 0– 6.9 years). During the study period, 985 (32.8%) participants died, of whom majority (363, 36.9%) died from cardiovascular causes (Table 2). The annual all-cause mortality rate over the study period was 64.1 per 1000 individuals (95% CI 60.2–68.3) per year and that of cardiovascular mortality was 23.8 per 1000 individuals (95% CI 21.5–26.4) per year (Fig. 1). There was a progressive increase in annual mortality rate with advancing CKD stages from 37.0 per 1000 individuals (95% CI 32.5–42.3) among patients with stage 3A to 57.5 per 1000 individuals (95% CI 51.6–64.2) among those with stage 3B; 98.3 per 1000 individuals (95%CI 87.3–110.6) among those with stage 4; and 198.5 per 1000 individuals (95% CI 169.2–233.0) among those with stage 5. There was also a progressive increase in annual mortality rate with increasing amount of albuminuria at baseline from 31.3 per 1000 individuals (95% CI 24.5–39.9) among patients with no albuminuria to 45.7 per 1000 individuals (95% CI 40.5–51.6) among those with microalbuminuria and 80.8 per 1000 individuals (95%CI 73.3–89.1) among those with macroalbuminuria.
Table 2.
Causes of death
| Causes of death | n = 985 (%) |
|---|---|
| Cardiovascular | 363(36.9) |
| Ischaemic heart diseases | 250 |
| Cerebrovascular diseases | 69 |
| Hypertensive heart diseases | 15 |
| Other heart diseases | 29 |
| Respiratory | 189(19.2) |
| Influenza and pneumonia | 147 |
| Malignant neoplasm of trachea, bronchus and lung | 33 |
| Other respiratory diseases | 9 |
| Renal | 120(12.2) |
| Nephritis, nephrotic syndrome and nephrosis | 107 |
| Unspecified disorder of kidney and ureter | 8 |
| Malignant neoplasm of kidney and renal pelvis | 5 |
| Gastrointestinal | 82(8.3) |
| Malignant neoplasm of liver and intraheptic ducts | 28 |
| Malignant neoplasm of colon, rectum and anus | 14 |
| Malignant neoplasm of pancreas | 8 |
| Malignant neoplasm of stomach | 8 |
| Chronic liver disease and cirrhosis | 6 |
| Peptic ulcer | 4 |
| Others | 14 |
| Endocrine | 88(8.9) |
| Diabetes mellitus | 88 |
| Urological | 66(6.7) |
| Other disorders of urethra and urinary tract | 42 |
| Urinary tract infection, site not specified | 13 |
| Malignant neoplasm of bladder | 6 |
| Malignant neoplasm of prostate | 5 |
| Others and unknown | 77(7.8) |
Figure 1.
Kaplan–Meier survival estimate.
The overall median time to death was 2.9 years (range 0–6.2 years). Those with CKD stage 3A had a median 3.3 years to death, longer compared with those in stage 3B (3 years), stage 4 (2.9 years) and stage 5 (1.8 years). Those without albuminuria had a median 3.5 years to death, followed by those with microalbuminuria (2.9 years) and macroalbuminuria (3.2 years).
Overall survival
The five year overall survival estimate for all patients was 72.9% (95% CI 71.3%–74.4%) There was a progressive decrease in five year overall survival with advancing CKD stages from 83.9% (95% CI 81.6%–85.9%) among patients with stage 3A to 75.1% (95% CI 72.4%–77.6%) among those with stage 3B; 62.2% (95% CI 58.2%–65.9%) among those with stage 4; and 37.6% (95% CI 31.3%–43.9%) among those with stage 5. There was also a progressive decrease in five year overall survival with increasing amount of albuminuria at baseline from 86.4% (95% CI 82.5%–89.5%) among patients with no albuminuria to 80.1% (95% CI 77.6%–82.3%) among those with microalbuminuria and 66.7% (95%CI 63.8%–69.5%) among those with macroalbuminuria.
Predictors of mortality
Univariate Cox's proportional hazards regression analysis for the study population showed significantly higher hazard ratios with increasing age and diabetes onset age, increasing creatinine level, albuminuria severity, advancing CKD stages, comorbid conditions, male gender and the use of drugs such as insulin and antiplatelet agents. There was an inverse association of mortality (significantly lower hazard ratios) with eGFR and the use of drugs such as ACEi and/or ARB, oral hypoglycaemic agents and statins (Table 3). Multivariate Cox's proportional hazards regression showed significant independent associations between death with increasing age, male gender, advancing CKD stage, albuminuria severity, comorbid conditions such as peripheral vascular disease, neuropathy and retinopathy and the use of antiplatelet agent (Table 4).
Table 3.
Univariate hazard ratios and 95% confidence intervals for mortality
| Covariates | Hazard ratio (95% confidence interval) | P value |
|---|---|---|
| Age | 1.06 (1.05–1.06) | <0.001 |
| Diabetes onset age | 1.04 (1.03–1.04) | <0.001 |
| Gender | ||
| Female | Reference | |
| Male | 1.15 (1.01–1.30) | 0.030 |
| Race | ||
| Chinese | Reference | |
| Malay | 0.80 (0.67–0.96) | 0.013 |
| Indian | 1.07 (0.84–1.36) | 0.582 |
| Others | 1.00 (0.72–1.38) | 0.987 |
| Duration of diabetes | 0.998 (0.989–1.01) | 0.681 |
| HBA1c | 0.978 (0.937–1.02) | 0.316 |
| Creatinine | 1.003 (1.002–1.003) | <0.001 |
| Glomerular filtration rate | 0.96 (0.96–0.97) | <0.001 |
| CKD stage | ||
| 3A | Reference | |
| 3B | 1.56 (1.31–1.85) | <0.001 |
| 4 | 2.68 (2.24–3.20) | <0.001 |
| 5 | 5.48 (4.45–6.75) | <0.001 |
| Albuminuria | ||
| None | Reference | |
| Microalbuminuria | 1.47 (1.12–1.92) | 0.006 |
| Macroalbuminuria | 2.60 (2.00–3.38) | <0.001 |
| Unknown | 3.39 (2.58–4.47) | <0.001 |
| Comorbid conditions | ||
| Hypertension | 1.05 (0.76–1.46) | 0.758 |
| Dyslipidaemia | 0.96 (0.64–1.44) | 0.846 |
| Ischaemic heart disease | 1.56 (1.36–1.79) | <0.001 |
| Cerebrovascular disease | 1.52 (1.28–1.81) | <0.001 |
| Retinopathy | 1.37 (1.15–1.64) | 0.001 |
| Peripheral vascular disease | 2.04 (1.65–2.53) | <0.001 |
| Neuropathy | 1.94 (1.51–2.50) | <0.001 |
| ACEi/ARB | ||
| None | Reference | |
| ACEi | 0.59 (0.50–0.69) | <0.001 |
| ARB | 0.60 (0.49–0.73) | <0.001 |
| Both | 0.68 (0.54–0.85) | 0.001 |
| Diabetes treatment | ||
| Diet | Reference | |
| Oral hypoglycaemic agents only | 0.82 (0.69–0.98) | 0.031 |
| Insulin only | 1.51 (1.18–1.93) | 0.001 |
| Both oral hypoglycaemic agents and insulin | 0.84 (0.68–1.05) | 0.120 |
| Statins | 0.84 (0.72–0.98) | 0.027 |
| Antiplatelet agent | 1.69 (1.49–1.92) | <0.001 |
Table 4.
Multivariate hazard ratios and 95% confidence intervals for mortality
| Covariates | Hazard ratio (95% confidence interval) | P value |
|---|---|---|
| Age | 1.06 (1.05–1.06) | <0.001 |
| Gender | ||
| Female | Reference | |
| Male | 1.38 (1.21–1.57) | <0.001 |
| CKD stage | ||
| 3A | Reference | |
| 3B | 1.37 (1.15–1.62) | <0.001 |
| 4 | 2.02 (1.69–2.43) | <0.001 |
| 5 | 4.24 (3.41–5.29) | <0.001 |
| Albuminuria | ||
| None | Reference | |
| Microalbuminuria | 1.45 (1.10–1.90) | 0.008 |
| Macroalbuminuria | 2.17 (1.66–2.84) | <0.001 |
| Unknown | 2.35 (1.77–3.11) | <0.001 |
| Comorbid conditions | ||
| Peripheral vascular disease | 2.04 (1.65–2.53) | 0.021 |
| Neuropathy | 1.49 (1.14–1.94) | 0.004 |
| Retinopathy | 1.32 (1.09–1.60) | 0.004 |
| Antiplatelet agent | 1.36 (1.19–1.55) | <0.001 |
Discussion
This study estimated the annual all-cause mortality rate of patients with diabetic kidney disease to be 64.1 per 1000 individuals and this rate can be used for future healthcare service provision planning. We have also provided the annual all-cause mortality rate by stages of CKD and albuminuria level and this would also help doctors in communicating the prognosis to their patients, and emphasize to them the importance of preventing progression to later stages of CKD.
Among those who died, more than one third died of cardiovascular causes and this is consistent with other studies that had confirmed chronic kidney disease as being an independent risk factor for the development of cardiovascular disease (CVD) and subsequent deaths from cardiac causes [5], [12], [13]. Thus, for these patients, it is even more important to optimize the control of their cardiovascular risk [14] and to regard them as the “highest risk” group for CVD, irrespective of the levels of traditional CVD risk factors [1].
The management of early stages of CKD is currently done in the primary care setting. In Singapore, primary healthcare is provided through a network of 18 outpatient polyclinics (public sector) and 1500 private medical practitioners clinics. Obstacles exist in the private sector such as the lack of patient trust, experience and communication with the specialist and the inability of the patient to afford a higher healthcare cost [15]. As for the public sector, it accounts for 45% of the chronic attendances while deploying only 14% of all resident general practitioners in Singapore [16] even though it was designed to meet 20% of the total demand for primary healthcare. This problem has been looked into by policymakers and changes have been made to lower the age cut-off for lower income patients to qualify for portable subsidies to be used in the private primary care sector [16].
Majority of patients in the study were already on ACEi and/or ARB but our study did not show the benefit of the use of ACEi and/or ARB in reducing all-cause mortality after adjustments. Our results differ from a recent large cohort study that showed that ACEi/ARB administration was associated with greater survival [17]. The study conducted by Molnar et al. reported that 30% of the original cohort (which included non-diabetes patients) had not been exposed to ACEi/ARBs [17] but our study comprised of only 15.4% that were naïve to ACEi/ARBs. This may explain why the association of ACEi and/or ARBs in our study became non-significant after adjustment.
We have also found that the mortality rate is higher with increasing albuminuria level and increasing CKD stages which are consistent with the new classification of CKD which classify CKD by cause, GFR category and albuminuria category [14]. These findings are similar to others studies in other populations [6], [18]. However, the use of this new classification has yet to be incorporated into the guidelines in primary care in Singapore and it may be timely to update it.
The use of antiplatelet agent was found to be associated with higher mortality but the use of aspirin is recommended for secondary prevention of cardiovascular events [14]. It is interesting to note that low dose aspirin therapy did not reduce atherosclerotic events of fatal and nonfatal ischaemic heart disease, stroke, and peripheral arterial disease in patients with eGFR <90 ml/min/1.73 m2 in a subanalysis from the JPAD trial [19]. Further research is needed to evaluate the safety of antiplatelet agent in patients with diabetic kidney disease.
Our study had the following strengths:
Firstly, we looked at a relatively large population of patients with diabetic nephropathy in a multi-ethnic Asian population with a follow up period of up to 6 years.
Secondly, the death data were obtained from the Death Registry where it is required by law for all deaths occurring in Singapore to be registered within 24 hours of occurrence.
Our study has its limitations. Firstly, there is now a new classification for CKD proposed by Kidney Disease: Improving Global Outcomes (DDGIO) [14]. However, this study was unable to use the latest classification as the cause of CKD was not captured and 17.1% of the patients did not do urine test for albumin at baseline. Secondly, we have only looked at baseline use of ACEi and/or ARBs and patients may have switched or stopped drugs over the years. A similar study showed that discontinuation rates of ACEI/ARB were high: only 66% of treated patients received renewed prescriptions on >50% of their follow-up visits, and only <10% of patients remained on ACEI/ARB therapy throughout all follow-up visits.9 This may affect the effect of these drugs on the mortality of CKD, therefore causing the effect to be non-significant after adjustments. Thirdly, the retrospective cohort study design would not allow us to explore variables that were not collected at the start of the study. Being an observational study, we could only assess association but not causation. Furthermore, renal biopsy results were not available and as such patients may have other coexisting renal diseases.
Lastly. we have used backwards elimination of non-significant variables which is prone to overfitting the data. However, the aim of the study was not to derive a predictive model for predicting mortality and thus we did not test the variables on a validation cohort.
Conclusion
We have estimated the annual mortality rate for patients with diabetic kidney disease by CKD stages and identified predictors of mortality. This study has affirmed the poor prognosis of these patients and an urgency to act to prevent the progression to later stages of CKD. We can also leverage on the use of information technology by incorporating the regression equation to determine the mortality rate for individual patient depending on his/her risk factors and look at how we can improve the modifiable ones.
References
- 1.National Kidney Foundation K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am J Kidney Dis. 2002;39(2 Suppl. 1):S1–266. 11904577 Epub 2002/03/21. [PubMed] [Google Scholar]
- 2.Zhang Q.L., Rothenbacher D. Prevalence of chronic kidney disease in population-based studies: systematic review. BMC Public Health. 2008;8:117. doi: 10.1186/1471-2458-8-117. 18405348 Epub 2008/04/15. Pubmed Central PMCID: PMC2377260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lozano R., Naghavi M., Foreman K., Lim S., Shibuya K., Aboyans V. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380(9859):2095–2128. doi: 10.1016/S0140-6736(12)61728-0. 23245604 Epub 2012/12/19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Perazella M.A., Khan S. Increased mortality in chronic kidney disease: a call to action. Am J Med Sci. 2006;331(3):150–153. doi: 10.1097/00000441-200603000-00007. 16538076 Epub 2006/03/16. [DOI] [PubMed] [Google Scholar]
- 5.Lim C.C., Teo B.W., Ong P.G., Cheung C.Y., Lim S.C., Chow K.Y. Chronic kidney disease, cardiovascular disease and mortality: a prospective cohort study in a multi-ethnic Asian population. Eur J Prev Cardiol. 2014;22(8):1018–1026. doi: 10.1177/2047487314536873. 24857889 Epub 2014/05/27. [DOI] [PubMed] [Google Scholar]
- 6.Matsushita K., van der Velde M., Astor B.C., Woodward M., Levey A.S., de Jong P.E. Association of estimated glomerular filtration rate and albuminuria with all-cause and cardiovascular mortality in general population cohorts: a collaborative meta-analysis. Lancet. 2010;375(9731):2073–2081. doi: 10.1016/S0140-6736(10)60674-5. 20483451 Epub 2010/05/21. Pubmed Central PMCID: PMC3993088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tonelli M., Wiebe N., Culleton B., House A., Rabbat C., Fok M. Chronic kidney disease and mortality risk: a systematic review. J Am Soc Nephrol. 2006;17(7):2034–2047. doi: 10.1681/ASN.2005101085. 16738019 Epub 2006/06/02. [DOI] [PubMed] [Google Scholar]
- 8.Woo K.T., Choong H.L., Wong K.S., Tan H.B., Chan C.M. The contribution of chronic kidney disease to the global burden of major noncommunicable diseases. Kidney Int. 2012;81(10):1044–1045. doi: 10.1038/ki.2012.39. 22543907 Epub 2012/05/01. [DOI] [PubMed] [Google Scholar]
- 9.Loh P.T., Toh M.P., Molina J.A., Vathsala A. Ethnic disparity in prevalence of diabetic kidney disease in an Asian primary healthcare cluster. Nephrology (Carlton) 2015;20(3):216–223. doi: 10.1111/nep.12379. 25495003 Epub 2014/12/17. [DOI] [PubMed] [Google Scholar]
- 10.NHG Corporate Yearbook 2014/2015. Singapore: National Healthcare Group; Group Corporate Communications, National Healthcare Group; 88 p.
- 11.Heng B.H., Sun Y., Cheah J.T., Jong M. The Singapore National Healthcare Group Diabetes Registry – descriptive epidemiology of type 2 diabetes mellitus. Ann Acad Med Singapore. 2010;39(5):348–352. 20535422 Epub 2010/06/11. [PubMed] [Google Scholar]
- 12.Sarnak M.J., Levey A.S., Schoolwerth A.C., Coresh J., Culleton B., Hamm L.L. Kidney disease as a risk factor for development of cardiovascular disease: a statement from the American Heart Association Councils on Kidney in Cardiovascular Disease, High Blood Pressure Research, Clinical Cardiology, and Epidemiology and Prevention. Circulation. 2003;108(17):2154–2169. doi: 10.1161/01.CIR.0000095676.90936.80. 14581387 Epub 2003/10/29. [DOI] [PubMed] [Google Scholar]
- 13.Go A.S., Chertow G.M., Fan D., McCulloch C.E., Hsu C. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. NEJM. 2004;351(13):1296–1305. doi: 10.1056/NEJMoa041031. 15385656 [DOI] [PubMed] [Google Scholar]
- 14.Group KDIGOKBPW KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int. 2013:1–163. doi: 10.1038/ki.2013.243. [DOI] [PubMed] [Google Scholar]
- 15.George P.P., Oh C.M., Loh P.T., Heng B.H., Lim F.S. Right-siting chronic kidney disease care-a survey of general practitioners in Singapore. Ann Acad Med Singapore. 2013;42(12):646–656. 24463826 Epub 2014/01/28. [PubMed] [Google Scholar]
- 16.Sng Q.S. Ministry of Health, Singapore; Singapore: 2011. Primary Care Survey 2010 Profile of Primary Care Patients. [Google Scholar]
- 17.Molnar M.Z., Kalantar-Zadeh K., Lott E.H., Lu J.L., Malakauskas S.M., Ma J.Z. Angiotensin-converting enzyme inhibitor, angiotensin receptor blocker use, and mortality in patients with chronic kidney disease. J Am Coll Cardiol. 2014;63(7):650–658. doi: 10.1016/j.jacc.2013.10.050. 24269363 Epub 2013/11/26. Pubmed Central PMCID: PMC3944089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.van der Velde M., Matsushita K., Coresh J., Astor B.C., Woodward M., Levey A. Lower estimated glomerular filtration rate and higher albuminuria are associated with all-cause and cardiovascular mortality. A collaborative meta-analysis of high-risk population cohorts. Kidney Int. 2011;79(12):1341–1352. doi: 10.1038/ki.2010.536. 21307840 Epub 2011/02/11. [DOI] [PubMed] [Google Scholar]
- 19.Saito Y., Morimoto T., Ogawa H., Nakayama M., Uemura S., Doi N. Low-dose aspirin therapy in patients with type 2 diabetes and reduced glomerular filtration rate: subanalysis from the JPAD trial. Diabetes Care. 2011;34(2):280–285. doi: 10.2337/dc10-1615. 21270185 Epub 2011/01/29. Pubmed Central PMCID: PMC3024334. [DOI] [PMC free article] [PubMed] [Google Scholar]