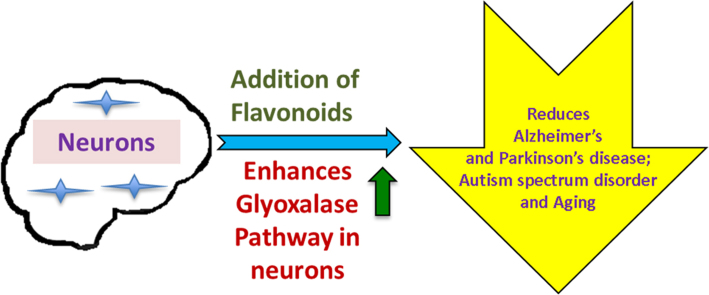
Abstract
The glyoxalase pathway functions to detoxify reactive dicarbonyl compounds, most importantly methylglyoxal. The glyoxalase pathway is an antioxidant defense mechanism that is essential for neuroprotection. Excessive concentrations of methylglyoxal have deleterious effects on cells, leading to increased levels of inflammation and oxidative stress. Neurodegenerative diseases – including Alzheimer's, Parkinson's, Aging and Autism Spectrum Disorder – are often induced or exacerbated by accumulation of methylglyoxal. Antioxidant compounds possess several distinct mechanisms that enhance the glyoxalase pathway and function as neuroprotectants. Flavonoids are well-researched secondary plant metabolites that appear to be effective in reducing levels of oxidative stress and inflammation in neural cells. Novel flavonoids could be designed, synthesized and tested to protect against neurodegenerative diseases through regulating the glyoxalase pathway.
Abbreviations: OS, oxidative stress; ROS, reactive oxygen species; MG, methylglyoxal; glo 1, glyoxalase-1; glo 2, glyoxalase-2; AGEs, advanced glycation end products; ASD, autism spectrum disorder; GSH, reduced glutathione; cas-3, caspase-3; NeuN, neuronal specific nuclear protein; NF-κB, nuclear factor kappa-light-chain-enhancer of activated B cells; AD, Alzheimer's disease; Aβ, amyloid beta; PD, Parkinson's disease; HO-1, Heme oxygenase-1; Nrf2, nuclear factor erythroid 2-related factor 2; ARE, antioxidant response element; GCS, gamma-glutamyl-cysteine synthetase; PNO2, Paraoxygenase-2; ETC, Electron transport chain; MPTP, 1-methyl-4-phenyl 1,2,3,6 tetrahydropyridine; 6-OHDA, 6-Hydroxydopamine; MPP+, 1-methyl-4-phenylpyridinium; NFT, neurofibrillary tangles
Keywords: Glyoxalase pathway, Neuron, Flavonoid, Antioxidant, Neurodegenerative disease, Neuroprotection, Detoxification, Neurons viable
Graphical abstract
Highlights
-
•
Methylglyoxal can have deleterious effects on cells and tissue, leading to high levels of oxidative stress and disease.
-
•
Neurodegenerative disease has many hallmarks, including a disruption of endogenous antioxidant systems.
-
•
The body uses the glyoxalase pathway as an antioxidant defense system to detoxify reactive species and free radicals.
-
•
This Review shows the enhancement of this pathway through flavonoid treatment to prevent neurodegenerative disease.
1. Introduction
The gyloxalase pathway is a well-conserved antioxidant defense system found in all cells of the body [1], [2], [3], [4]. The glyoxalase pathway facilitates the neutralization of highly reactive and oxidizing dicarbonyl molecules, with methylglyoxal (MG) being the most critical target [5]. Carbonyl molecules target the brain due to its high rate of metabolism and low antioxidant defense capacity [6]. The brain also contains high concentrations of oxidizable substrates including polyunsaturated fats and metal ions [7]. Accumulation of MG damages and influences the cellular environment, leading to a state of chronic inflammation and oxidative stress [4], [5], [8], [9], [10], [11], [12]. The glyoxalase system is present in all cells in the body, but has especially important functions in the brain [3], [13]
Oxidative stress (OS) results from an imbalance between electrophilic prooxidative molecules and the capacity of antioxidants to reduce and neutralize the free radicals [14]. It is a chronic state of inflammation that prevents the proper function of integral cellular processes, causing irreversible damage to cells [10], [14], [15]. Excessive oxidative stress in the brain leads to accelerated aging, and impacts the severity of neurodegenerative diseases [16], [17]. High levels of oxidative stress influence the onset and progression of aging, Alzheimer's disease (AD), Parkinson's disease (PD), and autism spectrum disorder (ASD) [18], [19]. Efficient and active function of the glyoxalase pathway is critical to reducing oxidative stress mediated damage to brain cells [20], [21], [22], [23], [24]. Flavonoid antioxidant compounds possess the capacity to enhance the glyoxalase pathway through several distinct mechanisms, including modulation of critical signaling pathways involved in cell proliferation [25], [26], [27]
OS and inflammation gradually increase during aging [28], [29]. However, high levels of OS and inflammation can accrue over time and are contributing factors to aging and neurodegenerative diseases including AD, PD, ASD, dementia, and psychiatric disorders [1], [30]. Hallmarks of these diseases are also shared in glyoxalase pathway dysfunction: increased reactive oxygen species (ROS) production, apoptosis, and oxidation of molecules [2], [9], [31], [32]. The onset and progression of neurodegenerative disease can be influenced by substituents of the glyoxalase pathway. For example, inhibition of glo 1 reduces neuronal viability and increases accumulation of advanced glycation endproducts (AGEs), while overexpression of glo 1 reduces formation of ROS [4], [21], [33], [34]. Aging is correlated with a decrease in glo 1 concentration and activity [16], [35]. Depletion of GSH by MG can prevent astrocytic detoxification of reactive molecules and lead to an increase in oxidized proteins, lipids, and amino acids [21], [36]. Increasing the capacity and efficiency of the glyoxalase pathway appears to be an effective means of reducing the onset and severity of aging and neurodegenerative disease [37].
1.1. Glyoxalase pathway
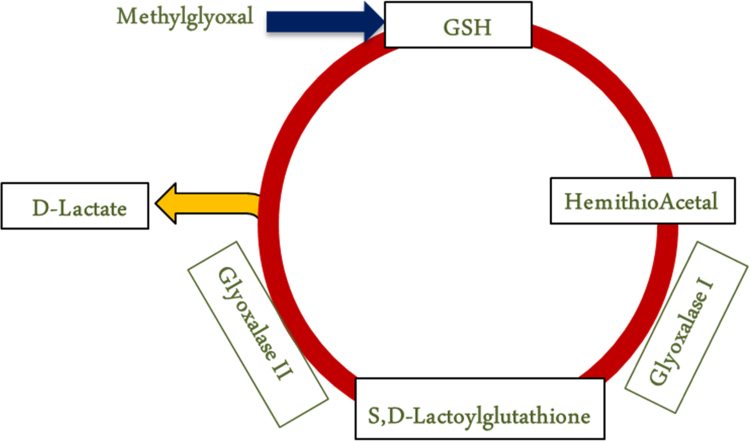
The glyoxalase pathway consists of proteins glyoxalase 1 (glo 1) and glyoxalase 2 (glo 2), reduced glutathione (GSH), and a dicarbonyl substrate (Fig. 1) [4], [16], [21]. The pathway is a series of two reactions that neutralize MG to produce D-lactate. MG spontaneously reacts with GSH to form a hemithioacetal. It is catalyzed by glo 1 into an intermediate compound S,D-lactylglutathione. Glo 2 catalyzes the final reaction producing D-lactate and recycling GSH into the pathway [21]. The rate of glyoxalase activity varies based on the type, location, and environment of the cell [30], [38], [39]. The glyoxalase pathway is a dynamic system able to respond to the constantly changing cellular environment. The glyoxalase pathway is in every cell, and is implicated in cancer cell proliferation, maintenance of blood glucose, liver enzymes, and cardiovascular and renal function [40].
Fig. 1.
The glyoxalase pathway functions to detoxify methylglyoxal. Methylglyoxal reacts with reduced glutathione (GSH) to form a hemithioacetal (HTA). Glo 1 uses the hemithioacetal as a substrate to form S,D-lactylglutathione. Glo-2 reacts with the resulting intermediate compound, and the pathway produces D-lactate. This step also reduces glutathione and recycles it into the pathway.
MG is a byproduct of glycolysis and the metabolism of proteins and lipids [5]. MG is an electrophile and highly reactive glycating agent, able to irreversibly modify proteins, lipids, and nucleic acids, forming advanced glycation end products (AGEs) [5], [10]. Structural modifications to molecules can significantly reduce function, and may lead to degradation by immune cells [41]. MG is stable molecule, and membrane permeable in certain forms. Excess MG is able to leak into surrounding cells and tissues [42]. In a functioning glyoxalase system, MG is degraded and prevented from accumulating [21]. Under a state of dysfunction - caused by damage to pathway constituents, high levels of metabolism, or high levels of carbonyl precursors present - MG will be produced at a higher level and disrupt macromolecular functions [58]. Accumulation of MG has many deleterious effects that lead to a cellular environment with high levels of inflammation and oxidative stress (OS) [4].
MG is directly able to cause impairment to the glyoxalase system [5]. ROS can be directly produced during its formation and degradation [43], [44], [45]. MG can also deplete the concentration of antioxidant enzymes [46]. MG is also able to modulate important signaling pathways, resulting in excessive ROS, inflammation, apoptosis, and ultimately chronic oxidative stress, leading to death of brain cells, tissues, and disease [5].
MG can neutralize enzymes that are able to catalyze reactions and scavenge substrates, including GSH [1], [5], [39], [40], [42]. MG can also increase NADPH oxidase activity, preventing the reduction of GSH and it's recycling back into the glyoxalase pathway [1], [6], [39], [40], [42]. GSH protects against OS by directly scavenging ROS, and regulates redox signaling [6], [43] It also plays a vital role in DNA and protein repair and synthesis, metal ion metabolism, and cellular survival [36], [47]. It is one of the most important endogenous antioxidants, and proper levels are paramount to a cell's health and normal functioning.
2. Diseases of glyoxalase pathway
Impaired function of the glyoxalase pathway – especially in the brain - has very serious ramifications for cells and tissue. Aging, Alzheimer's, and Parkinson's, (Fig. 2) are neurodegenerative diseases that can be caused or influenced by elevated levels of oxidative stress [4], [48], [49]. These diseases are complex and multifactorial, and can be a cause and consequence of disrupted glyoxalase pathway function. Increased levels of oxidative stress can be a result of glyoxalase system impairment [19], [50], [51] Psychiatric disorders like ASD, schizophrenia, anxiety, bipolar disorder, and depression can also be a cause/effect of impaired glyoxalase function [52], [53], [54] The glyoxlase pathway exerts control over antioxidant defense mechanisms that are paramount for homeostasis of the redox environment of the cell [55], [56] Disrupted function of the glyoxalase pathway can lead to an inflammatory environment contributing to the pathogenesis of neurodegenerative disease [23], [40]. MG can cause mutations of DNA and nucleic acids, culminating in telomere shortening, loss of heterochromatin, altered gene expression patterns, and mitochondrial dysfunction [23], [57], [58]
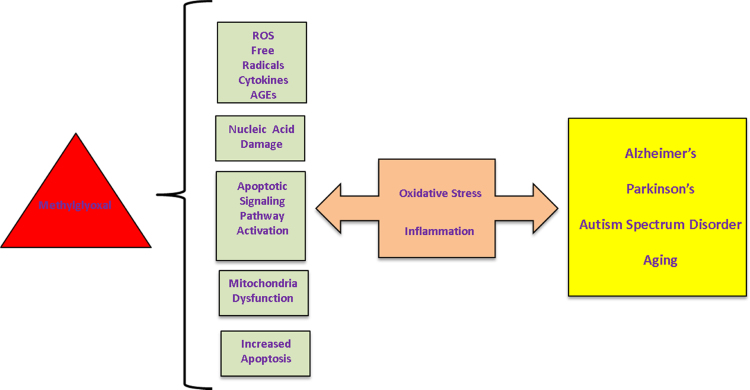
Fig. 2.
Methylglyoxal is a highly reactive compound with many deleterious effects on neurons. MG mediates the increased production of ROS, free radicals, pro-inflammatory cytokines, and advanced glycation end products, has damaging effects on mitochondria function and nucleic acids, and increases apoptosis through multiple means, including signaling pathway activation. The resulting increase in oxidative stress and inflammation plays a major role in aging and development of neurodegenerative disease including Alzheimer's, Parkinson's, Autism Spectrum Disorder, and Huntington's.
Oxidative stress results from the disequilibrium between levels of prooxidant molecules produced and the ability of a biological system to detoxify and neutralize the reactive molecules [14]. OS is a chronic, self-perpetuating state of damage to cells and increased inflammation resulting from the corresponding immune response [27], [43]. Glyoxalase pathway dysfunction can result in the accumulation of MG in cells [3]. The build up of MG has a direct link to the propagation of OS and inflammation, and is an influencing factor in neurodegenerative disease [8], [23], [48]
The main source of damage from OS is ROS mediated inflammation [43]. ROS and free radicals have unpaired electrons, and act as nucleophiles to attack macromolecules. The donor molecule gives its electrons to ROS [14]. The molecules attacked by ROS have structural modifications, which causes the molecules to have limited or no function [5]. Under normal conditions, ROS are used in cell signaling and defense against pathogens by macrophages; their damaging effects are caused when they have accumulated past a normal amount [10]. Glyoxalase cycle dysfunction is one perpetrator to blame for the increased ROS production.
AGEs can be formed from modified proteins, lipids, or nucleic acids [59]. Structural changes can reduce biological activity of macromolecules, and are often identified as misfolded or damaged and labeled for destruction [59], [60]. Nucleic acid (and mtDNA) can undergo strand breaks, impaired repair mechanisms, and permanent mutations [61]. Lipids are easily oxidizable, and membranes, being rich in lipids, are prone to these modifications [43].
Research has shown a link between MG accumulation, glyoxalase system activity, and disease [21]. Glyoxalase activity is differentially expressed based on cell type and state. Glyoxalase activity has found to increase with age, but subjects over 50 years old exhibit a decline in glyoxalase I [62]. The low glo 1 activity in aging leads to a higher level of dicarbonyl compounds, and it was also discovered that decreasing glo 1 levels correlated with an accumulation of AGEs [35]. Elevated levels of MG leads to inflammation, OS, apoptosis, and DNA damage [40], [63]
It has been shown the pathological hallmarks of AD and PD are colocalized to AGEs [41], [63], [64]. In a comparison of glyoxalase activity between healthy and AD brain tissue, the AD group had a significantly lowered glo 1 activity, at the mRNA and protein level [35].
MG is highly reactive and also cytotoxic. MG inhibits cell growth, and induces cell death at higher concentrations [65]. MG prevented proper hippocampal neurogenesis, adversely impacting neural differentiation, survival, and proliferation, it is believed due to reduced hippocampal BDNF levels [66]. MG concentration can act as a biomarker of severity of disease [23]. AD patients were found to have increased MG in CSF compared to healthy aged controls [64].
3. Aging
Aging is a progressive decline in physiological and metabolic functions of an organism [10]. The process is characterized by chronic, low level inflammation that progresses over time. The rate of aging can be accelerated and influenced by genetic and environmental factors. Age is also the biggest risk factor for Alzheimer's and Parkinson's [19]. Age is correlated with an increase in ROS formation, oxidized proteins and lipids, and apoptosis [14]. Also, during normal aging OS will increase while glutathione activity decreases. Decreasing glo 1 levels are strongly correlated with increasing levels of AGEs, and glo 1 levels drop in accordance to age [16].
The extent of damages can only be viewed posthumously, requiring the determination of disease progression through biomarkers of oxidative stress and disease. Markers denoting oxidized substrates, nuclear abnormalities are directly related to the extent and progression of aging [15].
4. Alzheimer's disease
Alzheimer's disease is the most common neurodegenerative disorder, and the leading cause of dementia in the elderly [67]. It is a multifactorial disease characterized by progressive neural loss of the hippocampus and cortex, memory and learning impairment, and changes in behavior and personality [19], [68]. It has pathogenic hallmarks of beta amyloid (Aβ) plaques and neurofibrillary tangles (NFT) [69]. Cognitive impairment reflects synapse loss in dentate gyrus of hippocampus, and neuron loss in frontal and parietal lobe of cortex [70], [71], [72]
The amyloid precursor protein (APP) is a membrane protein thought to be involved in plasticity, synapse formation and repair, and export of metal ions [68]. The APP present in the brain can be cleaved by three different secretases [73], [74]. Cleavage of APP first by alpha secretase and then gamma-secretase results in a functional protein; while cleavage by beta-secretase results in Aβ. The product formed from this improper cleavage – beta amyloid - will aggregate into plaques, and disrupt function of normal cells [19], [74]. These Aβ plaques can be modified by MG and AGEs, forming crosslinks, affording them stability and defense against protease cleavage [75], [76]. Aβ plaques are very stable and have long lives - The progression of AD is preceded 15 years by appearance of Aβ deposits [23], [41] Aβ plaques will disrupt neuron function, and induce ROS production, ultimately leading to inflammation, damage to cell/tissue, and apoptosis [48], [69], [77], [78]
Aβ aggregates will clump around neurons and prevent their proper function [67]. The Aβ plaques also attract and activate microglia, causing them to cluster and localize around the plaques [21], [67]. These immune cells release signals inducing cellular toxicity [68]. The Aβ plaques cause additional production of AGEs, which will reduce the activity of enzymes and proteins (especially in mitochondria) [41]. These AGEs will lead to increased ROS production, which increases the production of APP [23], [68]. Aβ plaques can also disrupt redox signaling by interacting with metal ions in active sites of macromolecule, preventing proper function [48].
Impairment of the glyoxalase system can have a direct impact on the severity of AD [22], [73]. Studies have shown glyoxalase I levels decrease with increased aging and severity of AD [14]. The imbalance of antioxidant affects causes a shift to a state of OS. Accumulation of MG and AGEs in the Alzheimer's disease brain is very deleterious [75]. Tissue from AD brain shows high amounts of AGEs and oxidized lipids and proteins, which is a marker of inflammation [41]. There is a correlation with the amount of MG/AGEs localized to specific brain regions and severity of AD [75].
NFT are filaments of bundled proteins formed from a disruption in the microtubule network of cells [67], [74]. Microtubule associated protein tau is responsible for promoting and stabilizing microtubule formation in cells, however hyperphosphorylation of tau destabilizes and disrupts the proper assembly of microtubules [79], [80]. Proteins aggregate and oligomerize forming toxic NFT, causing the death of neurons in the area [81].
Increased MG levels, apoptosis, and OS contribute to production of Aβ and hyperphosphorylation of tau [5]. MG can also disrupt cell signaling pathways that control kinases and phosphatases used to regulate phosphorylation of tau [1], [59] Aβ has an influential and contributing role in the production of NFT, and they are both stable molecules prone to glycation [76]. MG derived AGEs aggregate in NFT and beta amyloid plaques [19]. AGE crosslinking in Aβ affords it insolubility and protease resistance [75], [76]. MG modified Aβ in plaques has a longer half-life, allowing it to accumulate more AGE modifications, which causes more Aβ to form [9], [67]
5. Parkinson's disease (PD)
Parkinson's is the second common neurodegenerative disease. It is characterized by degeneration of dopamine producing neurons in the substantia nigra [19]. This leads to a decrease of dopamine levels in areas of the brain associated with movement, caused by deregulation in ganglion cell circuits [81], [82], [83], [84], [85], [86], [87], [88]. The disease is characterized by motor deficiencies - including tremors, rigidity, and slowness of movement – and cognitive deficiencies [49]. Pathological markers of Parkinson's include the accumulation of alpha synuclein into Lewy bodies [57], [84]. The degeneration of dopaminergic neurons and oxidation of dopamine causes altered mitochondrial respiration, inducing a state of oxidative stress in neural tissue [49], [89]
Alpha synuclein (AS) is a protein located in presynaptic terminals of neurons that functions in recycling and storage of neurotransmitters [17]. Under conditions of inflammation and oxidative stress, AS proteins misfold and accumulate into aggregates [81], [90]. The aggregates of misfolded AS oligimorize into Lewy bodies. These aggregates are cytotoxic, disrupt connections between neurons, and deplete levels of neurotransmitters [81]. AS also reacts with dopamine quinones leading to accumulation of toxic fibrils in the dopaminergic neurons [80], [89], [90]. Accumulation of AS and Lewy bodies have a detrimental impact on mitochondria activity, causing an elevation of ROS production and deficit in metabolic activity [57].
Neural dopamine can become oxidized if there are high levels of MG derived AGEs [49]. These dopamine quinones have impaired activity, and contribute to the degeneration of neurons [18], [90]. MG accumulation can lead to production of ROS and depletion of NADPH, which is critical for reducing glutathione for use in the glyoxalase pathway [81], [91], [92]. The decline in synthesis of dopamine also causes disruption in vesicle transport, and makes the cell prone to damage and mtDNA mutations [84].
There is a correlation between progression of disease and biomarkers of oxidative stress [93], [94]. Post mortem studies of PD brains show high levels of oxidized substrates, and colocalization of AGEs to Lewy bodies [89]. AD and PD have different clinical pathologies but share similar causes and symptoms and Aβ plaques can be commonly found in PD brains [19]. Patients with PD have been found to have depleted levels of GSH, and disruption of GSH metabolism has been found to progress neurological disorders [36].
6. Autism spectrum disorder
Autism Spectrum Disorder is a multifactorial neurodevelopmental disorder categorized by impairment in communication, language, social behaviors and relationships [24]. The basis for ASD is still misunderstood, but there is evidence of cellular and metabolic dysfunction influenced by mitochondrial activity [95]. There are also physical abnormalities and alterations in ASD brains. Over 100 genes contribute to ASD, mutations in any of these can lead to ASD [20]. All genes participate in different brain functions controlled by the brain areas, which affect emotional formation, learning and memory, cognitive control, and social orientation [96].
Autistic brains also have a lowered level of viable GABA producing Purkinje neuron cells [97]. MG derived AGEs and ROS will modify the Purkinje neurons, leading to their ultimate death [98]. The OS exhibited in ASD can be a cause of loss of these integral neuron cells [53]. Combined with the high amounts of lipid peroxidation, the OS exhibited in ASD brains could be alleviated by glyoxalase dependent MG detoxification [99], [100]
DNA and mtDNA mutations and abnormalities are common in ASD [97], [101]. These can cause impaired electron transport chain (ETC) and mitochondria function (membrane potential/polarization, molecule transport, mito protein translocation, and apoptosis) [102], [103] ASD is also categorized by an abnormal immune response; this can have negative effects on brain growth factors, development, and neural transmitters [52], [104]. Patients with autism demonstrated activated micro/astro glia and increased levels of proapoptotic cytokines [52]. Patients of ASD had significantly lower ratios of mitochondria proteins bcl-2/bak, which is an indicator of increased cell death and decreased function [24].
Abnormal neural brain maturation found in ASD is influenced by mitochondria dysfunction and MG mediated cellular signaling [97], [101]. ASD can lead to chronic immune activation, causing OS in the ASD brain [101]. These can be caused by disequilibrium in MG and glyoxalase signaling [105]. Patients with autism have lower reduced glutathione levels, however it is not known if it is due to a deficit in synthesis or regeneration of glutathione [100].
7. Flavonoid function
Antioxidants are compounds - when present at a lower concentration compared oxidizable substrate - that delay or prevent oxidation of the substrate [14], [106]. Antioxidants act as nucleophiles to reduce an oxidative molecule to prevent its interaction with another molecule [14], [107]. Endogenous antioxidants produced by the body function in prevention and neutralization of ROS and free radicals, repair of damaged macromolecules, and redox signaling [14]. Exogenous antioxidants consumed through food and drink also play an important role in cellular defense and survival, and have shown to aid the body in combating oxidative stress and inflammation [108], [109]
Flavonoids are secondary plant metabolites commonly found in fruits and vegetables [25]. Flavonoids are a family of polyphenol antioxidants that are effective in combating high levels of OS [10], [14]. Flavonoids are distinguished by the presence of multiple phenol rings, C to C double bonds, and hydroxyl groups [26]. These structural characteristics confer the antioxidant function of flavonoids, and the number and location of hydroxyl groups influence the biological activity of the flavonoids [110], [106]. The lipophilicity is also influenced by structure, which allows some flavonoids to favorably pass through the blood brain barrier [111], [112], [113], [114] The hydroxyl groups are critical for antioxidant activity, and scavenge free radicals and ROS by donation of a proton [114].
This class of antioxidant molecules possesses several distinct mechanisms of protection from oxidative stress. Flavonoids can directly scavenge and neutralize ROS and free radicals, increase intracellular GSH, prevent glutamate mediated Ca+2 influx, and modulation of signaling pathways involved in cellular survival [4], [25], [26] Directly neutralizing ROS prevents the oxidation of proteins, amino acids, lipids, metal ions, and other macromolecules. Oxidative modification irreversibly changes structure and prevents normal function of macromolecules. The presences of flavonoids are able to protect these molecules from MG mediated modification into AGEs. Glutathione is a major constituent of the glyoxalase pathway, and one of the most important endogenous antioxidants for neutralization of dicarbonyl compounds and maintaining redox balance in cells [26]. Flavonoid treatment was found to increase GSH concentration, and increased mRNA transcript levels of both GSH constituent subunits [4], [26]. GSH is also an essential substrate of astrocytic detoxification in the brain [47]. GSH is also critical for prevention of glutamate mediated apoptosis [115]. Elevated levels of glutamate are cytotoxic, and lead to apoptosis via influx of Ca+2. Excess glutamate depletes GSH, leading to a decrease in activity of glo 1 [25]. Flavonoids have shown to reduce intracellular Ca+2 influx in the presence of toxic levels of glutamate [25].
Flavonoid molecules can regulate signaling pathways to modulate cellular, immune, and metabolic processes [4]. Flavonoids are able to modulate a variety of pathways including NF-κB, MAPK, ERK, and Nrf2 [116], [117] Flavonoid molecules can modulate and reduce expression of proapoptotic and proinflammatory products of genes [118], [119]. ROS are used as signaling molecules during immune responses, and the presence of antioxidants can prevent ROS mediated phosphorylation of molecules and pathway targets, preventing their activation and transcription [109]. Flavonoids can also inhibit activation of kinases and phosphatases that would contribute to apoptotic cell death [120], [121],4]. After a flavonoid is oxidized by a free radical, the resulting quinones are involved in signaling pathways involved in cellular antioxidant and repair activities (Fig. 3).
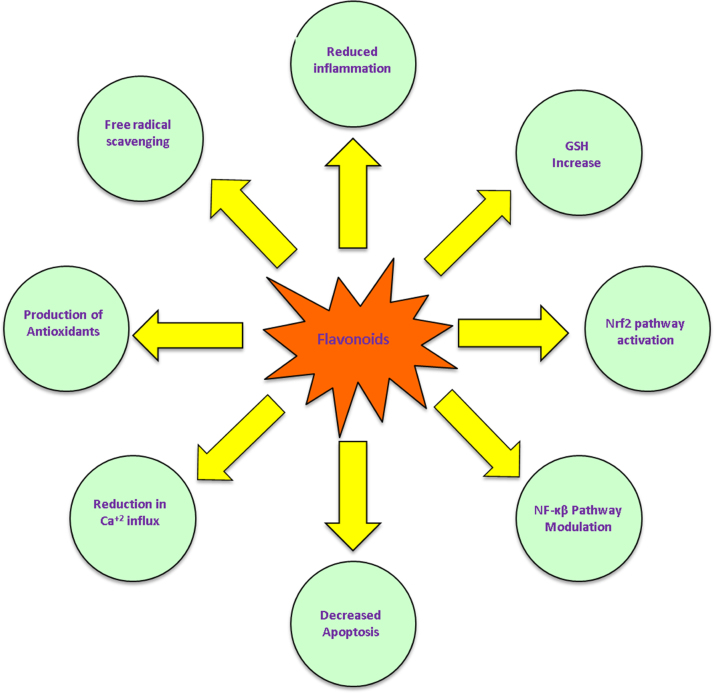
Fig. 3.
Flavonoid antioxidants possess several distinct of cellular neuroprotection. Flavonoids reduced the amount of inflammation and oxidative stress in the cellular environment through multiple means. Flavonoid compounds directly scavenge free radicals and ROS, increase intracellular concentration of GSH and antioxidant molecules, modulate NF-κB signaling pathways to reduce apoptosis, and reduce glutamate mediated Ca+2 intracellular influx. These actions of flavonoids directly enhance the glyoxalase pathway.
Flavonoids can have a direct impact [122] and influence on the function of the gyloxalase pathway. Flavonoids can bind and scavenge free radicals, and also increase the intracellular levels of GSH, while flavonoids are able to scavenge free radicals, in physiological concentrations they are not effectively able to scavenge free radicals [25]. The most effective form of neuroprotection by flavonoids is preventing formation of free radicals by modulation of cell signaling pathways [109], [123], [124]. Flavonoid antioxidant treatment can lower the intracellular levels of free radicals and ROS, and also enhance the performance of the glyoxalase system by modulating signaling pathways involved in cellular proliferation and survival, glutathione synthesis and expression of antioxidative proteins [4], [10], [14], [25], [121]
Morin is a flavonoid that has shown to have effective anti-inflammatory and anti-tumor function [118]. Morin was able to block the activation of NF-κB pathway by ROS and inflammatory cytokines, preventing a signaling cascade resulting in cell death [121]. Morin exerted its control over the signaling pathway by inhibiting TNF induced NF-κB activation by inhibiting degradation of IκBα, and morin was also able to inhibit TNF mediated p65 nuclear translocation [120], [125]. Morin inhibited phosphorylation of Akt in a breast cancer cell line, preventing metastases and tumor proliferation [116]. Morin was shown to decrease survival of cancer cells, while increasing viability of normal endothelial cells [116]. These compounds are involved in the pAkt - NF-κB signaling pathways in neuronal cells to enhance the glyoxalase expression [4].
Flavonoids have shown effectiveness in modulation of glyoxalase pathway and MG detoxification. Our previous research has shown treatment with catechin, morin, and quercetin was able to attenuate the effects of MG toxicity while retaining cellular function. The flavonoids increased glo 1 activity and GSH concentration, while reducing the concentration of MG [4], [126]
While a lack of flavonoids does not cause any disease, Exogenous antioxidants can influence cellular health and offer protection against inflammatory and degenerative diseases. A correlation exists between flavonoid consumption and low levels of dementia and neural pathology [127]. Intake of flavonoids can have a protective effect on neural cells in many diseased states [127], [128]. Silymarin and naringin are flavonoids that have shown efficacy in protection against excitotoxicity in dopaminergic neurons. Silymarin protected mice against 1-methyl-4-phenylpyridinium (MPP+) induced toxicity by attenuating production of inflammatory cytokines, and prevented mitochondrial dysfunction [127]. Naringin protected neural cells from toxicity mediated by 6-Hydroxydopamine (6-OHDA), mediated by an increase in Nrf2 activation [127]. Morin has also shown to mitigate the damage caused by ischemia and stroke by downregulating expression and release of proinflammatory cytokines [118]. A grape powder extract was shown to reduce anxiety-like behavior, depression, and memory impairments caused by elevated OS [129].
8. Mechanistic interference
When under states of stress and cytotoxicity, cells initiate antioxidant defense mechanisms. Nuclear factor erythroid 2-related factor 2 (Nrf2) activation leads to the transcription of proteins involved in counteracting oxidative damage [56]. The Nrf2 pathway is activated during elevated OS. Unstimulated Nrf2 is bound to Keap1 in the cytoplasm, and subsequently ubiquitinated and degraded [130]. Under states of OS, ROS disrupt the association between Keap1 and Nrf2. Nrf2 is released from the complex, and translocates to the nucleus to bind the antioxidant response element (ARE), leading to the production of antioxidant molecules [55]. (Fig. 4) Gene products function in an antioxidant, anti-inflammatory, and neuroprotective fashion [119]. Among the products are glo 1, heme oxygenase-1 (HO-1), gamma-glutamyl-cysteine synthetase (GCS), and glutathione-S-transferase.
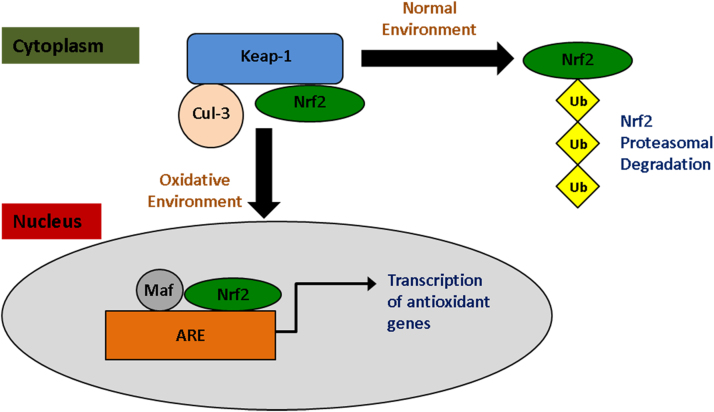
Fig. 4.
Inactive Nrf2 is bound to Keap1 and Cullin-3 in the cytoplasm. Cullin-3 ubiquitinates Nrf2 and is targeted for proteosomal degradation. Elevated levels of electrophiles and ROS disrupt the ability of Cullin-3 to degrade Nrf2. Nrf2 builds up in the cytoplasm, and translocates to the nucleus. It forms a complex with Maf and binds to ARE, inducing the transcription of antioxidant defense molecules to protect the cell from cytotoxicity.
Keap 1 is a negative regulator of Nrf2. Keap 1 and Cullin-3 contain Nrf2 in the cytoplasm of cells. Cullin-3 ubiquitinates Nrf2 and it is transported to proteasome for degradation. High levels of OS and free radicals phosphorylate targets on Keap 1 and Cullin-3 and disrupt the Keap1-Cullin-3 degradation system. Nrf2 is not degraded, and builds up in the cytoplasm until it is translocated to the nucleus and binds to ARE [131]. Nrf2 is critical for mediating expression of protective genes in response to MG induced OS and toxicity, and Nrf2 expression has been showed to suppress accumulation of AGEs [56]. Neurons treated with a Nrf2 activator were protected from MG mediated damage [132]. The Nrf2 signaling pathway increased intracellular GSH levels, and increased the glyoxalase pathway's detoxification of MG protecting cells from OS mediated damage [133]. Neuron cells treated with N-acetyl serotonin showed enhanced nuclear translocation of Nrf2 from the cytoplasm [134]. Nrf2 activators have shown benefits in PD animal models, Nrf2 expression prevented 1-methyl-4-phenyl 1,2,3,6 tetrahydropyridine (MPTP) induced toxicity in cells [135]. Flavonoids are able to indirectly induce Nrf2 expression by activation of kinase pathways resulting in phosphorylation of Nrf2 and induction of dependent genes [56], [136] Nrf2 also provided a stress-responsive defense against AGEs and lead to transcriptional control of glyoxalase [56] and a temporal dynamic reciprocal regulation of Nrf2 and glo 1 was observed during disease development [137]. Mangiferrin also upregulated glo 1 through activating Nrf2/ARE signaling pathway [138].
Flavonoids are able to modulate proinflammatory signaling pathways to prevent OS and apoptosis [139]. Paraoxygenase-2 (PNO2) is an enzyme involved in neuroprotection by preventing OS mediated damage in mitochondria. Flavonoids can modulate the JNK/AP-1 pathway to increase expression of PNO2 [111]. Flavonoids are able to inhibit expression of TNF-α by modulating NF-κB, inducing expression of antioxidant molecules like PNO2. PNO2 is primarily located in the mitochondria. PNO2 exerts its neuroprotection primarily through protecting against mitochondrial mediated oxidative stress [112].
Flavonoids can directly scavenge free radicals, but they have limited accessibility to the brain; antioxidant concentration in the brain is too low to have a significant impact on direct scavenging. Flavonoids exert their protective effects by modulation of cell signaling pathways and activation of cellular antioxidant defense mechanisms [112]. Flavonoids also inhibit AGE formation by preventing formation/presence of dicarbonyl compounds, and also attenuate damage [118]. Flavonoids were found to reduce MG mediated OS, and enhance the glyoxalase pathway activity [126].
9. Conclusion
The glyoxalase pathway is an integral part of the body's antioxidant system. Dysregulation can have deleterious and catastrophic events, leading to high levels of OS, and also neurodegenerative disease. This seemingly innocuous pathway found in all cells of our body can be responsible for production and formation of toxic intermediaries that alter a cell's normal function and leading to Alzheimer's disease, Parkinson's disease, ASD and Aging. Flavonoids were found to be involved in the reduction of oxidative stress through mechanisms regulated by the glyoxalase pathway. Flavonoid compounds have shown the ability to scavenge ROS and regulate cell signaling pathways integral for antioxidant defense mechanisms and cellular survival. The glyoxalase pathway is a promising drug target for neurodegenerative diseases. Drug discovery [4], [140], [141], [142], [143], [144] and delivery [145], [146], [147] processes could be explored in this target against neurodegenerative diseases. Novel flavonoids could be designed, synthesized, and tested to protect neurodegenerative diseases through glyoxalase pathway.
Acknowledgements
Funding is supported in part by a Nebraska Research Initiative Award (2014) to PN.
Acknowledgments
Notes
The authors declare no competing financial interest
References
- 1.Rabbani N., Thornalley P.J. Dicarbonyl stress in cell and tissue dysfunction contributing to ageing and disease. Biochem. Biophys. Res. Commun. 2015;458(2):221–226. doi: 10.1016/j.bbrc.2015.01.140. [DOI] [PubMed] [Google Scholar]
- 2.Edagwa B., Wang Y., Narayanasamy P. Synthesis of azide derivative and discovery of glyoxalase pathway inhibitor against pathogenic bacteria. Bioorg. Med. Chem. Lett. 2013;23(22):6138–6140. doi: 10.1016/j.bmcl.2013.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sousa Silva M., Gomes R.A., Ferreira A.E., Ponces Freire A., Cordeiro C. The glyoxalase pathway: the first hundred years… and beyond. Biochem. J. 2013;453(1):1–15. doi: 10.1042/BJ20121743. [DOI] [PubMed] [Google Scholar]
- 4.Frandsen J., Narayanasamy P. Flavonoid enhances the glyoxalase pathway in cerebellar neurons to retain cellular functions. Sci. Rep. 2017;7:5126. doi: 10.1038/s41598-017-05287-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Allaman I., Belanger M., Magistretti P.J. Methylglyoxal, the dark side of glycolysis. Front. Neurosci. 2015;9:23. doi: 10.3389/fnins.2015.00023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gu F., Chauhan V., Chauhan A. Glutathione redox imbalance in brain disorders. Curr. Opin. Clin. Nutr. Metab. Care. 2015;18(1):89–95. doi: 10.1097/MCO.0000000000000134. [DOI] [PubMed] [Google Scholar]
- 7.Wu L., Davies G.F., Roesler W.J., Juurlink B.H. Regulation of the glyoxalase pathway in human brain microvascular endothelium: effects of troglitazone and tertiary butylhydroperoxide. Endothelium. 2002;9(4):273–278. doi: 10.1080/10623320214734. [DOI] [PubMed] [Google Scholar]
- 8.Desai K.M., Chang T., Wang H., Banigesh A., Dhar A., Liu J., Untereiner A., Wu L. Oxidative stress and aging: is methylglyoxal the hidden enemy? Can. J. Physiol. Pharmacol. 2010;88(3):273–284. doi: 10.1139/Y10-001. [DOI] [PubMed] [Google Scholar]
- 9.Manini P., Panzella L., Tedesco I., Petitto F., Russo G.L., Napolitano A., Palumbo A., d'Ischia M. Tetrahydrobiisoquinoline derivatives by reaction of dopamine with glyoxal: a novel potential degenerative pathway of catecholamines under oxidative stress conditions. Chem. Res. Toxicol. 2004;17(9):1190–1198. doi: 10.1021/tx034268q. [DOI] [PubMed] [Google Scholar]
- 10.Campos P.B., Paulsen B.S., Rehen S.K. Accelerating neuronal aging in in vitro model brain disorders: a focus on reactive oxygen species. Front. Aging Neurosci. 2014;6:292. doi: 10.3389/fnagi.2014.00292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fujimoto M., Uchida S., Watanuki T., Wakabayashi Y., Otsuki K., Matsubara T., Suetsugi M., Funato H., Watanabe Y. Reduced expression of glyoxalase-1 mRNA in mood disorder patients. Neurosci. Lett. 2008;438(2):196–199. doi: 10.1016/j.neulet.2008.04.024. [DOI] [PubMed] [Google Scholar]
- 12.Hambsch B. Altered glyoxalase 1 expression in psychiatric disorders: cause or consequence? Semin. Cell Dev. Biol. 2011;22(3):302–308. doi: 10.1016/j.semcdb.2011.02.005. [DOI] [PubMed] [Google Scholar]
- 13.Skapare E., Konrade I., Liepinsh E., Makrecka M., Zvejniece L., Svalbe B., Vilskersts R., Dambrova M. Glyoxalase 1 and glyoxalase 2 activities in blood and neuronal tissue samples from experimental animal models of obesity and type 2 diabetes mellitus. J. Physiol. Sci. 2012;62(6):469–478. doi: 10.1007/s12576-012-0224-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pisoschi A.M., Pop A. The role of antioxidants in the chemistry of oxidative stress: a review. Eur. J. Med. Chem. 2015;97:55–74. doi: 10.1016/j.ejmech.2015.04.040. [DOI] [PubMed] [Google Scholar]
- 15.Nuzzo D., Picone P., Caruana L., Vasto S., Barera A., Caruso C., Di Carlo M. Inflammatory mediators as biomarkers in brain disorders. Inflammation. 2014;37(3):639–648. doi: 10.1007/s10753-013-9780-2. [DOI] [PubMed] [Google Scholar]
- 16.Kuhla B., Boeck K., Luth H.J., Schmidt A., Weigle B., Schmitz M., Ogunlade V., Munch G., Arendt T. Age-dependent changes of glyoxalase I expression in human brain. Neurobiol. Aging. 2006;27(6):815–822. doi: 10.1016/j.neurobiolaging.2005.04.006. [DOI] [PubMed] [Google Scholar]
- 17.Kurz A., Rabbani N., Walter M., Bonin M., Thornalley P., Auburger G., Gispert S. Alpha-synuclein deficiency leads to increased glyoxalase I expression and glycation stress. Cell Mol. Life Sci. 2011;68(4):721–733. doi: 10.1007/s00018-010-0483-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hipkiss A. On the relationship between energy metabolism, proteostasis, aging and Parkinson's disease: possible causative role of Methylglyoxal and Alleviative potential of Carnosine. Aging Dis. 2016;8 doi: 10.14336/AD.2016.1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xie A., Gao J., Xu L., Meng D. Shared mechanisms of neurodegeneration in Alzheimer's disease and Parkinson's disease. Biomed. Res. Int. 2014;2014:648740. doi: 10.1155/2014/648740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Barua M., Jenkins E.C., Chen W., Kuizon S., Pullarkat R.K., Junaid M.A. Glyoxalase I polymorphism rs2736654 causing the Ala111Glu substitution modulates enzyme activity--implications for autism. Autism Res. 2011;4(4):262–270. doi: 10.1002/aur.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Belanger M., Yang J., Petit J.M., Laroche T., Magistretti P.J., Allaman I. Role of the glyoxalase system in astrocyte-mediated neuroprotection. J. Neurosci. 2011;31(50):18338–18352. doi: 10.1523/JNEUROSCI.1249-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.More S.S., Vartak A.P., Vince R. Restoration of glyoxalase enzyme activity precludes cognitive dysfunction in a mouse model of Alzheimer's disease. ACS Chem. Neurosci. 2013;4(2):330–338. doi: 10.1021/cn3001679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Angeloni C., Zambonin L., Hrelia S. Role of methylglyoxal in Alzheimer's disease. Biomed. Res. Int. 2014;2014:238485. doi: 10.1155/2014/238485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wei H., Alberts I., Li X. The apoptotic perspective of autism. Int. J. Dev. Neurosci. 2014;36:13–18. doi: 10.1016/j.ijdevneu.2014.04.004. [DOI] [PubMed] [Google Scholar]
- 25.Ishige K., Schubert D., Sagara Y. Flavonoids protect neuronal cells from oxidative stress by three distinct mechanisms. Free Radic. Biol. Med. 2001;30(4):433–446. doi: 10.1016/s0891-5849(00)00498-6. [DOI] [PubMed] [Google Scholar]
- 26.Myhrstad M.C., Carlsen H., Nordstrom O., Blomhoff R., Moskaug J.O. Flavonoids increase the intracellular glutathione level by transactivation of the gamma-glutamylcysteine synthetase catalytical subunit promoter. Free Radic. Biol. Med. 2002;32(5):386–393. doi: 10.1016/s0891-5849(01)00812-7. [DOI] [PubMed] [Google Scholar]
- 27.di Penta A., Moreno B., Reix S., Fernandez-Diez B., Villanueva M., Errea O., Escala N., Vandenbroeck K., Comella J.X., Villoslada P. Oxidative stress and proinflammatory cytokines contribute to demyelination and axonal damage in a cerebellar culture model of neuroinflammation. PLoS One. 2013;8(2):e54722. doi: 10.1371/journal.pone.0054722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Walsh M.E., Shi Y., Van Remmen H. The effects of dietary restriction on oxidative stress in rodents. Free Radic. Biol. Med. 2014;66:88–99. doi: 10.1016/j.freeradbiomed.2013.05.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jang Y.C., Liu Y., Hayworth C.R., Bhattacharya A., Lustgarten M.S., Muller F.L., Chaudhuri A., Qi W., Li Y., Huang J.Y., Verdin E., Richardson A., Van Remmen H. Dietary restriction attenuates age-associated muscle atrophy by lowering oxidative stress in mice even in complete absence of CuZnSOD. Aging Cell. 2012;11(5):770–782. doi: 10.1111/j.1474-9726.2012.00843.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Xue M., Rabbani N., Thornalley P.J. Glyoxalase in ageing. Semin. Cell Dev. Biol. 2011;22(3):293–301. doi: 10.1016/j.semcdb.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 31.Jeong C.H., Joo S.H. Downregulation of reactive oxygen species in apoptosis. J. Cancer Prev. 2016;21(1):13–20. doi: 10.15430/JCP.2016.21.1.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang H., Sun N., Li X., Li K., Tian J., Li J. Diallyl trisulfide induces osteosarcoma cell apoptosis through reactive oxygen species-mediated downregulation of the PI3K/Akt pathway. Oncol. Rep. 2016;35(6):3648–3658. doi: 10.3892/or.2016.4722. [DOI] [PubMed] [Google Scholar]
- 33.Kuhla B., Lüth H.J., Haferburg D., Weick M., Reichenbach A., Arendt T., Münch G. Pathological effects of glyoxalase I inhibition in SH-SY5Y neuroblastoma cells. J. Neurosci. Res. 2006;83(8):1591–1600. doi: 10.1002/jnr.20838. [DOI] [PubMed] [Google Scholar]
- 34.Yao D., Brownlee M. Hyperglycemia-induced reactive oxygen species increase expression of the receptor for advanced glycation end products (RAGE) and RAGE ligands. Diabetes. 2010;59(1):249–255. doi: 10.2337/db09-0801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kuhla B., Boeck K., Schmidt A., Ogunlade V., Arendt T., Munch G., Luth H.J. Age- and stage-dependent glyoxalase I expression and its activity in normal and Alzheimer's disease brains. Neurobiol. Aging. 2007;28(1):29–41. doi: 10.1016/j.neurobiolaging.2005.11.007. [DOI] [PubMed] [Google Scholar]
- 36.Smeyne M., Smeyne R.J. Glutathione metabolism and Parkinson's disease. Free Radic. Biol. Med. 2013;62:13–25. doi: 10.1016/j.freeradbiomed.2013.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gaballah H.H., Zakaria S.S., Elbatsh M.M., Tahoon N.M. Modulatory effects of resveratrol on endoplasmic reticulum stress-associated apoptosis and oxido-inflammatory markers in a rat model of rotenone-induced Parkinson's disease. Chem. Biol. Interact. 2016;251:10–16. doi: 10.1016/j.cbi.2016.03.023. [DOI] [PubMed] [Google Scholar]
- 38.Radjei S., Gareil M., Moreau M., Leblanc E., Schnebert S., Friguet B., Nizard C., Petropoulos I. The glyoxalase enzymes are differentially localized in epidermis and regulated during ageing and photoageing. Exp. Dermatol. 2016;25(6):492–494. doi: 10.1111/exd.12995. [DOI] [PubMed] [Google Scholar]
- 39.Rabbani N., Thornalley P.J. The glyoxalase system--from microbial metabolism, through ageing to human disease and multidrug resistance. Semin. Cell Dev. Biol. 2011;22(3):261. doi: 10.1016/j.semcdb.2011.02.014. [DOI] [PubMed] [Google Scholar]
- 40.Rabbani N., Xue M., Thornalley P.J. Dicarbonyls and glyoxalase in disease mechanisms and clinical therapeutics. Glycoconj. J. 2016;33(4):513–525. doi: 10.1007/s10719-016-9705-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wong A., Lüth H.J., Deuther-Conrad W., Dukic-Stefanovic S., Gasic-Milenkovic J., Arendt T., Münch G. Advanced glycation endproducts co-localize with inducible nitric oxide synthase in Alzheimer's disease. Brain Res. 2001;920(1–2):32–40. doi: 10.1016/s0006-8993(01)02872-4. [DOI] [PubMed] [Google Scholar]
- 42.Rabbani N., Xue M., Thornalley P.J. Methylglyoxal-induced dicarbonyl stress in aging and disease: first steps towards glyoxalase 1-based treatments. Clin. Sci. 2016;130(19):1677–1696. doi: 10.1042/CS20160025. [DOI] [PubMed] [Google Scholar]
- 43.Cobb C.A., Cole M.P. Oxidative and nitrative stress in neurodegeneration. Neurobiol. Dis. 2015;84:4–21. doi: 10.1016/j.nbd.2015.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pacher P., Mackie K. Interplay of cannabinoid 2 (CB2) receptors with nitric oxide synthases, oxidative and nitrative stress, and cell death during remote neurodegeneration. J. Mol. Med. 2012;90(4):347–351. doi: 10.1007/s00109-012-0884-1. [DOI] [PubMed] [Google Scholar]
- 45.Kupershmidt L., Okun Z., Amit T., Mandel S., Saltsman I., Mahammed A., Bar-Am O., Gross Z., Youdim M.B. Metallocorroles as cytoprotective agents against oxidative and nitrative stress in cellular models of neurodegeneration. J. Neurochem. 2010;113(2):363–373. doi: 10.1111/j.1471-4159.2010.06619.x. [DOI] [PubMed] [Google Scholar]
- 46.Choi C.H., Park S.J., Jeong S.Y., Yim H.S., Kang S.O. Methylglyoxal accumulation by glutathione depletion leads to cell cycle arrest in Dictyostelium. Mol. Microbiol. 2008;70(5):1293–1304. doi: 10.1111/j.1365-2958.2008.06497.x. [DOI] [PubMed] [Google Scholar]
- 47.Dringen R., Brandmann M., Hohnholt M.C., Blumrich E.M. Glutathione-dependent detoxification processes in astrocytes. Neurochem Res. 2015;40(12):2570–2582. doi: 10.1007/s11064-014-1481-1. [DOI] [PubMed] [Google Scholar]
- 48.Chen Z., Zhong C. Oxidative stress in Alzheimer's disease. Neurosci. Bull. 2014;30(2):271–281. doi: 10.1007/s12264-013-1423-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Blesa J., Trigo-Damas I., Quiroga-Varela A., Jackson-Lewis V.R. Oxidative stress and Parkinson's disease. Front. Neuroanat. 2015;9:91. doi: 10.3389/fnana.2015.00091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lassmann H. Mechanisms of neurodegeneration shared between multiple sclerosis and Alzheimer's disease. J. Neural Transm. 2011;118(5):747–752. doi: 10.1007/s00702-011-0607-8. [DOI] [PubMed] [Google Scholar]
- 51.Benedetto A., Au C., Aschner M. Manganese-induced dopaminergic neurodegeneration: insights into mechanisms and genetics shared with Parkinson's disease. Chem. Rev. 2009;109(10):4862–4884. doi: 10.1021/cr800536y. [DOI] [PubMed] [Google Scholar]
- 52.Reus G.Z., Fries G.R., Stertz L., Badawy M., Passos I.C., Barichello T., Kapczinski F., Quevedo J. The role of inflammation and microglial activation in the pathophysiology of psychiatric disorders. Neuroscience. 2015;300:141–154. doi: 10.1016/j.neuroscience.2015.05.018. [DOI] [PubMed] [Google Scholar]
- 53.Smaga I., Niedzielska E., Gawlik M., Moniczewski A., Krzek J., Przegalinski E., Pera J., Filip M. Oxidative stress as an etiological factor and a potential treatment target of psychiatric disorders. Part 2. depression, anxiety, schizophrenia and autism. Pharmacol. Rep. 2015;67(3) doi: 10.1016/j.pharep.2014.12.015. (569-80) [DOI] [PubMed] [Google Scholar]
- 54.Moniczewski A., Gawlik M., Smaga I., Niedzielska E., Krzek J., Przegalinski E., Pera J., Filip M. Oxidative stress as an etiological factor and a potential treatment target of psychiatric disorders. Part 1. Chemical aspects and biological sources of oxidative stress in the brain. Pharmacol. Rep. 2015;67(3):560–568. doi: 10.1016/j.pharep.2014.12.014. [DOI] [PubMed] [Google Scholar]
- 55.Choi E.M., Suh K.S., Kim Y.J., Hong S.M., Park S.Y., Chon S. Glabridin alleviates the toxic effects of methylglyoxal on osteoblastic MC3T3-E1 cells by increasing expression of the glyoxalase system and Nrf2/HO-1 signaling and protecting mitochondrial function. J. Agric. Food Chem. 2016;64(1):226–235. doi: 10.1021/acs.jafc.5b05157. [DOI] [PubMed] [Google Scholar]
- 56.Xue M., Rabbani N., Momiji H., Imbasi P., Anwar M.M., Kitteringham N., Park B.K., Souma T., Moriguchi T., Yamamoto M., Thornalley P.J. Transcriptional control of glyoxalase 1 by Nrf2 provides a stress-responsive defence against dicarbonyl glycation. Biochem. J. 2012;443(1):213–222. doi: 10.1042/BJ20111648. [DOI] [PubMed] [Google Scholar]
- 57.Subramaniam S.R., Chesselet M.F. Mitochondrial dysfunction and oxidative stress in Parkinson's disease. Prog. Neurobiol. 2013;106–107:17–32. doi: 10.1016/j.pneurobio.2013.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Di Loreto S., Zimmitti V., Sebastiani P., Cervelli C., Falone S., Amicarelli F. Methylglyoxal causes strong weakening of detoxifying capacity and apoptotic cell death in rat hippocampal neurons. Int. J. Biochem. Cell Biol. 2008;40(2):245–257. doi: 10.1016/j.biocel.2007.07.019. [DOI] [PubMed] [Google Scholar]
- 59.Pinkas A., Aschner M. Advanced glycation end-products and their receptors: related pathologies, recent therapeutic strategies, and a potential model for future neurodegeneration studies. Chem. Res. Toxicol. 2016;29(5):707–714. doi: 10.1021/acs.chemrestox.6b00034. [DOI] [PubMed] [Google Scholar]
- 60.Lv L., Shao X., Chen H., Ho C.T., Sang S. Genistein inhibits advanced glycation end product formation by trapping methylglyoxal. Chem. Res. Toxicol. 2011;24(4):579–586. doi: 10.1021/tx100457h. [DOI] [PubMed] [Google Scholar]
- 61.Mikhed Y., Daiber A., Steven S. Mitochondrial oxidative stress, mitochondrial DNA damage and their role in age-related vascular dysfunction. Int. J. Mol. Sci. 2015;16(7):15918–15953. doi: 10.3390/ijms160715918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kuhla B., Boeck K., Lüth H.J., Schmidt A., Weigle B., Schmitz M., Ogunlade V., Münch G., Arendt T. Age-dependent changes of glyoxalase I expression in human brain. Neurobiol. Aging. 2006;27(6):815–822. doi: 10.1016/j.neurobiolaging.2005.04.006. [DOI] [PubMed] [Google Scholar]
- 63.Li X.H., Du L.L., Cheng X.S., Jiang X., Zhang Y., Lv B.L., Liu R., Wang J.Z., Zhou X.W. Glycation exacerbates the neuronal toxicity of β-amyloid. Cell Death Dis. 2013;4:e673. doi: 10.1038/cddis.2013.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kuhla B., Luth H.J., Haferburg D., Boeck K., Arendt T., Munch G. Methylglyoxal, glyoxal, and their detoxification in Alzheimer's disease. Ann. N. Y. Acad. Sci. 2005;1043:211–216. doi: 10.1196/annals.1333.026. [DOI] [PubMed] [Google Scholar]
- 65.Allaman I., Bélanger M., Magistretti P.J. Methylglyoxal, the dark side of glycolysis. Front. Neurosci. 2015;9:23. doi: 10.3389/fnins.2015.00023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Chun H.J., Lee Y., Kim A.H., Lee J. Methylglyoxal causes cell death in neural progenitor cells and impairs adult hippocampal neurogenesis. Neurotox. Res. 2016;29(3):419–431. doi: 10.1007/s12640-015-9588-y. [DOI] [PubMed] [Google Scholar]
- 67.Allaman I., Gavillet M., Belanger M., Laroche T., Viertl D., Lashuel H.A., Magistretti P.J. Amyloid-beta aggregates cause alterations of astrocytic metabolic phenotype: impact on neuronal viability. J. Neurosci. 2010;30(9):3326–3338. doi: 10.1523/JNEUROSCI.5098-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.McGeer P.L., McGeer E.G. The amyloid cascade-inflammatory hypothesis of Alzheimer disease: implications for therapy. Acta Neuropathol. 2013;126(4):479–497. doi: 10.1007/s00401-013-1177-7. [DOI] [PubMed] [Google Scholar]
- 69.Chang Y.T., Chang W.N., Tsai N.W., Huang C.C., Kung C.T., Su Y.J., Lin W.C., Cheng B.C., Su C.M., Chiang Y.F., Lu C.H. The roles of biomarkers of oxidative stress and antioxidant in Alzheimer's disease: a systematic review. Biomed. Res. Int. 2014;2014:182303. doi: 10.1155/2014/182303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Villar-Cheda B., Dominguez-Meijide A., Valenzuela R., Granado N., Moratalla R., Labandeira-Garcia J.L. Aging-related dysregulation of dopamine and angiotensin receptor interaction. Neurobiol. Aging. 2014;35(7):1726–1738. doi: 10.1016/j.neurobiolaging.2014.01.017. [DOI] [PubMed] [Google Scholar]
- 71.Mielke M.M., Bandaru V.V., Haughey N.J., Xia J., Fried L.P., Yasar S., Albert M., Varma V., Harris G., Schneider E.B., Rabins P.V., Bandeen-Roche K., Lyketsos C.G., Carlson M.C. Serum ceramides increase the risk of Alzheimer disease: the Women's Health and Aging Study II. Neurology. 2012;79(7):633–641. doi: 10.1212/WNL.0b013e318264e380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Car H., Zendzian-Piotrowska M., Fiedorowicz A., Prokopiuk S., Sadowska A., Kurek K. The role of ceramides in selected brain pathologies: ischemia/hypoxia, Alzheimer disease. Post. Hig. Med. Dosw (Online) 2012;66:295–303. doi: 10.5604/17322693.999024. [DOI] [PubMed] [Google Scholar]
- 73.More S.S., Vartak A.P., Vince R. The butter flavorant, diacetyl, exacerbates beta-amyloid cytotoxicity. Chem. Res. Toxicol. 2012;25(10):2083–2091. doi: 10.1021/tx3001016. [DOI] [PubMed] [Google Scholar]
- 74.Sabogal-Guaqueta A.M., Munoz-Manco J.I., Ramirez-Pineda J.R., Lamprea-Rodriguez M., Osorio E., Cardona-Gomez G.P. The flavonoid quercetin ameliorates Alzheimer's disease pathology and protects cognitive and emotional function in aged triple transgenic Alzheimer's disease model mice. Neuropharmacology. 2015;93:134–145. doi: 10.1016/j.neuropharm.2015.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ko S.Y., Ko H.A., Chu K.H., Shieh T.M., Chi T.C., Chen H.I., Chang W.C., Chang S.S. The possible mechanism of advanced glycation end products (AGEs) for Alzheimer's disease. PLoS One. 2015;10(11):e0143345. doi: 10.1371/journal.pone.0143345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Li X.H., Du L.L., Cheng X.S., Jiang X., Zhang Y., Lv B.L., Liu R., Wang J.Z., Zhou X.W. Glycation exacerbates the neuronal toxicity of beta-amyloid. Cell Death Dis. 2013;4:e673. doi: 10.1038/cddis.2013.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Yu L., Wang S., Chen X., Yang H., Li X., Xu Y., Zhu X. Orientin alleviates cognitive deficits and oxidative stress in Abeta1-42-induced mouse model of Alzheimer's disease. Life Sci. 2015;121:104–109. doi: 10.1016/j.lfs.2014.11.021. [DOI] [PubMed] [Google Scholar]
- 78.Xing S., Shen D., Chen C., Wang J., Yu Z. Early induction of oxidative stress in a mouse model of Alzheimer's disease with heme oxygenase activity. Mol. Med. Rep. 2014;10(2):599–604. doi: 10.3892/mmr.2014.2252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Li X.H., Xie J.Z., Jiang X., Lv B.L., Cheng X.S., Du L.L., Zhang J.Y., Wang J.Z., Zhou X.W. Methylglyoxal induces tau hyperphosphorylation via promoting AGEs formation. Neuromol. Med. 2012;14(4):338–348. doi: 10.1007/s12017-012-8191-0. [DOI] [PubMed] [Google Scholar]
- 80.Hwang O. Role of oxidative stress in Parkinson's disease. Exp. Neurobiol. 2013;22(1):11–17. doi: 10.5607/en.2013.22.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Segura-Aguilar J., Kostrzewa R.M. Neurotoxin mechanisms and processes relevant to Parkinson's disease: an update. Neurotox. Res. 2015;27(3):328–354. doi: 10.1007/s12640-015-9519-y. [DOI] [PubMed] [Google Scholar]
- 82.Cieri D., Brini M., Cali T. Emerging (and converging) pathways in Parkinson's disease: keeping mitochondrial wellness. Biochem. Biophys. Res. Commun. 2016 doi: 10.1016/j.bbrc.2016.08.153. [DOI] [PubMed] [Google Scholar]
- 83.He H., Wang S., Tian J., Chen L., Zhang W., Zhao J., Tang H., Zhang X., Chen J. Protective effects of 2,3,5,4'-tetrahydroxystilbene-2-O-beta-D-glucoside in the MPTP-induced mouse model of Parkinson's disease: involvement of reactive oxygen species-mediated JNK, P38 and mitochondrial pathways. Eur. J. Pharmacol. 2015;767:175–182. doi: 10.1016/j.ejphar.2015.10.023. [DOI] [PubMed] [Google Scholar]
- 84.Celardo I., Martins L.M., Gandhi S. Unravelling mitochondrial pathways to Parkinson's disease. Br. J. Pharmacol. 2014;171(8):1943–1957. doi: 10.1111/bph.12433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Gaweda-Walerych K., Zekanowski C. Integrated pathways of parkin control over mitochondrial maintenance - relevance to Parkinson's disease pathogenesis. Acta Neurobiol. Exp. 2013;73(2):199–224. doi: 10.55782/ane-2013-1931. [DOI] [PubMed] [Google Scholar]
- 86.Lehmann S., Martins L.M. Insights into mitochondrial quality control pathways and Parkinson's disease. J. Mol. Med. 2013;91(6):665–671. doi: 10.1007/s00109-013-1044-y. [DOI] [PubMed] [Google Scholar]
- 87.Soreq L., Ben-Shaul Y., Israel Z., Bergman H., Soreq H. Meta-analysis of genetic and environmental Parkinson's disease models reveals a common role of mitochondrial protection pathways. Neurobiol. Dis. 2012;45(3):1018–1030. doi: 10.1016/j.nbd.2011.12.021. [DOI] [PubMed] [Google Scholar]
- 88.Duke D.C., Moran L.B., Kalaitzakis M.E., Deprez M., Dexter D.T., Pearce R.K., Graeber M.B. Transcriptome analysis reveals link between proteasomal and mitochondrial pathways in Parkinson's disease. Neurogenetics. 2006;7(3):139–148. doi: 10.1007/s10048-006-0033-5. [DOI] [PubMed] [Google Scholar]
- 89.Dias V., Junn E., Mouradian M.M. The role of oxidative stress in Parkinson's disease. J. Park. Dis. 2013;3(4):461–491. doi: 10.3233/JPD-130230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Niranjan R. The role of inflammatory and oxidative stress mechanisms in the pathogenesis of Parkinson's disease: focus on astrocytes. Mol. Neurobiol. 2014;49(1):28–38. doi: 10.1007/s12035-013-8483-x. [DOI] [PubMed] [Google Scholar]
- 91.Takahashi K. Non-motor symptoms in premotor phase of Parkinson disease. Rinsho Shinkeigaku. 2013;53(11):974–976. doi: 10.5692/clinicalneurol.53.974. [DOI] [PubMed] [Google Scholar]
- 92.Hashimoto M., Takahara D., Hirata Y., Inoue K., Miyachi S., Nambu A., Tanji J., Takada M., Hoshi E. Motor and non-motor projections from the cerebellum to rostrocaudally distinct sectors of the dorsal premotor cortex in macaques. Eur. J. Neurosci. 2010;31(8):1402–1413. doi: 10.1111/j.1460-9568.2010.07151.x. [DOI] [PubMed] [Google Scholar]
- 93.Ho L., Lange G., Zhao W., Wang J., Rooney R., Patel D.H., Fobler M.M., Helmer D.A., Elder G., Shaughness M.C., Ahlers S.T., Russo S.J., Pasinetti G.M. Select small nucleolar RNAs in blood components as novel biomarkers for improved identification of comorbid traumatic brain injury and post-traumatic stress disorder in veterans of the conflicts in Afghanistan and Iraq. Am. J. Neurodegener. Dis. 2014;3(3):170–181. [PMC free article] [PubMed] [Google Scholar]
- 94.Hashimoto K. Brain-derived neurotrophic factor (BDNF) and its precursor proBDNF as diagnostic biomarkers for major depressive disorder and bipolar disorder. Eur. Arch. Psychiatry Clin. Neurosci. 2015;265(1):83–84. doi: 10.1007/s00406-014-0557-x. [DOI] [PubMed] [Google Scholar]
- 95.Tang G., Gutierrez Rios P., Kuo S.H., Akman H.O., Rosoklija G., Tanji K., Dwork A., Schon E.A., Dimauro S., Goldman J., Sulzer D. Mitochondrial abnormalities in temporal lobe of autistic brain. Neurobiol. Dis. 2013;54:349–361. doi: 10.1016/j.nbd.2013.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Gabriele S., Lombardi F., Sacco R., Napolioni V., Altieri L., Tirindelli M.C., Gregorj C., Bravaccio C., Rousseau F., Persico A.M. The GLO1 C332 (Ala111) allele confers autism vulnerability: family-based genetic association and functional correlates. J. Psychiatr. Res. 2014;59:108–116. doi: 10.1016/j.jpsychires.2014.07.021. [DOI] [PubMed] [Google Scholar]
- 97.Legido A., Jethva R., Goldenthal M.J. Mitochondrial dysfunction in autism. Semin. Pediatr. Neurol. 2013;20(3):163–175. doi: 10.1016/j.spen.2013.10.008. [DOI] [PubMed] [Google Scholar]
- 98.Maher P. Methylglyoxal, advanced glycation end products and autism: is there a connection? Med. Hypotheses. 2012;78(4):548–552. doi: 10.1016/j.mehy.2012.01.032. [DOI] [PubMed] [Google Scholar]
- 99.Meguid N.A., Dardir A.A., Abdel-Raouf E.R., Hashish A. Evaluation of oxidative stress in autism: defective antioxidant enzymes and increased lipid peroxidation. Biol. Trace Elem. Res. 2011;143(1):58–65. doi: 10.1007/s12011-010-8840-9. [DOI] [PubMed] [Google Scholar]
- 100.Ghanizadeh A., Akhondzadeh S., Hormozi M., Makarem A., Abotorabi-Zarchi M., Firoozabadi A. Glutathione-related factors and oxidative stress in autism, a review. Curr. Med. Chem. 2012;19(23):4000–4005. doi: 10.2174/092986712802002572. [DOI] [PubMed] [Google Scholar]
- 101.Ghanizadeh A., Berk M., Farrashbandi H., Alavi Shoushtari A., Villagonzalo K.A. Targeting the mitochondrial electron transport chain in autism, a systematic review and synthesis of a novel therapeutic approach. Mitochondrion. 2013;13(5):515–519. doi: 10.1016/j.mito.2012.10.001. [DOI] [PubMed] [Google Scholar]
- 102.Ryan B.J., Hoek S., Fon E.A., Wade-Martins R. Mitochondrial dysfunction and mitophagy in Parkinson's: from familial to sporadic disease. Trends Biochem. Sci. 2015;40(4):200–210. doi: 10.1016/j.tibs.2015.02.003. [DOI] [PubMed] [Google Scholar]
- 103.Anderson G., Maes M. Neurodegeneration in Parkinson's disease: interactions of oxidative stress, tryptophan catabolites and depression with mitochondria and sirtuins. Mol. Neurobiol. 2014;49(2):771–783. doi: 10.1007/s12035-013-8554-z. [DOI] [PubMed] [Google Scholar]
- 104.Das U.N. Autism as a disorder of deficiency of brain-derived neurotrophic factor and altered metabolism of polyunsaturated fatty acids. Nutrition. 2013;29(10):1175–1185. doi: 10.1016/j.nut.2013.01.012. [DOI] [PubMed] [Google Scholar]
- 105.Distler M.G., Palmer A.A. Role of Glyoxalase 1 (Glo1) and methylglyoxal (MG) in behavior: recent advances and mechanistic insights. Front. Genet. 2012;3:250. doi: 10.3389/fgene.2012.00250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Anouar el H., Raweh S., Bayach I., Taha M., Baharudin M.S., Di Meo F., Hasan M.H., Adam A., Ismail N.H., Weber J.F., Trouillas P. Antioxidant properties of phenolic Schiff bases: structure-activity relationship and mechanism of action. J. Comput. Aided Mol. Des. 2013;27(11):951–964. doi: 10.1007/s10822-013-9692-0. [DOI] [PubMed] [Google Scholar]
- 107.Niki E. Assessment of antioxidant capacity in vitro and in vivo. Free Radic. Biol. Med. 2010;49(4):503–515. doi: 10.1016/j.freeradbiomed.2010.04.016. [DOI] [PubMed] [Google Scholar]
- 108.Rahman I. Dietary polyphenols mediated regulation of oxidative stress and chromatin remodeling in inflammation. Nutr. Rev. 2008;Suppl 1(66):S42–S45. doi: 10.1111/j.1753-4887.2008.00067.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Forman H.J., Davies K.J., Ursini F. How do nutritional antioxidants really work: nucleophilic tone and para-hormesis versus free radical scavenging in vivo. Free Radic. Biol. Med. 2014;66:24–35. doi: 10.1016/j.freeradbiomed.2013.05.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Gottlieb M., Leal-Campanario R., Campos-Esparza M.R., Sanchez-Gomez M.V., Alberdi E., Arranz A., Delgado-Garcia J.M., Gruart A., Matute C. Neuroprotection by two polyphenols following excitotoxicity and experimental ischemia. Neurobiol. Dis. 2006;23(2):374–386. doi: 10.1016/j.nbd.2006.03.017. [DOI] [PubMed] [Google Scholar]
- 111.Suganthy N., Devi K.P., Nabavi S.F., Braidy N., Nabavi S.M. Bioactive effects of quercetin in the central nervous system: focusing on the mechanisms of actions. Biomed. Pharmacother. 2016;84:892–908. doi: 10.1016/j.biopha.2016.10.011. [DOI] [PubMed] [Google Scholar]
- 112.Costa L.G., Garrick J.M., Roque P.J., Pellacani C. Mechanisms of neuroprotection by quercetin: counteracting oxidative stress and more. Oxid. Med. Cell Longev. 2016;2016:2986796. doi: 10.1155/2016/2986796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Dal Belo C.A., Lucho A.P., Vinade L., Rocha L., Seibert Franca H., Marangoni S., Rodrigues-Simioni L. In vitro antiophidian mechanisms of Hypericum brasiliense choisy standardized extract: quercetin-dependent neuroprotection. Biomed. Res. Int. 2013;2013:943520. doi: 10.1155/2013/943520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Justino G.C., Rodrigues M., Florencio M.H., Mira L. Structure and antioxidant activity of brominated flavonols and flavanones. J. Mass Spectrom. 2009;44(10):1459–1468. doi: 10.1002/jms.1630. [DOI] [PubMed] [Google Scholar]
- 115.Costa S.L., Silva V.D., Dos Santos Souza C., Santos C.C., Paris I., Munoz P., Segura-Aguilar J. Impact of plant-derived flavonoids on neurodegenerative diseases. Neurotox. Res. 2016;30(1):41–52. doi: 10.1007/s12640-016-9600-1. [DOI] [PubMed] [Google Scholar]
- 116.Jin H., Lee W.S., Eun S.Y., Jung J.H., Park H.S., Kim G., Choi Y.H., Ryu C.H., Jung J.M., Hong S.C., Shin S.C., Kim H.J. Morin, a flavonoid from Moraceae, suppresses growth and invasion of the highly metastatic breast cancer cell line MDA-MB‑231 partly through suppression of the Akt pathway. Int. J. Oncol. 2014;45(4):1629–1637. doi: 10.3892/ijo.2014.2535. [DOI] [PubMed] [Google Scholar]
- 117.Jiang W., Luo T., Li S., Zhou Y., Shen X.Y., He F., Xu J., Wang H.Q. Quercetin protects against Okadaic acid-induced injury via MAPK and PI3K/Akt/GSK3β signaling pathways in HT22 hippocampal neurons. PLoS One. 2016;11(4):e0152371. doi: 10.1371/journal.pone.0152371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Campos-Esparza M.R., Sánchez-Gómez M.V., Matute C. Molecular mechanisms of neuroprotection by two natural antioxidant polyphenols. Cell Calcium. 2009;45(4):358–368. doi: 10.1016/j.ceca.2008.12.007. [DOI] [PubMed] [Google Scholar]
- 119.Serafini M., Peluso I., Raguzzini A. Flavonoids as anti-inflammatory agents. Proc. Nutr. Soc. 2010;69(3):273–278. doi: 10.1017/S002966511000162X. [DOI] [PubMed] [Google Scholar]
- 120.Manna S.K., Aggarwal R.S., Sethi G., Aggarwal B.B., Ramesh G.T. Morin (3,5,7,2',4'-Pentahydroxyflavone) abolishes nuclear factor-kappaB activation induced by various carcinogens and inflammatory stimuli, leading to suppression of nuclear factor-kappaB-regulated gene expression and up-regulation of apoptosis. Clin. Cancer Res. 2007;13(7):2290–2297. doi: 10.1158/1078-0432.CCR-06-2394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Kim J.M., Lee E.K., Park G., Kim M.K., Yokozawa T., Yu B.P., Chung H.Y. Morin modulates the oxidative stress-induced NF-kappaB pathway through its anti-oxidant activity. Free Radic. Res. 2010;44(4):454–461. doi: 10.3109/10715761003610737. [DOI] [PubMed] [Google Scholar]
- 122.Vauzour D., Vafeiadou K., Rodriguez-Mateos A., Rendeiro C., Spencer J.P. The neuroprotective potential of flavonoids: a multiplicity of effects. Genes Nutr. 2008;3(3–4):115–126. doi: 10.1007/s12263-008-0091-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Spencer J.P. The interactions of flavonoids within neuronal signalling pathways. Genes Nutr. 2007;2(3):257–273. doi: 10.1007/s12263-007-0056-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Mansuri M.L., Parihar P., Solanki I., Parihar M.S. Flavonoids in modulation of cell survival signalling pathways. Genes Nutr. 2014;9(3):400. doi: 10.1007/s12263-014-0400-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Zhang Z.T., Cao X.B., Xiong N., Wang H.C., Huang J.S., Sun S.G., Wang T. Morin exerts neuroprotective actions in Parkinson disease models in vitro and in vivo. Acta Pharmacol. Sin. 2010;31(8):900–906. doi: 10.1038/aps.2010.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Frandsen J., Narayanasamy P. Flavonoid enhances the glyoxalase pathway in cerebellar neurons to retain cellular functions. Sci. Rep. 2017;7(1):5126. doi: 10.1038/s41598-017-05287-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Costa S.L., Silva V.D., Dos Santos Souza C., Santos C.C., Paris I., Muñoz P., Segura-Aguilar J. Impact of plant-derived flavonoids on neurodegenerative diseases. Neurotox Res. 2016;30(1):41–52. doi: 10.1007/s12640-016-9600-1. [DOI] [PubMed] [Google Scholar]
- 128.Ohlow M.J., Sohre S., Granold M., Schreckenberger M., Moosmann B. Why have clinical trials of antioxidants to prevent neurodegeneration Failed? - A cellular investigation of novel phenothiazine-type antioxidants reveals competing objectives for pharmaceutical neuroprotection. Pharm. Res. 2017;34(2):378–393. doi: 10.1007/s11095-016-2068-0. [DOI] [PubMed] [Google Scholar]
- 129.Allam F., Dao A.T., Chugh G., Bohat R., Jafri F., Patki G., Mowrey C., Asghar M., Alkadhi K.A., Salim S. Grape powder supplementation prevents oxidative stress-induced anxiety-like behavior, memory impairment, and high blood pressure in rats. J. Nutr. 2013;143(6):835–842. doi: 10.3945/jn.113.174649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Na H.K., Surh Y.J. Modulation of Nrf2-mediated antioxidant and detoxifying enzyme induction by the green tea polyphenol EGCG. Food Chem. Toxicol. 2008;46(4):1271–1278. doi: 10.1016/j.fct.2007.10.006. [DOI] [PubMed] [Google Scholar]
- 131.Kobayashi A., Kang M.I., Okawa H., Ohtsuji M., Zenke Y., Chiba T., Igarashi K., Yamamoto M. Oxidative stress sensor Keap1 functions as an adaptor for Cul3-based E3 ligase to regulate proteasomal degradation of Nrf2. Mol. Cell Biol. 2004;24(16):7130–7139. doi: 10.1128/MCB.24.16.7130-7139.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Nishimoto S., Koike S., Inoue N., Suzuki T., Ogasawara Y. Activation of Nrf2 attenuates carbonyl stress induced by methylglyoxal in human neuroblastoma cells: increase in GSH levels is a critical event for the detoxification mechanism. Biochem. Biophys. Res. Commun. 2017;483(2):874–879. doi: 10.1016/j.bbrc.2017.01.024. [DOI] [PubMed] [Google Scholar]
- 133.Satoh T., McKercher S.R., Lipton S.A. Nrf2/ARE-mediated antioxidant actions of pro-electrophilic drugs. Free Radic. Biol. Med. 2013;65:645–657. doi: 10.1016/j.freeradbiomed.2013.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Suo H., Wang P., Tong J., Cai L., Liu J., Huang D., Huang L., Wang Z., Huang Y., Xu J., Ma Y., Yu M., Fei J., Huang F. NRSF is an essential mediator for the neuroprotection of trichostatin A in the MPTP mouse model of Parkinson's disease. Neuropharmacology. 2015;99:67–78. doi: 10.1016/j.neuropharm.2015.07.015. [DOI] [PubMed] [Google Scholar]
- 135.Kumar H., Koppula S., Kim I.S., More S.V., Kim B.W., Choi D.K. Nuclear factor erythroid 2-related factor 2 signaling in Parkinson disease: a promising multi therapeutic target against oxidative stress, neuroinflammation and cell death. CNS Neurol. Disord. Drug Targets. 2012;11(8):1015–1029. doi: 10.2174/1871527311211080012. [DOI] [PubMed] [Google Scholar]
- 136.Abed D.A., Goldstein M., Albanyan H., Jin H., Hu L. Discovery of direct inhibitors of Keap1-Nrf2 protein-protein interaction as potential therapeutic and preventive agents. Acta Pharm. Sin. B. 2015;5(4):285–299. doi: 10.1016/j.apsb.2015.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Mastrocola R. AGEs and neurodegeneration: the Nrf2/glyoxalase-1interaction. Oncotarget. 2017;8(4):5645–5646. doi: 10.18632/oncotarget.14232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Liu Y.W., Cheng Y.Q., Liu X.L., Hao Y.C., Li Y., Zhu X., Zhang F., Yin X.X. Mangiferin upregulates glyoxalase 1 through activation of Nrf2/ARE signaling in central neurons cultured with high glucose. Mol. Neurobiol. 2017;54(6):4060–4070. doi: 10.1007/s12035-016-9978-z. [DOI] [PubMed] [Google Scholar]
- 139.Bai D., Ueno L., Vogt P.K. Akt-mediated regulation of NFkappaB and the essentialness of NFkappaB for the oncogenicity of PI3K and Akt. Int. J. Cancer. 2009;125(12):2863–2870. doi: 10.1002/ijc.24748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Choi S.R., Larson M.A., Hinrichs S.H., Bartling A.M., Frandsen J., Narayanasamy P. Discovery of bicyclic inhibitors against menaquinone biosynthesis. Futur. Med. Chem. 2016;8(1):11–16. doi: 10.4155/fmc.15.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Choi S.R., Larson M.A., Hinrichs S.H., Narayanasamy P. Development of potential broad spectrum antimicrobials using C-symmetric 9-fluorenone alkyl amine. Bioorg. Med. Chem. Lett. 2016;26(8):1997–1999. doi: 10.1016/j.bmcl.2016.02.087. [DOI] [PubMed] [Google Scholar]
- 142.Narayanasamy P. MEP pathway: a novel Pathway for new antibiotics. Chem. Sci. J. 2015:6. [Google Scholar]
- 143.Narayanasamy P., Eoh H., Brennan P.J., Crick D.C. Synthesis of 4-diphosphocytidyl-2-C-methyl-d-erythritol 2-phosphate and kinetic studies of Mycobacterium tuberculosis IspF. Chem. Biol. 2010;17(2):117–122. doi: 10.1016/j.chembiol.2010.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Choi S.R., Frandsen J., Narayanasamy P. Novel long-chain compounds with both immunomodulatory and MenA inhibitory activities against Staphylococcus aureus and its biofilm. Sci. Rep. 2017;7:40077. doi: 10.1038/srep40077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Narayanasamy P., Switzer B.L., Britigan B.E. Prolonged-acting, multi-targeting gallium nanoparticles potently inhibit growth of both HIV and mycobacteria in co-infected human macrophages. Sci. Rep. 2015;5:8824. doi: 10.1038/srep08824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Choi S.R., Britigan B.E., Narayanasamy P. Ga(III) nanoparticles inhibit growth of both TB and HIV and release of IL-6 and IL-8 in Co-infected macrophages. Antimicrob. Agents Chemother. 2017 doi: 10.1128/AAC.02505-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Choi S.R., Britigan B.E., Moran D.M., Narayanasamy P. Gallium nanoparticles facilitate phagosome maturation and inhibit growth of virulent Mycobacterium tuberculosis in macrophages. PLoS One. 2017;12(5):e0177987. doi: 10.1371/journal.pone.0177987. [DOI] [PMC free article] [PubMed] [Google Scholar]