Abstract
Oxidative stress is known to play an important role in the pathogenesis of a number of diseases. In particular, it is linked to the etiology of Alzheimer’s disease (AD), an age-related neurodegenerative disease and the most common cause of dementia in the elderly. Histopathological hallmarks of AD are intracellular neurofibrillary tangles and extracellular formation of senile plaques composed of the amyloid-beta peptide (Aβ) in aggregated form along with metal-ions such as copper, iron or zinc. Redox active metal ions, as for example copper, can catalyze the production of Reactive Oxygen Species (ROS) when bound to the amyloid-β (Aβ). The ROS thus produced, in particular the hydroxyl radical which is the most reactive one, may contribute to oxidative damage on both the Aβ peptide itself and on surrounding molecule (proteins, lipids, …). This review highlights the existing link between oxidative stress and AD, and the consequences towards the Aβ peptide and surrounding molecules in terms of oxidative damage. In addition, the implication of metal ions in AD, their interaction with the Aβ peptide and redox properties leading to ROS production are discussed, along with both in vitro and in vivo oxidation of the Aβ peptide, at the molecular level.
Abbreviations: 4-HNE, 4-HydroxyNonenal; AD, Alzheimer’s Disease; AICD, Amino-terminal APP Intra Cellular Domain; ApoE, Apolipoprotein E; APP, Amyloid Precursor Protein; ATP, Adenosine TriPhosphate; Aβ, Amyloid beta peptide; AβDP, Aβ-Degrading Proteases; CNS, Central Nervous System; CSF, CerebroSpinal Fluid; CTF, CarboxyTerminal Fragment; CYP27A1, sterol-27-hydroxylase (cytochrome P450); CYP46A1, cholesterol-24-hydroxylase (cytochrome P450); DNA, DeoxyriboNucleic Acid; ENDOR, Electron Nuclear Double Resonance; ESI-MS, ElectroSpray Ionisation Mass Spectrometry; GlcNAc, N-acetyl-D-glucosamine; HYSCORE, Hyperfine Sublevel Correlation; ITC, IsoThermal Calorimetry; LRP1, Low density lipoprotein receptor-related protein 1; MALDI-TOF, Matrix-Assisted Laser Desorption Ionisation – Time Of Flight; MCO, Metal-Catalyzed Oxidation; MS/MS, tandem Mass Spectrometry; NMR, Nuclear Magnetic Resonance; PSEN1, PSEN2, genes encoding for Presenilin-1 and -2; RNA, RiboNucleic Acid; ROS, Reactive Oxygen Species; SH-SY5Y, neuroblastoma cell line; SOD, SuperOxide Dismutase; XAS, X-ray Absorption Spectroscopy
Keywords: Oxidative stress, Amyloid beta peptide, Metal-ions, Reactive oxygen species, Oxidative damages
Graphical abstract
Highlights
-
•
Oxidative stress plays a role in Alzheimer’s disease (AD), a multifactorial disease leading to loss of cognitive functions.
-
•
Metal ions can bind the amyloid beta peptide (Aβ) and are involved in the production of reactive oxygen species (ROS).
-
•
Oxidation targets neuronal membrane biomolecules and leads to disruption of membrane integrity.
-
•
Aβ is damaged during ROS production, with consequences regarding aggregation, ROS production and cell toxicity.
1. Introduction
Energy conversion is one of the very fundamental process of life. Energy conversion is there since the origin of life and the basic mechanism, i.e. the use of movement of ions across a semipermeable membrane (chemiosmosis), is present in all living organisms. Also, the overall design of the enzyme that converts the ion gradient into chemical energy in form of ATP is the same throughout the living beings [1].
Electron transfer reactions are used to form the ion gradient across a membrane. In other words, these are redox reactions, in which electrons are passed in a chain from a first donor via several intermediates to a final acceptor. For humans, animals and a lot of other beings, the final electron acceptor is dioxygen. An advantage of this final acceptor is its high redox potential and hence the high energy in the reaction:
| O2 + 4 e- + 4H+ → 2H2O | (1) |
The electron donors are in principle the food we take up. Thus the energy we need for living stems from a redox reaction between food (and its transformed products) and O2. In reaction (1), O2 accepts four electrons and four protons to produce two molecules of water. In reaction (2), a partial O2 reduction produces the superoxide anion (O2•–), hydrogen peroxide (H2O2) and the hydroxyl radical (HO•).
| (2) |
These intermediates are potentially dangerous, because they are either very reactive, and hence difficult to control (like HO•), or they are precursors that easily form very reactive and uncontrollable species (like O2•– + NO → peroxynitrite). While thermodynamically favored, O2 reaction with organic electron donors are kinetically prevented by the triplet ground state of O2. Thus the reaction (1) can be well controlled as such that little partial reduction (reaction (2)) occurs. The partially reduced oxygen species O2•–, H2O2, HO• belong to the family of compounds called reactive oxygen species (ROS). ROS are broadly defined as oxygen-containing chemicals with reactive properties [2]. The life in aerobic environment and with O2 as a final electron acceptor results in a constant production of ROS in our body. ROS are produced enzymatically (for instance in macrophages to kill invaders) or non-enzymatically, as a side reaction. Latter is the case in the respiratory chain, where the overall physiological reaction is (1), but “unwanted” side reactions leak ROS. Due to the importance of energy conversion, most ROS produced in the body come from the respiratory chain and are hence potentially dangerous. Thus several enzymes and small compounds exist to control the levels of ROS. Generally, ROS are kept at a low level but not fully eliminated. As they have messenger function, their total suppression is detrimental. Accumulation of too high levels of ROS is dangerous and defined as oxidative stress. ROS accumulation can occur either by an overproduction or an insufficient elimination of ROS.
Elimination can occur by different mechanisms and is performed by an antioxidant compound. By definition, an antioxidant compound is an endogenous or exogenous molecule that "when present in low concentrations compared to that of an oxidizable substrate significantly delays or inhibits the oxidation of the substrate" [3]. Diverse mechanisms are possible like i) scavenging of ROS, ii) quenching of ROS sources and iii) regeneration of endogenous antioxidants [4].
Considering the central role of oxygen, the various systems of production and elimination of ROS and their regulations, it is not astonishing that oxidative stress has been observed in a multitude of diseases. Moreover, oxidative stress can enter into a vicious cycle, as the produced ROS can destroy biomolecules, which may lead to higher ROS accumulation. For instance, when ROS attack metalloproteins, it can lead to the release of redox-competent metal ions with a subsequent increase of ROS production (see below).
In neurodegenerative diseases like Alzheimer’s and Parkinson’s, the brains show oxidative damage and oxidative stress often seem to be implicated in many of them. The brain might be particularly sensitive to oxidative damage upon oxidative stress due to the very high dioxygen consumption of the brain (20% of the total body consumption). But not only that, Halliwell listed 13 points called “problems of the brain”, that could explain the high sensitivity, including the somehow surprising modest antioxidant defense of the brain [5]. Although the occurrence of oxidative stress in several neurodegenerative diseases is relatively well established, the question of “cause or consequence” is much more difficult to answer. The question is important as the time point when oxidative stress occurs in the etiology is key for the validity/efficiency as a therapeutic target.
2. Linking oxidative stress and AD
2.1. Definition of AD and hallmarks
In 1907, Aloïs Alzheimer related in the article “Über eine eigenartige Erkankung der Hirnrinde” (“On an unusual Illness of the Cerebral Cortex”) the uncommon case of a 51-year-old patient who was suffering from memory loss, disorientation, hallucinations and cognitive impairment. After the death of the patient, post-mortem examination showed an atrophic brain with “striking changes of the neurofibrils” and “minute military foci” caused by the “deposition of a special substance in the cortex” [6]. One century later, this “unusual illness” named Alzheimer’s Disease (AD) has become the most widespread neurodegenerative disease whose etiology is still unknown [7]. According to the World Alzheimer Report [8], 46.8 million people were suffering from dementia worldwide in 2015; this number is expected to almost double every 20 years. Approximately 5–8% of individuals over age 65, 15–20% over age 75, and 25–50% over age 85 are affected by dementia [9]. The major prevalence is in Asia (22.9 million people) while Europe and the Americas account for 10.5 and 9.4 million people, respectively. AD is the most common form of dementia, accounting for 50–75% of all dementias [9].
AD is characterized by a progressive deterioration of cognitive functions that can be linked to a significant reduction of the volume of the brain in AD patients as compared to healthy patients [10]. The atrophy results from the degeneration of synapses and the death of neurons, in particular in hippocampus [11], the brain region playing a role in memory and spatial orientation. The age is the highest risk factor for AD, the risk of developing the disease reaching 50% for individuals beyond age 85 [9]. Women are more susceptible than men to suffer from AD, because of their higher life expectancy, and because the decrease in estrogen levels due to menopause could increase the risk of developing AD [12].
Apart from the global reduction in the brain volume, one of the hallmarks of AD is the presence of amyloid plaques in brain, caused by the “deposition of a special substance in the cortex”, as firstly described by Aloïs Alzheimer. These plaques, also named senile plaques, are found in the extracellular space of AD brain and are particularly present in the hippocampus region. They are mainly composed of a peptide, named Amyloid-β (Aβ), that is aggregated and forms mostly β-sheet rich fibrils [13]. Another hallmark of the disease is the presence of intracellular neurofibrillary tangles in the brain [14], also observed in Parkinson’s disease (PD) [15] and composed of hyperphosphorylated Tau protein [16]. This microtubule-associated protein normally interacts with tubulin to stabilize microtubules. In AD and PD, Aβ would cause an activation of p38 MAPK in cell that leads to the abnormal phosphorylation of Tau [17]. This latter induces accumulation as paired helical filaments that aggregate inside neurons in neurofibrillary tangles, making the microtubules unstable and causing the loss of neuron functionality.
2.2. Aβ and the amyloid plaques formation
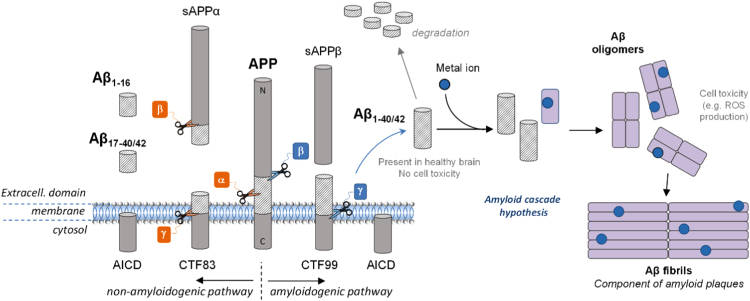
The Aβ peptide is a 38- to 43- amino acid residue peptide whose 1-letter code sequence is DAEFRHDSGYEVHHQKLVFFAEDVGSNKGAIIGLMVGGVVIAT. It is generated after enzymatic cleavage by β- and γ-secretases of APP, the Amyloid Precursor Protein, a type-1 trans-membrane protein expressed in various tissues, especially in the central nervous system (CNS) [18]. Its major neuronal isoform encompasses 695 amino acid residues [19]. Although its physiological function is still unclear, APP would play an important role in brain development, memory and synaptic plasticity [19]. The metabolism of APP can follow two different pathways (Fig. 1). In the non-amyloidogenic one (predominant), APP is first cleaved by α-secretase and then by γ-secretase to form truncated Aβ17–40/42 (P3) peptides or by β-secretase to lead to the formation of the truncated Aβ1–16 peptide. In the amyloidogenic one, which occurs to a minor extent, APP is cleaved consecutively by β- and γ-secretases leading to the formation of full-length Aβ peptides (mainly Aβ1–40/42). Both pathways also lead first to the formation of amino-terminal fragments (secreted APP (sAPP) α or β) and carboxyterminal fragments (CTF83 or CTF99) and then to the formation of the amino-terminal APP intracellular domain (AICD) [20]. The latter one is involved in nuclear signalization. [19] Depending on the exact location of the cleavage by γ-secretase, several lengths of peptide can be released, from Aβ1–38 to Aβ1–43. However, the most abundant species produced in the brain are Aβ1–40 and to a lesser extent Aβ1–42. A third way of APP cleavage has been recently discovered [21]. It involves η-secretase that cleaves APP at amino acids 504–505 and leads to the generation of the higher molecular mass carboxy-terminal fragments Aη-α and Aη-β, after second cleavage by α- and β-secretase, respectively. The first one, Aη-α, contains the Aβ1–16 peptide in its sequence and was reported to be neurotoxic.
Fig. 1.
A schematic view of APP proteolytic cleavage. In the non-amyloidogenic pathway, APP is first cleaved by α-secretase and then by γ-secretase to form truncated Aβ17–40/42 peptides or by β-secretase leading to the formation of the truncated Aβ1–16. In the amyloidogenic pathway, APP is cleaved consecutively by the β- and γ-secretases leading to the formation of full-length Aβ1–40/42 peptides. According to the amyloid cascade hypothesis, the Aβ peptide would be further able to interact with metal ions present in the brain and form oligomers and then fibrils, found in the senile plaques in vivo.
Thus, Aβ peptides are the product of a minor pathway of APP metabolism [22]. They are mainly produced intracellularly in vesicles like endosomes and released in the extracellular space of healthy brain during neuronal activity, without leading necessarily to Alzheimer’s pathology. Aβ is subject to a proteolytic degradation by Aβ-degrading proteases (AβDPs), which regulates Aβ levels in the brain [23]. Its function in the brain is still unknown, although Aβ could play a role in synaptic plasticity and memory [24].
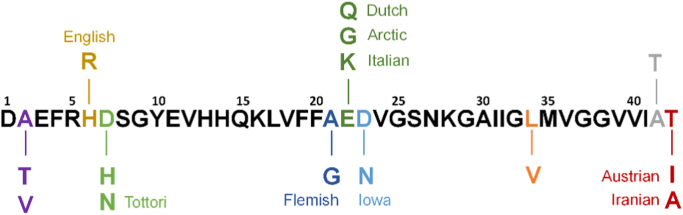
There are two major forms of AD: the sporadic or late-onset form, the most common one, and the familial or early-onset form, representing less than 5% of the cases [25]. Individuals living with Down’s syndrome (also called trisomy 21) have an increased risk of early-onset AD because they carry an extra copy of chromosome 21 in which is located the gene responsible for APP formation [26]. Mutations of several genes (including PSEN1 and PSEN2) coding for APP, Presenilin 1 and Presenilin 2 (two sub-units of γ-secretase), identified as causative genes, have been found to cause mainly early-onset AD, while ApoE (involved in Aβ clearance) is considered as being the most common high genetic risk factor for late-onset AD [25], [27]. The mutations on both PSEN1 and PSEN2 lead to a higher Aβ production, PSEN1 mutations specifically conducting to an increased Aβ1–42 formation [25]. Sixty-five mutations of APP are indexed in the Alzheimer Disease & Frontotemporal Dementia Mutation Database, with only 15 being non-pathogenic [28]. As APP mutations can occur in the Aβ domain, APP proteolysis by both β- and γ-secretases can lead to the formation of mutated Aβ peptides (the most frequent ones are presented in Fig. 2). The mutations are divided in three categories: mutations at the β-secretase cleavage site (N-term), at the γ-secretase cleavage site (C-term) and in the mid-domain amyloid-β region [29]. The mutations at the γ-secretase cleavage site can alter the cleavage position and lead to an increase of the Aβ1–42/Aβ1–40 ratio. The mutations at the β-secretase cleavage site increase the rate of APP proteolysis by the β-secretase. The mutations in the mid-domain of Aβ region in APP alters Aβ assembly by increasing the propensity of Aβ to form oligomers and fibrils [30].
Fig. 2.
Most frequent familial AD mutations occurring on Aβ1–43. The amino acid residues mutated and the names of the mutations are colored. (1-letter code). (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.). [25].
AD is a multifactorial disease and the multiple mechanisms related to the disease are unclear. However, since Aβ has been found in healthy brain in soluble form but in aggregated form in AD patient brain [13], a hypothesis has been proposed to explain the formation of the senile plaques. The amyloid cascade hypothesis (Fig. 1) formulated in the early 1990s [31], [32], [33], [34] has become a dominant model for AD pathogenesis [35], although still controversial [36], [37]. The hypothesis proposed that an abnormal extracellular increase of Aβ levels in brain could lead to Aβ aggregation into β-sheet rich structures [38]. Aggregation starts with the formation of oligomers species that are reorganized into protofibrils and fibrils, found in amyloid plaques. Oligomers accumulated in AD patient brains [39] are suggested to be the more toxic species for cells [40], [41] as they can in particular permeabilize cellular membranes, thus initiating a series of events leading to cell dysfunction and death [42]. According to this hypothesis, other events such as the intracellular formation of neurofibrillary tangles and the disruption of synaptic functions would result from this early and key event. Factors influencing this cascade are modulators and can have an important impact. Regarding oxidative stress, metal ions such as zinc, iron and copper are such modulators and they have been found in amyloid plaques [43]. Cu and Zn are excreted within the synaptic cleft of some neurons. They are supposed to play an important role in aggregation according to the amyloid cascade hypothesis [44], as they can bind Aβ and thus modulate the aggregation process. They act either on the kinetics or on the thermodynamics by impacting the morphology of the formed aggregates [45]. Furthermore, amyloid aggregates (low molecular weight) with entrapped redox-active metal ions such as copper ions are considered more toxic since they can produce ROS, deleterious for the Aβ peptide itself and for the surrounding biomolecules [46].
2.3. Oxidation of surrounding molecules
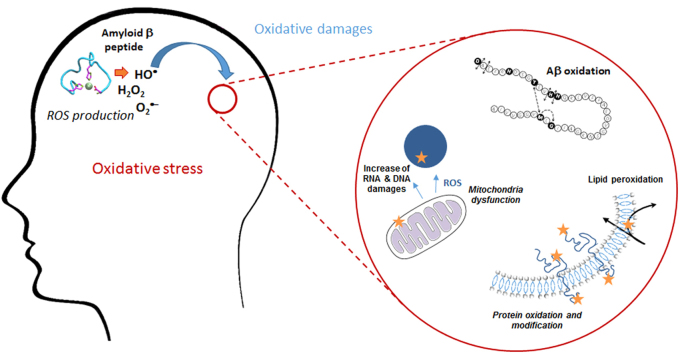
Oxidation of biomolecules in the context of AD is mainly related to neuronal membrane biomolecules and to a disruption of membrane integrity. It involves oxidation of lipids (among them, cholesterol), proteins and nucleic acids, and impairment of Aβ clearance by the low density lipoprotein receptor-related protein (LRP1) due to its oxidation. After a brief reminder of the existence of oxidative stress in AD, the consequences of the oxidation of biomolecules on membrane integrity and protein functionality will be addressed, in relation with AD pathogenesis.
2.3.1. Evidence of brain oxidative/nitrosative stress in AD
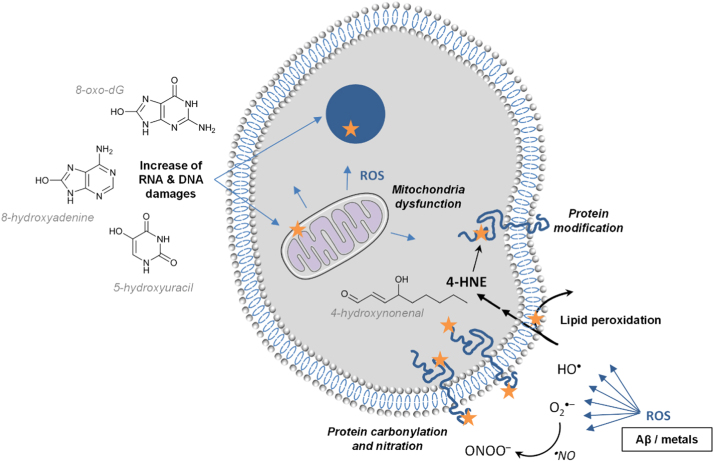
Several pieces of evidence suggest that oxidative stress and nitrosative stress play a key role in the pathogenesis of AD [47]. Oxidative stress occurs early in the course of AD, which would support its role in AD pathogenesis [48], in relation with the presence of Aβ. Indeed, elevated levels of Aβ1–40 and Aβ1–42 have been reported to be associated with increased levels of oxidation products from proteins, lipids and nucleic acids in AD hippocampus and cortex (Fig. 3) [49]. By contrast, brain regions with low Aβ levels (e.g., cerebellum) did not present high concentrations of oxidative stress markers [50], [51], [52]. More recently, it has been confirmed that protein and lipid oxidation was observed in brain regions rich in Aβ, where redox proteomics allowed identification of oxidized proteins in early stages of the disease [53]. In addition to ROS production by Aβ peptides in the presence of metal ions (see the section “Aβ peptide and ROS production” below), mitochondria dysfunction has also been involved in AD pathogenesis, via mitochondrial ROS generation [54], [55]. Biomarkers of oxidative stress in the AD brain have been well documented, with markers of protein, lipid, DNA and RNA oxidation [56]. Thus, protein oxidation has been classically evidenced by increased levels of carbonylated proteins, especially in the hippocampus and parietal cortex, i.e. in the brain areas the most involved in AD [51]. In human brain, membrane proteins were more oxidatively damaged than cytoplasmic proteins [57]. Protein modification also occurred by indirect oxidation due to reaction with 4-hydroxynonenal (4-HNE), a lipid peroxidation product, and by nitration. The latter process leads to a nitrosative stress due to reaction of proteins with peroxinitrite (ONOO–, that results from reaction of superoxide radicals with nitric oxide), and increases the susceptibility of brain proteins to proteosomal degradation [58]. Regarding lipid oxidation, increased concentrations of 4-HNE have been reported in the brain regions showing the typical histopathologic alterations of AD (i.e., hippocampus) [59]. Oxidative modification of lipoic acid by 4-HNE was detected in AD brain [60], and 4-HNE-lysine adducts were increased not only in neurons containing neurofibrillary tangles but also in “apparently” normal pyramidal neurons located in the hippocampal tissue sections [61]. Oxidation of nuclear and mitochondrial DNA has also been reported in AD, with increased levels of oxidized bases (i.e., 8-oxo-2-dehydroguanine, 8-hydroxyadenine, 5-hydroxyuracil) in temporal, parietal and frontal lobes [62], [63]. Increased levels of 8-hydroxyguanine have even been detected in the hippocampus of patients with a preclinical stage of AD [64]. This oxidative stress, especially oxidative DNA damage, has been detected not only associated with the most vulnerable regions, but also in peripheral AD blood cells [65]. RNA oxidation also occurred, especially mRNA oxidation in the frontal cortex [66].
Fig. 3.
Induced oxidative stress in cell of AD brain regions of high Aβ levels, where Aβ-metals is one of the production source for ROS. 4-HNE = 4-hydroxynonenal; 8-oxo-dG = 8-oxo-dehydroguanine. Orange star indicates oxidative damages. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.).
2.3.2. Consequences of the oxidation of biomolecules on membrane integrity and protein functionality
Alteration of functional integrity of neuronal membranes in AD could result from interactions between amyloid-forming proteins and membranes, leading to membrane permeabilization via several hypothetic mechanisms such as transmembrane oligomeric pore structures [67]. Besides this process, oxidative stress by itself could be responsible for a disruption of membrane integrity. As an example, lipid peroxidation could be involved in a loss of phospholipid asymmetry in synaptosomal membranes [68]. Indeed, this asymmetry is maintained by the ATP-dependent enzyme aminophospholipid-translocase or flippase, whose activity depends on at least one critical cysteine residue, possibly oxidized by 4-HNE. This lipid peroxidation product can conjugate with several membrane proteins, resulting in alterations of their structure and function, with a consequent neurotoxicity in AD brain [69]. Proteins involved in glycolysis and ATP production could thus become dysfunctional, and this impairment of brain energy metabolism, secondary to oxidative stress, seems to be a key event in AD [70]. Reciprocally, decreased ATP levels could result in electron leakage and increased mitochondrial ROS production, thereby generating another source of oxidative stress in AD [71]. Several proteins directly involved in glucose metabolism and ATP synthesis have been reported to be inactivated by oxidation in AD brain (e.g., fructose biphosphate aldolase, triose phosphate isomerase, glyceraldehyde phosphate dehydrogenase, phosphoglucose mutase, enolase, pyruvate kinase) [72]. ATP synthase itself could be oxidatively modified and consequently inactivated in AD brain, the α subunit of the enzyme being a target for oxidative damage at the very early stages of AD [73], [74]. In advanced stages of AD, ATP synthase activity was also decreased in AD brain [75]. This decreased activity would result from a direct binding between Aβ and ATP synthase and from inhibition of O-GlcNAcylation of the Thr432 residue on the ATP synthase subunit α [76]. Oxidation-induced impairment of enzymes involved in ATP production could be related with transportation abnormalities and dysfunction of intracellular glucose catabolism in AD [77]. Interestingly, the alterations of metabolic disorders could be supported by the link between AD and diabetes [78]. Accordingly, it has been recently shown that mTOR (whose signaling pathway plays a key role in regulating cell growth as well as lipid and glucose metabolism), aberrantly activated in AD from early stages, would be involved in AD neurodegeneration, via an inhibition of both insulin signaling and alteration of protein homeostasis [79]. Similarly, in the triple transgenic mouse model of AD (3xTg-AD) that develops both Aβ and Tau pathologies in an age-dependent manner, oxidative and nitrosative stresses have been suggested to contribute to impairment of insulin signaling in AD brain [80].
Oxidative stress could be involved in the clearance of Aβ. It has thus been hypothesized that Aβ would oxidize LRP1, leading to accumulation of the neurotoxic peptide Aβ in the brain. Indeed, LRP1 is a multifunctional protein that is notably in charge of the efflux of Aβ from the brain to the blood, across the blood-brain barrier [81], [82], and LRP1 activity is decreased in AD [83]. Thus, Aβ, by oxidizing LRP1, would lead to disruption of its own clearance [84]. LRP1 oxidation has been evidenced by the presence of 4-HNE-LRP1 adducts in AD hippocampus. Such alteration of Aβ clearance would lead to an increased Aβ accumulation in the brain, which could be a determinant factor in AD pathogenesis.
Protein Tau also constitutes a target for oxidative stress in AD. As an example, 4-HNE is able to induce modifications of protein Tau conformation, which supports the involvement of oxidative stress (notably induced by Aβ) in the pathogenesis of AD, by favoring neurofibrillary tangles formation [85]. Nitration of protein Tau could also promote a conformation change that may favor fibril assembly. It constitutes an early event in AD, since the appearance of nitrated Tau in neurofibrillary tangles appears essentially before the maturation of Tau inclusions [86]. Moreover, due to the role of protein Tau both in microtubule dynamics and in the protection of neuronal genomic DNA and of cytoplasmic and nuclear RNA towards ROS-induced damage, Tau alteration would lead to increased DNA and RNA oxidation [87]. Some authors have suggested that the DNA repair proteins might be inactivated by oxidative modifications, which could result in impaired DNA repair capabilities via the base excision repair pathway [88], [89]. It is noteworthy that oxidation of DNA can result, in addition to base oxidation, in DNA strand breaks, which could contribute to neurodegeneration by favoring the formation of neurofibrillary tangles [90].
Cholesterol in cell membranes, more specifically in microdomains rich in cholesterol named lipid rafts, is able to bind to APP, thereby promoting its insertion into the phospholipid monolayers; this induces the activity of the β-secretase, thus favoring the amyloidogenic pathway, by accumulation of Aβ1–42 peptide [91]. More precisely, esterified cholesterol (and not free cholesterol) enhanced Aβ formation [92], so that the balance between free and esterified cholesterol constitutes a modulator of amyloidogenesis. Cholesterol can be oxidized in vivo, to form oxysterols that represent a way to eliminate excess cholesterol from the brain; this way prevent cholesterol accumulation, since the brain cannot degrade cholesterol. Oxysterols can thus equilibrate the local synthesis of sterols in brain [93]. It is noteworthy that oxysterols could modify specific sites on Aβ, e.g. at Lys16, which could increase Aβ aggregation and neurotoxicity. Among oxysterols, 3β-hydroxy-5-oxo-5,6-secocholestan-6-al, that can be converted into its aldol form, can bind to an amine of Aβ to lead to a Schiff base [94]. This covalent modification of Aβ increases its amyloidogenicity [95], by decreasing the aggregation critical concentration and favoring the formation of spherical aggregates [96] that are neurotoxic [97]. Another oxysterol, named 24-hydroxycholesterol, is produced in the brain by action of the cholesterol 24-hydroxylase (CYP46A1) and can cross the blood brain barrier [98]. This oxysterol is involved in the regulation of cholesterol homeostasis in the brain, by inducing apoE-mediated efflux of cholesterol in astrocytes via a liver X receptor (LXR)-controlled pathway, which may be a process involved in the pathogenesis of AD [99]. It has been observed that another oxysterol, 27-hydroxycholesterol, produced in the brain by CYP27A1, mostly goes from the circulation to the brain by crossing the blood brain barrier [100]. Consequently, two main opposite fluxes of oxysterols coexist, i.e. 24-hydroxycholesterol from the brain and 27-hydroxycholesterol into the brain, so that the balance between 24-hydroxy- and 27-hydroxy-cholesterol would be of importance for amyloidogenesis [101]. The increased ratio of 27-hydroxycholesterol to 24-hydroxycholesterol observed in AD brains supports this hypothesis [102]. In SH-SY5Y cells (i.e., a human neuroblastoma cell line and a classical model for AD pathology), it has been suggested that 24-hydroxycholesterol would favor the processing of APP to the non-amyloidogenic pathway [103]. Nevertheless, a comprehensive in vitro analysis of APP and α-, β- and γ-secretases has been performed by Gamba et al. [104] in a human neuroblastoma cell line (SK-N-BE) treated with 1 µM of 24-hydroxy- or 27-hydroxycholesterol after differentiation into neuron-like cells. Under these conditions, both oxysterols induced an overexpression of APP and an increased β-secretase activity, leading to amyloidogenesis. The contradictory results obtained by Gamba et al. and Prasanthi et al. [103] could be related to the differences in the oxysterol concentrations tested (1 µM vs. 5–25 µM, respectively) and to the cell treatment with oxysterols (after retinoic acid-driven differentiation to a neuron-like phenotype vs. a direct challenging, respectively). The conditions with 1 µM oxysterols seem much closer to the actual amounts recovered from normal and AD brains and thus more patho-physiologically relevant. Interestingly, plasma level of 24-hydroxycholesterol, via its relation to the mass of metabolically active neuronal cells, could be used as a marker of brain atrophy in AD patients [105]. In addition to 24-hydroxy- and 27-hydroxy-cholesterol of enzymatic origin, other oxysterols (including 7-ketocholesterol, 7α-hydroxycholesterol,4β-hydroxycholesterol, 5α,6α-epoxycholesterol, and 5β,6β-epoxycholesterol) deriving from cholesterol autooxidation were detected in post-mortem human AD brain and the change of their levels was associated with AD progression [106].
Finally, it is noteworthy that the genotype of apoE, the main cholesterol-carrier protein in brain, impacts oxidative stress, since plasma from AD apoε4 carriers was more oxidized than plasma from AD non-apoε4 carriers [107], [108]. This genotype would influence cholesterol metabolism and formation of oxysterols [109]. Of note, apoE structure could play a role since apoE2 has two Cys residues, whereas apoE3 has only one Cys and apoE4 has no free thiol group; therefore, the lower number of Cys residues in apo E4 would lead to a lesser protection against oxidative stress. Oxidation of apoE, evidenced by analysis of oxidative stress-related modifications of the cerebrospinal fluid (CSF) proteome, could thus affect thiol-mediated antioxidant activity, which would allow excess oxidative damage to the lipoprotein particles and promote Aβ protein aggregation [110].
3. Metal implication in AD
3.1. Role of metals in brain
Like other tissues, the brain contains several essential d-block metal ions, such as Fe, Zn, Cu, Mn, Mo, Cr, Co and non-essential metals. In general, the brain belongs to the organs with the highest d-block metal content per weight. The content of the most abundant d-block ions Fe, Zn and Cu are 0.3 g, 0.1 g and 0.004 g per kg brain, respectively [111]. These three metal ions seem to be the most relevant regarding Aβ and/or oxidative stress. Fe, Cu and Zn ions are generally bound to proteins, in order to control their reactivity. They have most often the role in metalloproteins of catalytic center, electron transfer site or structural component. Only Zn occurs at higher concentration in non-protein bound forms at certain places, where it seems to play the role of a messenger. The metabolism of these ions is tightly controlled by a machinery that is able to sense the metal concentration, to perform metal transport in the blood or through the membranes, to provide the metal ion during protein folding and maturation, and to stock metal ions. It is well documented that conditions leading to too high or too little metal ions content can be lethal. This is the case for Wilson’s and Menkes’ diseases. First is a Cu-overload, second a Cu deficiency genetic disease. In either of these diseases, the brain is highly affected, in line with its high metal content [112], [113].
3.2. Misregulation in AD
There is a large body of evidence for metal ion misregulation in AD, in particular for Cu, Zn and Fe [114], [115]. If the misregulation is an early or late event in AD is still a matter of research. A well-established fact is the accumulation of Cu, Zn and Fe in the amyloid plaques, a hallmark of AD [116]. Interestingly, human amyloid plaques accumulate much higher metal concentrations than plaques in AD model mice [117]. APP is also implicated in metal metabolism, as it can promote iron efflux of neurons [118], under the control of an iron-responsible element [119]. Moreover, its transcription was reported as being promoted by Cu. Zn and Cu-binding sites where reported in vitro [120], [121], [122]. There are also a multitude of metalloproteins and transporters affected in AD, as well as metal concentrations, metal repartition and homeostasis (for recent review see [114]). Importantly, an increase in loosely bound Cu in human AD brains compared to healthy subjects was reported [123]. It is well established that such loosely bound Cu and Fe can promote oxidative stress [5].
3.3. Metals and oxidative stress
Metal ions, in particular Cu, Fe and Mn, play a central role in oxidative stress. They are implicated in the production and defense of oxidative stress. Free or loosely bound Cu and Fe are very efficient catalysts of ROS production. They can be reduced to Cu(I) or Fe(II) by physiological relevant reducing agents (like glutathione or ascorbate) and can then react with dioxygen or hydrogen peroxide to form superoxide and hydroxyl radicals, respectively [5]. On the other hand, the same metal ions are also present in the catalytic center of antioxidant enzymes, like Cu in SOD1 or Fe in catalase, where they destroy the superoxide anion and H2O2, respectively. This clearly shows the importance of the coordination chemistry. Depending on the coordination site, Cu and Fe can be pro-oxidants or antioxidants. Hence it becomes clear how important the control of these metal ions metabolism is, in terms of concentration, transport, storage and incorporation into active sites. In case of failure of Fe and Cu homeostasis, free or loosely bound Fe and Cu concentrations can increase, which are often competent to catalyze the production of ROS [111]. Such Cu and Fe can also bind to off-target biomolecules and disturb their function, which could also contribute to increased oxidative stress.
4. Aβ peptide and ROS production
4.1. Coordination of Aβ with metal ions
As described above, metal ions such as zinc, iron and copper are present in the brain. They are necessary and required to regulate the neuronal activity in the synapses and are involved in biological functions of metallo-proteins. In several diseases such as AD, the metal ion homeostasis is disrupted and the concentration and distribution are far from the physiological ones. In particular, Cu and Zn levels can reach up to three times the normal levels observed in healthy brains [124]. Moreover, high content of these metal ions is found in amyloid plaques extracted from AD brains [43]. As they can bind to Aβ under physiological concentrations, their coordination modes are of interest to understand their role in AD.
4.1.1. Zn(II) coordination to the Aβ peptide
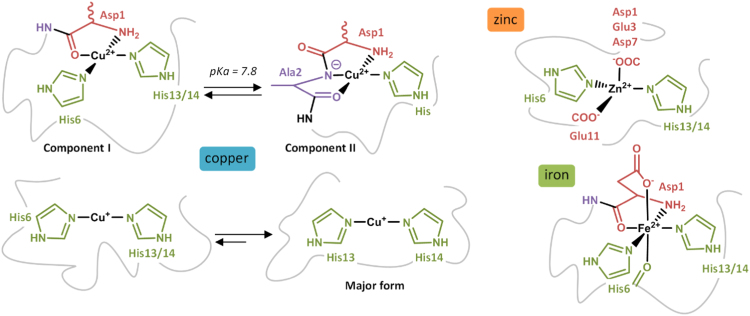
Zn ion exists only as Zn(II) and its coordination to Aβ is still not well-established. [125], [126], [127] Although it is consensual that a complex 1:1 is formed [126], the nature of the amino acid residues involved in the coordination sphere is still under debate. A novel binding model has recently been proposed, based on Nuclear Magnetic Resonance (NMR) and X-ray Absorption Spectroscopy (XAS) studies of Zn coordination with mutated and N-terminal acetylated peptides [128]. Zn(II) would be bound by imidazole rings of His6 and either His13 or His14 residues, the carboxylate group of Glu11 and the carboxylate group of Asp1, Glu3 or Asp7 (Fig. 4). Zn(II) affinity for Aβ has been investigated by isothermal calorimetry (ITC) and competition studies, leading to an affinity constant in the 105 M-1 range, which would permit Zn-Aβ in vivo interaction [129], [130].
Fig. 4.
Schematic representation of copper, zinc and iron coordination to Aβ. For Cu(II), only equatorial binding sites are shown.
4.1.2. Cu(II) coordination to the Aβ peptide
Copper is a redox-active ion, physiologically occurring mainly in two redox states: Cu(I) and Cu(II). The Cu(II) coordination to Aβ has been widely studied for years and was challenging as several species are formed depending on the pH. Numerous studies have been realized in the past decade and the results have been recently reviewed [125], [131], [132], [133], leading to a consensual model with different Cu(II) binding modes depending on the pH. The two major binding modes, called components I and II, observed around physiological pH, are shown in Fig. 4. For components I, it is now well-established that Cu(II) is bound to the NH2 terminus, to the adjacent CO function from Asp1-Ala2 and to imidazole rings of His6 and either His13 or His14 [134], [135], [136], [137], [138]. For component II, two distinct models have been proposed. In the first one, Cu(II) is bound to the carbonyl function from Ala2-Glu3 and to the imidazole rings of the three His residues [136], [138]. In the second one, Cu(II) is bound to the N-terminal amine of Asp1, to the amidyl function of Asp1-Ala2, to the carbonyl group of Ala2 and to the imidazole ring of one His residue [134], [135], [139]. Although reminiscent to the structure of Cu in the Cu,Zn-SOD, the first model does not explain the effect of pH on the coordination as all the residues involved in Cu(II) coordination that can undergo deprotonation are already deprotonated. The second model explains the change of Cu(II) binding mode that occurs around pH 7.8 with the deprotonation of the Asp1-Ala2 amide function, leading to its coordination. Furthermore, Electron Nuclear Double Resonance (ENDOR), Hyperfine Sublevel Correlation (HYSCORE) and NMR studies highlight the involvement of both the NH2 terminus of Asp1 and the deprotonated Asp1-Ala2 amide bond, favoring the second model (illustrated in Fig. 4) [131]. A carboxylate group has also been proposed to be involved in apical position for several components, coming from Asp1 [134], [135], [136] or from Glu3, Asp7 and Glu11 carboxylates in equilibrium with Asp1 for component I [135]. Numerous studies on Cu(II) affinity for Aβ have been reported (for reviews, see Arena & al. [140] and Zawisza & al. [133]). Depending on the method used, two ranges of affinity constants have been reported: 109−1010 M−1 for potentiometry and ITC studies, and 107−108 M−1 for Tyr10 fluorescence studies. This difference has been explained in a more recent paper, proposing that the affinity constant calculated from Tyr10 fluorescence experiments was underestimated because the inner-filter effect was not correctly taken into account [141]. Furthermore, a Cu(II) affinity constant in the 109 M−1 range has also been evaluated based on competition studies, in line with the affinity values from potentiometry and ITC [142].
4.1.3. Cu(I) coordination to the Aβ peptide
Cu(I) coordination with Aβ has been investigated more recently than Cu(II) coordination and the involvement of histidine residues is now consensual. Several binding models are suggested, two of them being most populated. The first model proposes a linear binding of histidine residues to Cu(I) with a dynamic exchange between His6, His13 and His14, while the second one involves an equilibrium between the His dyad and the His triad for Cu(I) coordination. NMR studies have shown the implication of the three histidine residues in the Cu(I) coordination with a dynamic exchange, in line with the two proposed models [135]. However, XAS studies [135], [143] and a comparison of synthetized Cu(I) complexes His-His dipeptides and Cu(I) complexes with truncated Aβ6–14 and Aβ10–14 peptides have validated the model involving a linear binding mode with 2 histidine residues [144], [145]. In addition, according to tandem mass spectrometry (MS/MS) studies on the Cu(I)-Aβ structure, the two histidine residues mostly involved in Cu(I) coordination would be His13 and His14 [146]. Thus, evidences suggest that Aβ is bound to Cu(I) by histidine residues in a linear fashion with a dynamic exchange between His6, His13 and His14, the major form being His13 and His14 dyad (Fig. 4). This is in line with affinity studies realized on three Cu(I) complexes with one His-Ala mutation on Aβ peptide (named H6A, H13A and H14A) [147], [148], [149] that point out a slightly lower affinity than for the native peptide, H6A having a stronger affinity than the other two mutants. These results indicate that Aβ only needs two histidine residues for binding Cu(I), His13-His14 dyad being the major form. To the best of our knowledge, only three studies have been carried out on Cu(I) affinity for Aβ, leading to three very different affinity constants of 1015 M-1 [148], 1010.4 M-1 [149] and 107 M-1 [147]. The two last values are the most realistic ones and actually in agreement, the difference coming from the value of the formation constant taken into account by the authors for the competitor used (i.e. ferrozine) for evaluating Cu(I) affinity for Aβ. More investigations have still to be done to determine more precisely the affinity constant and evaluate the biological relevance of the Cu(I)-peptide interaction.
4.1.4. Fe(II) coordination to the Aβ peptide
Very few structural studies on iron coordination, mainly as Fe(II) and Fe(III) ion, to Aβ have been reported. Fe(III) coordination to Aβ is not possible at physiological pH because of the formation of the highly stable Fe(III)(OH)3 precipitate [150]. For Fe(II)-Aβ coordination, to the best of our knowledge, only one study has been performed by using 1H, 13C and 2D NMR, highlighting the involvement of Asp1, Glu3, the three His but neither Tyr10 nor Met35 in Fe(II) sphere [151]. A comparison of the NMR data of component I of Cu(II)-Aβ (for which the coordination mode is well established) with the NMR data obtained for Fe(II)-Aβ, a preferred coordination mode has been proposed (Fig. 4). Both the terminal amine and the carboxylate group of Asp1, the Asp1-Ala2 and His6-Asp7C˭O peptide bonds, the imidazole ring of His6 as well as the one of either His13 or His14 are proposed to be involved in Fe(II)-Aβ. Further investigations would be needed in order to validate this proposition of binding mode and to evaluate its affinity for Aβ as well as to determine the affinity constant of Fe(II) for Aβ.
4.2. ROS production by Aβ-metals
Redox active metal ions such as copper and iron are known to be involved in ROS production. In the presence of a reducing agent, they can have a catalytic activity, by cycling between two redox states [152]. Cu and Fe can be coordinated to Aβ, as detailed above, and the resulting complex could be directly involved in ROS production, thus establishing a direct link between AD and oxidative stress. ROS production has been mostly studied with Cu-Aβ, because Fe-Aβ has a lower redox activity. [153] Iron is found in the amyloid plaques predominantly in a colloidal form (originating from ferritin), but histochemical studies indicate that it could also be bound to Aβ [154]. The coordination mode of Fe(II) with Aβ has been characterized (Fig. 4) [151] but Fe(III) does not form a stable complex with Aβ because it finally converts into Fe(III)(HO)3 and precipitates. Thus, the physiological stable formation of a binary Fe(III)-Aβ is unlikely. However, ROS production by Fe-Aβ still might be relevant as Fe(II)-Aβ is stable and the Fe(III) complex formed during ROS production might not have time to precipitate. As the involvement of iron bound to Aβ in ROS production is still unclear, we focus here only on Cu-Aβ.
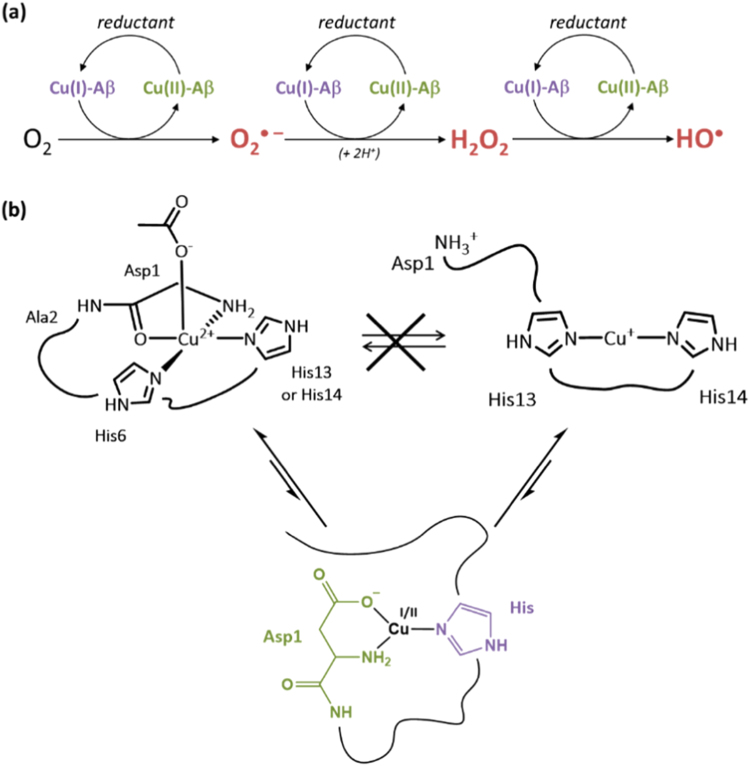
In the case of copper, the pro-oxidant role of the Cu-Aβ system is not clearly established because the complex is more active in ROS production than several biological relevant Cu-peptides or Cu-proteins [155] but less efficient than copper in buffer [4], [153], [155], [156], [157], [158]. However, latter is not very relevant biologically, as all Cu in biology is normally coordinated to a biomolecule. In vitro studies have shown that Cu-Aβ is able to catalyze the formation of H2O2 and HO• in the presence of O2 and a reducing agent such as ascorbate (Fig. 5a) [153], [155], [156], [159]. Although it was generally proposed that H2O2 production by Cu-Aβ occurs via a two-electron process, a recent study has highlighted the formation of superoxide as an intermediate in the production of H2O2 by Cu-Aβ and O2 [160].
Fig. 5.
(a) Mechanism of ROS production from a reductant and dioxygen catalyzed by the Cu-Aβ complex. The ROS produced are the superoxide anion (O2• −), hydrogen peroxide (H2O2) and the hydroxyl radical (HO•). (b) Top: Resting states that are the most populated states of Cu(II)-Aβ (left) and Cu(I)-Aβ (right). The redox reaction between these states is sluggish due to a high reorganization energy. Bottom: proposed Cu(I/II) environment in the catalytic in-between state [168].
Copper is redox-active and cycles between the +I and +II oxidative states when bound to Aβ. An electrochemistry study has shown that a preorganization mechanism was needed to allow the electron transfer for the oxidation of Cu(I) or the reduction of Cu(II) since the Cu(II) and Cu(I) coordination spheres are very different (Fig. 5b, top) [161]. The energy required for the rearrangement between the Cu(I) and Cu(II) geometries (linear and square-planar respectively) being very high, the electron transfer would rather proceed via a low-populated redox-competent state in which Cu(I) and Cu(II) binding modes are highly similar, thus inducing a low reorganization energy. This transient state, called here electrochemical in-between state, is in equilibrium with the resting states and represents about 1/1000 of all the species in solution.
The electron transfer during the metal-catalyzed ROS production has also been proposed to occur via a similar state, called here catalytic in-between state. The copper environment in that state as well as the reactivity towards the substrates (O2 or H2O2) have been investigated by computational studies [162], [163], [164], [165]. The nature of the amino acid residues involved in the catalytic in-between state has been studied with MS/MS by identifying the sites of oxidative damage on the peptide [166], since the latter is oxidized during the metal-catalyzed ROS production. By comparing the non-specific oxidations detected on Aβ28 after the radiation-induced ROS production with the copper-mediated oxidations of Aβ28, Asp1, His 13 and His14 have been found to be the metal-specific targeted amino acid residues. Furthermore, kinetic studies of the copper-mediated Aβ28 oxidation have shown that Asp1 would be the first amino acid residues damaged. Thus, in this study, the proposed ligands for both Cu(II) and Cu(I) coordination in the catalytic in-between state are Asp1, His 13 and His14. As they have been found to be the main targets for HO•, they are supposed to be the amino acid residues the closest from copper during the metal-catalyzed ROS production. A similar study performed with the full-length Aβ40 peptide has been reported recently, leading to the same conclusion [167].
Finally, in a recent paper, the evaluation of the ROS production by Cu bound to a wide series of modified peptides by fluorescence and UV–Vis–based methods has led to the proposition of a coordination model of the Cu-Aβ complex in the catalytic in-between state involved in ROS production [168]. The terminal amine and the carboxylate group of Asp1 as well as the imidazole group of one His are proposed to be involved in the coordination sphere of both Cu(I) and Cu(II), leading to the electron transfer with a minimal reorganization energy (Fig. 5b, bottom).
5. Oxidative damages undergone by the Aβ peptide
5.1. In vitro damage on Aβ residues during metal-catalyzed oxidation (MCO)
As discussed above, ROS are radicals and molecules deriving from the incomplete reduction of molecular oxygen. They are produced in small quantity during the in vivo metabolism of oxygen, through four successive 1-electron reductions of O2 leading to H2O formation. They are necessary to maintain the homeostasis in cells and play an important role in signaling [169] but are also reactive oxidants, able to damage biomolecules. In cells, endogenous enzymes are in charge of the antioxidant defense to prevent the ROS mediated damages. [46] The superoxide (O2•−) anion, produced by the one-electron reduction of dioxygen, is capable of inactivating few enzymes, [46] but has a poor reactivity with most of the bio-inorganic substrates due to low rate constant (usually below 102 L mol-1 s-1). [170], [171] Hydrogen peroxide (H2O2) is the product of the one-electron reduction of superoxide. It can oxidize proteins with thiol groups and is deleterious in the presence of redox-active metal ions such as iron and copper as it can produce the hydroxyl radical during the Fenton or Haber-Weiss reaction. H2O2 is regulated in vivo by two enzymes (catalase and glutathione peroxidase). The hydroxyl radical (HO•) is the result of the third one-electron reduction of oxygen, and can be produced in the presence of metal ions from H2O2. HO• has a very short half-life (10-9 s) compared with O2•− (10-6 s) and is thus the more reactive and deleterious ROS, [169] being able to oxidize the biomolecules such as proteins, lipids, DNA [172] because of its very high redox potential (E°' = 2.34 V [173]). To control the quantity of pro-oxidants (ROS) and prevent the damages on the biomolecules, the body has protecting mechanisms including enzymatic and chemical antioxidants. However, in some diseases such as AD [174], an imbalance may occur between pro-oxidants and antioxidants, due to a higher ROS production or a reduced activity of the enzymes responsible for the ROS degradation, leading to oxidative damages on biomolecules [175].
During the metal-catalyzed ROS production, the Aβ peptide undergoes oxidative damages. This is in line with the detection of oxidized Aβ in amyloid plaques in vivo [176]. Studies on single amino acid residue oxidations could allow a prediction on the residues targeted during the MCO of Aβ [177], [178], [179]. The physiological main targets for HO• are the sulfur-containing amino acids (methionine, cysteine), the basic amino acids (arginine, histidine, lysine) and the aromatic amino acids (phenylalanine, tyrosine, tryptophan) [180]. Table 1 provides the main oxidation products of these amino acid residues. Oxidation of Aβ28 by HO• produced by γ-radiolysis has shown that His and Phe residues are mainly targeted [166], in line with the oxidations reported previously for free amino acid residues. However, in the case of MCO of Aβ, the ROS are produced at the metal center. Thus, the oxidations are site-specific and can differ from the amino acid oxidations usually detected without metal ion.
Table 1.
Main oxidation products of the principal amino acid residues undergoing HO• attack [180].
| Amino acid residue | 3-letter abbreviation | Products of oxidation by HO• |
|---|---|---|
| Cysteine | Cys | Cysteic acid |
| Cystine | ||
| Methionine | Met | Methionine sulfoxide |
| Methionine sulfone | ||
| Arginine | Arg | 5-hydroxy-2-amino valeric acid |
| Histidine | His | 2-oxohistidine |
| Lysine | Lys | 3,4 or 5-hydroxylysine |
| Phenylalanine | Phe | 2-hydroxyphenylalanine |
| Tryptophan | Trp | N'-Formylkynurenine |
| Kynurenine | ||
| Tyrosine | Tyr | Dihydroxyphenylalanine (DOPA) |
| Dityrosine |
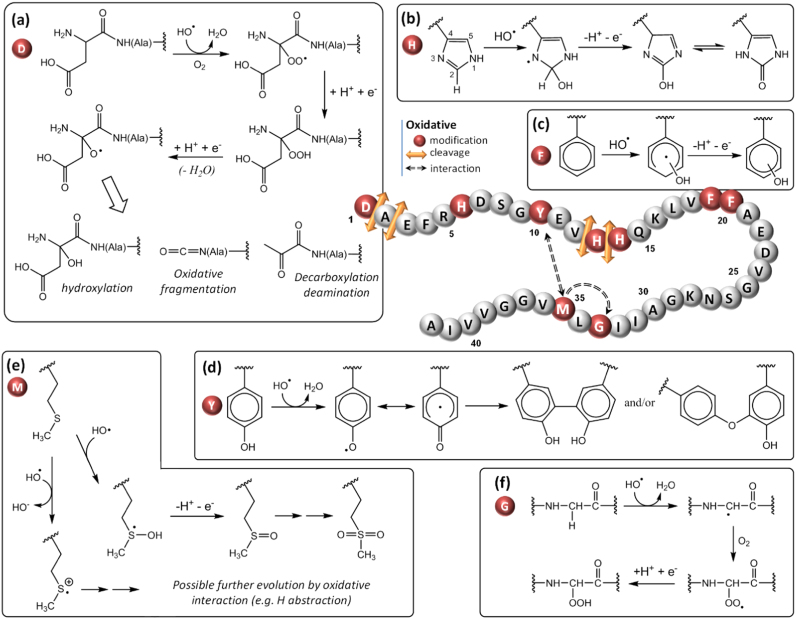
Several studies have reported the damages undergone by the Aβ peptide during the copper-mediated oxidation. The amino acid residues damaged are summarized in Fig. 6 and further described in the following paragraphs.
5.1.1. Oxidation of aspartate
The Aβ peptide has 3 aspartate residues at positions 1, 7 and 23. In the literature, only Asp1 has been found to be oxidized during MCO of Aβ. Asp1 is involved in the coordination of Cu(II) in the resting state [131], [132] and its involvement in the Cu sphere during ROS production has also been proposed [166], [168]. Thus, it would appear as a preferential target for the hydroxyl radical produced at the metal center. Several oxidative damages have been detected during MCO of Asp1 both in the presence of ascorbate [166], [167], [181] and of hydrogen peroxide [182]. Fig. 6a summarizes the oxidative mechanism leading to the formation of either pyruvate, isocyanate or 2-hydroxyaspartate function through the formation of an alkoxyl radical. The formation of a pyruvate function upon Asp1 oxidation was previously detected during MCO of Aβ [166], [181], [182] and would proceed through the α-amidation pathway [177]. The intermediate alkoxyl radical is generated from the hydroperoxide function by reaction that could involve the hydroperoxyl radical (HO2•), the protonated form of the superoxide anion [177]. Asp1 is also subject to a backbone cleavage on the α-position of the peptide [141], [153], leading to an isocyanate function in a reaction mechanism proceeding through the diamine pathway [177]. Asp1 was also found to convert into 2-hydroxyaspartate upon MCO, which corresponds to the formal addition of an oxygen atom [166].
Fig. 6.
Schematic view of the different oxidative modifications (red spheres), cleavages (orange arrows) and interactions (dashed arrows) undergone by the Aβ1–42 peptide during the copper-mediated oxidation. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.).
5.1.2. Oxidation of histidine
The Aβ peptide contains 3 histidine residues located at position 6, 13 and 14. They are involved in both Cu(II) and Cu(I) coordination in the resting states by their imidazole ring, and it has been proposed that they are involved in the Cu sphere during ROS production [166], [168]. Histidine residues s have been found oxidized into 2-oxohistidine (Fig. 6b) during MCO of Aβ bound to copper in the presence of ascorbate [156], [166], [167], [183], [184] or hydrogen peroxide [182]. The reaction mechanism of histidine oxidation by HO• radicals starts with an attack at the C-2 position of the imidazole ring [185], [186] (Fig. 6b). The resulting hydroxyhistidinyl radical generated would be further oxidized into 2-oxohistidine after reaction with Cu(II), that is itself reduced in Cu(I) [185]. His13 and His14 have been found to be more sensitive to oxidation, His6 being not detected on its oxidized form [166], [182], [184] or affected after longer oxidation time [183]. His13 and His14 were also found converted into dehydrooxohistidine after catalytic photooxygenation, in the absence of a metal ion [187].
5.1.3. Oxidation of phenylalanine
Three phenylalanines are present in the Aβ sequence at positions 4, 19 and 20. None of them is involved in the Cu(II) or Cu(I) coordination, nevertheless Phe19 and Phe20 have been found oxidized during MCO of Aβ in the presence of Cu(II) and ascorbate. [166], [167] Phe19 and Phe20 has been detected with the formal addition of an oxygen atom, likely oxidized into hydroxyphenylalanine (Fig. 6c). [177] This oxidation seems to occur after the oxidation of Asp1 which is involved in Cu binding [166].
5.1.4. Oxidation of tyrosine
Although the amino acid residues involved in copper coordination are more vulnerable to oxidation, non-coordinating amino acid residues can also be oxidized. It is the case for Tyr10 which is sensitive to oxidation and is responsible for the Aβ peptide cross-linking by dityrosine formation (Fig. 6d). [177] The latter process, induced by Cu(II), has been detected for Aβ in the presence of H2O2 [188]. MCO of Tyr10 into dityrosine was found to have an impact on aggregation as Aβ cross-linking was correlated with the formation of covalent oligomers [189], [190]. Furthermore, a study has proposed that Tyr10 acts as a gate that promotes the electron transfer from Met35 to Cu(II) for its reduction in Cu(I) [191]. However, this is in contradiction with the stability of Cu(II)-Aβ reported by several group.
5.1.5. Oxidation of methionine
Methionine is an amino acid residue very sensitive to oxidation. In vivo, the enzyme methionine sulfoxide reductase is responsible for the reduction of the methionine sulfoxide (Fig. 6e), a main oxidized form of the methionine [192]. Methionine can also be converted into sulfuranyl / hydroxysulfuranyl radical cation by a one-electron oxidation [193]. Reviews have reported about oxidation of the methionine of the Aβ peptide located at position 35 and its role in toxicity and oxidative stress [194], [195]. Although methionine is very sensitive to oxidation, its conversion into methionine sulfoxide occurs only after the oxidation of His13 and His14 during the in vitro MCO of Aβ in the presence of Cu(II)/ascorbate [183]. This highlights the site-specificity of the amino acid residue oxidation catalyzed by the bound copper. Met35 has also been found to promote Tyr10 oxidation [196] and to interact with Gly33, inducing its peroxidation by promoting the formation of a carbon-centered radical, leading to a hydroperoxide [158], [197]. However, this particular mechanisms would have to be confirmed by further independent studies. Fig. 6f shows the general mechanism of hydroperoxide formation after H abstraction by the hydroxyl radical. The sulfuranyl radical generated by primary oxidation of Met35 (Fig. 6e) is able to induce a similar H abstraction.
5.1.6. Other cleavages
Other oxidative cleavages have been reported for Aβ bound to Cu(II) in the presence of H2O2 such as the cleavage of the peptide bond of Asp1/Ala2, Ala2/Glu3, Val12/His13 or His13/His14 [182].
5.2. In vivo characterization of Aβ oxidation
Purification and characterization of Aβ peptides from in vivo samples is a crucial objective when investigating the etiology of AD. It is essential in order to ascertain the biological relevance of the results obtained in vitro. However, this is a challenging process from its beginning. The complexity of the biological samples (cerebrospinal fluid, brain tissue, or serum), their low content in Aβ peptides, the specific physicochemical properties of these peptides (i.e. tendency to aggregate and to bind to other proteins [198] and to adsorb to the surface of the laboratory tubes [199]), among others, make this purpose very arduous. Furthermore, it becomes more difficult again when the final aim is the identification of the oxidative damages potentially undergone by the Aβ peptides. First, the amount of oxidized Aβ that can be expected in a biological sample is very low. Second, oxidative modifications can be induced in the proteome as a consequence of the purification and characterization procedures; for instance, oxidation of methionine residue in protein can be the result of such a non-wanted oxidation, as this residue is one of the most sensitive one to oxidation. Thus, many control samples are needed to avoid such artifact.
A soft and selective purification method such as immunoprecipitation combined with gel electrophoresis, immunoassays and/or mass spectrometry (MS) are commonly used for the purification and characterization of Aβ peptides from biological sources. Regarding the extraction protocols, the capture of the target peptides by using antibodies coupled to protein G coated magnetic beads has extensively been used, giving successful results [200], [201], [202]. To this end, the Aβ peptides are typically immunocaptured by using the antibodies 6E10, 6C3 [203] (both N-terminal) and 4G8 (whose epitope lies within amino acids 18–22 of the Aβ sequence, UniProtKB P05067[672-713]). Several extractions with different antibodies on the same sample are usually performed, making possible to target different fragments of the Aβ sequence. This approach allows not to lose the Aβ peptides which have undergone changes in their primary structure, therefore potentially lacking the sequence specifically recognized by a single antibody. As already stated before, the analysis of the recovered peptides is usually made by means of gel-based and/or MS-based techniques, and it was by following the latter strategy that Näslund and coworkers discovered that methionine sulfoxide (MetO) in Aβ1–40 is abundant in senile plaques [204]. They reported the presence of this species in the brain tissue from an individual with sporadic AD by identifying the Aβ peptides with immunoblotting (6E10 antibody directed to amino acids 4–9 of Aβ [205]) and subsequently characterizing them by electrospray ionisation – mass spectrometry (ESI-MS) of the non-digested purified Aβ. Although an unambiguous identification of a modified peptide could be more complicated, this work allowed them to assign a species with +16 mass units as the MetO. More recently, and confirming the previous assignation, the MetO at position 35 was detected in CSF by Portelius and collaborators by using the 6E10, 4G8 and 11A50-B10 (reactive to the C-terminus) antibodies in a first immunoprecipitation step, which was directly followed by a mass spectrometric analysis [206]. MALDI-TOF MS measurements were performed and the data were accurately evaluated with an in-house developed software in this study. Together with the hypotheses coming from in vitro studies already developed in previous sections, the role of Met35 oxidation in the neurotoxicity of the Aβ peptides was demonstrated to be critical in J20 mice (a transgenic mouse model for AD) expressing a mutated Aβ1–42 where a Leu was in place of the Met35 residue [207].
The difficulties previously exposed for these kind of analyses and the fact that, for years, the broadly used detection techniques were immunoassays (not suitable for detection and characterization of chemical modifications on peptides) are probably some of the reasons explaining the low number of papers reporting about oxidized Aβ in vivo. During the last decade there has been a shift from gel-based to MS-based proteomic studies, thus overcoming some of the methodological issues [200], [208], [209]. Further targeted proteomics studies should be conducted in order to assess the chemical nature of Aβ oxidized species, which will shed light into the comprehension of the consequences associated to the disease.
6. Perspective and future research
An important feature in AD is the presence of oxidative damages in neuronal lipids and proteins in particular, which clearly links oxidative stress to AD. Oxidative stress can be an early event in the etiology of AD, since markers of oxidation appears in mild cognitive impairment brain regions [210], [211]. It can have different origins, but the overproduction of ROS is considered as a major contribution. Loosely bound metal ions like copper and iron are very efficient catalysts for the production of ROS and an increase in loosely bound Cu has been described in AD [123]. Cu ions bound to Aβ might also be contributing to the observed oxidative stress in AD.
A part of recent research is interested in the characterization of the oxidative damages undergone by the Aβ peptide itself, and the way the oxidized peptides coordinate metal ions to further produce ROS [166], [168], [212]. First interest lies in the understanding of the consequences of ROS attack towards surrounding molecules and the Aβ peptide itself. Regarding the latter, oxidative damages would have consequences on metal ion coordination with further impact on ROS production by the oxidized Aβ peptide and on Aβ aggregation. Oxygenation of Aβ was previously reported as attenuating the formation of β-sheet rich fibrils for the Aβ1–42 peptide [187]. Current efforts in our laboratory are focusing on the impact of MCO of Aβ regarding the aggregation process, and the possibility that oxidation may favor the formation of small oligomeric species, known to be more toxic than fibril ones. The reorganization of Cu binding site upon MCO of Aβ has also been found to promote ROS production [212]. Another way of research relies in the possibility of developing innovative therapeutic strategies to fight against AD, based on the better knowledge about the mechanisms of ROS production associated with AD. Lots of in vitro studies have been devoted to developing chelating molecules able to prevent in particular Cu(II) induced ROS production by Aβ, with no convincing results to date. The question of direct therapeutic chelation of copper ions to fight against AD is thus still in debate [213]. Some of the novel approaches is now including the presence of zinc along with copper in chelating strategies, zinc being present in brain in higher content [214], [215]. And in parallel, antibody-based therapeutic strategies fighting against Aβ aggregates are now emerging and seem to be promising [216]. In this context, all the efforts for a better knowledge of the molecular mechanisms involved in AD etiology and for developing novel therapeutic strategies are welcome.
Acknowledgements
The authors acknowledge the French agency for research (ANR, grant ANR-13-BSV5-0016) for financial support. The ERC aLzINK grant (ERC-StG-638712) is acknowledged for financial support.
References
- 1.Sousa F.L., Thiergart T., Landan G., Nelson-Sathi S., Pereira I.A.C., Allen J.F., Lane N., Martin W.F. Early bioenergetic evolution. Philos. Trans. R. Soc. B: Biol. Sci. 2013;368(1622) doi: 10.1098/rstb.2013.0088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gorrini C., Harris I.S., Mak T.W. Modulation of oxidative stress as an anticancer strategy. Nat. Rev. Drug Discov. 2013;12(12):931–947. doi: 10.1038/nrd4002. [DOI] [PubMed] [Google Scholar]
- 3.Halliwell B., Gutteridge J.M.C. Clarendon Press; Oxford: 1989. Free Radicals in Biology and Medicine. [Google Scholar]
- 4.Chassaing S., Collin F., Dorlet P., Gout J., Hureau C., Faller P. Copper and heme-mediated abeta toxicity: redox chemistry, abeta oxidations and anti-ROS compounds. Curr. Top. Med. Chem. 2012;12(22):2573–2595. doi: 10.2174/1568026611212220011. [DOI] [PubMed] [Google Scholar]
- 5.Halliwell B. Oxidative stress and neurodegeneration: where are we now? J. Neurochem. 2006;97(6):1634–1658. doi: 10.1111/j.1471-4159.2006.03907.x. [DOI] [PubMed] [Google Scholar]
- 6.Alzheimer A., Stelzmann R.A., Schnitzlein H.N., Murtagh F.R. An English translation of Alzheimer's 1907 paper, "Uber eine eigenartige Erkankung der Hirnrinde". Clin. Anat. 1995;8(6):429–431. doi: 10.1002/ca.980080612. [DOI] [PubMed] [Google Scholar]
- 7.Goedert M., Spillantini M.G. A century of Alzheimer's disease. Science. 2006;314(5800):777–781. doi: 10.1126/science.1132814. [DOI] [PubMed] [Google Scholar]
- 8.M. Prince, A. Wimo, M. Guerchet, G. Ali, Y. Wu, M. Prina, World Alzheimer Report 2015. The global impact of dementia. An analysis of prevalence, incidence, cost and trends, Alzheimer’s Disease International, London, 2015.
- 9.B. Duthey, Background paper 6.11: Alzheimer disease and other dementias, Priority Medicines for Europe and the World. "A public Health Approach to Innovation", 2004, pp. 1–74.
- 10.Mattson M.P. Pathways towards and away from Alzheimer's disease. Nature. 2004;430(7000):631–639. doi: 10.1038/nature02621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Soto-Rojas L.O., de la Cruz-López F., Torres M.A.O., Viramontes-Pintos A., del Carmen Cárdenas-Aguayo M., Meraz-Ríos M.A., Salinas-Lara C., Florán-Garduño B., Luna-Muñoz J. Neuroinflammation and alteration of the blood-brain barrier in Alzheimers disease. In: Zerr I., editor. Alzheimer's Disease – Challenges for the Future. InTech; 2015. [Google Scholar]
- 12.Janicki S.C., Schupf N. Hormonal influences on cognition and risk for Alzheimer disease. Curr. Neurol. Neurosci. Rep. 2010;10(5):359–366. doi: 10.1007/s11910-010-0122-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Glenner G.G., Wong C.W. Alzheimer's disease: initial report of the purification and characterization of a novel cerebrovascular amyloid protein. Biochem. Biophys. Res. Commun. 1984;120(3):885–890. doi: 10.1016/s0006-291x(84)80190-4. [DOI] [PubMed] [Google Scholar]
- 14.Minati L., Edginton T., Bruzzone M.G., Giaccone G. Current concepts in Alzheimer's disease: a multidisciplinary review. Am. J. Alzheimer'S. Dis. Other Dement. 2009;24(2):95–121. doi: 10.1177/1533317508328602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Goedert M. NEURODEGENERATION. Alzheimer's and Parkinson's diseases: the prion concept in relation to assembled Abeta, tau, and alpha-synuclein. Science. 2015;349(6248):1255555. doi: 10.1126/science.1255555. [DOI] [PubMed] [Google Scholar]
- 16.Grundke-Iqbal I., Iqbal K., Tung Y.C., Quinlan M., Wisniewski H.M., Binder L.I. Abnormal phosphorylation of the microtubule-associated protein tau (tau) in Alzheimer cytoskeletal pathology. Proc. Natl. Acad. Sci. USA. 1986;83(13):4913–4917. doi: 10.1073/pnas.83.13.4913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Giraldo E., Lloret A., Fuchsberger T., Vina J. Abeta and tau toxicities in Alzheimer's are linked via oxidative stress-induced p38 activation: protective role of vitamin E. Redox Biol. 2014;2:873–877. doi: 10.1016/j.redox.2014.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jakob‐Roetne R., Jacobsen H. Alzheimer's disease: from pathology to therapeutic approaches. Angew. Chem. Int. Ed. 2009;48(17):3030–3059. doi: 10.1002/anie.200802808. [DOI] [PubMed] [Google Scholar]
- 19.Nalivaeva N.N., Turner A.J. The amyloid precursor protein: a biochemical enigma in brain development, function and disease. FEBS Lett. 2013;587(13):2046–2054. doi: 10.1016/j.febslet.2013.05.010. [DOI] [PubMed] [Google Scholar]
- 20.Chow V.W., Mattson M.P., Wong P.C., Gleichmann M. An overview of APP processing enzymes and products. NeuroMol. Med. 2010;12(1):1–12. doi: 10.1007/s12017-009-8104-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Willem M., Tahirovic S., Busche M.A., Ovsepian S.V., Chafai M., Kootar S., Hornburg D., Evans L.D., Moore S., Daria A., Hampel H., Muller V., Giudici C., Nuscher B., Wenninger-Weinzierl A., Kremmer E., Heneka M.T., Thal D.R., Giedraitis V., Lannfelt L., Muller U., Livesey F.J., Meissner F., Herms J., Konnerth A., Marie H., Haass C. eta-Secretase processing of APP inhibits neuronal activity in the hippocampus. Nature. 2015;526(7573):443–447. doi: 10.1038/nature14864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Haass C., Schlossmacher M.G., Hung A.Y., Vigo-Pelfrey C., Mellon A., Ostaszewski B.L., Lieberburg I., Koo E.H., Schenk D., Teplow D.B., Selkoe D.J. Amyloid β-peptide is produced by cultured cells during normal metabolism. Nature. 1992;359(6393):322–325. doi: 10.1038/359322a0. [DOI] [PubMed] [Google Scholar]
- 23.Saido T., Leissring M.A. Proteolytic degradation of amyloid beta-protein. Cold Spring Harb. Perspect. Med. 2012;2(6):a006379. doi: 10.1101/cshperspect.a006379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Puzzo D., Arancio O. Amyloid-beta peptide: Dr. Jekyll or Mr. Hyde? J. Alzheimers Dis. 2013;33(Suppl. 1):S111–S120. doi: 10.3233/JAD-2012-129033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Barber R.C. The genetics of Alzheimer's disease. Scientifica (Cairo) 2012;2012:246210. doi: 10.6064/2012/246210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wiseman F.K., Al-Janabi T., Hardy J., Karmiloff-Smith A., Nizetic D., Tybulewicz V.L., Fisher E.M., Strydom A. A genetic cause of Alzheimer disease: mechanistic insights from Down syndrome. Nat. Rev. Neurosci. 2015 doi: 10.1038/nrn3983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nasica-Labouze J., Nguyen P.H., Sterpone F., Berthoumieu O., Buchete N.-V., Coté S., De Simone A., Doig A.J., Faller P., Garcia A. Amyloid β protein and Alzheimer’s disease: when computer simulations complement experimental studies. Chem. Rev. 2015;115(9):3518–3563. doi: 10.1021/cr500638n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cruts M., Theuns J., Van Broeckhoven C. Locus-specific mutation databases for neurodegenerative brain diseases. Human. Mutat. 2012;33(9):1340–1344. doi: 10.1002/humu.22117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Karran E., Mercken M., De Strooper B. The amyloid cascade hypothesis for Alzheimer's disease: an appraisal for the development of therapeutics. Nat. Rev. Drug Discov. 2011;10(9):698–712. doi: 10.1038/nrd3505. [DOI] [PubMed] [Google Scholar]
- 30.Holtzman D.M., Morris J.C., Goate A.M. Alzheimer’s disease: the challenge of the second century. Sci. Transl. Med. 2011;3(77) doi: 10.1126/scitranslmed.3002369. (77sr1-77sr1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hardy J., Allsop D. Amyloid deposition as the central event in the aetiology of Alzheimer's disease. Trends Pharmacol. Sci. 1991;12:383–388. doi: 10.1016/0165-6147(91)90609-v. [DOI] [PubMed] [Google Scholar]
- 32.Selkoe D.J. The molecular pathology of Alzheimer's disease. Neuron. 1991;6(4):487–498. doi: 10.1016/0896-6273(91)90052-2. [DOI] [PubMed] [Google Scholar]
- 33.Beyreuther K., Masters C.L. Amyloid precursor protein (APP) and beta A4 amyloid in the etiology of Alzheimer's disease: precursor-product relationships in the derangement of neuronal function. Brain Pathol. 1991;1(4):241–251. doi: 10.1111/j.1750-3639.1991.tb00667.x. [DOI] [PubMed] [Google Scholar]
- 34.Hardy J.A., Higgins G.A. Alzheimer's disease: the amyloid cascade hypothesis. Science. 1992;256(5054):184. doi: 10.1126/science.1566067. [DOI] [PubMed] [Google Scholar]
- 35.Selkoe D.J., Hardy J. The amyloid hypothesis of Alzheimer's disease at 25 years. EMBO Mol. Med. 2016;8:595–608. doi: 10.15252/emmm.201606210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Reitz C. Alzheimer's disease and the amyloid cascade hypothesis: a critical review. Int. J. Alzheimer’s Dis. 2012;2012 doi: 10.1155/2012/369808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Herrup K. The case for rejecting the amyloid cascade hypothesis. Nat. Neurosci. 2015:794–799. doi: 10.1038/nn.4017. [DOI] [PubMed] [Google Scholar]
- 38.Ding F., Borreguero J.M., Buldyrey S.V., Stanley H.E., Dokholyan N.V. Mechanism for the α-helix to β-hairpin transition. Protein.: Struct. Funct. Bioinforma. 2003;53(2):220–228. doi: 10.1002/prot.10468. [DOI] [PubMed] [Google Scholar]
- 39.Pham E., Crews L., Ubhi K., Hansen L., Adame A., Cartier A., Salmon D., Galasko D., Michael S., Savas J.N., Yates J.R., Glabe C., Masliah E. Progressive accumulation of amyloid-beta oligomers in Alzheimer's disease and in amyloid precursor protein transgenic mice is accompanied by selective alterations in synaptic scaffold proteins. FEBS J. 2010;277(14):3051–3067. doi: 10.1111/j.1742-4658.2010.07719.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Forloni G., Artuso V., La Vitola P., Balducci C. Oligomeropathies and pathogenesis of Alzheimer and Parkinson's diseases. Mov. Disord. 2016 doi: 10.1002/mds.26624. [DOI] [PubMed] [Google Scholar]
- 41.Deshpande A., Mina E., Glabe C., Busciglio J. Different conformations of amyloid β induce neurotoxicity by distinct mechanisms in human cortical neurons. J. Neurosci. 2006;26(22):6011–6018. doi: 10.1523/JNEUROSCI.1189-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Glabe C.G. Common mechanisms of amyloid oligomer pathogenesis in degenerative disease. Neurobiol. Aging. 2006;27(4):570–575. doi: 10.1016/j.neurobiolaging.2005.04.017. [DOI] [PubMed] [Google Scholar]
- 43.Lovell M.A., Robertson J.D., Teesdale W.J., Campbell J.L., Markesbery W.R. Copper, iron and zinc in Alzheimer's disease senile plaques. J. Neurol. Sci. 1998;158(1):47–52. doi: 10.1016/s0022-510x(98)00092-6. [DOI] [PubMed] [Google Scholar]
- 44.Tiiman A., Palumaa P., Tõugu V. The missing link in the amyloid cascade of Alzheimer’s disease – metal ions. Neurochem. Int. 2013;62(4):367–378. doi: 10.1016/j.neuint.2013.01.023. [DOI] [PubMed] [Google Scholar]
- 45.Faller P., Hureau C., Berthoumieu O. Role of metal ions in the self-assembly of the Alzheimer’s amyloid-β peptide. Inorg. Chem. 2013;52(21):12193–12206. doi: 10.1021/ic4003059. [DOI] [PubMed] [Google Scholar]
- 46.Bayir H. Reactive oxygen species. Crit. Care Med. 2005;33(12):S498–S501. doi: 10.1097/01.ccm.0000186787.64500.12. [DOI] [PubMed] [Google Scholar]
- 47.Butterfield D.A., Bader Lange M.L., Sultana R. Involvements of the lipid peroxidation product, HNE, in the pathogenesis and progression of Alzheimer's disease. Biochim. Biophys. Acta. 2010;1801(8):924–929. doi: 10.1016/j.bbalip.2010.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wang X., Wang W., Li L., Perry G., Lee H.G., Zhu X. Oxidative stress and mitochondrial dysfunction in Alzheimer's disease. Biochim. Biophys. Acta. 2014;1842(8):1240–1247. doi: 10.1016/j.bbadis.2013.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Butterfield D.A., Lauderback C.M. Lipid peroxidation and protein oxidation in Alzheimer's disease brain: potential causes and consequences involving amyloid beta-peptide-associated free radical oxidative stress. Free Radic. Biol. Med. 2002;32(11):1050–1060. doi: 10.1016/s0891-5849(02)00794-3. [DOI] [PubMed] [Google Scholar]
- 50.Sultana R., Boyd-Kimball D., Poon H.F., Cai J., Pierce W.M., Klein J.B., Merchant M., Markesbery W.R., Butterfield D.A. Redox proteomics identification of oxidized proteins in Alzheimer's disease hippocampus and cerebellum: an approach to understand pathological and biochemical alterations in AD. Neurobiol. Aging. 2006;27(11):1564–1576. doi: 10.1016/j.neurobiolaging.2005.09.021. [DOI] [PubMed] [Google Scholar]
- 51.Hensley K., Hall N., Subramaniam R., Cole P., Harris M., Aksenov M., Aksenova M., Gabbita S.P., Wu J.F., Carney J.M. Brain regional correspondence between Alzheimer's disease histopathology and biomarkers of protein oxidation. J. Neurochem. 1995;65(5):2146–2156. doi: 10.1046/j.1471-4159.1995.65052146.x. [DOI] [PubMed] [Google Scholar]
- 52.Butterfield D.A., Yatin S.M., Varadarajan S., Koppal T. Amyloid beta-peptide-associated free radical oxidative stress, neurotoxicity, and Alzheimer's disease. Methods Enzymol. 1999;309:746–768. doi: 10.1016/s0076-6879(99)09050-3. [DOI] [PubMed] [Google Scholar]
- 53.Butterfield D.A. The 2013 SFRBM discovery award: selected discoveries from the butterfield laboratory of oxidative stress and its sequela in brain in cognitive disorders exemplified by Alzheimer disease and chemotherapy induced cognitive impairment. Free Radic. Biol. Med. 2014;74:157–174. doi: 10.1016/j.freeradbiomed.2014.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhao Y., Zhao B. Oxidative stress and the pathogenesis of Alzheimer's disease. Oxid. Med. Cell. Longev. 2013;2013:316523. doi: 10.1155/2013/316523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Selfridge J.E., E L., Lu J., Swerdlow R.H. Role of mitochondrial homeostasis and dynamics in Alzheimer's disease. Neurobiol. Dis. 2013;51:3–12. doi: 10.1016/j.nbd.2011.12.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Butterfield D.A., Reed T., Newman S.F., Sultana R. Roles of amyloid beta-peptide-associated oxidative stress and brain protein modifications in the pathogenesis of Alzheimer's disease and mild cognitive impairment. Free Radic. Biol. Med. 2007;43(5):658–677. doi: 10.1016/j.freeradbiomed.2007.05.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Granold M., Moosmann B., Staib-Lasarzik I., Arendt T., Del Rey A., Engelhard K., Behl C., Hajieva P. High membrane protein oxidation in the human cerebral cortex. Redox Biol. 2015;4:200–207. doi: 10.1016/j.redox.2014.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gow A.J., Duran D., Malcolm S., Ischiropoulos H. Effects of peroxynitrite-induced protein modifications on tyrosine phosphorylation and degradation. FEBS Lett. 1996;385(1–2):63–66. doi: 10.1016/0014-5793(96)00347-x. [DOI] [PubMed] [Google Scholar]
- 59.Markesbery W.R., Lovell M.A. Four-hydroxynonenal, a product of lipid peroxidation, is increased in the brain in Alzheimer's disease. Neurobiol. Aging. 1998;19(1):33–36. doi: 10.1016/s0197-4580(98)00009-8. [DOI] [PubMed] [Google Scholar]
- 60.Hardas S.S., Sultana R., Clark A.M., Beckett T.L., Szweda L.I., Murphy M.P., Butterfield D.A. Oxidative modification of lipoic acid by HNE in Alzheimer disease brain. Redox Biol. 2013;1:80–85. doi: 10.1016/j.redox.2013.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sayre L.M., Zelasko D.A., Harris P.L., Perry G., Salomon R.G., Smith M.A. 4-Hydroxynonenal-derived advanced lipid peroxidation end products are increased in Alzheimer's disease. J. Neurochem. 1997;68(5):2092–2097. doi: 10.1046/j.1471-4159.1997.68052092.x. [DOI] [PubMed] [Google Scholar]
- 62.Mecocci P., MacGarvey U., Beal M.F. Oxidative damage to mitochondrial DNA is increased in Alzheimer's disease. Ann. Neurol. 1994;36(5):747–751. doi: 10.1002/ana.410360510. [DOI] [PubMed] [Google Scholar]
- 63.Gabbita S.P., Lovell M.A., Markesbery W.R. Increased nuclear DNA oxidation in the brain in Alzheimer's disease. J. Neurochem. 1998;71(5):2034–2040. doi: 10.1046/j.1471-4159.1998.71052034.x. [DOI] [PubMed] [Google Scholar]
- 64.Lovell M.A., Soman S., Bradley M.A. Oxidatively modified nucleic acids in preclinical Alzheimer's disease (PCAD) brain. Mech. Ageing Dev. 2011;132(8–9):443–448. doi: 10.1016/j.mad.2011.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Coppede F., Migliore L. DNA damage in neurodegenerative diseases. Mutat. Res. 2015;776:84–97. doi: 10.1016/j.mrfmmm.2014.11.010. [DOI] [PubMed] [Google Scholar]
- 66.Shan X., Lin C.L. Quantification of oxidized RNAs in Alzheimer's disease. Neurobiol. Aging. 2006;27(5):657–662. doi: 10.1016/j.neurobiolaging.2005.03.022. [DOI] [PubMed] [Google Scholar]
- 67.Butterfield S.M., Lashuel H.A. Amyloidogenic protein-membrane interactions: mechanistic insight from model systems. Angew. Chem. Int. Ed. Engl. 2010;49(33):5628–5654. doi: 10.1002/anie.200906670. [DOI] [PubMed] [Google Scholar]
- 68.Castegna A., Lauderback C.M., Mohmmad-Abdul H., Butterfield D.A. Modulation of phospholipid asymmetry in synaptosomal membranes by the lipid peroxidation products, 4-hydroxynonenal and acrolein: implications for Alzheimer's disease. Brain Res. 2004;1004(1–2):193–197. doi: 10.1016/j.brainres.2004.01.036. [DOI] [PubMed] [Google Scholar]
- 69.Subramaniam R., Roediger F., Jordan B., Mattson M.P., Keller J.N., Waeg G., Butterfield D.A. The lipid peroxidation product, 4-hydroxy-2-trans-nonenal, alters the conformation of cortical synaptosomal membrane proteins. J. Neurochem. 1997;69(3):1161–1169. doi: 10.1046/j.1471-4159.1997.69031161.x. [DOI] [PubMed] [Google Scholar]
- 70.Tramutola A., Lanzillotta C., Perluigi M., Butterfield D.A. Oxidative stress, protein modification and Alzheimer disease. Brain Res. Bull. 2016 doi: 10.1016/j.brainresbull.2016.06.005. [DOI] [PubMed] [Google Scholar]
- 71.Aluise C.D., Robinson R.A., Cai J., Pierce W.M., Markesbery W.R., Butterfield D.A. Redox proteomics analysis of brains from subjects with amnestic mild cognitive impairment compared to brains from subjects with preclinical Alzheimer's disease: insights into memory loss in MCI. J. Alzheimers Dis. : JAD. 2011;23(2):257–269. doi: 10.3233/JAD-2010-101083. [DOI] [PubMed] [Google Scholar]
- 72.Perluigi M., Sultana R., Cenini G., Domenico F. Di, Memo M., Pierce W.M., Coccia R., Butterfield D.A. Redox proteomics identification of 4-hydroxynonenal-modified brain proteins in Alzheimer's disease: role of lipid peroxidation in Alzheimer's disease pathogenesis. Proteom. Clin. Appl. 2009;3(6):682–693. doi: 10.1002/prca.200800161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Reed T., Perluigi M., Sultana R., Pierce W.M., Klein J.B., Turner D.M., Coccia R., Markesbery W.R., Butterfield D.A. Redox proteomic identification of 4-hydroxy-2-nonenal-modified brain proteins in amnestic mild cognitive impairment: insight into the role of lipid peroxidation in the progression and pathogenesis of Alzheimer's disease. Neurobiol. Dis. 2008;30(1):107–120. doi: 10.1016/j.nbd.2007.12.007. [DOI] [PubMed] [Google Scholar]
- 74.Terni B., Boada J., Portero-Otin M., Pamplona R., Ferrer I. Mitochondrial ATP-synthase in the entorhinal cortex is a target of oxidative stress at stages I/II of Alzheimer's disease pathology. Brain Pathol. 2010;20(1):222–233. doi: 10.1111/j.1750-3639.2009.00266.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Schagger H., Ohm T.G. Human diseases with defects in oxidative phosphorylation. 2. F1F0 ATP-synthase defects in Alzheimer disease revealed by blue native polyacrylamide gel electrophoresis. Eur. J. Biochem. 1995;227(3):916–921. doi: 10.1111/j.1432-1033.1995.tb20219.x. [DOI] [PubMed] [Google Scholar]
- 76.Cha M.Y., Cho H.J., Kim C., Jung Y.O., Kang M.J., Murray M.E., Hong H.S., Choi Y.J., Choi H., Kim D.K., Kim J., Dickson D.W., Song H.K., Cho J.W., Yi E.C., Jin S.M., Mook-Jung I. Mitochondrial ATP synthase activity is impaired by suppressed O-GlcNAcylation in Alzheimer's disease. Hum. Mol. Genet. 2015;24(22):6492–6504. doi: 10.1093/hmg/ddv358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Chen Z., Zhong C. Decoding Alzheimer's disease from perturbed cerebral glucose metabolism: implications for diagnostic and therapeutic strategies. Prog. Neurobiol. 2013;108:21–43. doi: 10.1016/j.pneurobio.2013.06.004. [DOI] [PubMed] [Google Scholar]
- 78.Butterfield D.A., Di Domenico F., Barone E. Elevated risk of type 2 diabetes for development of Alzheimer disease: a key role for oxidative stress in brain. Biochim. Biophys. Acta. 2014;1842(9):1693–1706. doi: 10.1016/j.bbadis.2014.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Di Domenico F., Barone E., Perluigi M., Butterfield D.A. The triangle of death in Alzheimer's disease brain: the aberrant cross-talk among energy metabolism, mammalian target of rapamycin signaling, and protein homeostasis revealed by redox proteomics. Antioxid. Redox Signal. 2016 doi: 10.1089/ars.2016.6759. [DOI] [PubMed] [Google Scholar]
- 80.Barone E., Di Domenico F., Cassano T., Arena A., Tramutola A., Lavecchia M.A., Coccia R., Butterfield D.A., Perluigi M. Impairment of biliverdin reductase-A promotes brain insulin resistance in Alzheimer disease: a new paradigm. Free Radic. Biol. Med. 2016;91:127–142. doi: 10.1016/j.freeradbiomed.2015.12.012. [DOI] [PubMed] [Google Scholar]
- 81.Ito S., Ohtsuki S., Kamiie J., Nezu Y., Terasaki T. Cerebral clearance of human amyloid-beta peptide (1-40) across the blood-brain barrier is reduced by self-aggregation and formation of low-density lipoprotein receptor-related protein-1 ligand complexes. J. Neurochem. 2007;103(6):2482–2490. doi: 10.1111/j.1471-4159.2007.04938.x. [DOI] [PubMed] [Google Scholar]
- 82.Sagare A., Deane R., Bell R.D., Johnson B., Hamm K., Pendu R., Marky A., Lenting P.J., Wu Z., Zarcone T., Goate A., Mayo K., Perlmutter D., Coma M., Zhong Z., Zlokovic B.V. Clearance of amyloid-beta by circulating lipoprotein receptors. Nat. Med. 2007;13(9):1029–1031. doi: 10.1038/nm1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Jeynes B., Provias J. Evidence for altered LRP/RAGE expression in Alzheimer lesion pathogenesis. Curr. Alzheimer Res. 2008;5(5):432–437. doi: 10.2174/156720508785908937. [DOI] [PubMed] [Google Scholar]
- 84.Owen J.B., Sultana R., Aluise C.D., Erickson M.A., Price T.O., Bu G., Banks W.A., Butterfield D.A. Oxidative modification to LDL receptor-related protein 1 in hippocampus from subjects with Alzheimer disease: implications for Abeta accumulation in AD brain. Free Radic. Biol. Med. 2010;49:1798–1803. doi: 10.1016/j.freeradbiomed.2010.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Liu Q., Smith M.A., Avila J., DeBernardis J., Kansal M., Takeda A., Zhu X., Nunomura A., Honda K., Moreira P.I., Oliveira C.R., Santos M.S., Shimohama S., Aliev G., de la Torre J., Ghanbari H.A., Siedlak S.L., Harris P.L., Sayre L.M., Perry G. Alzheimer-specific epitopes of tau represent lipid peroxidation-induced conformations. Free Radic. Biol. Med. 2005;38(6):746–754. doi: 10.1016/j.freeradbiomed.2004.11.005. [DOI] [PubMed] [Google Scholar]
- 86.Horiguchi T., Uryu K., Giasson B.I., Ischiropoulos H., LightFoot R., Bellmann C., Richter-Landsberg C., Lee V.M., Trojanowski J.Q. Nitration of tau protein is linked to neurodegeneration in tauopathies. Am. J. Pathol. 2003;163(3):1021–1031. doi: 10.1016/S0002-9440(10)63462-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Violet M., Delattre L., Tardivel M., Sultan A., Chauderlier A., Caillierez R., Talahari S., Nesslany F., Lefebvre B., Bonnefoy E., Buee L., Galas M.C. A major role for Tau in neuronal DNA and RNA protection in vivo under physiological and hyperthermic conditions. Front. Cell. Neurosci. 2014;8:84. doi: 10.3389/fncel.2014.00084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Coppede F., Migliore L. Evidence linking genetics, environment, and epigenetics to impaired DNA repair in Alzheimer's disease. J. Alzheimer's Dis. : JAD. 2010;20(4):953–966. doi: 10.3233/JAD-2010-1415. [DOI] [PubMed] [Google Scholar]
- 89.Weissman L., Jo D.G., Sorensen M.M., de Souza-Pinto N.C., Markesbery W.R., Mattson M.P., Bohr V.A. Defective DNA base excision repair in brain from individuals with Alzheimer's disease and amnestic mild cognitive impairment. Nucleic Acids Res. 2007;35(16):5545–5555. doi: 10.1093/nar/gkm605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Su J.H., Deng G., Cotman C.W. Neuronal DNA damage precedes tangle formation and is associated with up-regulation of nitrotyrosine in Alzheimer's disease brain. Brain Res. 1997;774(1–2):193–199. doi: 10.1016/s0006-8993(97)81703-9. [DOI] [PubMed] [Google Scholar]
- 91.Beel A.J., Sakakura M., Barrett P.J., Sanders C.R. Direct binding of cholesterol to the amyloid precursor protein: an important interaction in lipid-Alzheimer's disease relationships? Biochim. Biophys. Acta. 2010;1801(8):975–982. doi: 10.1016/j.bbalip.2010.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Puglielli L., Konopka G., Pack-Chung E., Ingano L.A., Berezovska O., Hyman B.T., Chang T.Y., Tanzi R.E., Kovacs D.M. Acyl-coenzyme A: cholesterol acyltransferase modulates the generation of the amyloid beta-peptide. Nat. Cell Biol. 2001;3(10):905–912. doi: 10.1038/ncb1001-905. [DOI] [PubMed] [Google Scholar]
- 93.Bjorkhem I., Cedazo-Minguez A., Leoni V., Meaney S. Oxysterols and neurodegenerative diseases. Mol. Asp. Med. 2009;30(3):171–179. doi: 10.1016/j.mam.2009.02.001. [DOI] [PubMed] [Google Scholar]
- 94.Usui K., Hulleman J.D., Paulsson J.F., Siegel S.J., Powers E.T., Kelly J.W. Site-specific modification of Alzheimer's peptides by cholesterol oxidation products enhances aggregation energetics and neurotoxicity. Proc. Natl. Acad. Sci. USA. 2009;106(44):18563–18568. doi: 10.1073/pnas.0804758106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Murray I.V., Liu L., Komatsu H., Uryu K., Xiao G., Lawson J.A., Axelsen P.H. Membrane-mediated amyloidogenesis and the promotion of oxidative lipid damage by amyloid beta proteins. J. Biol. Chem. 2007;282(13):9335–9345. doi: 10.1074/jbc.M608589200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Zhang Q., Powers E.T., Nieva J., Huff M.E., Dendle M.A., Bieschke J., Glabe C.G., Eschenmoser A., Wentworth P., Jr., Lerner R.A., Kelly J.W. Metabolite-initiated protein misfolding may trigger Alzheimer's disease. Proc. Natl. Acad. Sci. USA. 2004;101(14):4752–4757. doi: 10.1073/pnas.0400924101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Klein W.L., Stine W.B., Jr., Teplow D.B. Small assemblies of unmodified amyloid beta-protein are the proximate neurotoxin in Alzheimer's disease. Neurobiol. Aging. 2004;25(5):569–580. doi: 10.1016/j.neurobiolaging.2004.02.010. [DOI] [PubMed] [Google Scholar]
- 98.Bjorkhem I. Crossing the barrier: oxysterols as cholesterol transporters and metabolic modulators in the brain. J. Intern. Med. 2006;260(6):493–508. doi: 10.1111/j.1365-2796.2006.01725.x. [DOI] [PubMed] [Google Scholar]
- 99.Abildayeva K., Jansen P.J., Hirsch-Reinshagen V., Bloks V.W., Bakker A.H., Ramaekers F.C., de Vente J., Groen A.K., Wellington C.L., Kuipers F., Mulder M. 24(S)-hydroxycholesterol participates in a liver X receptor-controlled pathway in astrocytes that regulates apolipoprotein E-mediated cholesterol efflux. J. Biol. Chem. 2006;281(18):12799–12808. doi: 10.1074/jbc.M601019200. [DOI] [PubMed] [Google Scholar]
- 100.Heverin M., Meaney S., Lutjohann D., Diczfalusy U., Wahren J., Bjorkhem I. Crossing the barrier: net flux of 27-hydroxycholesterol into the human brain. J. Lipid Res. 2005;46(5):1047–1052. doi: 10.1194/jlr.M500024-JLR200. [DOI] [PubMed] [Google Scholar]
- 101.Gamba P., Testa G., Sottero B., Gargiulo S., Poli G., Leonarduzzi G. The link between altered cholesterol metabolism and Alzheimer's disease. Ann. N. Y. Acad. Sci. 2012;1259:54–64. doi: 10.1111/j.1749-6632.2012.06513.x. [DOI] [PubMed] [Google Scholar]
- 102.Heverin M., Bogdanovic N., Lutjohann D., Bayer T., Pikuleva I., Bretillon L., Diczfalusy U., Winblad B., Bjorkhem I. Changes in the levels of cerebral and extracerebral sterols in the brain of patients with Alzheimer's disease. J. Lipid Res. 2004;45(1):186–193. doi: 10.1194/jlr.M300320-JLR200. [DOI] [PubMed] [Google Scholar]
- 103.Prasanthi J.R., Huls A., Thomasson S., Thompson A., Schommer E., Ghribi O. Differential effects of 24-hydroxycholesterol and 27-hydroxycholesterol on beta-amyloid precursor protein levels and processing in human neuroblastoma SH-SY5Y cells. Mol. Neurodegener. 2009;4:1. doi: 10.1186/1750-1326-4-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Gamba P., Guglielmotto M., Testa G., Monteleone D., Zerbinati C., Gargiulo S., Biasi F., Iuliano L., Giaccone G., Mauro A., Poli G., Tamagno E., Leonarduzzi G. Up-regulation of beta-amyloidogenesis in neuron-like human cells by both 24- and 27-hydroxycholesterol: protective effect of N-acetyl-cysteine. Aging Cell. 2014;13(3):561–572. doi: 10.1111/acel.12206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Leoni V., Caccia C. Oxysterols as biomarkers in neurodegenerative diseases. Chem. Phys. Lipids. 2011;164(6):515–524. doi: 10.1016/j.chemphyslip.2011.04.002. [DOI] [PubMed] [Google Scholar]
- 106.Testa G., Staurenghi E., Zerbinati C., Gargiulo S., Iuliano L., Giaccone G., Fanto F., Poli G., Leonarduzzi G., Gamba P. Changes in brain oxysterols at different stages of Alzheimer's disease: their involvement in neuroinflammation. Redox Biol. 2016;10:24–33. doi: 10.1016/j.redox.2016.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Chico L., Simoncini C., Lo Gerfo A., Rocchi A., Petrozzi L., Carlesi C., Volpi L., Tognoni G., Siciliano G., Bonuccelli U. Oxidative stress and APO E polymorphisms in Alzheimer's disease and in mild cognitive impairment. Free Radic. Res. 2013;47(8):569–576. doi: 10.3109/10715762.2013.804622. [DOI] [PubMed] [Google Scholar]
- 108.Fernandes M.A., Proenca M.T., Nogueira A.J., Grazina M.M., Oliveira L.M., Fernandes A.I., Santiago B., Santana I., Oliveira C.R. Influence of apolipoprotein E genotype on blood redox status of Alzheimer's disease patients. Int. J. Mol. Med. 1999;4(2):179–186. doi: 10.3892/ijmm.4.2.179. [DOI] [PubMed] [Google Scholar]
- 109.Jenner A.M., Lim W.L., Ng M.P., Wenk M.R., Shui G., Sharman M.J., Gandy S.E., Martins R.N. The effect of APOE genotype on brain levels of oxysterols in young and old human APOE epsilon2, epsilon3 and epsilon4 knock-in mice. Neuroscience. 2010;169(1):109–115. doi: 10.1016/j.neuroscience.2010.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Di Domenico F., Pupo G., Giraldo E., Badia M.C., Monllor P., Lloret A., Schinina M.E., Giorgi A., Cini C., Tramutola A., Butterfield D.A., Vina J., Perluigi M. Oxidative signature of cerebrospinal fluid from mild cognitive impairment and Alzheimer disease patients. Free Radic. Biol. Med. 2016;91:1–9. doi: 10.1016/j.freeradbiomed.2015.12.004. [DOI] [PubMed] [Google Scholar]
- 111.Faller P., Hureau C. A bioinorganic view of Alzheimer's disease: when misplaced metal ions (re)direct the electrons to the wrong target. Chemistry. 2012;18(50):15910–15920. doi: 10.1002/chem.201202697. [DOI] [PubMed] [Google Scholar]
- 112.Lutsenko S., Bhattacharjee A., Hubbard A.L. Copper handling machinery of the brain. Metallomics : Integr. Biomet. Sci. 2010;2(9):596–608. doi: 10.1039/c0mt00006j. [DOI] [PubMed] [Google Scholar]
- 113.Delangle P., Mintz E. Chelation therapy in Wilson's disease: from D-penicillamine to the design of selective bioinspired intracellular Cu(I) chelators. Dalton Trans. 2012;41(21):6359–6370. doi: 10.1039/c2dt12188c. [DOI] [PubMed] [Google Scholar]
- 114.Barnham K.J., Bush A.I. Biological metals and metal-targeting compounds in major neurodegenerative diseases. Chem. Soc. Rev. 2014;43(19):6727–6749. doi: 10.1039/c4cs00138a. [DOI] [PubMed] [Google Scholar]
- 115.Bonda D.J., Lee H.G., Blair J.A., Zhu X., Perry G., Smith M.A. Role of metal dyshomeostasis in Alzheimer's disease. Metallomics : Integr. Biomet. Sci. 2011;3(3):267–270. doi: 10.1039/c0mt00074d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Miller L.M., Wang Q., Telivala T.P., Smith R.J., Lanzirotti A., Miklossy J. Synchrotron-based infrared and X-ray imaging shows focalized accumulation of Cu and Zn co-localized with beta-amyloid deposits in Alzheimer's disease. J. Struct. Biol. 2006;155(1):30–37. doi: 10.1016/j.jsb.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 117.Leskovjan A.C., Lanzirotti A., Miller L.M. Amyloid plaques in PSAPP mice bind less metal than plaques in human Alzheimer's disease. Neuroimage. 2009;47(4):1215–1220. doi: 10.1016/j.neuroimage.2009.05.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Duce J.A., Tsatsanis A., Cater M.A., James S.A., Robb E., Wikhe K., Leong S.L., Perez K., Johanssen T., Greenough M.A., Cho H.H., Galatis D., Moir R.D., Masters C.L., McLean C., Tanzi R.E., Cappai R., Barnham K.J., Ciccotosto G.D., Rogers J.T., Bush A.I. Iron-export ferroxidase activity of beta-amyloid precursor protein is inhibited by zinc in Alzheimer's disease. Cell. 2010;142(6):857–867. doi: 10.1016/j.cell.2010.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Rogers J.T., Randall J.D., Cahill C.M., Eder P.S., Huang X., Gunshin H., Leiter L., McPhee J., Sarang S.S., Utsuki T., Greig N.H., Lahiri D.K., Tanzi R.E., Bush A.I., Giordano T., Gullans S.R. An iron-responsive element type II in the 5'-untranslated region of the Alzheimer's amyloid precursor protein transcript. J. Biol. Chem. 2002;277(47):45518–45528. doi: 10.1074/jbc.M207435200. [DOI] [PubMed] [Google Scholar]
- 120.Bellingham S.A., Lahiri D.K., Maloney B., La Fontaine S., Multhaup G., Camakaris J. Copper depletion down-regulates expression of the Alzheimer's disease amyloid-beta precursor protein gene. J. Biol. Chem. 2004;279(19):20378–20386. doi: 10.1074/jbc.M400805200. [DOI] [PubMed] [Google Scholar]
- 121.Dahms S.O., Konnig I., Roeser D., Guhrs K.H., Mayer M.C., Kaden D., Multhaup G., Than M.E. Metal binding dictates conformation and function of the amyloid precursor protein (APP) E2 domain. J. Mol. Biol. 2012;416(3):438–452. doi: 10.1016/j.jmb.2011.12.057. [DOI] [PubMed] [Google Scholar]
- 122.Multhaup G., Schlicksupp A., Hesse L., Beher D., Ruppert T., Masters C.L., Beyreuther K. The amyloid precursor protein of Alzheimer's disease in the reduction of copper(II) to copper(I) Science. 1996;271(5254):1406–1409. doi: 10.1126/science.271.5254.1406. [DOI] [PubMed] [Google Scholar]
- 123.James S.A., Volitakis I., Adlard P.A., Duce J.A., Masters C.L., Cherny R.A., Bush A.I. Elevated labile Cu is associated with oxidative pathology in Alzheimer disease. Free Radic. Biol. Med. 2012;52(2):298–302. doi: 10.1016/j.freeradbiomed.2011.10.446. [DOI] [PubMed] [Google Scholar]
- 124.Kozlowski H., Luczkowski M., Remelli M., Valensin D. Copper, zinc and iron in neurodegenerative diseases (Alzheimer's, Parkinson's and prion diseases) Coord. Chem. Rev. 2012;256(19–20):2129–2141. [Google Scholar]
- 125.Migliorini C., Porciatti E., Luczkowski M., Valensin D. Structural characterization of Cu 2+, Ni 2+ and Zn 2+ binding sites of model peptides associated with neurodegenerative diseases. Coord. Chem. Rev. 2012;256(1):352–368. [Google Scholar]
- 126.Tõugu V., Palumaa P. Coordination of zinc ions to the key proteins of neurodegenerative diseases: aβ, APP, α-synuclein and PrP. Coord. Chem. Rev. 2012;256(19):2219–2224. [Google Scholar]
- 127.Zirah S., Kozin S.A., Mazur A.K., Blond A., Cheminant M., Segalas-Milazzo I., Debey P., Rebuffat S. Structural changes of region 1-16 of the Alzheimer disease amyloid beta-peptide upon zinc binding and in vitro aging. J. Biol. Chem. 2006;281(4):2151–2161. doi: 10.1074/jbc.M504454200. [DOI] [PubMed] [Google Scholar]
- 128.Alies B., Conte-Daban A., Sayen Sp, Collin F., Kieffer I., Guillon E., Faller P., Hureau C. Zinc (II) binding site to the amyloid-β peptide: insights from spectroscopic studies with a wide series of modified peptides. Inorg. Chem. 2016;55(20):10499–10509. doi: 10.1021/acs.inorgchem.6b01733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Noel S., Bustos Rodriguez S., Sayen S., Guillon E., Faller P., Hureau C. Use of a new water-soluble Zn sensor to determine Zn affinity for the amyloid-beta peptide and relevant mutants. Metallomics : Integr. Biomet. Sci. 2014;6(7):1220–1222. doi: 10.1039/c4mt00016a. [DOI] [PubMed] [Google Scholar]
- 130.Talmard C., Bouzan A., Faller P. Zinc binding to amyloid-beta: isothermal titration calorimetry and Zn competition experiments with Zn sensors. Biochemistry. 2007;46(47):13658–13666. doi: 10.1021/bi701355j. [DOI] [PubMed] [Google Scholar]
- 131.Hureau C., Dorlet P. Coordination of redox active metal ions to the amyloid precursor protein and to amyloid-β peptides involved in Alzheimer disease. Part 2: dependence of Cu(II) binding sites with Aβ sequences. Coord. Chem. Rev. 2012;256(19–20):2175–2187. [Google Scholar]
- 132.Hureau C. Coordination of redox active metal ions to the amyloid precursor protein and to amyloid-β peptides involved in Alzheimer disease. Part 1: an overview. Coord. Chem. Rev. 2012;256(19–20):2164–2174. [Google Scholar]
- 133.Zawisza I., Rózga M., Bal W. Affinity of copper and zinc ions to proteins and peptides related to neurodegenerative conditions (Aβ, APP, α-synuclein, PrP) Coord. Chem. Rev. 2012;256(19):2297–2307. [Google Scholar]
- 134.Dorlet P., Gambarelli S., Faller P., Hureau C. Pulse EPR Spectroscopy Reveals The Coordination Sphere Of Copper(II) ions in the 1–16 amyloid-β peptide: a key role of the first two n-terminus residues. Angew. Chem. Int. Ed. 2009;48(49):9273–9276. doi: 10.1002/anie.200904567. [DOI] [PubMed] [Google Scholar]
- 135.Hureau C., Coppel Y., Dorlet P., Solari P.L., Sayen S., Guillon E., Sabater L., Faller P. Deprotonation of the Asp1- Ala2 peptide bond induces modification of the dynamic copper (II) environment in the amyloid‐β peptide near physiological pH. Angew. Chem. Int. Ed. 2009;48(50):9522–9525. doi: 10.1002/anie.200904512. [DOI] [PubMed] [Google Scholar]
- 136.Drew S.C., Masters C.L., Barnham K.J. Alanine-2 carbonyl is an oxygen ligand in Cu2+ coordination of Alzheimer’s disease amyloid-β peptide− relevance to N-terminally truncated forms. J. Am. Chem. Soc. 2009;131(25):8760–8761. doi: 10.1021/ja903669a. [DOI] [PubMed] [Google Scholar]
- 137.Drew S.C., Barnham K.J. The heterogeneous nature of Cu2+ interactions with Alzheimer’s amyloid-β peptide. Acc. Chem. Res. 2011;44(11):1146–1155. doi: 10.1021/ar200014u. [DOI] [PubMed] [Google Scholar]
- 138.Drew S.C., Noble C.J., Masters C.L., Hanson G.R., Barnham K.J. Pleomorphic copper coordination by Alzheimer’s disease amyloid-β peptide. J. Am. Chem. Soc. 2009;131(3):1195–1207. doi: 10.1021/ja808073b. [DOI] [PubMed] [Google Scholar]
- 139.Alies B., Eury H., Bijani C., Rechignat L., Faller P., Hureau C. pH-dependent Cu(II) coordination to amyloid-β peptide: impact of sequence alterations, including the H6R and D7N familial mutations. Inorg. Chem. 2011;50(21):11192–11201. doi: 10.1021/ic201739n. [DOI] [PubMed] [Google Scholar]
- 140.Arena G., Pappalardo G., Sovago I., Rizzarelli E. Copper(II) interaction with amyloid-β: affinity and speciation. Coord. Chem. Rev. 2012;256(1):3–12. [Google Scholar]
- 141.Alies B., Renaglia E., Rozga M., Bal W., Faller P., Hureau C. Cu(II) affinity for the Alzheimer's peptide: tyrosine fluorescence studies revisited. Anal. Chem. 2013;85(3):1501–1508. doi: 10.1021/ac302629u. [DOI] [PubMed] [Google Scholar]
- 142.Conte-Daban A., Borghesani V., Sayen S., Guillon E., Journaux Y., Gontard G., Lisnard L., Hureau C. Link between affinity and cu(ii) binding sites to amyloid-beta peptides evaluated by a new water-soluble UV–visible ratiometric dye with a moderate Cu(II) affinity. Anal. Chem. 2017;89(3):2155–2162. doi: 10.1021/acs.analchem.6b04979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Shearer J., Szalai V.A. The amyloid-beta peptide of Alzheimer's disease binds Cu(I) in a linear bis-his coordination environment: insight into a possible neuroprotective mechanism for the amyloid-beta peptide. J. Am. Chem. Soc. 2008;130(52):17826–17835. doi: 10.1021/ja805940m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Himes R.A., Park G.Y., Barry A.N., Blackburn N.J., Karlin K.D. Synthesis and X-ray absorption spectroscopy structural studies of Cu (I) complexes of histidylhistidine peptides: the predominance of linear 2-coordinate geometry. J. Am. Chem. Soc. 2007;129(17):5352–5353. doi: 10.1021/ja0708013. [DOI] [PubMed] [Google Scholar]
- 145.Himes R.A., Park G.Y., Siluvai G.S., Blackburn N.J., Karlin K.D. Structural studies of copper (i) complexes of amyloid‐β peptide fragments: formation of two‐coordinate bis (histidine) complexes. Angew. Chem. Int. Ed. 2008;47(47):9084–9087. doi: 10.1002/anie.200803908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Lu Y., Prudent M., Qiao L., Mendez M.A., Girault H.H. Copper(i) and copper(ii) binding to β-amyloid 16 (Aβ16) studied by electrospray ionization mass spectrometry. Metallomics. 2010;2(7) doi: 10.1039/c004693k. [DOI] [PubMed] [Google Scholar]
- 147.Alies B., Badei B., Faller P., Hureau C. Reevaluation of copper(i) affinity for amyloid-β peptides by competition with ferrozine—an unusual copper(i) indicator. Chem. – Eur. J. 2012;18(4):1161–1167. doi: 10.1002/chem.201102746. [DOI] [PubMed] [Google Scholar]
- 148.Feaga H.A., Maduka R.C., Foster M.N., Szalai V.A. Affinity of Cu+ for the copper-binding domain of the amyloid-β peptide of Alzheimer’s disease. Inorg. Chem. 2011;50(5):1614–1618. doi: 10.1021/ic100967s. [DOI] [PubMed] [Google Scholar]
- 149.Young T.R., Kirchner A., Wedd A.G., Xiao Z. An integrated study of the affinities of the Aβ16 peptide for Cu (I) and Cu (II): implications for the catalytic production of reactive oxygen species. Metallomics. 2014;6(3):505–517. doi: 10.1039/c4mt00001c. [DOI] [PubMed] [Google Scholar]
- 150.Valensin D., Migliorini C., Valensin G., Gaggelli E., La Penna G., Kozlowski H., Gabbiani C., Messori L. Exploring the reactions of beta-amyloid (Abeta) peptide 1-28 with Al(III) and Fe(III) ions. Inorg. Chem. 2011;50(15):6865–6867. doi: 10.1021/ic201069v. [DOI] [PubMed] [Google Scholar]
- 151.Bousejra-ElGarah F., Bijani C., Coppel Y., Faller P., Hureau C. Iron(II) binding to amyloid-β, the Alzheimer’s peptide. Inorg. Chem. 2011;50(18):9024–9030. doi: 10.1021/ic201233b. [DOI] [PubMed] [Google Scholar]
- 152.Halliwell B. Oxidative stress and neurodegeneration: where are we now? J. Neurochem. 2006;97(6):1634–1658. doi: 10.1111/j.1471-4159.2006.03907.x. [DOI] [PubMed] [Google Scholar]
- 153.Nakamura M., Shishido N., Nunomura A., Smith M.A., Perry G., Hayashi Y., Nakayama K., Hayashi T. Three histidine residues of amyloid-β peptide control the redox activity of copper and iron. Biochemistry. 2007;46(44):12737–12743. doi: 10.1021/bi701079z. [DOI] [PubMed] [Google Scholar]
- 154.Smith M.A., Harris P.L., Sayre L.M., Perry G. Iron accumulation in Alzheimer disease is a source of redox-generated free radicals. Proc. Natl. Acad. Sci. USA. 1997;94(18):9866–9868. doi: 10.1073/pnas.94.18.9866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Guilloreau L., Combalbert S., Sournia-Saquet A., Mazarguil H., Faller P. Redox chemistry of copper–amyloid-β: the generation of hydroxyl radical in the presence of ascorbate is linked to redox-potentials and aggregation state. ChemBioChem. 2007;8(11):1317–1325. doi: 10.1002/cbic.200700111. [DOI] [PubMed] [Google Scholar]
- 156.Nadal R.C., Rigby S.E.J., Viles J.H. Amyloid β–Cu2+ complexes in both monomeric and fibrillar forms do not generate H2O2 catalytically but quench hydroxyl radicals. Biochemistry. 2008;47(44):11653–11664. doi: 10.1021/bi8011093. [DOI] [PubMed] [Google Scholar]
- 157.Baruch-Suchodolsky R., Fischer B. Soluble amyloid β1-28-copper(I)/copper(II)/iron(II) complexes are potent antioxidants in cell-free systems. Biochemistry. 2008;47(30):7796–7806. doi: 10.1021/bi800114g. [DOI] [PubMed] [Google Scholar]
- 158.Hureau C., Faller P. Aβ-mediated ROS production by Cu ions: structural insights, mechanisms and relevance to Alzheimer's disease. Biochimie. 2009;91(10):1212–1217. doi: 10.1016/j.biochi.2009.03.013. [DOI] [PubMed] [Google Scholar]
- 159.Dikalov S.I., Vitek M.P., Mason R.P. Cupric–amyloid β peptide complex stimulates oxidation of ascorbate and generation of hydroxyl radical. Free Radic. Biol. Med. 2004;36(3):340–347. doi: 10.1016/j.freeradbiomed.2003.11.004. [DOI] [PubMed] [Google Scholar]
- 160.Reybier K., Ayala S., Alies B., Rodrigues J.V., Bustos Rodriguez S., La Penna G., Collin F., Gomes C.M., Hureau C., Faller P. Free superoxide is an intermediate in the production of H2O2 by copper(I)-abeta peptide and O2. Angew. Chem. Int. Ed. Engl. 2016;55(3):1085–1089. doi: 10.1002/anie.201508597. [DOI] [PubMed] [Google Scholar]
- 161.Balland V., Hureau C., Saveant J.-M. Electrochemical and homogeneous electron transfers to the Alzheimer amyloid-beta copper complex follow a preorganization mechanism. Proc. Natl. Acad. Sci. USA. 2010;107(40):17113–17118. doi: 10.1073/pnas.1011315107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.La Penna G., Hureau C., Andreussi O., Faller P. Identifying, By first-principles simulations, Cu[amyloid-β] species making fenton-type reactions in Alzheimer’s disease. J. Phys. Chem. B. 2013;117(51):16455–16467. doi: 10.1021/jp410046w. [DOI] [PubMed] [Google Scholar]
- 163.Mirats A., Alí-Torres J., Rodríguez-Santiago L., Sodupe M., La Penna G. Dioxygen activation in the Cu–amyloid β complex. Phys. Chem. Chem. Phys. 2015;17(41):27270–27274. doi: 10.1039/c5cp04025f. [DOI] [PubMed] [Google Scholar]
- 164.Furlan S., Hureau C., Faller P., La Penna G. Modeling copper binding to the amyloid-β peptide at different pH: toward a molecular mechanism for Cu reduction. J. Phys. Chem. B. 2012;116(39):11899–11910. doi: 10.1021/jp308977s. [DOI] [PubMed] [Google Scholar]
- 165.Prosdocimi T., De Gioia L., Zampella G., Bertini L. On the generation of OH·radical species from H2O2 by Cu (I) amyloid beta peptide model complexes: a DFT investigation. J. Biol. Inorg. Chem. 2016;21(2):197–212. doi: 10.1007/s00775-015-1322-y. [DOI] [PubMed] [Google Scholar]
- 166.Cassagnes L.-E., Hervé V., Nepveu F., Hureau C., Faller P., Collin F. The Catalytically Active Copper-Amyloid-Beta State: coordination Site Responsible for Reactive Oxygen Species Production. Angew. Chem. Int. Ed. 2013;52:11110–11113. doi: 10.1002/anie.201305372. [DOI] [PubMed] [Google Scholar]
- 167.Cheignon C., Hureau C., Collin F. Real-time evolution of Aβ40 metal-catalyzed oxidation reveals Asp1 as the main target and a dependence on metal binding site. Inorg. Chim. Acta. 2017 (In press) [Google Scholar]
- 168.Cheignon C., Jones M., Atrian-Blasco E., Kieffer I., Faller P., Collin F., Hureau C. Identification of key structural features of the elusive Cu-A[small beta] complex that generates ROS in Alzheimer's disease. Chem. Sci. 2017;8(7):5107–5118. doi: 10.1039/c7sc00809k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Devasagayam T., Tilak J., Boloor K., Sane K.S., Ghaskadbi S.S., Lele R. Free radicals and antioxidants in human health: current status and future prospects. JAPI. 2004;52(794804):4. [PubMed] [Google Scholar]
- 170.Gardès-Albert M., Jore D. Aspects physicochimiques des radicaux libres centrés sur l'oxygène. In: Delattre J., Beaudeux J.-L., Bonnefont-Rousselot D., editors. Radicaux Libres et Stress Oxydant : Aspects Biologiques et Pathologiques. Lavoisier; Paris: 2005. pp. 6–23. [Google Scholar]
- 171.Bielski B.H., Cabelli D.E., Arudi R.L., Ross A.B. Reactivity of HO2/O–2 radicals in aqueous solution. J. Phys. Chem. Ref. Data. 1985;14(4):1041–1100. [Google Scholar]
- 172.L.M. Dorfman, G.E. Adams, Reactivity of the hydroxyl radical in aqueous solutions, DTIC Document, 1973.
- 173.Wardman P. Reduction potentials of one-electron couples involving free radicals in aqueous solution. J. Phys. Chem. Ref. Data. 1989;18(4):1637–1755. [Google Scholar]
- 174.Markesbery W.R. Oxidative stress hypothesis in Alzheimer's disease. Free Radic. Biol. Med. 1997;23(1):134–147. doi: 10.1016/s0891-5849(96)00629-6. [DOI] [PubMed] [Google Scholar]
- 175.Butterfield D.A., Drake J., Pocernich C., Castegna A. Evidence of oxidative damage in Alzheimer's disease brain: central role for amyloid β-peptide. Trends Mol. Med. 2001;7(12):548–554. doi: 10.1016/s1471-4914(01)02173-6. [DOI] [PubMed] [Google Scholar]
- 176.Näslund J., Schierhorn A., Hellman U., Lannfelt L., Roses A.D., Tjernberg L.O., Silberring J., Gandy S.E., Winblad B., Greengard P. Relative abundance of Alzheimer A beta amyloid peptide variants in Alzheimer disease and normal aging. Proc. Natl. Acad. Sci. USA. 1994;91(18):8378–8382. doi: 10.1073/pnas.91.18.8378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Stadtman E.R., Levine R.L. Free radical-mediated oxidation of free amino acids and amino acid residues in proteins. Amino Acids. 2003;25(3–4):207–218. doi: 10.1007/s00726-003-0011-2. [DOI] [PubMed] [Google Scholar]
- 178.Stadtman E. Oxidation of free amino acids and amino acid residues in proteins by radiolysis and by metal-catalyzed reactions. Annu. Rev. Biochem. 1993;62(1):797–821. doi: 10.1146/annurev.bi.62.070193.004053. [DOI] [PubMed] [Google Scholar]
- 179.Stadtman E.R. Role of oxidized amino acids in protein breakdown and stability. Methods Enzymol. 1995;258:379–393. doi: 10.1016/0076-6879(95)58057-3. [DOI] [PubMed] [Google Scholar]
- 180.Bonnefont-Rousselot D., Beaudeux J.-L., Delattre J. Oxydation des acides aminés et des protéines. In: Delattre J., Beaudeux J.-L., Bonnefont-Rousselot D., editors. Radicaux Libres et Stress Oxydant : Aspects Biologiques et Pathologiques. Lavoisier; Paris: 2005. pp. 147–167. [Google Scholar]
- 181.Inoue K., Nakagawa A., Hino T., Oka H. Screening assay for metal-catalyzed oxidation inhibitors using liquid chromatography–mass spectrometry with an n-terminal β-amyloid peptide. Anal. Chem. 2009;81(5):1819–1825. doi: 10.1021/ac802162n. [DOI] [PubMed] [Google Scholar]
- 182.Kowalik-Jankowska T., Ruta M., Wiśniewska K., Łankiewicz L., Dyba M. Products of Cu(II)-catalyzed oxidation in the presence of hydrogen peroxide of the 1–10, 1–16 fragments of human and mouse β-amyloid peptide. J. Inorg. Biochem. 2004;98(6):940–950. doi: 10.1016/j.jinorgbio.2004.03.001. [DOI] [PubMed] [Google Scholar]
- 183.Schöneich C., Williams T.D. Cu(II)-catalyzed oxidation of β-amyloid peptide targets His13 and His14 over His6: detection of 2-Oxo-histidine by HPLC-MS/MS. Chem. Res. Toxicol. 2002;15(5):717–722. doi: 10.1021/tx025504k. [DOI] [PubMed] [Google Scholar]
- 184.Inoue K., Garner C., Ackermann B.L., Oe T., Blair I.A. Liquid chromatography/tandem mass spectrometry characterization of oxidized amyloid beta peptides as potential biomarkers of Alzheimer's disease. Rapid Commun. Mass Spectrom. 2006;20(5):911–918. doi: 10.1002/rcm.2395. [DOI] [PubMed] [Google Scholar]
- 185.Schöneich C. Mechanisms of metal-catalyzed oxidation of histidine to 2-oxo-histidine in peptides and proteins. J. Pharm. Biomed. Anal. 2000;21(6):1093–1097. doi: 10.1016/s0731-7085(99)00182-x. [DOI] [PubMed] [Google Scholar]
- 186.Schoneich C. Selective Cu2+/ascorbate-dependent oxidation of alzheimer's disease beta-amyloid peptides. Ann. N. Y. Acad. Sci. 2004;1012:164–170. doi: 10.1196/annals.1306.013. [DOI] [PubMed] [Google Scholar]
- 187.Taniguchi A., Sasaki D., Shiohara A., Iwatsubo T., Tomita T., Sohma Y., Kanai M. Attenuation of the aggregation and neurotoxicity of amyloid-beta peptides by catalytic photooxygenation. Angew. Chem. 2014;53(5):1382–1385. doi: 10.1002/anie.201308001. [DOI] [PubMed] [Google Scholar]
- 188.Atwood C.S., Perry G., Zeng H., Kato Y., Jones W.D., Ling K.-Q., Huang X., Moir R.D., Wang D., Sayre L.M., Smith M.A., Chen S.G., Bush A.I. Copper mediates dityrosine cross-linking of Alzheimer's amyloid-β. Biochemistry. 2004;43(2):560–568. doi: 10.1021/bi0358824. [DOI] [PubMed] [Google Scholar]
- 189.Gunn A.P., Roberts B.R., Bush A.I. Rapid generation of dityrosine cross-linked Aβ oligomers via Cu-redox cycling. In: Sigurdsson E.M., Calero M., Gasset M., editors. Amyloid Proteins. Humana Press; Totowa, NJ: 2012. pp. 3–10. [Google Scholar]
- 190.Al-Hilaly Y.K., Williams T.L., Stewart-Parker M., Ford L., Skaria E., Cole M., Bucher W.G., Morris K.L., Sada A.A., Thorpe J.R. A central role for dityrosine crosslinking of Amyloid-β in Alzheimer’s disease. Acta Neuropathol. Commun. 2013;1(1) doi: 10.1186/2051-5960-1-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Barnham K.J., Haeffner F., Ciccotosto G.D., Curtain C.C., Tew D., Mavros C., Beyreuther K., Carrington D., Masters C.L., Cherny R.A., Cappai R., Bush A.I. Tyrosine gated electron transfer is key to the toxic mechanism of Alzheimer's disease beta-amyloid. FASEB J. 2004;18(12):1427–1429. doi: 10.1096/fj.04-1890fje. [DOI] [PubMed] [Google Scholar]
- 192.Moskovitz J. Methionine sulfoxide reductase system in health and disease. Austin J. Pharmacol. Ther. 2014;2(3):3. [Google Scholar]
- 193.Schöneich C., Pogocki D., Hug G.L., Bobrowski K. Free radical reactions of methionine in peptides: mechanisms relevant to β-amyloid oxidation and Alzheimer's disease. J. Am. Chem. Soc. 2003;125(45):13700–13713. doi: 10.1021/ja036733b. [DOI] [PubMed] [Google Scholar]
- 194.Schöneich C. Methionine oxidation by reactive oxygen species: reaction mechanisms and relevance to Alzheimer's disease. Biochim Biophys. Acta. 2005;1703(2):111–119. doi: 10.1016/j.bbapap.2004.09.009. [DOI] [PubMed] [Google Scholar]
- 195.Butterfield D.A., Sultana R. Methionine-35 of aβ (1–42): importance for oxidative stress in Alzheimer disease. J. Amino Acids. 2011;2011 doi: 10.4061/2011/198430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Ali F.E., Separovic F., Barrow C.J., Cherny R.A., Fraser F., Bush A.I., Masters C.L., Barnham K.J. Methionine regulates copper/hydrogen peroxide oxidation products of Aβ. J. Pept. Sci. 2005;11(6):353–360. doi: 10.1002/psc.626. [DOI] [PubMed] [Google Scholar]
- 197.Kanski J., Varadarajan S., Aksenova M., Butterfield D.A. Role of glycine-33 and methionine-35 in Alzheimer’s amyloid β-peptide 1–42-associated oxidative stress and neurotoxicity, Biochimica et Biophysica Acta. Mol. Basis Dis. 2002;1586(2):190–198. doi: 10.1016/s0925-4439(01)00097-7. [DOI] [PubMed] [Google Scholar]
- 198.Slemmon J.R., Meredith J., Guss V., Andreasson U., Andreasen N., Zetterberg H., Blennow K. Measurement of Abeta1-42 in cerebrospinal fluid is influenced by matrix effects. J. Neurochem. 2012;120(2):325–333. doi: 10.1111/j.1471-4159.2011.07553.x. [DOI] [PubMed] [Google Scholar]
- 199.Perret-Liaudet A., Pelpel M., Tholance Y., Dumont B., Vanderstichele H., Zorzi W., Elmoualij B., Schraen S., Moreaud O., Gabelle A., Thouvenot E., Thomas-Anterion C., Touchon J., Krolak-Salmon P., Kovacs G.G., Coudreuse A., Quadrio I., Lehmann S. Risk of Alzheimer's disease biological misdiagnosis linked to cerebrospinal collection tubes. J. Alzheimer's Dis. : JAD. 2012;31(1):13–20. doi: 10.3233/JAD-2012-120361. [DOI] [PubMed] [Google Scholar]
- 200.Bros P., Delatour V., Vialaret J., Lalere B., Barthelemy N., Gabelle A., Lehmann S., Hirtz C. Quantitative detection of amyloid-beta peptides by mass spectrometry: state of the art and clinical applications. Clin. Chem. Lab. Med. 2015;53(10):1483–1493. doi: 10.1515/cclm-2014-1048. [DOI] [PubMed] [Google Scholar]
- 201.Rogeberg M., Wettergreen M., Nilsson L.N., Fladby T. Identification of amyloid beta mid-domain fragments in human cerebrospinal fluid. Biochimie. 2015;113:86–92. doi: 10.1016/j.biochi.2015.03.022. [DOI] [PubMed] [Google Scholar]
- 202.Lesne S.E., Sherman M.A., Grant M., Kuskowski M., Schneider J.A., Bennett D.A., Ashe K.H. Brain amyloid-beta oligomers in ageing and Alzheimer's disease. Brain. 2013;136(Pt 5):1383–1398. doi: 10.1093/brain/awt062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Youmans K.L., Tai L.M., Kanekiyo T., Stine W.B., Jr., Michon S.C., Nwabuisi-Heath E., Manelli A.M., Fu Y., Riordan S., Eimer W.A., Binder L., Bu G., Yu C., Hartley D.M., LaDu M.J. Intraneuronal Abeta detection in 5xFAD mice by a new Abeta-specific antibody. Mol. Neurodegener. 2012;7:8. doi: 10.1186/1750-1326-7-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Naslund J., Schierhorn A., Hellman U., Lannfelt L., Roses A.D., Tjernberg L.O., Silberring J., Gandy S.E., Winblad B., Greengard P. Relative abundance of Alzheimer A beta amyloid peptide variants in Alzheimer disease and normal aging. Proc. Natl. Acad. Sci. USA. 1994;91(18):8378–8382. doi: 10.1073/pnas.91.18.8378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Kim K.S., Miller D.L., Sapienza V.J., Chen C.-M.J., Bai C., Gundke-Iqbal I., Currie J.R., Wisniewski H.M. Production and characterization of monoclonal antibodies reactive to synthetic cerebrovascular amyloid peptide. Neurosci. Res. Commun. 1988;2:121–130. [Google Scholar]
- 206.Portelius E., Westman-Brinkmalm A., Zetterberg H., Blennow K. Determination of beta-amyloid peptide signatures in cerebrospinal fluid using immunoprecipitation-mass spectrometry. J. Proteome Res. 2006;5(4):1010–1016. doi: 10.1021/pr050475v. [DOI] [PubMed] [Google Scholar]
- 207.Butterfield D.A., Galvan V., Lange M.B., Tang H., Sowell R.A., Spilman P., Fombonne J., Gorostiza O., Zhang J., Sultana R., Bredesen D.E. In vivo oxidative stress in brain of Alzheimer disease transgenic mice: requirement for methionine 35 in amyloid beta-peptide of APP. Free Radic. Biol. Med. 2010;48(1):136–144. doi: 10.1016/j.freeradbiomed.2009.10.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Inoue K., Garner C., Ackermann B.L., Oe T., Blair I.A. Liquid chromatography/tandem mass spectrometry characterization of oxidized amyloid beta peptides as potential biomarkers of Alzheimer's disease. Rapid Commun. Mass Spectrom. 2006;20(5):911–918. doi: 10.1002/rcm.2395. [DOI] [PubMed] [Google Scholar]
- 209.Brinkmalm A., Portelius E., Ohrfelt A., Brinkmalm G., Andreasson U., Gobom J., Blennow K., Zetterberg H. Explorative and targeted neuroproteomics in Alzheimer's disease. Biochim Biophys. Acta. 2015;1854(7):769–778. doi: 10.1016/j.bbapap.2015.01.009. [DOI] [PubMed] [Google Scholar]
- 210.Arce-Varas N., Abate G., Prandelli C., Martinez C., Cuetos F., Menendez M., Marziano M., Cabrera-Garcia D., Fernandez-Sanchez M.T., Novelli A., Memo M., Uberti D. Comparison of extracellular and intracellular blood compartments highlights redox alterations in Alzheimer's and mild cognitive impairment patients. Curr. Alzheimer Res. 2017;14(1):112–122. doi: 10.2174/1567205013666161010125413. [DOI] [PubMed] [Google Scholar]
- 211.Butterfield D.A., Poon H.F., Clair D., St, Keller J.N., Pierce W.M., Klein J.B., Markesbery W.R. Redox proteomics identification of oxidatively modified hippocampal proteins in mild cognitive impairment: insights into the development of Alzheimer's disease. Neurobiol. Dis. 2006;22(2):223–232. doi: 10.1016/j.nbd.2005.11.002. [DOI] [PubMed] [Google Scholar]
- 212.Cheignon C., Faller P., Testemale D., Hureau C., Collin F. Metal-catalyzed oxidation of Abeta and the resulting reorganization of Cu binding sites promote ROS production. Metallomics : Integr. Biomet. Sci. 2016;8(10):1081–1089. doi: 10.1039/c6mt00150e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Drew S.C. The case for abandoning therapeutic chelation of copper ions in Alzheimer's Disease. Front Neurosci. 2017;11:317. doi: 10.3389/fnins.2017.00317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Conte-Daban A., Day A., Faller P., Hureau C. How Zn can impede Cu detoxification by chelating agents in Alzheimer's disease: a proof-of-concept study. Dalton Trans. 2016;45(39):15671–15678. doi: 10.1039/c6dt02308h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Atrian-Blasco E., Conte-Daban A., Hureau C. Mutual interference of Cu and Zn ions in Alzheimer’s disease: perspectives at a molecular level. Dalton Trans. 2017;46:12750. doi: 10.1039/c7dt01344b. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Sevigny J., Chiao P., Bussiere T., Weinreb P.H., Williams L., Maier M., Dunstan R., Salloway S., Chen T., Ling Y., O'Gorman J., Qian F., Arastu M., Li M., Chollate S., Brennan M.S., Quintero-Monzon O., Scannevin R.H., Arnold H.M., Engber T., Rhodes K., Ferrero J., Hang Y., Mikulskis A., Grimm J., Hock C., Nitsch R.M., Sandrock A. The antibody aducanumab reduces Abeta plaques in Alzheimer's disease. Nature. 2016;537(7618):50–56. doi: 10.1038/nature19323. [DOI] [PubMed] [Google Scholar]