Abstract
The onset of acute myocardial ischemia (MI) is accompanied by a rapid increase in electrical instability and often fatal ventricular arrhythmias. This study investigated that whether oxytocin (OT) can modulate ischemia-induced arrhythmias and considered relationships between the severity of arrhythmia and the electrocardiogram parameters during ischemia. OT (0.0001–1 μg) was administrated intraperitoneally 30 min before ischemia. To examine receptor involved, a selective OT-receptor antagonist, atosiban (ATO), was infused 10 min before OT. OT caused a significant and biphasic dose-dependent reduction in ectopic heart activity and arrhythmia score. OT doses that reduced ventricular arrhythmia elicited significant increase in QT interval. OT attenuated the electrophysiological changes associated with MI and there was significant direct relationship between QRS duration and arrhythmia score. ATO treatment reduced beneficial effects of OT on arrhythmogenesis. Nevertheless, ATO failed to alter OT effects on premature ventricular contractions. We assume that the ability of OT to modulate the electrical activity of the heart may play an important role in the antiarrhythmic actions of OT.
Key words: Arrhythmias, electrocardiography, myocardial ischemia, oxytocin
INTRODUCTION
Acute occlusion of an epicardial coronary artery leading to myocardial ischemia (MI) and subsequent necrosis is accompanied by a rapid appearance of electrical instability and often fatal ventricular arrhythmias.[1] Prevention of lethal ventricular arrhythmias is a major problem and important objective of therapeutic intervention during MI.[2]
Oxytocin (OT) is one of the cardiac peptides that recently has been focused in cardiovascular functions.[3,4] OT can be synthesized and released by the heart, and G-protein-coupled OT receptors exist in cardiac cells.[5]
OT is also able to modulate electrical and mechanical activities of atria and exert negative inotropic and chronotropic effects on rat heart both in vivo and in vitro,[3,6] and its protective action on the heart has been proven.[7,8,9,10] In addition, electrocardiogram (ECG) changes suggestive of cardiac ischemia have been shown in patients undergoing cesarean section (CS),[11,12] which has been attributed to OT.[13,14] Nevertheless, studies on the role of OT in cardiovascular function are limited and the mechanism(s) by which OT induces its effects has not been fully established.[4]
Since arrhythmias are one of the major deleterious consequences of prolonged MI and regarding a dose-dependent effect of OT, this study tests the hypothesis that pretreatment with OT prevents ischemia-induced arrhythmia. In this study, for the first time, the effects of various doses of OT and the role of OT receptor on electrophysiological changes during experimental MI and the relationship between ECG and antiarrhythmic effect of OT were investigated.
METHODS
Animals
Male Sprague-Dawley rats weighing 300 ± 20 g were housed under standard conditions (20°C–22°C room temperatures, 12 h light–dark cycle, 40%–50% humidity, free access to food and water). The experiment was approved by the Animal Ethics Committee of Medicine School of Tehran University of Medical Sciences.
Method of arrhythmias induction
Regional ischemia was induced by occlusion of the left anterior descending coronary artery (LAD).[7] After tracheotomy, animals were mechanically ventilated with room air supplemented with O2 (60–70 strokes/min, stroke volume 1.2 ml/100 g body weight). Body temperature was maintained at 37°C ± 1°C. A standard limb lead II ECG was obtained by needle electrodes inserted subcutaneously and arterial blood pressure was monitored through carotid artery using a computerized data acquisition system (PowerLab data acquisition system, four channels, ADInstruments). Then, left thoracotomy was performed between the fourth and fifth ribs.
Experimental protocol
The animals were divided into eight groups and all of them underwent to 25 min ischemia [Figure 1].
Figure 1.
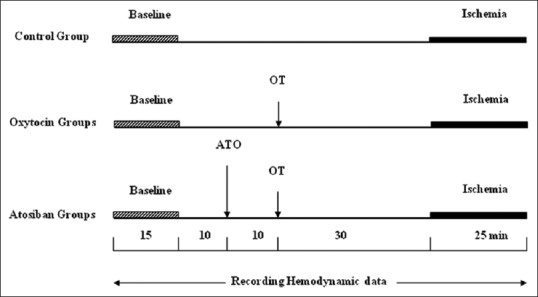
Illustration of the experimental protocolsassessed.
Control group (ischemia, n = 11): animals were subjected to LAD occlusion. Saline was administered intraperitoneally 30 min before the occlusion. OT groups (n = 6-8): treatment with OT in which dose-response effects of OT (0.0001, 0.001, 0.01, 0.1 and 1 μg/0.5 mL, i. p., 30 min before LAD occlusion) (Sigma Chemical Co. St. Louis, MO, USA) was assessed. Atosiban (ATO) groups (n = 6–9): to determine the role of OT receptor in low and high phase of biphasic dose-response protection and in a dose of maximum effect, ATO (Sigma Chemical Co. St. Louis, MO, USA) was tested in 3 groups (OT 0.001, 0.01, and 0.1 μg). ATO was given (10−6M, i. p) 10 min before the OT treatment.
Electrocardiogram parameters
Each ECG was analyzed for QRS complex, ST-segment, R-R, P-R, and QT interval changes. The QT interval was not corrected for heart rate (HR) since it is not HR dependent in rats.[15]
Classification of ischemic arrhythmias
The severity of arrhythmias was quantified by using a scoring system. Here, definition of arrhythmia was based on criteria described in the Lambeth Conventions (score A, Curtis).[16] Arterial blood pressure tracings were used to confirm ectopic activity type, particularly to distinguish between the polymorphic ventricular tachycardia (VT) and ventricular fibrillation (VF). In VT, arterial blood pressure tracing could still be seen, whereas in VF, it rapidly dropped almost to zero [Figure 2]. The antiarrhythmic response for each dose (AStest) was expressed as the percent protection (%Ptest) relative to the arrhythmia score in the control group (Ascontrol) according to the equation below. The arrhythmia score in saline-treated rats was defined as 0% response (AScontrol = 4.9 ± 0.1, mean ± standard error of mean [SEM], n = 11) and the maximum possible response (100%) was defined as the complete suppression of arrhythmias (AS = 0).
Figure 2.
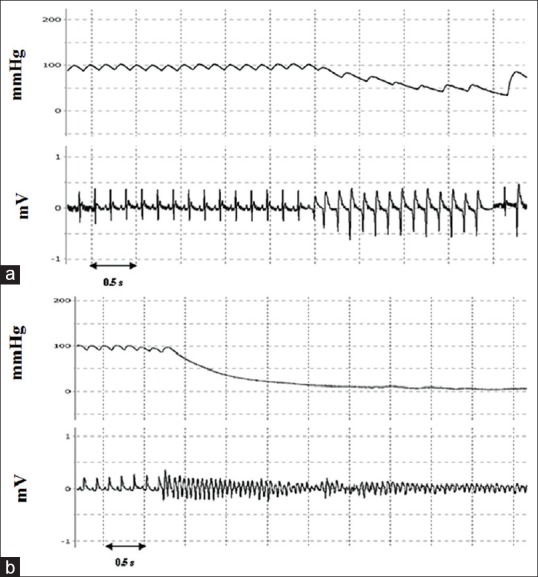
Simultaneous recording of blood pressure and electrocardiogram from a control rat during ischemia. (a) Ventricular tachycardia, (b) ventricular fibrillation, with accompanying drops in blood pressure toward zero
%Ptest = 100 − 100× (AStest/AScontrol)
Exclusion criteria
Data were excluded from the final analysis if mean arterial blood pressure was <60 mmHg at the end of surgical procedure or in case of inadequate blood gas.
Statistical analysis
First, all variables were tested for normal distribution using Kolmogorov test. Parametric tests (repeated measures ANOVA, and ANOVA with Dunnett and Duncan) as well as the nonparametric tests (Kruskal–Wallis and Fridman with Dunn) were used to analysis data. Furthermore, Pearson correlation coefficient was used to determine the linear relationship between different variables. The incidences of VF were compared using the Fisher's exact test. All data are expressed as means ± SEM; P < 0.05 are considered statistically significant.
RESULTS
OT significantly reduced ischemia-induced arrhythmia with lower changes of hemodynamic values than those in control group with exception a significant drop of mean arterial pressure (MAP) in the 0.1 and 0.0001 μg OT-treated groups 10, 15 min, and 1 min, respectively, after occlusion. In the control animals, the MAP and HR were dropped (33.5 mmHg and 42 beat/min (P < 0.05) respectively), 5 min after the coronary occlusion.
Electrophysiological effects of oxytocin
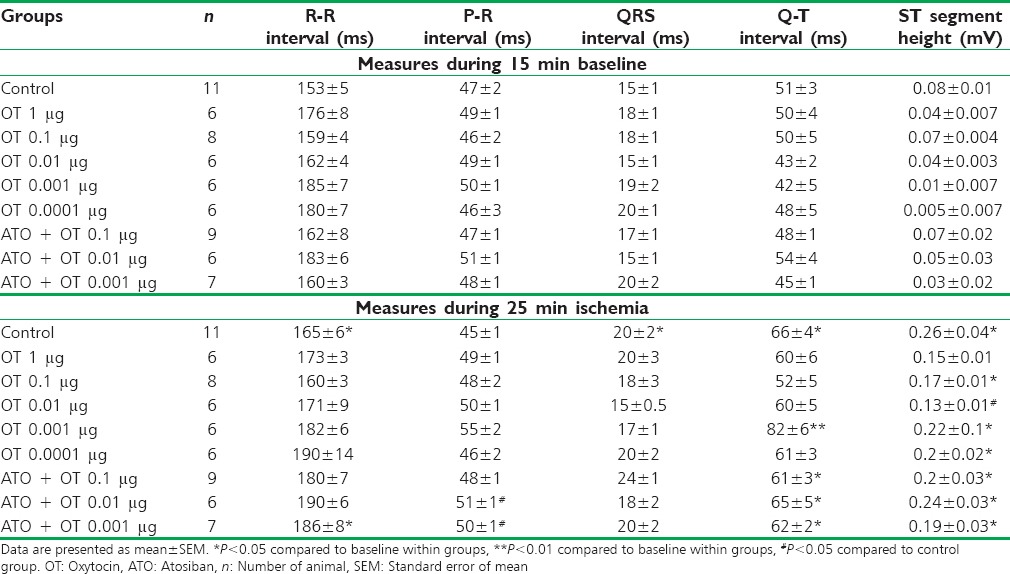
There was no significant difference in ECG parameters between control and OT groups at the baseline. Ischemia has markedly prolonged the R-R interval, elevated the ST segment, and increased QRS duration and QT interval as compared to the baseline [Table 1]. Administration of OT 0.001 μg significantly prolonged the QT interval; however, there were no significant changes in cardiac rate (R-R interval) or ventricular conduction (QRS duration). OT also reduced ST segment elevation, and this effect was significant in OT 0.01 μg group.
Table 1.
Alteration of electrocardiogram produced by coronary artery occlusion
Oxytocin effects on ischemic arrhythmias
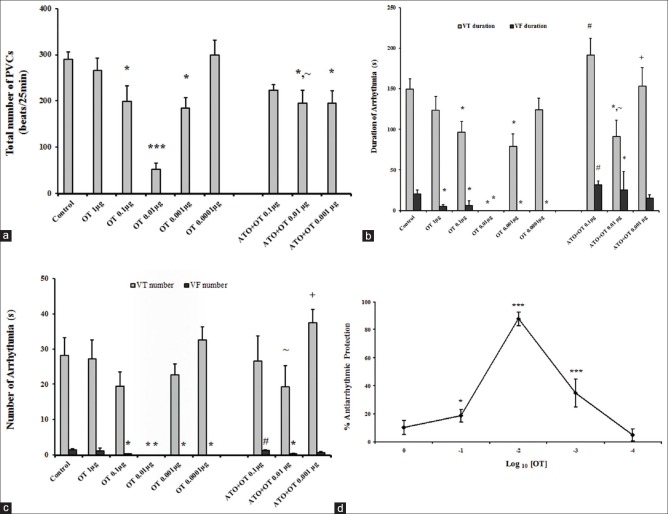
In control group, ischemia caused arrhythmias in the form of VT and VF with 28% mortality rate. Premature ventricular contraction (PVC) and VT were seen in all animals [Figure 3] whereas 64% experienced VF and the average arrhythmia score (AS) was 4.9 ± 0.1 [Table 2].
Figure 3.
Dose-response curves for oxytocin-induced suppression of ischemia-induced arrhythmias (a) number of premature ventricular contractions. (b) Sum of durations of ventricular fibrillation and ventricular tachycardia. (c) Sum of episodes of ventricular fibrillation and ventricular tachycardia in control and the treatment groups. Data are mean ± standard error of mean *P < 0.05, oxytocin versus control. #P < 0.05, ~P < 0.05, and +P <0.05 compared to oxytocin 0.1, oxytocin 0.01, and oxytocin 0.001 μg, respectively. (d) Antiarrhythmic dose-response curves of oxytocin relative to the arrhythmia score in the control group, as described in the methods section. Each point represents the mean percent protection ± standard error of mean *P < 0.05 and ***P < 0.001
Table 2.
Distribution of the arrhythmia score, the incidence of ventricular fibrillation during ischemia in control, oxytocin, and atosiban groups
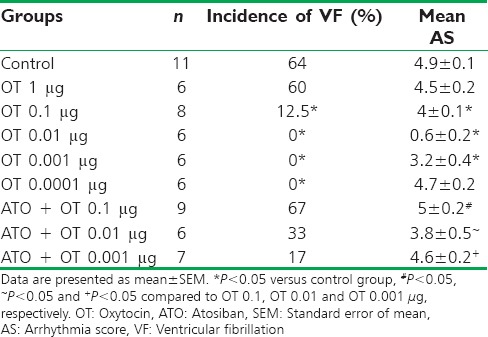
OT treatment reduces number and durations of any type of arrhythmias [Figure 3a–c]. Total number of PVCs and the duration of VT and VF were significantly decreased in OT 0.01 μg group compared with control group so that VT and VF incidence were reduced from 100% and 64% in the control to 0% in OT 0.01 μg treated animals, respectively. In spite of a significant drop in VF duration, high and low dose of OT (1 and 0.0001 μg) was ineffective in protecting against PVC and VT.
Arrhythmia score
OT effect on arrhythmia severity (AS) is shown as a percent protection in Figure 3d. Arrhythmia severity showed a significant decrease when OT was given in doses of 0.001, 0.01 and 0.1 μg [Table 2]. The most profound antiarrhythmic protection was observed with the dose of 0.01 μg [Figure 3d].
Effects of atosiban on oxytocin-induced antiarrhythmic protection
ATO suppressed the OT protective effect [Figure 3a–c] and markedly increased the incidence of VF [Table 2], but it cannot prevent beneficial effects of OT on PVC [Figure 3a]. During ischemia, two out of nine rats in ATO + OT 0.1 were died due to irreversible VF (mortality rate was 22.2%).
Correlation between QRS interval and severity of arrhythmia
There was significant direct correlation between the two variables (R2 = 0.316, P < 0.05). There was also a significant relationship between QRS interval duration and the AS (R2 = 0.128, P < 0.05). Similar direct correlation was seen between AS and incidence of VF (R2 = 0.33, P < 0.05) [Figure 4a–c].
Figure 4.
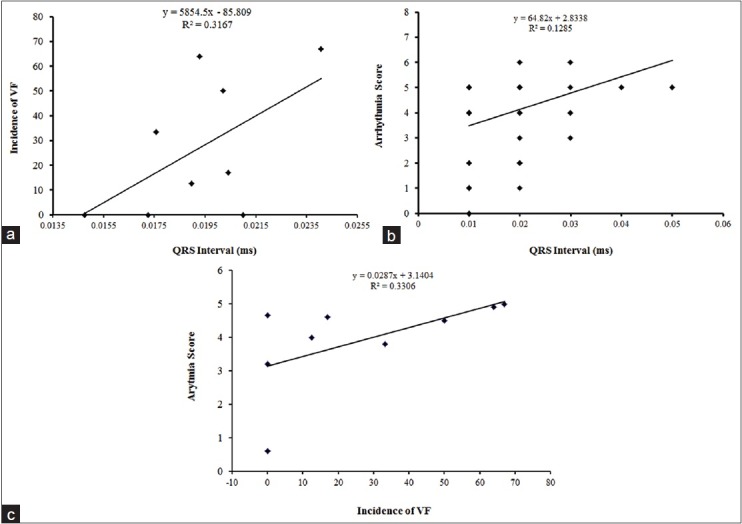
The relationships between the incidence of ventricular fibrillation (a) and arrhythmia score (b) versus the QRS duration during ischemia. There was significant correlation between the two variables. (c) Direct correlation between arrhythmia score and incidence of ventricular fibrillation (R2 = 0.330, P < 0.05)
DISCUSSION
The present study shows that treatment with nonhypotensive doses of OT before the coronary occlusion results in protection against ischemia-induced arrhythmias in anesthetized rats. Arrhythmia score which is a better indicator of antiarrhythmic effects was significantly decreased in OT 0.001 and 0.01 μg and at a dose of 0.01 μg, OT produced almost complete antiarrhythmic protection (87.6% maximal protection; AS = 0 equivalent to 100% protection).
Our results confirmed the findings of Feldman et al. who used OT (1U/kg) to block cyclopropane-epinephrine arrhythmias in dog[17] and Panisset and Beaulnes who demonstrated an OT antiarrhythmic action on arrhythmias induced by electrical stimulation of rabbit heart.[18] OT also suppresses the induced cardiac arrhythmia during cyclopropane anesthesia for parturition.[19] Here, we found that number of PVC, number, and duration of VT were significantly and biphasic lower in OT groups. OT could also significantly prevented VF. The antifibrillatory action of OT has also been reported by Melville and Varma,[20] Beaulnes et al.,[21] and Covino.[22]
On the basis of our results and other studies assumed that OT antiarrhythmic effect is independent of the experimental models of inducing arrhythmia.
During dose-response studies, we noticed that OT had a biphasic dose-response curve (U-shaped). OT at the highest dose (1 μg) did not reduce PVC, VT, and arrhythmia score (AS). This unfavorable effect may be mediated through an interaction between exogenous OT and vasopressin receptors since OT activates V1 receptors at high concentrations. On the other hand, suppressing effect of OT on VF at any given dose cannot be interpreted through this mechanism. Because MI is a major cause of sudden death due to VF[23] and OT has a life-saving potential against ischemia-induced arrhythmias; therefore, it should be specific notice to antifibrillatory effect of this neuropeptide.
We find that OT reduced arrhythmia severity in a dose-dependent, biphasic manner where low and high protective doses located in descending and ascending limb of the U-shaped curve and optimal efficacy was in between. Szabadi described this concept that the receptor systems display biphasic dose-responses when a single agonist has differential affinities for two opposing receptor subtypes.[24] Since ATO reduced the beneficial effects of OT on ischemia-induced arrhythmias, the mechanism of OT in both phases of dose-response curve appears to be related to the OT receptor activity.
Since ATO cannot prevent beneficial effects of OT on PVC, we may consider that the effect of OT on low-frequency arrhythmia was independent of OT receptor activation and other mechanisms should be involved. In addition, VF was longer in ATO + OT 0.1 μg than in control group. It is likely that by blockade of the OT receptor, concentration of OT in this group can reach a level which is able to stimulate vasopressin receptors which are also present in the heart.
The second major finding was the prevention of electrophysiological changes typically associated with MI by OT. Svanström et al. have reported that the cardiovascular and ECG changes often observed during CS are related to OT administration.[11] Jonsson observed magnitude of changes in ECG, suggestive of MI, is dose-dependent in healthy women undergoing regional anesthesia.[25] Controversially, Katz confirmed the OT antiarrhythmic action in man.[26,27] In spite of many investigations, there is still a question of whether or not the ECG changes and subjective symptoms observed during CS are important and relevant as signs of MI.[11]
The present data show that ischemia has marked effects on ECG parameters, in particular causing an increase in the R-R interval, duration of the QRS complex, and ST elevation, although there was no significant effect on the P-R interval. The significant increase in the QT interval was seen in the control group during ischemia compared to preocclusion values.
Under ischemic condition, as a result of intracellular ATP depletion and accumulation of ischemic metabolites, the KATP channels is likely to open, resulting in the membrane depolarization and action potential shortening.[23] Since opening of KATP channels is limited to an ischemic area, cellular events lead to electrical heterogeneity within the heart and generating injury currents between ischemic and normal cells, which is potentially proarrhythmic.[28] Although some studies have reported shortened action potential duration (APD) in abnormal tissues,[29,30] other studies have shown contradictory results that regional ischemia in humans and experimental animals created QT prolongation.[31,32,33]
It is generally believed that the APD in the ventricles determines the QT interval. Wu et al. have concluded that although opening of the KATP channel shorten the APD, it does not shorten the QT interval.[33] They reported that because APD in the nonischemic region may lengthen after the creation of ischemia, the QT interval on surface ECG also lengthens. Therefore, the APD shortening in ischemic region is not associated with QT shortening.[33] In addition, the slowed conduction and repolarization delay in the rat's heart could be partly responsible for QT interval prolongation.[34] In the present experiment, increase in the QRS intervals hints that ischemia widen the QT interval as a consequence of slowed ventricular conduction in the control group.
It has previously been suggested that OT might lead to proarrhythmia in circumstances favoring QT interval increase.[35] In contrast, our study demonstrated that despite prolonged QT interval in OT groups, they are resistant against ischemia-induced arrhythmia and noticeably prevented VF.
OT also attenuated the S-T segment elevation induced by ischemia, that agrees with Klassen et al.[36] Because the duration of the QRS complex reflects the propagation of the action potential through the ventricles, the observation that OT has no effect on the duration of the QRS complex suggests that OT has little effect on the rate of propagation of the action potential. We supposed that cardiac protection of OT might be due to effectiveness in restoring impaired ventricular conduction.
Since the PR interval is prolonged by inhibition the fast and slow inward current,[37] this would appear to rule out inhibition of these currents as the mechanism of antiarrhythmic action of OT. Because the changes in the PR interval were slight and unrelated to the antiarrhythmic effects of OT.
Moreover, direct correlation was seen between changes in AS and incidence of VF and QRS interval prolongation, suggesting that this is the main possible mechanism involved in our observations; however, exact molecular mechanism of OT cannot be concluded from the results of this study.
CONCLUSION
Based on antifibrillatory action and absence of significant hemodynamic effects, OT can be a potentially important agent for clinical application. Accordingly, understanding the mechanism(s) underlying this protection and whether similar antiarrhythmic aspects are present in humans under ischemic condition remain to be investigated.
Financial support and sponsorship
This study was financially supported by Tehran University of Medical Sciences project number: 6066-300386 (Tehran, Iran).
Conflicts of interest
There are no conflicts of interest.
Acknowledgment
This study was financially supported by Tehran University of Medical Sciences project number: 6066-300386 (Tehran, Iran).
REFERENCES
- 1.Opitz CF, Mitchell GF, Pfeffer MA, Pfeffer JM. Arrhythmias and death after coronary artery occlusion in the rat. Continuous telemetric ECG monitoring in conscious, untethered rats. Circulation. 1995;92:253–61. doi: 10.1161/01.cir.92.2.253. [DOI] [PubMed] [Google Scholar]
- 2.Leprán I, Baczkó I, Varró A, Papp JG. ATP-sensitive potassium channel modulators: Both pinacidil and glibenclamide produce antiarrhythmic activity during acute myocardial infarction in conscious rats. J Pharmacol Exp Ther. 1996;277:1215–20. [PubMed] [Google Scholar]
- 3.Costa-E-Sousa RH, Pereira-Junior PP, Oliveira PF, Olivares EL, Werneck-de-Castro JP, Mello DB, et al. Cardiac effects of oxytocin: Is there a role for this peptide in cardiovascular homeostasis? Regul Pept. 2005;132:107–12. doi: 10.1016/j.regpep.2005.09.011. [DOI] [PubMed] [Google Scholar]
- 4.Gutkowska J, Jankowski M, Mukaddam-Daher S, McCann SM. Oxytocin is a cardiovascular hormone. Braz J Med Biol Res. 2000;33:625–33. doi: 10.1590/s0100-879x2000000600003. [DOI] [PubMed] [Google Scholar]
- 5.Jankowski M, Hajjar F, Kawas SA, Mukaddam-Daher S, Hoffman G, McCann SM, et al. Rat heart: A site of oxytocin production and action. Proc Natl Acad Sci U S A. 1998;95:14558–63. doi: 10.1073/pnas.95.24.14558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mukaddam-Daher S, Yin YL, Roy J, Gutkowska J, Cardinal R. Negative inotropic and chronotropic effects of oxytocin. Hypertension. 2001;38:292–6. doi: 10.1161/01.hyp.38.2.292. [DOI] [PubMed] [Google Scholar]
- 7.Houshmand F, Faghihi M, Zahediasl S. Biphasic protective effect of oxytocin on cardiac ischemia/reperfusion injury in anaesthetized rats. Peptides. 2009;30:2301–8. doi: 10.1016/j.peptides.2009.09.010. [DOI] [PubMed] [Google Scholar]
- 8.Ondrejcakova M, Ravingerova T, Bakos J, Pancza D, Jezova D. Oxytocin exerts protective effects on in vitro myocardial injury induced by ischemia and reperfusion. Can J Physiol Pharmacol. 2009;87:137–42. doi: 10.1139/Y08-108. [DOI] [PubMed] [Google Scholar]
- 9.Houshmand F, Faghihi M, Zahediasl S. Role of atrial natriuretic Peptide in oxytocin induced cardioprotection. Heart Lung Circ. 2015;24:86–93. doi: 10.1016/j.hlc.2014.05.023. [DOI] [PubMed] [Google Scholar]
- 10.Moghimian M, Faghihi M, Karimian SM, Imani A, Houshmand F, Azizi Y. Role of central oxytocin in stress-induced cardioprotection in ischemic-reperfused heart model. J Cardiol. 2013;61:79–86. doi: 10.1016/j.jjcc.2012.08.021. [DOI] [PubMed] [Google Scholar]
- 11.Svanström MC, Biber B, Hanes M, Johansson G, Näslund U, Bålfors EM, et al. Signs of myocardial ischaemia after injection of oxytocin: A randomized double-blind comparison of oxytocin and methylergometrine during caesarean section. Br J Anaesth. 2008;100:683–9. doi: 10.1093/bja/aen071. [DOI] [PubMed] [Google Scholar]
- 12.Thomas JS, Koh SH, Cooper GM. Haemodynamic effects of oxytocin given as i.v. Bolus or infusion on women undergoing caesarean section. Br J Anaesth. 2007;98:116–9. doi: 10.1093/bja/ael302. [DOI] [PubMed] [Google Scholar]
- 13.Guillon A, Leyre S, Remérand F, Taihlan B, Perrotin F, Fusciardi J, et al. Modification of tp-e and QTc intervals during caesarean section under spinal anaesthesia. Anaesthesia. 2010;65:337–42. doi: 10.1111/j.1365-2044.2010.06246.x. [DOI] [PubMed] [Google Scholar]
- 14.Liou SC, Chen C, Wong SY, Wong KM. Ventricular tachycardia after oxytocin injection in patients with prolonged Q-T interval syndrome – Report of two cases. Acta Anaesthesiol Sin. 1998;36:49–52. [PubMed] [Google Scholar]
- 15.Rees SA, Curtis MJ. Specific IK1 blockade: A new antiarrhythmic mechanism? Effect of RP58866 on ventricular arrhythmias in rat, rabbit, and primate. Circulation. 1993;87:1979–89. doi: 10.1161/01.cir.87.6.1979. [DOI] [PubMed] [Google Scholar]
- 16.Curtis M, Walker M. Quantification of arrhythmias using scoring systems: An examination of seven scores in an in vivo model of regional myocardial ischaemia. Cardiovasc Res. 1988;22:656–65. doi: 10.1093/cvr/22.9.656. [DOI] [PubMed] [Google Scholar]
- 17.Feldman SA, Forgaard DM, Morris LE. Compatibility of a synthetic oxytocin (syntocinon) with anesthesia in dogs. Anesthesiology. 1958;19:787–91. doi: 10.1097/00000542-195811000-00011. [DOI] [PubMed] [Google Scholar]
- 18.Panisset JC, Beaulnes A. The effect of oxytocin on experimental cardiac arrhythmias. Rev Can Biol. 1961;20:47–53. [PubMed] [Google Scholar]
- 19.Morris LE, Thornton MJ, Harris JW. Comparison of the effect of pituitrin, pitocin, and ergonovine on cardiac rhythm during cyclopropane anesthesia for parturition. Am J Obstet Gynecol. 1952;63:171–4. doi: 10.1016/s0002-9378(16)38996-7. [DOI] [PubMed] [Google Scholar]
- 20.Melville KI, Varma DR. Synthetic oxytocin as an antagonist of experimental cardiac anoxic changes in rabbits. Br J Pharmacol Chemother. 1961;17:218–23. doi: 10.1111/j.1476-5381.1961.tb01281.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Beaulnes A, Panisset JC, Brodeur J, Beltrami E, Gariepy G. Arrhythmias in isolated atria and ventricles and in the intact animal; antiarrhythmic effects of some biological polypeptides. Circ Res. 1964;15(Suppl 2):210–4. [PubMed] [Google Scholar]
- 22.Covino BG. Cardiac effects of synthetic oxytocin (syntocinon) Am Heart J. 1963;66:627–31. doi: 10.1016/0002-8703(63)90317-x. [DOI] [PubMed] [Google Scholar]
- 23.Billman GE, Englert HC, Schölkens BA. HMR 1883, a novel cardioselective inhibitor of the ATP-sensitive potassium channel. Part II: Effects on susceptibility to ventricular fibrillation induced by myocardial ischemia in conscious dogs. J Pharmacol Exp Ther. 1998;286:1465–73. [PubMed] [Google Scholar]
- 24.Szabadi E. A model of two functionally antagonistic receptor populations activated by the same agonist. J Theor Biol. 1977;69:101–12. doi: 10.1016/0022-5193(77)90390-3. [DOI] [PubMed] [Google Scholar]
- 25.Jonsson M, Hanson U, Lidell C, Nordén-Lindeberg S. ST depression at caesarean section and the relation to oxytocin dose. A randomised controlled trial. BJOG. 2010;117:76–83. doi: 10.1111/j.1471-0528.2009.02356.x. [DOI] [PubMed] [Google Scholar]
- 26.Katz RL. Antiarrhythmic action of synthetic oxytocin in anesthetized man. Experientia. 1963;19:160–1. doi: 10.1007/BF02171612. [DOI] [PubMed] [Google Scholar]
- 27.Katz RL. Antiarrhythmic and cardiovascular effects of synthetic oxytocin. Anesthesiology. 1964;25:653–61. doi: 10.1097/00000542-196409000-00013. [DOI] [PubMed] [Google Scholar]
- 28.Coronel R. Heterogeneity in extracellular potassium concentration during early myocardial ischaemia and reperfusion: Implications for arrhythmogenesis. Cardiovasc Res. 1994;28:770–7. doi: 10.1093/cvr/28.6.770. [DOI] [PubMed] [Google Scholar]
- 29.Gasser RN, Vaughan-Jones RD. Mechanism of potassium efflux and action potential shortening during ischaemia in isolated mammalian cardiac muscle. J Physiol. 1990;431:713–41. doi: 10.1113/jphysiol.1990.sp018356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hamada K, Yamazaki J, Nagao T. Shortening of monophasic action potential duration during hyperkalemia and myocardial ischemia in anesthetized dogs. Jpn J Pharmacol. 1998;76:149–54. doi: 10.1254/jjp.76.149. [DOI] [PubMed] [Google Scholar]
- 31.Clements-Jewery H, Andrag E, Hearse DJ, Curtis MJ. Complex adrenergic and inflammatory mechanisms contribute to phase 2 ventricular arrhythmias in anaesthetized rats. Br J Pharmacol. 2009;156:444–53. doi: 10.1111/j.1476-5381.2008.00054.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kenigsberg DN, Khanal S, Kowalski M, Krishnan SC. Prolongation of the QTc interval is seen uniformly during early transmural ischemia. J Am Coll Cardiol. 2007;49:1299–305. doi: 10.1016/j.jacc.2006.11.035. [DOI] [PubMed] [Google Scholar]
- 33.Wu S, Hayashi H, Lin SF, Chen PS. Action potential duration and QT interval during pinacidil infusion in isolated rabbit hearts. J Cardiovasc Electrophysiol. 2005;16:872–8. doi: 10.1111/j.1540-8167.2005.40811.x. [DOI] [PubMed] [Google Scholar]
- 34.Farkas A, Curtis MJ. Does QT widening in the langendorff-perfused rat heart represent the effect of repolarization delay or conduction slowing? J Cardiovasc Pharmacol. 2003;42:612–21. doi: 10.1097/00005344-200311000-00006. [DOI] [PubMed] [Google Scholar]
- 35.Charbit B, Funck-Brentano C, Samain E, Jannier-Guillou V, Albaladejo P, Marty J. QT interval prolongation after oxytocin bolus during surgical induced abortion. Clin Pharmacol Ther. 2004;76:359–64. doi: 10.1016/j.clpt.2004.06.005. [DOI] [PubMed] [Google Scholar]
- 36.Klassen GA, Rubin JW, McGregor M. The effect of synthetic oxytocin on impaired atrioventricular conduction. Am J Cardiol. 1963;12:523–6. doi: 10.1016/0002-9149(63)90191-7. [DOI] [PubMed] [Google Scholar]
- 37.Farkas A, Curtis MJ. Limited antifibrillatory effectiveness of clinically relevant concentrations of class I antiarrhythmics in isolated perfused rat hearts. J Cardiovasc Pharmacol. 2002;39:412–24. doi: 10.1097/00005344-200203000-00013. [DOI] [PubMed] [Google Scholar]