Abstract
Attention deficit/hyperactivity disorder (ADHD) is one of the most common psychiatric disorders in children with several complications. This study was conducted to investigate the effect of adding ferrous sulfate to methylphenidate in decreasing ADHD symptoms. This study was a double-blind, randomized clinical trial. In this study, 42 nonanemic children with ADHD and serum ferritin below 30 mg/ml were enrolled according to convenience sampling and randomly assigned to two groups of 21 each, cases and controls. The two groups were matched for age and sex. The case group was administered with ferrous sulfate 5 mg/kg in addition to methylphenidate up to 1 mg/kg and the control group with methylphenidate alone. The scores on child symptoms inventory-4 (CSI-4) were recorded at baseline and after 2 months of treatment. Data were analyzed by t-test, Pearson's correlation coefficient, and repeated measures ANOVA in SPSS 16. The scores on CSI-4 decreased significantly at month 2 in both groups (P < 0.001). The scores on attention deficit and hyperactivity subscales of the CSI-4 were significantly lower in the case group than the control group (P < 0.05). The total score on CSI-4 decreased more markedly in the case group (P < 0.04). Use of ferrous sulfate plus methylphenidate can be effective in reducing ADHD symptoms in nonanemic children with low serum ferritin.
Key words: Attention-deficit/hyperactivity disorder, ferrous sulfate, methylphenidate
INTRODUCTION
Attention-deficit/hyperactivity disorder (ADHD) is one of the most common reasons for referring to child psychiatry clinics worldwide[1] and one of the most common psychiatric disorders in Iran.[2] ADHD leads to certain complications such as hyperactivity, impulsivity, and inattention in children, which may cause academic failure and social functioning-related problems in this age group.[3] Furthermore, ADHD may be associated with certain health outcomes such as smoking and substance abuse in very young age, injuries caused by accident, having difficulty falling asleep, obesity,[4] and other unhealthy conditions such as nutritional deficiencies.[5] Although no definite etiology has yet been offered for development of ADHD, genetic, and environmental factors are considered possible causes.[6,7,8] Disturbed frontal-subcortical-cerebellar catecholaminergic circuit and abnormal dopamine transporters, which lead to impairment of neurotransmitters, are thought to be the molecular causes of ADHD. These causes require the use of stimulant drugs such as methylphenidate,[9] which is a drug of choice to treat ADHD in children[10] and increases norepinephrine and dopamine levels through inhibiting their resorption.[11] Some clinical trials have been conducted to investigate the efficacy of different psychotropic medications in children with ADHD.[12,13]
Moreover, food supplements are considered to be one of the complementary treatments for ADHD welcome by parents.[14] Iron is one of the supplements which is considered to be useful to treat ADHD[15] because iron plays an important role in the regulation of dopaminergic activity, which is associated with the pathogenesis and symptoms of ADHD.[16]
In 1997, Sever et al.[17] found that iron supplementation significantly increased serum ferritin levels and decreased ADHD symptom scores in children with ADHD, concluding that nonanemic children with ADHD may benefit from iron supplementation. However, inconsistent results regarding the relationship between iron deficiency and ADHD have also been reported.[18] Some studies reported that mean serum ferritin levels were lower in children with ADHD than controls.[16,19,20] Juneja et al.[16,19] reported that serum ferritin levels were inversely correlated with ADHD symptom severity. Moreover, studies[16,20] have reported significantly lower brain iron in the bilateral thalami of children with ADHD compared with healthy controls in magnetic resonance imaging (MRI). However, some researchers did not find any relationship between serum ferritin and ADHD.[21,22,23] Therefore, this issue needs further investigation; and more evidence is still being sought out to confirm the effect of certain supplements such as iron in treating ADHD.[24] We, therefore, conducted a randomized clinical trial to investigate the effect of adding ferrous sulfate to methylphenidate on ADHD symptoms in children.
SUBJECTS AND METHODS
This study is a double-blind, randomized clinical trial. In this study, 42 patients with ADHD referred to child psychiatry clinic affiliated with Shahrekord University of Medical Sciences in southwest Iran in 2014 were enrolled. The patients were enrolled if they were 5–15 years, suffered from ADHD and had ferritin levels under 30 mg/ml and normal serum hemoglobin levels. Suffering from other psychiatric disorders according to diagnostic and statistical manual for mental disorders version 4 (DSM-5), intake of iron supplements within the previous 3 months, mental retardation, history of seizure, motor tics, acute medical problems, and contraindications for methylphenidate such as cardiovascular disease, incidence of serious adverse drug events (ADEs), and lack of the parents' consent to their children participation in the study were considered the exclusion criteria.
In this study, convenience sampling was used to select the participants. The patients were randomly assigned to two groups of 21 each, cases and controls. The two groups were matched for age and sex. The participants were enrolled into the study after approval of the Shahrekord University of Medical Sciences Ethics Committee and the parents' written consent to their children participation in the study were provided. The case group were administered with ferrous sulfate tablet 5 mg/kg plus methylphenidate up to 1 mg/kg daily.[15] The control group was administered with methylphenidate alone. Dose of methylphenidate was titrated through a phone call to parents once a week. The patients were examined for vital signs and ADEs once per week.
The body's iron reserve was estimated by measurement of complete blood count and ferritin level. Normal serum ferritin levels (≥30 mg/kg) were considered to represent adequate iron reserves. The measurements were conducted at baseline and week 8 of the study.
In this study, the child symptom inventory-4 (CSI-4) parent version was used. CSI-4 is an instrument routinely used to screen for psychiatric disorders and has been developed according to the criteria of DSM-4.
The CSI-4 parent version consists of 18 items for ADHD, nine of which address inattention and the rest do hyperactivity/impulsivity, representing three subtypes of ADHD, predominantly hyperactive/impulsive, predominantly inattentive, and combined. The reliability of this scale has been reported to be 0.92, and the subscales were found to have acceptable validity and reliability in other studies including one study conducted in Iran.[25,26]
The CSI-4 parent version was completed at baseline and week 8 of the treatment. The data were analyzed by t-test, Pearson's correlation coefficient, and repeated measures ANOVA in SPSS for Windows, Version 16.0. Chicago, SPSS Inc.
RESULTS
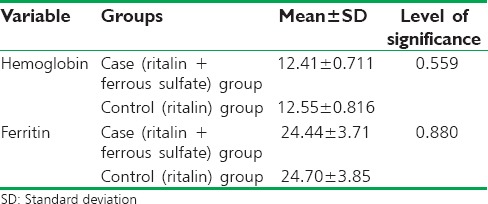
The mean age of the patients was 8.95 ± 2.83 years in the case group and 7.57 ± 1.91 years in the control group with no significant difference according to t-test (P = 0.07). Therefore, the groups were age-matched. In the control group, two (9.5%) girls and 19 (90.5%) boys were enrolled. In the case group, five (23.8%) girls and 16 (76.2%) boys were enrolled. There was no significant difference in gender between the two groups according to Chi-square (P = 0.2). Therefore, the two groups were gender-matched. Mean hemoglobin and ferritin levels after 2 months of treatment are shown in Table 1.
Table 1.
Comparison of hemoglobin and ferritin after the study between the two groups
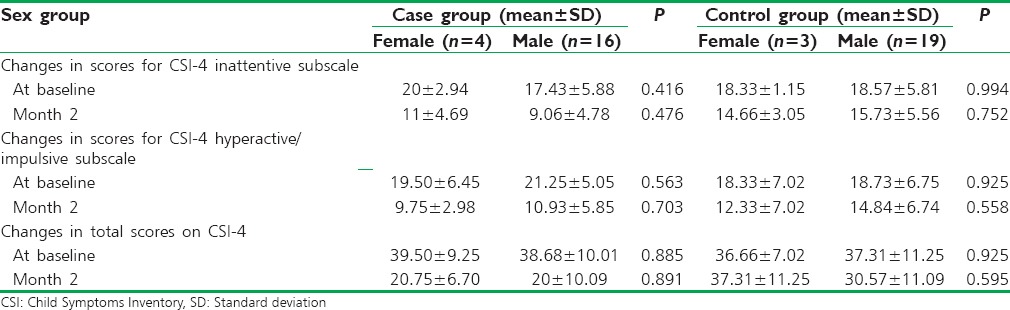
As shown in Table 2, there was no significant difference in the mean total scores on CSI-4 and inattentive and hyperactive/impulsive subtypes between sex groups at baseline and after 2 months of treatment (P > 0.05).
Table 2.
Comparison of changes in the total scores on Child Symptoms Inventory-4 and inattentive and hyperactive/impulsive subtypes based on sex group
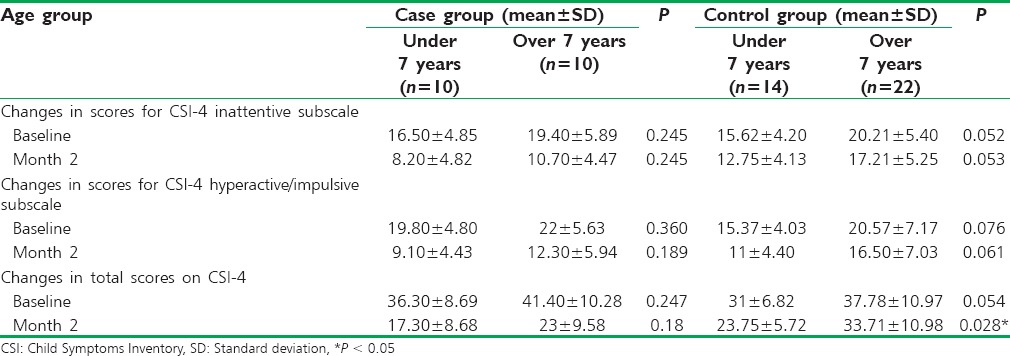
There was no significant difference in the mean total scores on CSI-4 and inattentive and hyperactive/impulsive subtypes among age groups at baseline and 2 months after the treatment (P > 0.05), except for total CSI-4 score of age groups after 2 months of treatment in control group [Table 3].
Table 3.
Comparison of changes in the total scores on Child Symptoms Inventory-4 and inattentive and hyperactive/impulsive subtypes based on the age group
Comparison of the scores on CSI-4 between the two groups at baseline indicated no statistically significant difference (P > 0.05). In the control and case groups, a statistically significant decrease was observed in the scores on CSI-4 after 2 months of treatment compared to baseline (P < 0.001) [Figure 1].
Figure 1.
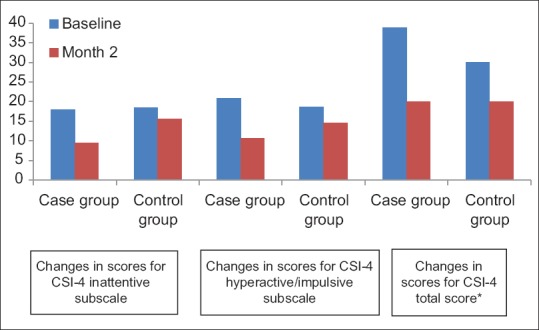
Comparison of total scores on child symptoms inventory-4 * and inattentive and hyperactive/impulsive subtypes between the two groups. *P<0.05
Comparison of the scores on CSI-4 inattentive subscale at the end of month 2 indicates a significant difference between the two groups as the mean scores were markedly lower in the case group than the control group (P < 0.001). Regarding hyperactive/impulsive subscale, at the end of month 2, the scores of the case group were markedly lower than those of the control group (P = 0.04). In both groups, the scores on total CSI-4 decreased at the end of the month 2 yet more markedly in the case group (P = 0.002).
DISCUSSION
The results of the present study showed that adding ferrous sulfate to methylphenidate significantly increased serum ferritin level and improved symptoms of ADHD in nonanemic children with ADHD and low serum ferritin. Consistent with our results, studies of Konofal et al.[15] and Sever et al.[17] showed that after iron supplementation, the serum ferritin levels of ADHD patients increased significantly and ADHD symptoms improved. Based on these data, it appears that iron supplementation may benefit ADHD; however, more studies with larger sample size should be conducted to confirm these results.
The meta-analysis conducted by Wang et al., on 10 case–control studies investigating serum ferritin levels in a total of 2191 participants and 1196 ADHD cases, indicated that serum ferritin levels were lower in ADHD cases than healthy controls.[18]
Iron plays a crucial role in the development of the brain.[27] Iron deficiency with or without anemia during childhood has a negative impact on cognition, behavior, and motor skills of children.[28]
Serum ferritin, an intracellular protein that stores iron, is a reliable indicator of iron stores in body tissues; however, whether the serum ferritin is a good indicator of iron stores in the brain is debated.[29] Compared with serum iron, serum ferritin is a more sensitive marker which can be detected at the early stage of iron deficiency even in nonanemic cases.
Iron is a major cofactor of tyrosine hydroxylase enzyme and a rate-limiting step in dopamine synthesis.[30] Iron deficiency is associated with decreased dopamine transporter density and activity, resulting in increased extracellular dopamine as well as reduced dopamine receptors in the striatum.[29,30] Lower thalamic iron levels were found in children with ADHD compared to controls, which indicates that decreased iron levels in thalamus may be implicated in the etiology of ADHD.[16]
However, several limitations of our study should be taken into consideration. First, the sample size was small, which may reduce the power of our analyses. Further studies with larger numbers of participants should be conducted to clarify the effect of iron supplementation on serum ferritin and ADHD symptoms. Second, in this study, we only used ferritin as an indicator of serum iron levels. Further studies should consider combining more indices reflecting blood iron status (transferrin, total iron binding capacity). Third, we used a fixed dose of ferrous sulfate/kg body weight. More studies with different doses of ferrous sulfate are recommended. Fourth, serum ferritin may not completely reflect actual iron levels in the brain. Using MRI, Adisetiyo et al.[20] found lower levels of iron in the striatum and thalamus of medication-naïve patients with ADHD. However, serum ferritin levels were not significantly different between medciation-naïve patients with ADHD and healthy controls. To assess brain iron levels more precisely, studies using noninvasive methods such as brain MRI are recommended to assess brain iron directly.
Use of ferrous sulfate plus methylphenidate can be effective in decreasing ADHD symptoms in children. Regarding the findings, we can infer that use of ferrous sulfate alongside methylphenidate in nonanemic children with ADHD and iron deficiency caused a decrease in the scores on CSI-4 and hence ADHD symptoms especially at the end of the 2nd month of treatment. Therefore, the psychiatrists in health-care centers are recommended to prescribe ferrous sulfate.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Willcutt EG. The prevalence of DSM-IV attention-deficit/hyperactivity disorder: A meta-analytic review. Neurotherapeutics. 2012;9:490–9. doi: 10.1007/s13311-012-0135-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Safavi P, Ganji F, Bidad A. Prevalence of attention-deficit hyperactivity disorder in students and needs modification of mental health services in Shahrekord, Iran in 2013. J Clin Diagn Res. 2016;10:LC25–8. doi: 10.7860/JCDR/2016/14481.7671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Association American Psychiatric. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. Washington, DC: American Psychiatric Association; 2013. [Google Scholar]
- 4.Nigg JT. Attention-deficit/hyperactivity disorder and adverse health outcomes. Clin Psychol Rev. 2013;33:215–28. doi: 10.1016/j.cpr.2012.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Millichap JG, Yee MM. The diet factor in attention-deficit/hyperactivity disorder. Pediatrics. 2012;129:330–7. doi: 10.1542/peds.2011-2199. [DOI] [PubMed] [Google Scholar]
- 6.Golmirzaei J, Namazi S, Amiri S, Zare S, Rastikerdar N, Hesam AA, et al. Evaluation of attention-deficit hyperactivity disorder risk factors. Int J Pediatr. 2013;2013:953103. doi: 10.1155/2013/953103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Polańska K, Jurewicz J, Hanke W. Exposure to environmental and lifestyle factors and attention-deficit/hyperactivity disorder in children – A review of epidemiological studies. Int J Occup Med Environ Health. 2012;25:330–55. doi: 10.2478/S13382-012-0048-0. [DOI] [PubMed] [Google Scholar]
- 8.Froehlich TE, Anixt JS, Loe IM, Chirdkiatgumchai V, Kuan L, Gilman RC, et al. Update on environmental risk factors for attention-deficit/hyperactivity disorder. Curr Psychiatry Rep. 2011;13:333–44. doi: 10.1007/s11920-011-0221-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Biederman J, Faraone SV. Attention-deficit hyperactivity disorder. Lancet. 2005;366:237–48. doi: 10.1016/S0140-6736(05)66915-2. [DOI] [PubMed] [Google Scholar]
- 10.Machado FS, Caetano SC, Hounie AG, Scivoletto S, Muszkat M, Gattás IG, et al. Methylphenidate use in children with attention deficit hyperactivity disorder. Rev Saude Publica. 2015;49:32. doi: 10.1590/S0034-8910.2015049005966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.De Sousa A, Kalra G. Drug therapy of attention deficit hyperactivity disorder: Current trends. Mens Sana Monogr. 2012;10:45–69. doi: 10.4103/0973-1229.87261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Safavi P, Dehkordi AH, Ghasemi N. Comparison of the effects of methylphenidate and the combination of methylphenidate and risperidone in preschool children with attention-deficit hyperactivity disorder. J Adv Pharm Technol Res. 2016;7:144–8. doi: 10.4103/2231-4040.191425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Safavi P, Hasanpour-Dehkordi A, AmirAhmadi M. Comparison of risperidone and aripiprazole in the treatment of preschool children with disruptive behavior disorder and attention deficit-hyperactivity disorder: A randomized clinical trial. J Adv Pharm Technol Res. 2016;7:43–7. doi: 10.4103/2231-4040.177203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Searight HR, Robertson K, Smith T, Perkins S, Searight BK. Complementary and alternative therapies for pediatric attention deficit hyperactivity disorder: A descriptive review. ISRN Psychiatry. 2012;2012:804127. doi: 10.5402/2012/804127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Konofal E, Lecendreux M, Deron J, Marchand M, Cortese S, Zaïm M, et al. Effects of iron supplementation on attention deficit hyperactivity disorder in children. Pediatr Neurol. 2008;38:20–6. doi: 10.1016/j.pediatrneurol.2007.08.014. [DOI] [PubMed] [Google Scholar]
- 16.Cortese S, Azoulay R, Castellanos FX, Chalard F, Lecendreux M, Chechin D, et al. Brain iron levels in attention-deficit/hyperactivity disorder: A pilot MRI study. World J Biol Psychiatry. 2012;13:223–31. doi: 10.3109/15622975.2011.570376. [DOI] [PubMed] [Google Scholar]
- 17.Sever Y, Ashkenazi A, Tyano S, Weizman A. Iron treatment in children with attention deficit hyperactivity disorder. A preliminary report. Neuropsychobiology. 1997;35:178–80. doi: 10.1159/000119341. [DOI] [PubMed] [Google Scholar]
- 18.Wang Y, Huang L, Zhang L, Qu Y, Mu D. Iron status in attention-deficit/Hyperactivity disorder: A Systematic review and meta-analysis. PLoS One. 2017;12:e0169145. doi: 10.1371/journal.pone.0169145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Juneja M, Jain R, Singh V, Mallika V. Iron deficiency in Indian children with attention deficit hyperactivity disorder. Indian Pediatr. 2010;47:955–8. doi: 10.1007/s13312-010-0160-9. [DOI] [PubMed] [Google Scholar]
- 20.Adisetiyo V, Jensen JH, Tabesh A, Deardorff RL, Fieremans E, Di Martino A, et al. Multimodal MR imaging of brain iron in attention deficit hyperactivity disorder: A noninvasive biomarker that responds to psychostimulant treatment? Radiology. 2014;272:524–32. doi: 10.1148/radiol.14140047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Percinel I, Yazici KU, Ustundag B. Iron deficiency parameters in children and adolescents with attention-deficit/Hyperactivity disorder. Child Psychiatry Hum Dev. 2016;47:259–69. doi: 10.1007/s10578-015-0562-y. [DOI] [PubMed] [Google Scholar]
- 22.Hariri M, Azadbakht L. Magnesium, iron, and zinc supplementation for the treatment of attention deficit hyperactivity disorder: A Systematic review on the recent literature. Int J Prev Med. 2015;6:83. doi: 10.4103/2008-7802.164313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hack M, Taylor HG, Schluchter M, Andreias L, Drotar D, Klein N, et al. Behavioral outcomes of extremely low birth weight children at age 8 years. J Dev Behav Pediatr. 2009;30:122–30. doi: 10.1097/DBP.0b013e31819e6a16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Najafi M, Foladchang M, Alizadeh H, Mohammadifar MA. Prevalence of attention deficit hyperactivity disorder, conduct disorder and oppositional defiant disorder. Res Except Child. 2009;9:239–54. [Google Scholar]
- 25.Beard JL. Why iron deficiency is important in infant development. J Nutr. 2008;138:2534–6. doi: 10.1093/jn/138.12.2534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jáuregui-Lobera I. Iron deficiency and cognitive functions. Neuropsychiatr Dis Treat. 2014;10:2087–95. doi: 10.2147/NDT.S72491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Oner O, Oner P, Bozkurt OH, Odabas E, Keser N, Karadag H, et al. Effects of zinc and ferritin levels on parent and teacher reported symptom scores in attention deficit hyperactivity disorder. Child Psychiatry Hum Dev. 2010;41:441–7. doi: 10.1007/s10578-010-0178-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sachdev P. The neuropsychiatry of brain iron. J Neuropsychiatry Clin Neurosci. 1993;5:18–29. doi: 10.1176/jnp.5.1.18. [DOI] [PubMed] [Google Scholar]
- 29.Erikson KM, Jones BC, Beard JL. Iron deficiency alters dopamine transporter functioning in rat striatum. J Nutr. 2000;130:2831–7. doi: 10.1093/jn/130.11.2831. [DOI] [PubMed] [Google Scholar]
- 30.Lozoff B. Early iron deficiency has brain and behavior effects consistent with dopaminergic dysfunction. J Nutr. 2011;141:740S–6S. doi: 10.3945/jn.110.131169. [DOI] [PMC free article] [PubMed] [Google Scholar]


