Abstract
To overcome the limitations of the conventionally used methods for evaluation of orally administered colon-targeted delivery systems, a novel dissolution method using probiotics has been recently reported. In the present study, universal suitability of this medium composed of five different probiotics is established. Different delivery systems – mini tablets, liquisolid compacts, and microspheres coated with different polysaccharides – were prepared and subjected to sequential dissolution testing in medium with and without microbiota. The results obtained from fluid thioglycollate medium (FTM)-based probiotic medium for all the polysaccharide-based formulations showed statistically similar dissolution profile to that in the rat and goat cecal content media. Hence, it can be concluded that the developed FTM-based probiotic medium, once established, may eliminate the need for further animal sacrifice in the dissolution testing of polysaccharide-based colon-targeted delivery system.
Key words: Fluid thioglycollate medium, liquisolid compacts, mini tablets, polysaccharides, probiotics
INTRODUCTION
Dissolution testing of polysaccharide-based oral colon-targeted delivery systems is a consistent challenge due to the varying pH, viscosity, presence of diverse microbiota, and motility conditions in the upper GI segments that the system needs to traverse before reaching the colon.[1] This challenge is further intensified by the diverse strategies used in the formulation of colon-targeted delivery systems, their release being designed to be facilitated by stimuli such as pH,[2,3] pH and time,[2] presence of enzymes,[4] time,[6,7,8,9] using prodrug based,[7] pressure controlled,[10] and microbially triggered delivery systems.[11,12,13,14,15,16,17,18] The most commonly reported medium to evaluate all these types of delivery systems is the one that utilizes colon contents of rodents.[5] However, this medium has some serious limitations including lack of reproducibility, high cost, complexity of procedure, and vivisection.[6,15] Other dissolution media include the use of human fecal slurries and goat cecal contents, for which the element of reproducibility is even less.
Recently, we reported a unique, simple, inexpensive, and animal-sparing dissolution medium using probiotic culture for establishing the release pattern of polysaccharide-based colon-specific delivery system.[15] Fluid thioglycollate medium (FTM) containing probiotic (BIOMIX-I) composed of strains of Lactobacillus acidophilus, Lactobacillus rhamnosus, Bifidobacterium longum, Bifidobacterium bifidum, and Saccharomyces boulardii was used as dissolution medium.[15] The results obtained using this medium were found to be comparable with those obtained using the conventional rat cecal content medium. As the previous study was limited to spheroids as delivery system, the present study was conducted on diverse delivery systems, namely, mini matrix tablets, liquisolid tablets, and microspheres using different polysaccharides, i.e., guar gum, pectin, and xanthan gum to prove the wider application of developed medium. Sulfasalazine (SFZ) was selected for the study as it is a commonly used drug for ulcerative colitis. Successful application of this medium for such diverse systems is expected to establish its usefulness and avoid the sacrifice of animals.
MATERIALS AND METHODS
Materials
SFZ was purchased from Swapnroop Drugs and Pharmaceuticals, India. Probiotic (BIOMIX-I) was a gift from Unique Biotech, Hyderabad, India. Eudragit® S100 was received from Evonik Pharma, India. Castor oil, guar gum, sodium hydroxide pellets, hydrochloric acid, and isopropyl alcohol were procured from Loba Chemie, India. Microcrystalline cellulose PH 101 (MCC PH 101) was from Jackson Laboratories, India. Aerosil 200 was purchased from Central Drug House, India and talc from Qualikems Fine, India. Millipore water was used throughout the study. Hydrochloric acid, potassium dihydrogen orthophosphate, sodium hydroxide, Tween-80, Span 80, formalin, acetic acid, and concentrated sulfuric acid were supplied by S. D. Fine Chemicals, India. Pectin was purchased from Alliance Global, Delhi, India whereas ethyl cellulose was from Dhariyal Polymers Pvt Ltd, India. Amylose was from Otto Chemie Pvt. Ltd., India.
Formulation of sulfasalazine tablets
Tablets were prepared using direct compression method. “V-” cone blender was used to achieve uniform mixing of the blend. Compression was carried out using rotary tablet machine (Trover Pharmamach, India). Unit formula composition of the tablets is shown in Table 1.
Table 1.
Unit formula composition of prepared tablets

Coating of sulfasalazine tablets
Coating solutions of different polysaccharides, i.e., guar gum, xanthan gum, pectin, and inulin were prepared by dispersing 5 g of each gum separately in 100 mL of distilled water. This was followed by homogenization using Remi Elektrotechnik Ltd, India, at 50 rpm for 2 h. In the case of inulin, coating material was dissolved in hot water at 65°C and then homogenized for 2 h. Tablets were coated in a pan coater at a speed of 25 rpm. Aqueous suspensions of polysaccharides (1% w/v) were used. The coater was operated in “exhaust temperature control” mode, meaning that the unit self-adjusted the inlet air temperature to reach and maintain the exhaust temperature at the set target value. Coating was done till 20% weight gain was achieved.
Preparation of sulfasalazine liquisolid compacts
Carrier materials, i.e., guar gum, pectin, and Eudragit® S-100 were blended with nonvolatile liquid, i.e., Tween 80, in varying proportions. Tween-80 was selected as nonvolatile liquid vehicle as the drug has maximum solubility in it. The blend was kept aside for 20 min to ensure complete absorption of Tween-80. This was followed by equilibration of Aerosil® 200 to the blend as coating material, which helped in further adsorption of the unadsorbed liquid from the wet particles. Addition of coating material has been reported to enhance the flow property of liquisolid powder.[19] The blend was then sieved through sieve #24. Other tableting excipients such as talc and magnesium stearate were added. Compression was carried out at the pressure of 20 kN. The composition of various liquisolid formulations is shown in Table 2.
Table 2.
Unit formula composition for Liquisolid tablets

Preparation of Sulfasalazine-loaded microspheres
SFZ-loaded microspheres were prepared by emulsion polymerization technique.[18] An aqueous dispersion of guar gum-xanthan gum (1:0.5 w/w) was prepared by dissolving 20 g of guar gum and 10 g of xanthan gum in a specified volume of water containing the drug and allowed to swell for 2 h. This mixture was then dispersed in 100 g of castor oil containing 3 g of Span 80 with constant stirring at 4000 rpm. This was followed by addition of 0.2 mL of concentrated sulfuric acid and 2.0 mL of glutaraldehyde with constant stirring at 50°C. The prepared microspheres were collected by centrifugation at 2500 rpm, then washed with 4–5 fractions of isopropyl alcohol, and dried in a vacuum desiccator for 48 h. Free-flowing powder consisting of spherical micron-sized particles was obtained. Formula used in the preparation of SFZ microspheres is given in Table 3.
Table 3.
List of ingredients and their quantities used in formulation of SFZ microspheres

Preparation of fluid thioglycollate dissolution medium using probiotic culture
FTM was prepared using the procedure given by Singh et al., 2015.[15] Composition of FTM is shown in Table 4 which is similar to that reported in our previous study.[15] FTM (8.94 g) was mixed in 300 mL of distilled water by stirring. Mixture was autoclaved at 15 lbs pressure and temperature of 121°C for 15 min. Medium was then inoculated by adding 325 mg of the probiotic mixture and incubated for 48 h at 35°C in an anaerobic environment.[15] Composition of probiotic blend is given in Table 5.[15]
Table 4.
Composition of FTM (Formula/L)
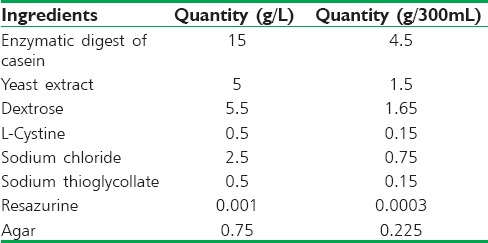
Table 5.
Composition of 325 mg of probiotic blend
Dissolution studies of developed formulations
All the dissolution studies reported here (except that using goat cecal contents) were carried out as stated in our previous report.[15] The procedures are described in brief.
Dissolution studies of prepared formulations using probiotic culture
Studies were conducted in USP dissolution apparatus I (LAB INDIA DS 8000, India) at 100 ± 4 rpm and a temperature of 37°C ± 0.5°C. For the first 2 h, the study was conducted in 200 mL of 1.2 pH HCl solution at 100 rpm at 37°C ± 0.5°C. The pH of the medium was then adjusted to 6.8 and volume made up to 900 mL. Study was continued in this medium for 3 h. The pH of the medium was then adjusted to 7.4 at the end of 5 h period and purged with carbon dioxide gas for 10 min to remove of any undissolved oxygen. Probiotic culture in FTM (100 mL) was then added and the final volume was made up to 1000 mL in each flask. Purging of CO2 was continued till the end of the study. Samples (5 mL) were withdrawn at hourly intervals for 1–14 h followed by 2 hourly intervals till 24 h with simultaneous replacement by fresh medium. The samples were filtered through 0.22 μ membrane filters. The suitably diluted solutions were analyzed at 359 nm by high-performance liquid chromatography. All the studies were performed in triplicate and the mean data recorded. For negative control, the procedure was conducted in exactly similar manner except the addition of probiotic culture in the FTM (medium without microbiota).
Dissolution studies of prepared formulations using 4.5% w/v rat cecal contents
Dissolution studies of the prepared formulation were conducted in a medium containing 4.5% w/v rat cecal contents. Procedure followed was the same as that reported above in “Dissolution studies of prepared formulations using probiotic culture section” up to the 5th h after which rat cecal contents were added in place of the FTM.
Cecal contents were obtained from Sprague Dawley rats (IAEC Protocol Number: LPU/LSPS/IAEC/CPCSEA/MEETING No. 2/] PROTOCOL No. 4). Rats of either sex, weighing 250-300 g, were bought from the National Institute of Pharmaceutical Education and Research, Mohali, India. They were kept in standard laboratory conditions at 25°C ± 2°C under 12 h light and dark cycle and were given rat chow diet and water ad libitum. Acclimatization of animals to laboratory conditions for 7 days was done before the start of the study.
Fifteen minutes before the end of the 5th h of dissolution studies, six animals were sacrificed. Cecal contents were isolated after opening their abdomens. Weighed amounts of cecal contents were transferred to 300 mL of pH 6.8 phosphate buffer with constant purging of CO2. To each of the dissolution vessels, 100 mL of cecal slurries was added and pH of the solution adjusted to 7.4 to maintain a final concentration of 0.45% w/v and the study was continued as per procedure mentioned in “Dissolution studies of prepared formulations using probiotic culture section”. Sampling and analysis procedures were carried out as reported in “Dissolution studies of prepared formulations using probiotic culture section”. For negative control, the procedure was conducted in exactly similar manner except the addition of rat cecal contents (medium without microbiota).
Dissolution studies of prepared formulations using 5.0% w/v human fecal slurries
Dissolution studies of the prepared formulation were conducted in medium containing 4.5% w/v rat cecal contents. The procedure followed was the same as reported above in “Dissolution studies of prepared formulations using probiotic culture section” up to the 5th h after which human fecal contents were added in place of the FTM.
At the end of 5th h, the media was purged with CO2 and 5% w/v of freshly prepared fecal slurries were added and the study was continued as per procedure mentioned in “Dissolution studies of prepared formulations using probiotic culture section”. Sampling and analysis procedures were carried out as reported in “Dissolution studies of prepared formulations using probiotic culture section”. For negative control, the procedure was conducted in exactly similar manner except the addition of human fecal slurries (medium without microbiota).
Dissolution studies of prepared formulations using 4.5% w/v goat cecal content
The procedure for conducting dissolution studies using goat cecal contents was similar to that conducted using rat cecal contents. Goat cecal contents were procured from slaughterhouse. Accurately weighed amount of cecal contents were taken to prepare 4.5% w/v slurries. These were added to dissolution medium at the end of 5th h and the study was continued as per procedure mentioned in “Dissolution studies of prepared formulations using probiotic culture section”. Sampling and analysis procedures were carried out as reported in “Dissolution studies of prepared formulations using probiotic culture section”. For negative control, the procedure was conducted in exactly similar manner except the addition of goat cecal contents (medium without microbiota).
RESULTS AND DISCUSSION
The most commonly used medium for evaluation of colon-targeted delivery systems is the one containing rat cecal contents which requires the sacrifice of laboratory animals. Limitations associated with this medium include sacrifice of animals, high cost, and batch to batch variability. The other commonly used media that avoid the sacrifice of animals are those using human fecal slurries and goat cecal contents, both of which suffer from high batch-to-batch variation.
To position the proposed FTM medium as a pragmatic alternative to all these reported media, comparative dissolution studies were carried out using probiotic culture in FTM, 4.5% w/v rat cecal contents, 5% w/v human fecal slurries, and 4.5% w/v goat cecal contents.
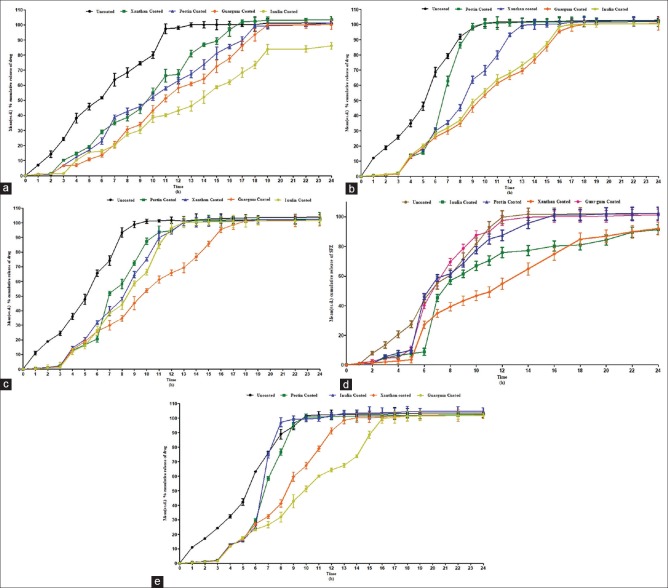
In the medium without microbiota, the release of the uncoated tablets and tablets coated with xanthan gum, pectin, guar gum, and inulin was found to be 45.8, 19.18, 16.96, 10.8, and 15.4%, respectively, up to 5 h [Figure 1]. Similar observations were recorded in case of media containing human fecal slurries, rat cecal content, probiotics medium, and goat cecal content up to 5 h. It was observed that the release from pectin-coated tablets was almost similar to those of guar gum-coated tablets. In the dissolution medium without microbiota, none of the tablets showed rapid drug release, whereas in all the media containing microbiota, the release was found to be quite rapid after the 5th h. This shows that polysaccharides were digested by the colonic bacteria present in the respective media. When similarity factor was applied between dissolution profiles of probiotic culture versus rat and goat cecal contents, it was found highly similar with a value of 54.42 and 51.51.
Figure 1.
(a) Mean (±standard deviation) % cumulative release of sulfasalazine from tablets using compendial method. (b) Mean (±standard deviation) % cumulative release of sulfasalazine from tablets using 5.0% w/v human fecal slurries. (c) Mean (±standard deviation) % cumulative release of sulfasalazine from tablets using 4.5% w/v rat cecal contents. (d) Mean (±standard deviation) % cumulative release of sulfasalazine from tablets using 4.5% goat cecal contents. (e) Mean (±standard deviation) % cumulative release of sulfasalazine from tablets using probiotic medium
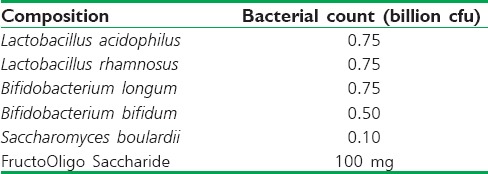
Similar results were observed with liquisolid compacts of SFZ [Figure 2]. Less than 10% drug release was observed in all the media. However, at the end of 5 h, a sudden burst in the release occurred in the medium containing microbiota. More than 70% drug release was observed within 12 h of the study in microbiota containing media. The formulation released less than 10% of SFZ in all the media in 5 h. No significant difference was observed in the dissolution profiles obtained using different media. Similar results were obtained in case of SFZ microspheres also [Figure 3].
Figure 2.
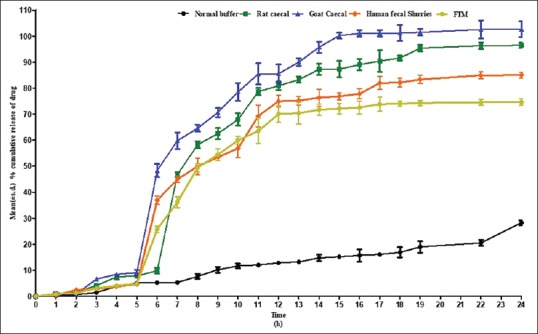
Mean (±standard deviation) % cumulative release of sulfasalazine from liquisolid tablets using different media
Figure 3.
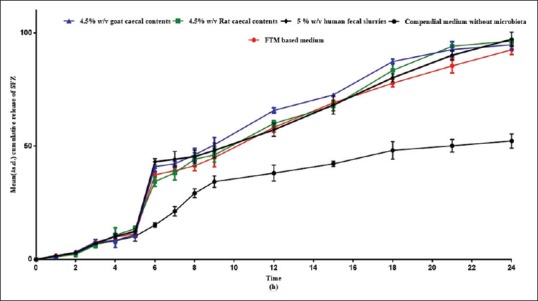
Mean (±standard deviation) % cumulative release of sulfasalazine from microspheres using different media
The wider use of medium containing cecal contents of rats over those containing enzymes clearly indicates that the use of a source of continuously replenishing wide array of enzymes is more predictive of the behavior in colonic milieu as compared to the limited supply of single enzyme.[20] Another essential requisite for the suitability of any medium is that its similarity to the colonic content in terms of numbers and types of bacteria which is lacking in the media using goat or human fecal slurries.[21,22] These limitations lead to the widespread use of rat cecal contents in the media for evaluation of polysaccharide-based colonic delivery systems.[6] This medium, however, requires the sacrifice of a large number of animals. In the past 5 years, efforts have been made to develop a medium containing probiotic that can provide an effective alternative to rat cecal content.[13,14,15] The present work has established that the use probiotic containing medium may be extended to various dosage forms prepared using different polysaccharides and is not limited by the nature of formulation or types of polysaccharides used.
Improved reproducibility of results with this medium may be attributed to the fixed and well-defined composition of the media in terms of species and concentration of the bacteria and composition of thioglycollates.
CONCLUSION
The FTM-based probiotic medium can be used for dissolution testing of polysaccharide-based colon-targeted delivery of drugs. The dissolution profiles are found to be more reproducible than those obtained using the methods that are currently in use. Its biggest advantage is that it eliminates the need for animal sacrifice and could be a suitable alternative for in vitro evaluation of polysaccharide-based colon-targeted delivery systems.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Tom Linson E. Theory and practice of site specific drug delivery. Adv Drug Deliv Rev. 1988;1:271–2. [Google Scholar]
- 2.Ibekwe VC, Fadda HM, McConnell EL, Khela MK, Evans DF, Basit AW, et al. Interplay between intestinal pH, transit time and feed status on the in vivo performance of pH responsive ileo-colonic release systems. Pharm Res. 2008;25:1828–35. doi: 10.1007/s11095-008-9580-9. [DOI] [PubMed] [Google Scholar]
- 3.Maestrelli F, Cirri M, Corti G, Mennini N, Mura P. Development of enteric-coated calcium pectinate microspheres intended for colonic drug delivery. Eur J Pharm Biopharm. 2008;69:508–18. doi: 10.1016/j.ejpb.2007.12.004. [DOI] [PubMed] [Google Scholar]
- 4.Lai H, Lin K, Zhang W, Zhang Z, Jie L, Wu Y, et al. Development of pH- and enzyme-controlled, colon-targeted, pulsed delivery system of a poorly water-soluble drug: Preparation and in vitro evaluation. Drug Dev Ind Pharm. 2010;36:81–92. doi: 10.3109/03639040903092335. [DOI] [PubMed] [Google Scholar]
- 5.Semdé R, Amighi K, Devleeschouwer MJ, Moës AJ. Effect of pectinolytic enzymes on the theophylline release from pellets coated with water insoluble polymers containing pectin HM or calcium pectinate. Int J Pharm. 2000;197:169–79. doi: 10.1016/s0378-5173(99)00465-2. [DOI] [PubMed] [Google Scholar]
- 6.Yang LB, Watanabe S, Shi Y, Liu F, Chu JS, Li J, et al. Design and in vitro/in vivo Evaluation of a Novel Colon-Specific Drug Delivery System (CODES™), AAPS Annual Meeting Poster Presentation (W4091); 21-25 October, 2001; Denver, CO. 2001. [Google Scholar]
- 7.Kotla NG, Gulati M, Singh SK, Shivapooja A. Facts, fallacies and future of dissolution testing of polysaccharide based colon-specific drug delivery. J Control Release. 2014;178:55–62. doi: 10.1016/j.jconrel.2014.01.010. [DOI] [PubMed] [Google Scholar]
- 8.Ahmed IS. Effect of simulated gastrointestinal conditions on drug release from pectin/ethylcellulose as film coating for drug delivery to the colon. Drug Dev Ind Pharm. 2005;31:465–70. doi: 10.1080/03639040500214704. [DOI] [PubMed] [Google Scholar]
- 9.Philip AK, Pathak K, Shakya P. Asymmetric membrane in membrane capsules: A means for achieving delayed and osmotic flow of cefadroxil. Eur J Pharm Biopharm. 2008;69:658–66. doi: 10.1016/j.ejpb.2007.12.011. [DOI] [PubMed] [Google Scholar]
- 10.Muraoka M, Hu Z, Shimokawa T, Sekino S, Kurogoshi R, Kuboi Y, et al. Evaluation of intestinal pressure-controlled colon delivery capsule containing caffeine as a model drug in human volunteers. J Control Release. 1998;52:119–29. doi: 10.1016/s0168-3659(97)00201-0. [DOI] [PubMed] [Google Scholar]
- 11.Prasad YV, Krishnaiah YS, Satyanarayana S. In vitro evaluation of guar gum as a carrier for colon-specific drug delivery. J Control Release. 1998;51:281–7. doi: 10.1016/s0168-3659(97)00181-8. [DOI] [PubMed] [Google Scholar]
- 12.Krishnaiah YS, Satyanarayana V, Dinesh Kumar B, Karthikeyan RS. In vitro drug release studies on guar gum-based colon targeted oral drug delivery systems of 5-fluorouracil. Eur J Pharm Sci. 2002;16:185–92. doi: 10.1016/s0928-0987(02)00081-7. [DOI] [PubMed] [Google Scholar]
- 13.Kotla NG, Gulati M, Singh SK, Basotra M, Choudhary Y. Media for in vitro Dissolution Testing of Polysaccharide Based Colon Targeted Formulations and Method Thereof. Indian Patent 2485/DEL/2013 A. 2013 [Google Scholar]
- 14.Kotla NG, Singh S, Maddiboyina B, Sunnapu O, Webster TJ. A novel dissolution media for testing drug release from a nanostructured polysaccharide-based colon specific drug delivery system: An approach to alternative colon media. Int J Nanomedicine. 2016;11:1089–95. doi: 10.2147/IJN.S97177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Singh SK, Yadav AK, Prudhviraj G, Gulati M, Kaur P, Vaidya Y, et al. Anovel dissolution method for evaluation of polysaccharide based colon specific delivery systems: A suitable alternative to animal sacrifice. Eur J Pharm Sci. 2015;73:72–80. doi: 10.1016/j.ejps.2015.03.012. [DOI] [PubMed] [Google Scholar]
- 16.Prudhviraj G, Vaidya Y, Singh SK, Yadav AK, Kaur P, Gulati M, et al. Effect of co-administration of probiotics with polysaccharide based colon targeted delivery systems to optimize site specific drug release. Eur J Pharm Biopharm. 2015;97:164–72. doi: 10.1016/j.ejpb.2015.09.012. [DOI] [PubMed] [Google Scholar]
- 17.Milojevic S, Newton JM, Cummings JH, Gibson GR, Botham RL, et al. Amylose as a coating for drug delivery to the colon: Preparation and in vitro evaluation using 5 –amino salicylic acid pellets. J Control Release. 1996;38:75–84. [Google Scholar]
- 18.Kaur R, Gulati M, Singh SK. Role of synbiotics in polysaccharide assisted colon targeted microspheres of mesalamine for the treatment of ulcerative colitis. Int J Biol Macromol. 2017;95:438–50. doi: 10.1016/j.ijbiomac.2016.11.066. [DOI] [PubMed] [Google Scholar]
- 19.Singh SK, Srinivasan KK, Gowthamarajan K, Prakash D, Gaikwad NB, Singare DS, et al. Influence of formulation parameters on dissolution rate enhancement of glyburide using liquisolid technique. Drug Dev Ind Pharm. 2012;38:961–70. doi: 10.3109/03639045.2011.634810. [DOI] [PubMed] [Google Scholar]
- 20.Yang L, Chu JS, Fix JA. Colon-specific drug delivery: New approaches and in vitro/in vivo evaluation. Int J Pharm. 2002;235:1–5. doi: 10.1016/s0378-5173(02)00004-2. [DOI] [PubMed] [Google Scholar]
- 21.Gibson GR, Cummings JH, Macfarlane GT. Use of a three-stage continuous culture system to study the effect of mucin on dissimilatory sulfate reduction and methanogenesis by mixed populations of human gut bacteria. Appl Environ Microbiol. 1988;54:2750–5. doi: 10.1128/aem.54.11.2750-2755.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Macfarlane S, Quigley ME, Hopkins MJ, Newton DF, Macfarlane GT. Polysaccharide degradation by human intestinal bacteria during growth under multi-substrate limiting conditions in a three-stage continuous culture system. FEMS Microbiol Ecol. 1998;26:231–43. [Google Scholar]