Abstract
Background:
Newer drug delivery systems such as transdermal patches using pain relieving or modifying agents emerged as a mainstream treatment protocol for management of pain on the outpatient basis. The administration of diclofenac 100 mg in the transdermal patch in the patients having dental pain due to periapical/periodontal infections was evaluated.
Materials and Methods:
Ninety patients of either gender, between 18 and 80 years were divided into 3 groups (Group A - oral medication, Group B - transdermal patch, Group C - intramuscular group). Patients at the Dental Department with pain from periapical/periodontal pathologies were explained about the procedure of analgesia. With written consent, 100 mg diclofenac sodium transdermal patches were prescribed to patients who opted their use in pain control for 2 consecutive days. A visual analog scale was provided for all patients assessing the pain intensity during the study.
Results:
Significant difference in the mean percentage reduction in visual analog scale (VAS) score among the three groups at day 1 and 2 (P < 0.001). Post hoc test showed that intramuscular (IM) and oral groups had significantly higher mean VAS score than patch group.
Conclusion:
Diclofenac administered through oral and IM routes showed significant improvement in pain relief when compared to the transdermal route. However, diclofenac transdermal patches have shown significant improvement in VAS score between the baseline and consecutive days and can be used in mild pain with lower adverse events.
Keywords: Alternative drug delivery, analgesia, tablet
INTRODUCTION
The most commonly encountered symptoms to the oral physician is the dental pain. This assumes pivotal role in the effective management and follow-up in terms of patient management. Pain is so closely dealt in dentistry that they cannot be separable.[1,2]
The spurt in new drug discovery, dosage, and the delivery systems lead to employing such advancements from research to practice. The innovations had their effects immediately in the medical field, but somehow it was less rapid in dentistry. Evidence-based clinical practice outcome have further enhanced their usage into the regular outpatient dental setup. The newer drug delivery systems have shown their advantage in being enhanced efficacy, better bioavailability with decreased adverse effects and also into the frequency of drug intake.[2] The expanded profile of nonsteroidal anti-inflammatory drugs (NSAIDs) into the clinical practice with their overzealous usage lead to untoward adverse reactions in terms of cardiac, renal, and gastrointestinal toxicity to reduce such occurrences, innovative method of drug delivery systems or with a change in the pharmacological profiles of the drugs were employed. Prescription of gastroprotective medication along with NSAID had become a common practice.[2,3,4,5,6]
Diclofenac sodium which is an aryl acetic acid derivative exerts its action by blocking COX-1 and COX-2 enzymes thereby it has an inhibitory effect in the synthesis of the chemical mediators such as prostaglandin E2, D2, F2, and thromboxane A2.
Transdermal drug delivery, a local drug delivery system has gained populace in the recent time. They provide an advantage of controlled drug delivery at the local site, either over the skin or in the buccal mucosa in case of the oral cavity. The incidence of adverse drug reaction with them is mild and predictable with better patient compliance.
Transdermal patches ensure simple, painless procedure of drug usage even in patients with needle phobia. They are on par with sustainable plasma levels comparable with that of oral medication. With the local drug delivery method, hepatic first-pass metabolism is bypassed. Offering increased flexibility in placing and removing the transdermal patch better patient compliance is achieved.[7,8]
This study was carried out to compare the efficacy of analgesia, occurrence of side effects from the drugs employed and patient compliance during the drug usage with diclofenac sodium trans dermal patch 100 mg, oral supplementation of diclofenac sodium 50 mg given as twice daily medication and intra muscular administration of 75 mg diclofenac sodium once daily for 2 days in patients having dental pain due to periapical or periodontal pathology.
MATERIALS AND METHODS
A total sample of 90 subjects of either gender, between 18 and 80 years attending Department of Oral Medicine and Radiology with dental pain and who were willing to be part of the study were included and divided randomly into 3 groups based on their preference and priority of the route of administration of the drug. As this was a pilot study, a sample size of 90 was chosen according to recommendations by Isaac and Michael.[9]
The study was cleared by ethical clearance committee with necessary approvals. The subjects were informed thoroughly about the study and could withdraw from the study at their choice when there is no relief of pain with the medication. (Ethical clearance number: PMVIDSandRC/IEC/OMR/PR 0051–15). This study was registered with clinical trial registry of United States with trial registry number NCT03221946. The groups included in the study were: [CONSORT checklist Figure 1].
Figure 1.
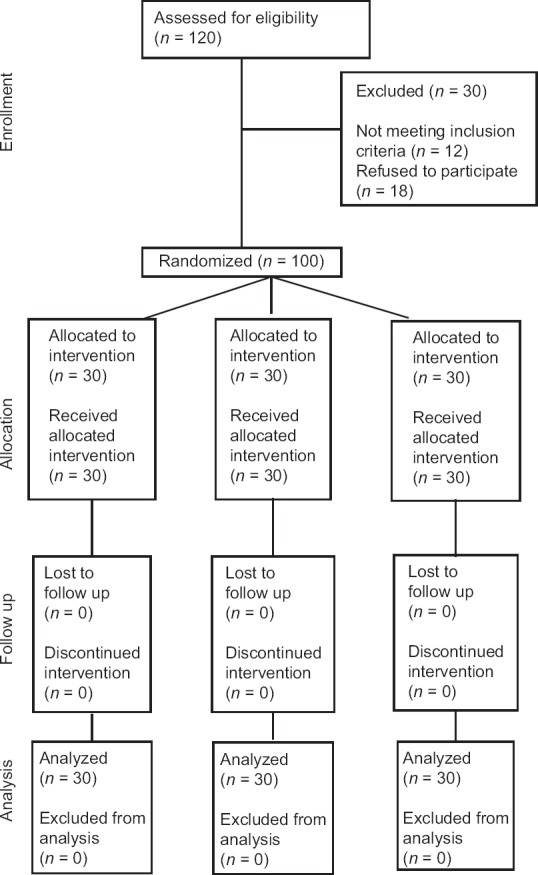
Requirement, allocation, and follow-up of participants
Group A that included 30 patients of either gender who were prescribed tablet Diclofenac sodium 50 mg orally twice daily for 2 days which is the preferred adult dose for dental pain disorders
Group B which included thirty patients who were prescribed Diclofenac sodium 100 mg transdermal patch to equate the oral dosage of 50 mg that is given twice daily once daily for 2 days (SPARSH PHARMA). It is a transparent patch that gave sustained release of the drug for 24 h at the local site applied. A total number of 2 patches were given to each patient 1/day. The patches were applied at a hairless area on the left or right shoulders which were subsequently replaced the next day to another area of application to avoid contact dermatitis in the area of application
Group C which included 30 patients of either gender who were given intra muscular injection of 75 mg which was the nearest available dosage to 100 mg availability in India at deltoid or gluteus muscle once daily for 2 days using sterile and aseptic precautions.
All the subjects were prescribed necessary antibiotics for a reduction in periapical/periodontal infections. The rescue medication tablet chosen was Paracetamol 325 mg, if any of the patients opted for further medication for pain relief. In addition, a visual analog scale was provided to all the subjects during their presentation to the hospital for two consecutive days for treatment. The scales were obtained in all the subjects individually assessing the intensity of pain after the medication intake during the study. Team of four doctors evaluated the patients in the study. Two physicians were assigned the task of data collection from the study participants and assigning them into the group chosen by the patients themselves. Two physicians had evaluated the patient's visual analog scale (VAS) scores clinically and administered the necessary medication to the patients and rest of the doctors had evaluated the patients at each visit, making a note of interobserver variability. The enquiry of following adverse effects was noted for all three groups.
Group A: Rash, Itch, Gastritis
Group B: Rash at the delivery site, adherence of patch
Group C: Pain at injection site, nerve palsy, hypersensitivity reactions.
The administration of rescue medication marked the end of the study in any individual. The data were then statistically evaluated using SPSS version 18 (IBM Corporation). The value of P < 0.05 was considered statistically significant. The intra-group analysis was performed using Repeated measures ANOVA with Bonferroni test. The inter-group analysis was performed using ANOVA with post hoc Tukey's test.
RESULTS
Distribution of three groups based on age and gender
Out of ninety patients from all the 3 groups, in the maximum number of patients fell in the age ranged between 18 and 28 (32.3%) which was statistically significant. A high number of males (58.1%) participated when compared to the female counterparts (41.9%) that were statistically significant [Table 1].
Table 1.
Distribution of three groups based on age and gender
Comparison of the visual analog scale score at baselines, day 1 and day 2 in each intervention group
Showed significant difference in the means of the score from baseline until day 2 in patch group (P < 0.001). Post hoc test revealed that baseline had significantly higher mean VAS scores than day 1 and 2; although, no significant difference was seen between day 1 and 2. Similarly, there was also a statistical difference in the mean score from baseline to day 2 in intramuscular (IM) and oral group (P < 0.001). A major difference lies in IM group in which, day 1 had higher mean VAS score than day 2, which recorded least scores compared to the other 2 groups. The baseline scores were higher in IM group due to the increased pain perception psychologically by the patients and preference route of drug administration in the form of injection by the patient for immediate relief from pain.
The oral group had significantly higher mean VAS scores than day 1 followed by day 2. Similar to IM group but slightly higher VAS scores on day 2 than scores of day 2 in IM group. Nevertheless, patch group showed higher VAS scores at day 2 when compared to both other groups [Table 2].
Table 2.
Comparison of visual analog scale score at baselines, day 1 and day 2 in each intervention group showed
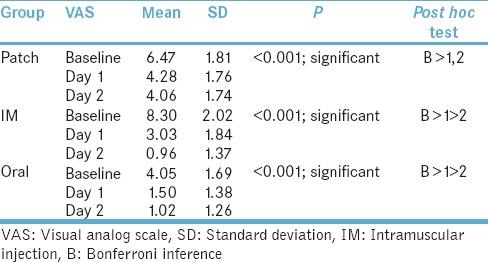
At the baseline, there was marked variation in the mean VAS score in all 3 groups at baseline (P < 0.001). Post hoc test showed that IM group had highest mean VAS score followed by Patch with least being an oral group. Day 1 showed a significant difference in the mean VAS score among the three groups (P < 0.001). Post hoc test showed that patch group had highest mean VAS score followed by IM group with least being an oral group. Day 2 showed a significant difference in the mean VAS score among the three groups (P < 0.001). Post hoc test showed that patch group had significantly higher mean VAS score than oral and IM groups. No significant difference in the mean VAS scores between oral and IM group.
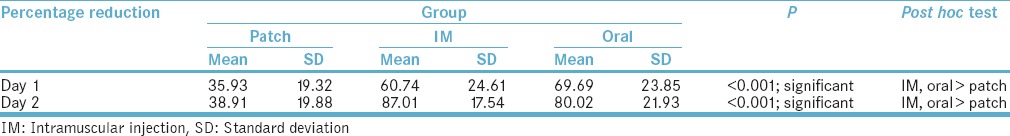
There was a significant difference in the mean percentage reduction in VAS score among the three groups at day 1 (P < 0.001). Post hoc test showed that IM and oral groups had significantly higher mean VAS score than Patch group. There was a significant difference in the mean percentage reduction in VAS score among the three groups at day 2 (P < 0.001). Post hoc test showed that IM and oral groups had significantly higher mean VAS score than patch group [Table 3].
Table 3.
Percentage reduction of pain on day 1 and day 2
DISCUSSION
NSAIDS are common prescription drugs for analgesia. In the recent years, with evidence on their overzealous usage and potential side effects had led to a search for a better alternative or modifications in the drug delivery mechanism. Rapid strides were made to reduce the incidence of side effects without compromising their efficacy. Once such ingress was topical NSAID delivery systems in health-care setting.[10]
NSAIDS when administered systemically, have been implicated in various adverse drug events due to their inherent mechanism of inhibiting prostaglandin synthesis thereby disrupting the general physiologic system.[11] Thus, the advantage of local drug application provided an alternative system of administration which may be beneficial in reducing the unfavorable reactions to a certain extent than the oral-systemic of drug delivery.[12] In addition, topical usage has the direct access, to the local area, controlled release, and ease of application leading to good adherence outcome and sustained plasma concentration.[13]
In the present study, there was a significant reduction in pain on day 2 when compared to day 1 in oral group and IM group. However, such significance was not found on day 1 and 2 in case of transdermal patch group. Bachalli et al. did a study in terms of pain reduction following extraction of third molars wherein oral administration of diclofenac sodium had shown a significant reduction in pain when compared to that administered transdermally. However, there was no statistical difference in the VAS score among the two routes.[1]
In the present study, there was statistically significant pain reduction in oral and IM groups when compared to transdermal patch group. Patches have shown good improvement in cases of mild pain. Predel et al. advocated diclofenac in the form of patches in cases of soft tissue injuries. They concluded that the patches were efficacious in blunt trauma.[14] Although parenteral drug delivery offers benefits like better accessibility to circulation and better absorption, these benefits go hand in hand with a rapid reduction of drug levels in the circulation.[15,16]
Hemant Bhaskar et al., concluded in a study on the orthodontic patient group having pain following premolar extractions. In the study, transdermal patches of diclofenac sodium of 100 mg, used as single application per day had given similar relief from pain as oral 150 mg tablets of the same drug. It was demonstrated that trans dermal patch drug delivery was found to be on par in efficacy with oral diclofenac supplementation in pain reduction. Over a half who had tablets orally had reduced pain over a day. The subjects who were prescribed transdermal patches had 65% reduction of pain during the immediate 2 postoperative days.[17]
The limitation that was observed with transdermal patches during the study was the ability of the patch to adhere to the site of application. Four patients out of 30 showed poor adhesiveness of patch.
CONCLUSION
Through this study, it is noteworthy that diclofenac transdermal patches have shown significant improvement in moderate pain cases, Diclofenac administered through oral and IM routes showed significant improvement in reduction of pain scores when compared to the transdermal route. However, transdermal patches have proved to be promising in moderate pain cases with superior patient compliance and were well-tolerated by the patients.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgments
We acknowledge the staff of the Department of Oral medicine and Radiology for helping us in the initial screening of the dental pain patients at the opd level and counseling them for appropriate treatment options.
REFERENCES
- 1.Bachalli PS, Nandakumar H, Srinath N. A comparitive study of diclofenac transdermal patch against oral diclofenac for pain control following removal of mandibular impacted third molars. J Maxillofac Oral Surg. 2009;8:167–72. doi: 10.1007/s12663-009-0041-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Monheim S. Local Anesthesia and Pain Control in Dental Practice. 7th ed. CBS publications; 1990. Jan, [Google Scholar]
- 3.Altman R, Bosch B, Brune K, Patrignani P, Young C. Advances in NSAID development: Evolution of diclofenac products using pharmaceutical technology. Drugs. 2015;75:859–77. doi: 10.1007/s40265-015-0392-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McGettigan P, Henry D. Cardiovascular risk with non-steroidal anti-inflammatory drugs: Systematic review of population-based controlled observational studies. PLoS Med. 2011;8:e1001098. doi: 10.1371/journal.pmed.1001098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McGettigan P, Henry D. Use of non-steroidal anti-inflammatory drugs that elevate cardiovascular risk: An examination of sales and essential medicines lists in low-middle-and high-income countries. PLoS Med. 2013;10:e1001388. doi: 10.1371/journal.pmed.1001388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Salvo F, Fourrier-Réglat A, Bazin F, Robinson P, Riera-Guardia N, Haag M, et al. Cardiovascular and gastrointestinal safety of NSAIDs: A systematic review of meta-analyses of randomized clinical trials. Clin Pharmacol Ther. 2011;89:855–66. doi: 10.1038/clpt.2011.45. [DOI] [PubMed] [Google Scholar]
- 7.Bajaj S, Whiteman A, Brandner B. Transdermal drug delivery in pain management. Anaesth Crit Care Pain. 2011;11:39–43. [Google Scholar]
- 8.Scheindlin S. Transdermal drug delivery: Past, present, future. Mol Interv. 2004;4:308–12. doi: 10.1124/mi.4.6.1. [DOI] [PubMed] [Google Scholar]
- 9.Isaac S, Michael WB. Handbook in Research and Evaluation. San Diego, CA: Educational and Industrial Testing Services; 1995. [Google Scholar]
- 10.McCarberg BH, Argoff CE. Topical diclofenac epolamine patch 1.3% for treatment of acute pain caused by soft tissue injury. Int J Clin Pract. 2010;64:1546–53. doi: 10.1111/j.1742-1241.2010.02474.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Laine L, Curtis SP, Cryer B, Kaur A, Cannon CP. MEDAL Steering Committee. Assessment of upper gastrointestinal safety of etoricoxib and diclofenac in patients with osteoarthritis and rheumatoid arthritis in the Multinational Etoricoxib and Diclofenac Arthritis Long-term (MEDAL) programme: A randomised comparison. Lancet. 2007;369:465–73. doi: 10.1016/S0140-6736(07)60234-7. [DOI] [PubMed] [Google Scholar]
- 12.Moore RA, Tramèr MR, Carroll D, Wiffen PJ, McQuay HJ. Quantitative systematic review of topically applied non-steroidal anti-inflammatory drugs. BMJ. 1998;316:333–8. doi: 10.1136/bmj.316.7128.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pandey S, Praveen SH, Udupa N. Formulation and evaluation of nimesulide transdermal drug delivery systems. Indian J Pharm Sci. 2000;62:376–9. [Google Scholar]
- 14.Predel HG, Koll R, Pabst H, Dieter R, Gallacchi G, Giannetti B, et al. Diclofenac patch for topical treatment of acute impact injuries: A randomised double blind placebo controlled multicentre study. Br J Sports Med. 2004;38:318–23. doi: 10.1136/bjsm.2003.005017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vaile JH, Davis P. Topical NSAIDs for musculoskeletal conditions. A review of the literature. Drugs. 1998;56:783–99. doi: 10.2165/00003495-199856050-00004. [DOI] [PubMed] [Google Scholar]
- 16.Brooks PM, Kean WF, Buchanun WW. The Clinical Pharmacology of Anti-Inflammatory Agents. Philadelphia: Taylor & Francis; 1986. [Google Scholar]
- 17.Bhaskar H, Kapoor P, Ragini Comparison of transdermal diclofenac patch with oral diclofenac as an analgesic modality following multiple premolar extractions in orthodontic patients: A cross over efficacy trial. Contemp Clin Dent. 2010;1:158–63. doi: 10.4103/0976-237X.72783. [DOI] [PMC free article] [PubMed] [Google Scholar]


