Abstract
Introduction:
Oxidative stress is a common factor in cataract. Considering the antioxidant properties of hesperidin as a flavanone glycoside from the flavonoid family with radioprotective effect, this study aimed to determine the protective effect of this flavanone glycoside on reducing oxidative stress in the eye lens tissue of mature rats caused by gamma irradiation.
Materials and Methods:
A total of 48 adult rats were randomly divided into six groups, namely, control, Dimethyl sulfoxide (DMSO), hesperidin, radiation, radiation + DMSO, and radiation + hesperidin. 15 Gy irradiation was carried out using Cobalt-60 teletherapy instrument with a source-to-surface distance of 80 cm at a dose rate of 98.5 cGy/min. 2 days following irradiation, we removed the rats' lenses and analyzed them to determine the effects of hesperidin.
Results:
The comparison of control and intervention groups after irradiation showed that malondialdehyde (MDA) level in the lens tissue was significantly higher in the irradiation groups than the control group. Furthermore, a significant difference between radiation and radiation + hesperidin groups were observed. The level of glutathione (GSH) in the lens tissue was significantly lower in the irradiation groups compared to the control group. Nonetheless, significant elevation of GSH in the radiation + hesperidin group compared to radiation group was seen.
Conclusions:
Radiation exposure reduced GSH and enhanced MDA levels in the lens tissue. However, GSH and MDA levels were modulated after hesperidin consumption. These results show the antioxidative properties of hesperidin in the lens and demonstrated that radiation complications such as cataract can be reduced by hesperidin through reducing oxidative stress.
Keywords: Cataract, hesperidin, oxidative stress, radiation
INTRODUCTION
Cataract is opacity in the normally transparent crystalline lens of the eye leading to reduced vision, which can eventually result in blindness.[1] In fact, it is the main cause of preventable blindness in the world[2] and a major health-care challenge worldwide, which is highly associated with age.[1] Cataract is observed in all countries, although it is more commonly observed in developing countries, especially in the African and Asian ones which are affected by different factors such as oxidative stress.[2]
Oxidative stress as a common factor in the majority of cataract cases[2] could be induced by radiation. Radiation effects could be compensated in different ways, some of the oxidative stress such as repair mechanisms[3] and radioprotective material,[4,5] but radiation exposure may lead to the production of strong oxidant species such as reactive oxygen species (ROS), which can lead to lipid, protein, and nucleic acid oxidation and subsequently, serious injuries. It is common knowledge that production of ROS reduces the total antioxidant capacity of the body. Some persons and tissues are more radiosensitive than other tissues, and ocular lens is one of the most sensitive tissues to radiation and ionizing radiation, which can lead to progressive cataract.[1] In radiotherapy, tumoral tissue receives a maximum dose of radiation (based on tissue tolerance threshold) through surrounding the normal tissue.[6]
Cellular health depends on the strength of the enzymatic and nonenzymatic antioxidant defense system including glutathione peroxidase (GSH-PX), superoxide dismutase (SOD), and catalase.[7] Gamma radiation through water radiolysis produces hydroxyl radical which is known as the most powerful free radical and initiator of oxidative stress. In addition, superoxide anion (in an anaerobic environment) and hydrogen peroxide can attack biological molecules encompasses proteins, lipids, and DNA which lead to the incidence of cataract and can prevent the restoration of epithelial cells of the lens.[7,8] Thus, various radiation intensities can result in cataract through crystal lens oxidation and ROS production,[9] but this injury in lens could be modulated by some substances such as zinc.[10]
Hesperidin is a flavanone glycoside[11] from the flavonoid family, with protective effects against reducing radiation-induced hepatic injury[12] and damage to peripheral blood lymphocytes caused by gamma radiation.[13] The effects of hesperidin on cardiac tissue, renal, and hepatic damages,[12] and different human cancer types[14] were evaluated in several studies. Given the antioxidant properties of hesperidin,[15] studying the effectiveness of hesperidin in reducing radiation-induced cataract is of great importance.
Due to the protective effect of such drugs on the treatment of cataract and considering the limited studies performed on the effectiveness of this substance in the prevention of cataract, this study aimed to investigate the protective effect of hesperidin on reducing oxidative stress caused by gamma irradiation in the eye lens tissue of laboratory rats.
MATERIALS AND METHODS
This experimental and fundamental research was performed on mature rats to determine the effect of hesperidin on prevention and reduction of radiation cataract in the rats' eyes in Babol University of Medical Sciences, 2013.
Experimental design
Forty-eight mature rats were randomly divided into six gender, race, and age-matched groups. All the rats were kept in individual cages at 22°C ± 3°C in 12 h of light and 12 h of dark with free access to food and water. The animals were kept in this condition for 1 week in order for compatibility with the environment. All the rats were randomly divided into one control and five experimental groups as follows:
Control group
Dimethyl sulfoxide (DMSO) group
Hesperidin group
Radiation group
Radiation plus DMSO group
Radiation plus hesperidin group.
In control group, the rats did not receive any radiation or medication; however, they were exposed to sham irradiation without any effect on their body. DMSO group received only DMSO (2%) orally for 7 consecutive days. Hesperidin group received only 100 mg/kg of hesperidin dissolved in DMSO orally for 7 consecutive days. Radiation group were undergone 15 Gy cranial gamma irradiation and Radiation plus DMSO group received DMSO (2%) orally before exposure to radiation for 7 consecutive days. Radiation plus Hesperidin group received 100 mg/kg of hesperidin dissolved in DMSO orally before irradiation for 7 consecutive days.
Irradiation
In this study, irradiation was carried out using Cobalt-60 teletherapy instrument (MODEL. Theratron 780E) from a source-to-surface distance of 80 cm at a dose of 98.5 cGy/min. First, we anesthetized the rats with ketamine (90 mg/kg) and xylazine (10 mg/kg) injection and placed them in the prone position. We covered the rats' eyes by a 2 mm thick damp towel to enhance lens dose to the maximum. The rats in the control group were anesthetized but not irradiated and received sham irradiation. 2 days following the irradiation, we anesthetized the rats with ketamine (90 mg/kg) and xylazine (10 mg/kg) injection and sacrificed them through intracardiac potassium chloride injection. Afterward, we enucleated their eyes and the lenses were removed with a posterior approach and then washed, weighed, broke, and froze them at −70°C.
Biochemical survey
We homogenized the rats' lenses in a mixture of 1 ml of 0.9% cold saline and 0.2 ml of 25% trichloroacetic acid, which was centrifuged at 5000 rpm for 15 min. Then, we measured glutathione (GSH) by clear upper supernatant and measured malondialdehyde (MDA) level through assessing sediment. We applied thiobarbituric acid (TBA) technique to determine MDA level.
Thereafter, we soluted 2.5 ml of 0.05 M sulfuric acid and 3 ml of 0.2% TBA in the sediment and incubated the mixture at 100°C for 30 min. We chilled the obtained sample, added 4 cc n-butanol to it, and stirred it rapidly. Then, we centrifuged the mixture at 3500 rpm for 10 min.
We used the standard curve to measure MDA concentration and applied tetra ethoxy propane as the criteria for providing the calibration curve, and Kuo and Hook's technique was used to determine GSH content.
In the next step, we mixed 0.5 ml of distilled water, 2 ml of 0.3 M Na2 HPO4, and 0.5 ml of 0.04% 5'-dithiobis-2-nitrobenzoic acid with 0.5 ml of the supernatant and incubated the mixture for 10 min. The experiment was conducted at room temperature. We read the absorbance of the resulting yellowish and compared it with the blank areas at 412 nm. GSH concentration was measured based on the standard curve. Finally, we used pure GSH as the criterion for creating the calibration curve.
Statistical analysis
To compare groups, ANOVA analysis was performed using SPSS 16 (SPSS Inc., Chicago, USA) and the value of P < 0.05 was considered for statistical significance. Values were expressed as mean ± standard deviation.
Ethical consideration
In the present study, moral considerations were respected for minimizing animal pain based on the guidelines for care and use of laboratory animals which were approved by the Ethical Committee of Babol University of Medical Sciences, Babol, Iran. The study was approved by the Ethical Committee of Babol University of Medical Sciences.
RESULTS
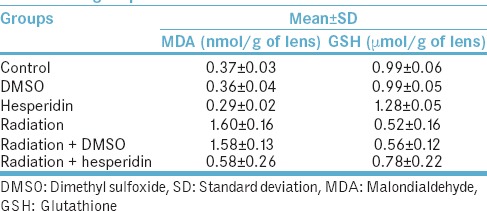
Two days after irradiation, the comparison between the control and intervention groups showed that MDA levels in the lens tissues were significantly higher in the irradiation groups than the control group (P < 0.05). The comparison of the control and intervention groups demonstrated that GSH level in the lens tissue was significantly lower in the irradiation groups compared to the control group (P < 0.05). Comparing biochemical parameters [Table 1] demonstrate a statistical significance in different groups (P < 0.05).
Table 1.
The mean levels of malondialdehyde and glutathione in different groups
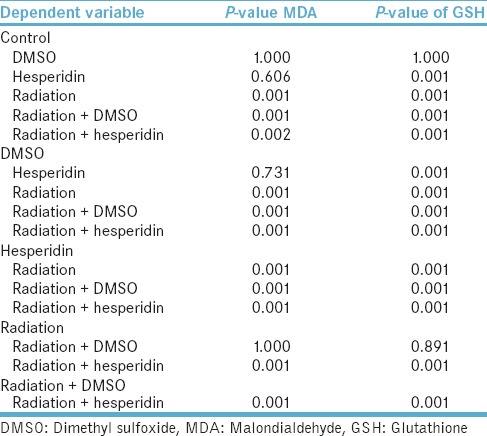
The one by one comparison between groups regarding MDA and GSH levels [Table 2] demonstrate statistical significance between radiation group in comparison with radiation + hesperidin and control groups. Furthermore, we can observe this statistical significance for GSH tissue levels.
Table 2.
The comparison between groups regarding malondialdehyde and glutathione levels
DISCUSSION
According to the present study, MDA level of the lens tissue of the rats exposed to total cranium single-dose irradiation of 15 Gy was significantly enhanced in comparison to the control group and the irradiation plus hesperidin one. On the other hand, the comparison between the control group and intervention groups revealed that GSH level in the lens tissue was significantly lower in the irradiation groups than the control group the irradiation plus hesperidin one.
Production of free radicals and ROS can be induced as a result of action and reaction between ionizing radiation and biological molecules. DNA, nucleic acids, lipids, proteins, and carbohydrates as cellular macromolecules can be damaged due to aggregation of free radicals and ROS. Exposed and absorbed doses, duration of exposure, interval after exposure, and sensitivity of the tissue to ionizing radiation are the main factors affecting the severity and extent of the damage.[16,17]
The incidence of cataract caused by ionizing radiation[18] is highly possible due to radiosensitivity of the ocular lens.[19] DNA damage in epithelial cells of the lens,[20] damage to cellular membranes,[21] and reduction of antioxidant defense of the lens[7] are the most important effects of ionizing radiation on the ocular lens, which lead to cataract by transforming the lens cells function and enhancing the dispersion of light in the lens.
In line with our findings, Shirazi et al.[9] and Tahamtan et al.[22] reported the radioprotective effect of melatonin on diminishing oxidative stress. They demonstrated that MDA level of the lens of the rats exposed to total cranium single-dose irradiation of 5 Gy and 8 Gy enhanced significantly in comparison with the control and irradiation plus melatonin groups. In addition, GSH levels of the lens were reduced significantly in irradiation group compared to control and irradiation plus melatonin groups. Heidari et al. reported a reduction in the rate of micronuclei polychromatic erythrocyte due to sulfur in hot spring[4] and Keramati Yazdi et al. reported the radioprotective ability of Zamzam water as an alkaline water.[23]
According to a study by Randazzo et al.,[2] the radioprotective effect of multi-component antioxidant compounds on rats receiving gamma irradiation of 15 Gy, cataract was observed in all groups after 180 days; nonetheless, the development of cataract (grade 6) was faster in the control group than the experimental groups on day 65.
The evaluation of radioprotective of curcumin on cataracts caused by ionizing radiation (15 Gy of gamma radiation) in rat eye lens, in a study by Ozgen et al.,[1] the incidence rate of cataract was calculated to be 100% in the radiation groups, which was reduced to 40% in the rats receiving curcumin (cataract was limited to grades 1 and 2). The level of antioxidant enzymes was decreased and MDA level was increased in the radiation groups, whereas the level of antioxidant enzymes was increased and MDA level was reduced in the curcumin plus irradiation group.
Furthermore, the effectiveness of sodium hydrogen-S-(3-amino-2-hydroxypropyl) phosphorothioate (WR-77913) in preventing cataract caused by irradiation done by Osgood et al.,[24] showed significant protein transformations in the unprotected rats who were exposed to gamma irradiation of 15.3 Gy. However, WR-77913 could prevent protein changes as well as lens hydration, which in turn, prevented the development of cataract. This result was confirmed in another study by Osgood et al.[24]
Protective role of Vitamin E on the levels of lipid peroxidation and antioxidant enzymatic activity in the lens of rats with cataract caused by gamma irradiation of 5 Gy was evaluated in a study by Karslioglu et al.[25] According to the obtained results of that study, the levels of MDA and GSH-PX activity in the rats receiving radiation without a protective agent was significantly higher than the control group, while SOD activity was significantly higher in the control group. Furthermore, SOD and GSH-PX activity were higher and MDA level was lower in the rats receiving Vitamin E + radiation than the radiation group.
Study of the effect of age and gender on cataract caused by ultraviolet radiation was performed by Lofgren et al.[26] They revealed which younger rats were more sensitive to radiation, and there was no significant difference between the two genders.
Evaluating the radioprotective effect of hesperidin on the rat liver by Kalpana et al.[12] showed reduced antioxidant enzymes levels due to radiation, increased peroxidative lipid index, DNA damage, and changed comet parameters. However, administration of hesperidin increased the level of the antioxidant enzyme and decreased peroxidative lipid index, DNA damage and comet parameters. These results were confirmed in studies by Pradeep et al.[27,28] Moreover, the effectiveness of radioprotective effect of hesperidin on peripheral blood lymphocytes (PBL) and liver, heart, and kidneys was proven in a study by Kalpana et al.,[13] and Pradeep et al. study,[29] respectively.
According to several studies, irradiation enhances MDA formation as the final product of lipid peroxidation.[7,9] Hesperidin as an antioxidant scavenges free radicals and reduces lipid peroxidation, protein and DNA damage. Hence, use of hesperidin before and after irradiation protects against radiation side effects.[25] We noted significant differences between the irradiated and hesperidin-treated rats regarding GSH (as an index of antioxidant and reducing the power of the lens) concentration. Congruent with our study, some studies demonstrated that irradiation reduced tissue GSH concentration.[25,30]
CONCLUSIONS
The study findings demonstrated that radiation exposure, reduce the GSH level and enhanced MDA level in the lens; however, these results were not observed in the administration of hesperidin. These results confirmed the antioxidative properties of hesperidin and revealed that radiation-induced complications such as cataract can be diminished by hesperidin consumption through reducing oxidative stress. Ultimately, administration of hesperidin during radiotherapy can protect ocular lenses against oxidative injuries caused by radiation. The present study on the antioxidant and radioprotective effect of hesperidin is in progress to elucidate their mode of action and a suitable dose of its administration. In terms of future applications, we will examine biochemical and environmental parameters in more details as we go forward.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgment
The study were funded by Deputy of research and technology, Babol University of Medical Sciences, Babol, Iran.
REFERENCES
- 1.Ozgen SÇ, Dökmeci D, Akpolat M, Karadaǧ CH, Gündüz O, Erbaş H, et al. The protective effect of curcumin on ionizing radiation-induced cataractogenesis in rats. Balkan Med J. 2012;29:358–63. doi: 10.5152/balkanmedj.2012.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Randazzo J, Zhang P, Makita J, Blessing K, Kador PF. Orally active multi-functional antioxidants delay cataract formation in streptozotocin (type 1) diabetic and gamma-irradiated rats. PLoS One. 2011;6:e18980. doi: 10.1371/journal.pone.0018980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shabestani Monfared A, Borzoueisileh S, Zabihi E, Amiri M, Abedian S. Predicting factors of radiosensitivity in individual radiotherapy. J Babol Univ Med Sci. 2015;17:67–73. [Google Scholar]
- 4.Heidari A, Monfared AS, Mozdarani H, Mahmoudzadeh A, Razzaghdoust A. Radioprotective effects of sulfur-containing mineral water of ramsar hot spring with high natural background radiation on mouse bone marrow cells. J Biomed Phys Eng. 2016;7:347–54. [PMC free article] [PubMed] [Google Scholar]
- 5.Rafat N, Monfared AS, Shahidi M, Pourfallah TA. The modulating effect of royal jelly consumption against radiation-induced apoptosis in human peripheral blood leukocytes. J Med Phys. 2016;41:52–7. doi: 10.4103/0971-6203.177281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barabino S, Raghavan A, Loeffler J, Dana R. Radiotherapy-induced ocular surface disease. Cornea. 2005;24:909–14. doi: 10.1097/01.ico.0000154235.64359.d3. [DOI] [PubMed] [Google Scholar]
- 7.Karslioglu I, Ertekin MV, Taysi S, Koçer I, Sezen O, Gepdiremen A, et al. Radioprotective effects of melatonin on radiation-induced cataract. J Radiat Res. 2005;46:277–82. doi: 10.1269/jrr.46.277. [DOI] [PubMed] [Google Scholar]
- 8.Brian G, Taylor H. Cataract blindness – Challenges for the 21st century. Bull World Health Organ. 2001;79:249–56. [PMC free article] [PubMed] [Google Scholar]
- 9.Shirazi A, Haddadi GH, Asadi-Amoli F, Sakhaee S, Ghazi-Khansari M, Avand A, et al. Radioprotective effect of melatonin in reducing oxidative stress in rat lenses. Cell J. 2011;13:79–82. [PMC free article] [PubMed] [Google Scholar]
- 10.Taysi S, Okumus S, Akyuz M, Uzun N, Aksoy A, Demir E, et al. Zinc administration modulates radiation-induced oxidative injury in lens of rat. Pharmacogn Mag. 2012;8:245–9. doi: 10.4103/0973-1296.103646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Weon JB, Ma JY, Yang HJ, Lee B, Yun BR, Ma CJ, et al. Qualitative and quantitative analysis of nine major compounds in the bozhougyiqi-tang using a high-performance liquid chromatography coupled with a diode array detector and electrospray ionization mass spectrometer. Pharmacogn Mag. 2013;9:271–82. doi: 10.4103/0973-1296.113291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kalpana KB, Devipriya N, Srinivasan M, Vishwanathan P, Thayalan K, Menon VP, et al. Evaluating the radioprotective effect of hesperidin in the liver of swiss albino mice. Eur J Pharmacol. 2011;658:206–12. doi: 10.1016/j.ejphar.2011.02.031. [DOI] [PubMed] [Google Scholar]
- 13.Kalpana KB, Devipriya N, Srinivasan M, Menon VP. Investigation of the radioprotective efficacy of hesperidin against gamma-radiation induced cellular damage in cultured human peripheral blood lymphocytes. Mutat Res. 2009;676:54–61. doi: 10.1016/j.mrgentox.2009.03.005. [DOI] [PubMed] [Google Scholar]
- 14.Sak K. Cytotoxicity of dietary flavonoids on different human cancer types. Pharmacogn Rev. 2014;8:122–46. doi: 10.4103/0973-7847.134247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Andrade JM, Passos Cdos S, Dresch RR, Kieling-Rubio MA, Moreno PR, Henriques AT, et al. Chemical analysis, antioxidant, antichemotactic and monoamine oxidase inhibition effects of some pteridophytes from brazil. Pharmacogn Mag. 2014;10:S100–9. doi: 10.4103/0973-1296.127354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Karbownik M, Reiter RJ. Antioxidative effects of melatonin in protection against cellular damage caused by ionizing radiation. Proc Soc Exp Biol Med. 2000;225:9–22. doi: 10.1177/153537020022500102. [DOI] [PubMed] [Google Scholar]
- 17.Shirazi A, Ghobadi G, Ghazi-Khansari M. A radiobiological review on melatonin: A novel radioprotector. J Radiat Res. 2007;48:263–72. doi: 10.1269/jrr.06070. [DOI] [PubMed] [Google Scholar]
- 18.Rini FJ, Worgul BV, Merriam GR., Jr Radiation cataracogenesis in rat lenses. Bull N Y Acad Med. 1986;62:744–53. [PMC free article] [PubMed] [Google Scholar]
- 19.Anwar MM, Moustafa MA. The effect of melatonin on eye lens of rats exposed to ultraviolet radiation. Comp Biochem Physiol C Toxicol Pharmacol. 2001;129:57–63. doi: 10.1016/s1532-0456(01)00180-6. [DOI] [PubMed] [Google Scholar]
- 20.Dynlacht JR, Tyree C, Valluri S, DesRosiers C, Caperell-Grant A, Mendonca MS, et al. Effect of estrogen on radiation-induced cataractogenesis. Radiat Res. 2006;165:9–15. doi: 10.1667/rr3481.1. [DOI] [PubMed] [Google Scholar]
- 21.Esterbauer H, Cheeseman KH. Determination of aldehydic lipid peroxidation products: Malonaldehyde and 4-hydroxynonenal. Methods Enzymol. 1990;186:407–21. doi: 10.1016/0076-6879(90)86134-h. [DOI] [PubMed] [Google Scholar]
- 22.Tahamtan R, Shabestani Monfared A, Tahamtani Y, Tavassoli A, Akmali M, Mosleh-Shirazi MA, et al. Radioprotective effect of melatonin on radiation-induced lung injury and lipid peroxidation in rats. Cell J. 2015;17:111–20. doi: 10.22074/cellj.2015.517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Keramati Yazdi F, Shabestani Monfared A, Tashakkorian H, Mahmoudzadeh A, Borzoueisileh S. Radioprotective effect of Zamzam (alkaline) water: A cytogenetic study. J Environ Radioact. 2017;167:166–9. doi: 10.1016/j.jenvrad.2016.10.019. [DOI] [PubMed] [Google Scholar]
- 24.Osgood TB, Menard TW, Clark JI, Krohn KA. Inhibition of lens opacification in x-irradiated rats treated with WR-77913. Invest Ophthalmol Vis Sci. 1986;27:1780–4. [PubMed] [Google Scholar]
- 25.Karslioglu I, Ertekin MV, Koçer I, Taysi S, Sezen O, Gepdiremen A, et al. Protective role of intramuscularly administered Vitamin E on the levels of lipid peroxidation and the activities of antioxidant enzymes in the lens of rats made cataractous with gamma-irradiation. Eur J Ophthalmol. 2004;14:478–85. [PubMed] [Google Scholar]
- 26.Löfgren S, Michael R, Söderberg PG. Impact of age and sex in ultraviolet radiation cataract in the rat. Invest Ophthalmol Vis Sci. 2003;44:1629–33. doi: 10.1167/iovs.01-0922. [DOI] [PubMed] [Google Scholar]
- 27.Pradeep K, Mi Hee C, Kyong Cheol K, Hae Jun P. Hesperidin and curdlan treatment ameliorates γ-radiation induced cellular damage and oxidative stress in the liver of Sprague-Dawley rats. Res J Pharm Biol Chem Sci. 2010;1:165–77. [Google Scholar]
- 28.Pradeep K, Park SH, Ko KC. Hesperidin a flavanoglycone protects against gamma-irradiation induced hepatocellular damage and oxidative stress in sprague-dawley rats. Eur J Pharmacol. 2008;587:273–80. doi: 10.1016/j.ejphar.2008.03.052. [DOI] [PubMed] [Google Scholar]
- 29.Pradeep K, Ko KC, Choi MH, Kang JA, Chung YJ, Park SH, et al. Protective effect of hesperidin, a citrus flavanoglycone, against γ-radiation-induced tissue damage in sprague-dawley rats. J Med Food. 2012;15:419–27. doi: 10.1089/jmf.2011.1737. [DOI] [PubMed] [Google Scholar]
- 30.Aghazadeh S, Azarnia M, Shirazi A, Mahdavi SR, Zangii BM. Melatonin as a protective agent in spinal cord damage after gamma irradiation. Rep Pract Oncol Radiother. 2007;12:95–9. [Google Scholar]


