Abstract
Angiogenesis is critical for oxygen and nutrient delivery to proliferating tumor cells. Therefore, as angiogenesis is required and vital for the tumor growth and metastasis. Antiangiogenic therapy is considered to be beneficial for tumor growth prevention due to starvation of tumor of oxygen and nutrients, but in some cases, the benefits are not permanent. Tyrosine kinase inhibitors and many other agents often target angiogenesis through inhibition of the vascular endothelial growth factor (VEGF) pathway. Although preclinical studies showed satisfactory outcomes in tumor growth inhibition, antiangiogenic therapy in the clinical setting may not be effective. The resistance observed in several tumor types through alternative angiogenic “escape” pathways contributes to restoration of tumor growth and may induce progression, enhancement of invasion, and metastasis. Therefore, activation of major compensatory angiogenic pathways, sustaining tumor angiogenesis during VEGF blockade contributing to the recurrence of tumor growth overcome antiangiogenic strategies. In this review, we summarize the novel mechanisms involved in evasive resistance to antiangiogenic therapies and represent different cancer types which have the ability to adapt to VEGF inhibition achieving resistance to antiangiogenic therapy through these adaptive mechanisms.
Keywords: Angiogenesis inhibitors, antiangiogenic resistance, metastasis, tumor growth restoration
INTRODUCTION
Cancer, a disease involving abnormal cell growth, has a potential to invade and spread to other parts of the body. There are no symptoms at initial stage. Some general symptoms including unintentional weight loss, fever, excessive fatigue, and some changes to the skin appear as the mass grows.
In 2012, 14.1 million new cases and 8.2 million deaths were projected to occur in 20 large “areas” of the world. Estimates of the worldwide incidence and mortality from several major cancers showed that the most commonly diagnosed cancers were lung (1.82 million), breast (1.67 million), and colorectal (1.36 million); the most common causes of cancer death were lung cancer (1.6 million deaths), liver cancer (745,000 deaths), and stomach cancer (723,000 deaths).[1]
Angiogenesis is critical for tumor growth and metastasis. Proliferating tumor cells activate angiogenesis to provide oxygen and nutrients for tumor cells.[2] Induction of angiogenesis obliges the balance of angiogenesis inducers and inhibitors toward a pro-angiogenic environment.
The less angiogenesis, the less tumor growth is observed. In 1972, Folkman hypothesized that by hindering blood supply, tumor could be starved into remission and suggested antiangiogenesis as a new anticancer strategy for the first time.[3] Until 2005, Folkman's laboratory discovered 12 angiogenesis inhibitors (AIs). AIs characterization as well as the isolation and cloning of vascular endothelial growth factor A (VEGFA) was a breakthrough in understanding of the mechanism of angiogenesis. Understanding the function of VEGFs and their receptors in angiogenesis led to the US Food and Drug Administration approval of bevacizumab (BVZ) (a monoclonal antibody for VEGFA) as the first antiangiogenic drug for colorectal cancer in 2004.[4] Consequently, many AIs were developed including monoclonal antibodies, angiogenesis peptide inhibitors, to small molecule drugs and microRNAs.[5]
Drug development is limited due to theraputic resistance. Overall survival dose not lengthen for a long time and there is no permanent cure for renal cancer cells (RCCs), breast and colon cancers.[6,7,8]
Adaptation of angiogenic tumors to antiangiogenic drugs through acquiring different means evading the treatment is a controversial hypothesis in many studies.[9]
Among evasive resistance, many alternative pathways are activated to assure tumor growth whereas the antiangiogenic target remains inhibited.[10]
The crucial mechanisms of evasive resistance consist of revascularization, tumor vasculature protection, accentuated invasiveness of tumor cells, and increased metastatic manner.
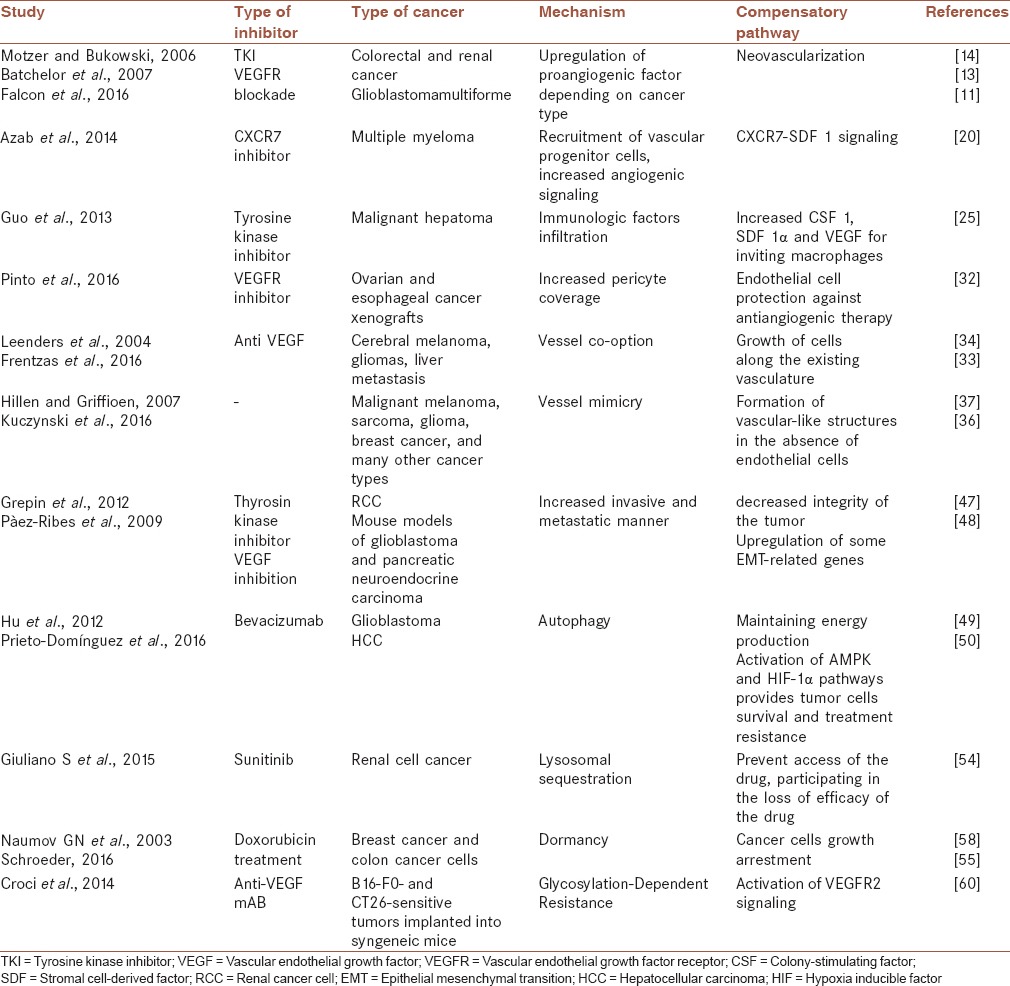
In this review, we elaborate the potential mechanisms of the transitory efficacy of the current AIs that summarized in Table 1, based on-clinical and preclinical investigations.
Table 1.
Some mechanisms of acquired resistance to antiangiogenic therapy
SOME MECHANISMS OF ANTIANGIOGENIC RESISTANCE
Upregulation of alternative angiogenic factors
Tumor vasculature blockade by angiogenic inhibitors leads to the release of many proangiogenic factors and cytokines such as placental growth factor (PGF), VEGF, Angiopoietin 1 (ANG1), and fibroblast growth factor (FGFs), granulocyte colony-stimulating factor (G-CSF) and stromal cell-derived factor 1 (SDF1).[11]
The circulating levels of FGF-1 and -2, angiopoietin-1, ephrin-A1 and A2 increased pancreatic tumors after anti-VEGF treatment. Upregulated Ephrin-A2 is observed in malignant tumors.[12]
Vascular endothelial growth factor receptor (VEGFR) blockade upregulates FGF-2, SDF-1, and circulating endothelial cells (ECs) in glioblastomamultiforme patients.[13]
In addition, increased levels of PIGF and VEGF have been observed in colorectal, and renal cancer patients received tyrosine kinase inhibitors.[14]
Edoglin (CD105), a transforming growth factor (TGF)-b coreceotor, has been shown to be highly expressed after anti-VEGF therapy in a pancreatic cancer model.[15] TGF-b/ALK1 signaling is facilitated due to upregulation of endoglin expression during tumor angiogenesis.[16]
Recruitment of vascular progenitor cells
The release of proangiogenic factors (PLGF, VEGF, ANG1, and FGFs) resulted from vascularization blockade leads to the recruitment of various bone marrow-derived cells (BMDCs) to elicit new blood vessels nourishing tumors. Proangiogenic BMDCs consist of stem cells involving in blood vessels production and vascular modulatory cells infiltrating into tumor stroma. ECs and pericytes compromised from their progenitors form blood vessels which are protected by pericyte envelop.[17] Vascular modulators include proangiogenic monocytes, such as tumour-associated macrophages (TAMs), and immature monocytic cells including TIE2+ monocytes, VEGFR1+ hemangiocytes, and CD11b+ myeloid cells.
Endothelial to mesenchymal transition depending on tumor characteristics leads to more angiogenic and invasive capacities.[18]
Antiangiogenic therapy induced hypoxia activates hypoxia inducible factor-1α (HIF-1α) in tumor cells contributing to SDF1, and VEGF secretion may promote movement and assemblage of endothelial progenitor cells (EPCs). Differentiated ECs from their progenitors (EPC) under VEGF and SDF1 chemotactic factors secretion, incorporate into newly forming blood vessels.[19]
SDF1 stimulates CXCR7 leading to proangiogenic cytokines secretions by EPCs to angiogenesis promotion. In multiple myeloma, CXCR7–SDF1 signaling is involved in migration and homing of angiogenic immune cells into areas of tumor growth.[20]
Immunologic factors infiltration and antiangiogenic resistance
Hypoxic conditions due to antiangiogenic therapy result in recruitment and expansion of myeloid derived suppressor cells (MDSCs), leading to a weakened antitumor response. MDSCs represent promising targets for therapy by regulation of T-cell exclusion through a variety of mechanisms. Neutrophils, T helper cells, and macrophages play important roles in resistance to antiangiogenic therapy. G-CSF expression stimulated by tumor infiltrating T helper type 17 cells and interleukin-17 (IL-17), results in recruitment of MDSC into the tumor tissue and tumor angiogenesis.[21] This is why Th17 cell function inhibition makes tumors sensitive to anti-VEGF therapies.[22]
Increased recruitment of neutrophils during anti-VEGF therapy promotes tumor progression and treatment resistance. Tumor progression with mesenchymal characteristics is partly mediated by increased neutrophil infiltration through the expression of S100A4.
Therefore, targeting granulocytes and S100A4 may be beneficial in inhibiting the tumor malignant phenotype and diminishing antiangiogenic therapy resistance.[23]
Macrophages performing the role “bridging cells” between the cells are contributed in vascular sprouting and therefore antiangiogenic resistance. A proinflammatory response induced by highly expressed IL-8 in VEGF-therapy resistant tumors can promote angiogenesis by recruiting proangiogenic CD11b+ myeloid cells.[24]
According to a study by Guo et al., it has been shown that there are increased CSF-1, SDF-1α, and VEGF which are intrinsic chemokines for inviting macrophages[25] in malignant hepatoma treated with sorafenib (a tyrosine kinase inhibitor). In other word, TAMs have crucial roles in tumor angiogenesis in hepatocellular carcinoma tumors. Bone marrow-derived TAMs are fundamental factors contributing to resistance to anti-VEGF therapy.[26]
Increased pericyte coverage
The tumor vessels which are heavily covered by pericytes have been shown a decreased sensitivity for AIs.[27] Increased pericyte coverage promotes EC survival after antiangiogenic treatment.[28] Reduction in tumor vascularity after anti-VEGF therapy is also accompanied by distinctive functional slim and tightly pericyte covered vessels protecting ECs from anti-VEGF therapy.[29] Pericytes mediate neovessel maturation and protect ECs from antiangiogenic therapy.[30]
Increased number of vessels covered with pericytes has been observed in a preclinical malignant glioma model treated with temozolomide (a chemotherapy drug) and sunitinib.[31]
Moreover, Ovarian and esophageal cancer xenografts treated with BVZ were accompanied with increased pericyte coverage around vessels contributing to EC maintenance and resistance to VEGFR inhibitors.[32]
Vessel co-option
A different strategy providing oxygen and nutrients for efficient tumor outgrowth is termed vessel co-option in which tumor cells give rise along the existing vasculature. No angiogenic growth factor is required for this process, and it has been observed after anti-VEGF treatment.[33]
Vessel co-option leads to sustained cerebral melanoma metastasis growth.[34] In fact, vessel co-option has been observed in several tumors such as gliomas and lung cancer.[35]
In addition, BVZ-treated patients with colorectal cancer liver metastases demonstrated a poor response to antiangiogenic therapy due to vessel co-option.
Moreover, vessel co-option has been reported in human breast cancer liver metastases, liver metastases nonsmall cell lung cancer, and lung metastases.[36]
In liver metastases, cancer cell motility mediated by the actin-related protein 2/3 complex is required for vessel co-option.
Vasculogenic mimicry
Whereas vasculogenesis is the process of blood vessel formation through a de novo production of ECs, angiogenesis is the formation of new blood vessels from preexisting ones. The formation of vascular-like structures providing tumors oxygen and nutrients under the process of vasculogenic mimicry has been described in different tumor types such as malignant melanoma, sarcoma, glioma, breast cancer, and many other cancer types.[37,38,39] Vasculogenic mimicry is deeply associated with poor patient survival.
Dedifferentiation of melanoma cells to form vasculature is a plausible mechanism induced by an ischemic microenvironment.[40]
According to the evidence from preclinical studies, antiangiogenic treatment with BVZ leads to increased vasculogenic mimicry.[41] The ability of cancer cells to form vasculature in the absence of ECs and anastomoses of these pseudovasculature with existing vasculature are crucial adaptation manners nourishing the tumor. Among the vasculogenic mimicry process, tumor cells are required to differentiate and gain ECs features such as expressing the endothelial markers VE-cadherin, TIE1, ephrin A2Mosaic vessels consisting of both cancer cells, and ECs lining the vessel walls have been observed in many cancer types.[42]
Increased capabilities for invasion and metastasis
When tumors genetically or pharmacologically prevented from angiogenesis, cancer cells switch on a distinctive invasive growth program. Increased intravasation due to decreased integrity of the tumor vasculature is an insidious resistance mechanism to antiangiogenic therapy.
Upregulation of some epithelial mesenchymal transition (EMT)-related genes, such as twist and snail, and shifting of the epithelial to mesenchymal markers promote tumor metastasis.[43,44]
In untreated glioblastomas (GBMs), single cancer cells invade normal brain tissue whereas impairment of angiogenesis results in migration of multicellular layers and then metastasis.[35,45,46]
RCC treated with BVZ demonstrated accelerated growth capacity, and distant metastasis was observed as a result of tumor cells invasive profile.[47]
In addition, VEGF inhibition showed enhanced invasiveness metastasis of primary tumors in mouse models of GBM and pancreatic neuroendocrine carcinoma.[48]
Autophagy
Autophagy, a reversible process having a prodeath or a prosurvival role in cancer, mediates antiangiogenic resistance.[49]
Autophagy, a cytoprotective adaptive response, provides a rescue mechanism for GBM cancer cells in unfavorable condition and maintains energy production leading to tumor growth and therapeutic resistance.[50] Activation of AMPK and HIF-1α pathways due to hypoxia-induced autophagy causes treatment resistance in GBM.[49]
These conflicting effects of autophagy on tumor cells are puzzling. According to the previous studies, autophagy is required for tamoxifen resistance. The activity of kinases confers resistance to tamoxifen. For example, a kinase called HSPB8 protects the cells against tamoxifen-induced death which results in tamoxifen resistance.[51] Autophagy induction is a mechanism of chemoresistance and is also observed in chemotherapeutic drug-treated esophageal cancer cells, enhancing the induction of apoptosis.[52]
Lysosomal sequestration
Sunitinib administration without any interruption leads to resistance of tumor cells due to increasing intracellular lysosomal sunitinib accumulation and activity.[53] It has been reported that lysosomal sequestration can prevent access of the drug to the kinase domain of tyrosine kinase receptors present in the cytoplasm, thus participating in the loss of efficacy of the drug.[53] Resistance to sunitinib through lysosomal sequestration has been observed in renal cell cancer patients although this resistance is transient. So that, targeting lysosomal function will overcome sunitinib resistance.[54]
Acquiring dormant and quiescent state
Tumor dormancy occurs with the counteraction of cell proliferation by apoptosis and impaired vascularization or immunosurveillance and cellular dormancy occurs with the cancer cells growth arrestment.[55]
Quiescence resulting in cancer cell survival after exposure to anticancer drugs contributes to disease recurrence.[56,57]
AIs induce long-term dormancy in tumor cells. Acquiring dormant state after antiangiogenic treatment and then recommencing the proliferation of tumor cells in the absence of angiogenic inhibitors lead to antiangiogenic resistance. Growth arrest due to active survival mechanisms providing dormant cells protection against chemotherapy and then doxorubicin resistance has been shown in breast cancer and colon cancer cells.[58]
Glycosylation-Dependent Resistance in multidrug resistance and epithelial mesenchymal transition
According to recent evidence, angiogenic receptor signaling can also become activated independent of ligand binding.
EMT is related to the acquisition of multidrug resistance (MDR) phenotype; in other words, there is interplay between these two apparently distinct processes. Glycosylation, a posttranslational modification, is required in both phenomena. Disease relapse through MDR mechanism is a fundamental cause of death in patients with small cell lung cancer, breast cancer, ovarian cancer, acute leukemia.[59]
Galectin-1 which is a glycan binding protein mediates the activation of VEGFR2 signaling after anti-VEGF intervention. This process is mediated by receptor glycosylation allowing the binding of galectin-1 that lead to VEGFR2 clustering and snoozed receptor internalization. Therefore, galectin-1permissive glycosylation was associated with resistance to anti-VEGF therapy.[60]
CONCLUSION
In spite of the primary promising results of angiogenic inhibitors, antiangiogenesis therapy encountered several challenges. Some cancers based on their stage of progression, genomic constitution and their microenvironment have the capacity to show refractory response to antiangiogenic agents. Although the majority of tumor types respond some cancer types avoid treatment through a variety of mechanisms.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, et al. Cancer incidence and mortality worldwide: Sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:E359–86. doi: 10.1002/ijc.29210. [DOI] [PubMed] [Google Scholar]
- 2.Carmeliet P. Angiogenesis in life, disease and medicine. Nature. 2005;438:932–6. doi: 10.1038/nature04478. [DOI] [PubMed] [Google Scholar]
- 3.Folkman J. Anti-angiogenesis: New concept for therapy of solid tumors. Ann Surg. 1972;175:409–16. doi: 10.1097/00000658-197203000-00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shih T, Lindley C. Bevacizumab: An angiogenesis inhibitor for the treatment of solid malignancies. Clin Ther. 2006;28:1779–802. doi: 10.1016/j.clinthera.2006.11.015. [DOI] [PubMed] [Google Scholar]
- 5.Lin Z, Zhang Q, Luo W. Angiogenesis inhibitors as therapeutic agents in cancer: Challenges and future directions. Eur J Pharmacol. 2016;793:76–81. doi: 10.1016/j.ejphar.2016.10.039. [DOI] [PubMed] [Google Scholar]
- 6.Montero AJ, Escobar M, Lopes G, Glück S, Vogel C. Bevacizumab in the treatment of metastatic breast cancer: Friend or foe? Curr Oncol Rep. 2012;14:1–11. doi: 10.1007/s11912-011-0202-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Allegra CJ, Yothers G, O’Connell MJ, Sharif S, Petrelli NJ, Colangelo LH, et al. Phase III trial assessing bevacizumab in stages II and III carcinoma of the colon: Results of NSABP protocol C-08. J Clin Oncol. 2011;29:11–6. doi: 10.1200/JCO.2010.30.0855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.de Gramont A, Van Cutsem E, Schmoll HJ, Tabernero J, Clarke S, Moore MJ, et al. Bevacizumab plus oxaliplatin-based chemotherapy as adjuvant treatment for colon cancer (AVANT): A phase 3 randomised controlled trial. Lancet Oncol. 2012;13:1225–33. doi: 10.1016/S1470-2045(12)70509-0. [DOI] [PubMed] [Google Scholar]
- 9.Miller KD, Sweeney CJ, Sledge GW., Jr Can tumor angiogenesis be inhibited without resistance? EXS. 2005:95–112. doi: 10.1007/3-7643-7311-3_7. [DOI] [PubMed] [Google Scholar]
- 10.Kadenhe-Chiweshe A, Papa J, McCrudden KW, Frischer J, Bae JO, Huang J, et al. Sustained VEGF blockade results in microenvironmental sequestration of VEGF by tumors and persistent VEGF receptor-2 activation. Mol Cancer Res. 2008;6:1–9. doi: 10.1158/1541-7786.MCR-07-0101. [DOI] [PubMed] [Google Scholar]
- 11.Falcon BL, Chintharlapalli S, Uhlik MT, Pytowski B. Antagonist antibodies to vascular endothelial growth factor receptor 2 (VEGFR-2) as anti-angiogenic agents. Pharmacol Ther. 2016;164:204–25. doi: 10.1016/j.pharmthera.2016.06.001. [DOI] [PubMed] [Google Scholar]
- 12.Zhuang G, Brantley-Sieders DM, Vaught D, Yu J, Xie L, Wells S, et al. Elevation of receptor tyrosine kinase EphA2 mediates resistance to trastuzumab therapy. Cancer Res. 2010;70:299–308. doi: 10.1158/0008-5472.CAN-09-1845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Batchelor TT, Sorensen AG, di Tomaso E, Zhang WT, Duda DG, Cohen KS, et al. AZD2171, a pan-VEGF receptor tyrosine kinase inhibitor, normalizes tumor vasculature and alleviates edema in glioblastoma patients. Cancer Cell. 2007;11:83–95. doi: 10.1016/j.ccr.2006.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Motzer RJ, Bukowski RM. Targeted therapy for metastatic renal cell carcinoma. J Clin Oncol. 2006;24:5601–8. doi: 10.1200/JCO.2006.08.5415. [DOI] [PubMed] [Google Scholar]
- 15.Bockhorn M, Tsuzuki Y, Xu L, Frilling A, Broelsch CE, Fukumura D. Differential vascular and transcriptional responses to anti-vascular endothelial growth factor antibody in orthotopic human pancreatic cancer xenografts. Clin Cancer Res. 2003;9:4221–6. [PubMed] [Google Scholar]
- 16.van Meeteren LA, Thorikay M, Bergqvist S, Pardali E, Stampino CG, Hu-Lowe D, et al. Anti-human activin receptor-like kinase 1 (ALK1) antibody attenuates bone morphogenetic protein 9 (BMP9)-induced ALK1 signaling and interferes with endothelial cell sprouting. J Biol Chem. 2012;287:18551–61. doi: 10.1074/jbc.M111.338103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Song S, Ewald AJ, Stallcup W, Werb Z, Bergers G. PDGFRbeta+ perivascular progenitor cells in tumours regulate pericyte differentiation and vascular survival. Nat Cell Biol. 2005;7:870–9. doi: 10.1038/ncb1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hida K, Ohga N, Akiyama K, Maishi N, Hida Y. Heterogeneity of tumor endothelial cells. Cancer Sci. 2013;104:1391–5. doi: 10.1111/cas.12251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bergers G, Hanahan D. Modes of resistance to anti-angiogenic therapy. Nat Rev Cancer. 2008;8:592–603. doi: 10.1038/nrc2442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Azab AK, Sahin I, Moschetta M, Mishima Y, Burwick N, Zimmermann J, et al. CXCR7-dependent angiogenic mononuclear cell trafficking regulates tumor progression in multiple myeloma. Blood. 2014;124:1905–14. doi: 10.1182/blood-2014-02-558742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sica A, Allavena P, Mantovani A. Cancer related inflammation: The macrophage connection. Cancer Lett. 2008;267:204–15. doi: 10.1016/j.canlet.2008.03.028. [DOI] [PubMed] [Google Scholar]
- 22.Chung AS, Wu X, Zhuang G, Ngu H, Kasman I, Zhang J, et al. An interleukin-17-mediated paracrine network promotes tumor resistance to anti-angiogenic therapy. Nat Med. 2013;19:1114–23. doi: 10.1038/nm.3291. [DOI] [PubMed] [Google Scholar]
- 23.Liang J, Piao Y, Holmes L, Fuller GN, Henry V, Tiao N, et al. Neutrophils promote the malignant glioma phenotype through S100A4. Clin Cancer Res. 2014;20:187–98. doi: 10.1158/1078-0432.CCR-13-1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Carbone C, Moccia T, Zhu C, Paradiso G, Budillon A, Chiao PJ, et al. Anti-VEGF treatment-resistant pancreatic cancers secrete proinflammatory factors that contribute to malignant progression by inducing an EMT cell phenotype. Clin Cancer Res. 2011;17:5822–32. doi: 10.1158/1078-0432.CCR-11-1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Guo C, Buranych A, Sarkar D, Fisher PB, Wang XY. The role of tumor-associated macrophages in tumor vascularization. Vasc Cell. 2013;5:20. doi: 10.1186/2045-824X-5-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Noy R, Pollard JW. Tumor-associated macrophages: From mechanisms to therapy. Immunity. 2014;41:49–61. doi: 10.1016/j.immuni.2014.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Thomas M, Kienast Y, Scheuer W, Bähner M, Kaluza K, Gassner C, et al. A novel angiopoietin-2 selective fully human antibody with potent anti-tumoral and anti-angiogenic efficacy and superior side effect profile compared to Pan-Angiopoietin-1/-2 inhibitors. PLoS One. 2013;8:e54923. doi: 10.1371/journal.pone.0054923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Franco M, Roswall P, Cortez E, Hanahan D, Pietras K. Pericytes promote endothelial cell survival through induction of autocrine VEGF-A signaling and Bcl-w expression. Blood. 2011;118:2906–17. doi: 10.1182/blood-2011-01-331694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kamba T, McDonald DM. Mechanisms of adverse effects of anti-VEGF therapy for cancer. Br J Cancer. 2007;96:1788–95. doi: 10.1038/sj.bjc.6603813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McCarty MF, Somcio RJ, Stoeltzing O, Wey J, Fan F, Liu W, et al. Overexpression of PDGF-BB decreases colorectal and pancreatic cancer growth by increasing tumor pericyte content. J Clin Invest. 2007;117:2114–22. doi: 10.1172/JCI31334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Norden AD, Drappatz J, Wen PY. Antiangiogenic therapies for high-grade glioma. Nat Rev Neurol. 2009;5:610–20. doi: 10.1038/nrneurol.2009.159. [DOI] [PubMed] [Google Scholar]
- 32.Pinto MP, Sotomayor P, Carrasco-Avino G, Corvalan AH, Owen GI. Escaping antiangiogenic therapy: Strategies employed by cancer cells. Int J Mol Sci. 2016;17 doi: 10.3390/ijms17091489. pii: E1489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Frentzas S, Simoneau E, Bridgeman VL, Vermeulen PB, Foo S, Kostaras E, et al. Vessel co-option mediates resistance to anti-angiogenic therapy in liver metastases. Nat Med. 2016;22:1294–302. doi: 10.1038/nm.4197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Leenders WP, Küsters B, Verrijp K, Maass C, Wesseling P, Heerschap A, et al. Antiangiogenic therapy of cerebral melanoma metastases results in sustained tumor progression via vessel co-option. Clin Cancer Res. 2004;10(18 Pt 1):6222–30. doi: 10.1158/1078-0432.CCR-04-0823. [DOI] [PubMed] [Google Scholar]
- 35.Du R, Lu KV, Petritsch C, Liu P, Ganss R, Passegué E, et al. HIF1alpha induces the recruitment of bone marrow-derived vascular modulatory cells to regulate tumor angiogenesis and invasion. Cancer Cell. 2008;13:206–20. doi: 10.1016/j.ccr.2008.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kuczynski EA, Yin M, Bar-Zion A, Lee CR, Butz H, Man S, et al. Co-option of liver vessels and not sprouting angiogenesis drives acquired sorafenib resistance in hepatocellular carcinoma. J Natl Cancer Inst. 2016;108:8. doi: 10.1093/jnci/djw030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hillen F, Griffioen AW. Tumour vascularization: Sprouting angiogenesis and beyond. Cancer Metastasis Rev. 2007;26:489–502. doi: 10.1007/s10555-007-9094-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.van der Schaft DW, Hillen F, Pauwels P, Kirschmann DA, Castermans K, Egbrink MG, et al. Tumor cell plasticity in Ewing sarcoma, an alternative circulatory system stimulated by hypoxia. Cancer Res. 2005;65:11520–8. doi: 10.1158/0008-5472.CAN-05-2468. [DOI] [PubMed] [Google Scholar]
- 39.Kuczynski, Elizabeth Anne. Doktorarbeit. Canada: Doktorarbeit University of Toronto; 2016. Mechanisms of reversible sorafenib resistance in hepatocellular carcinoma. [Google Scholar]
- 40.Hendrix MJ, Seftor EA, Hess AR, Seftor RE. Vasculogenic mimicry and tumour-cell plasticity: Lessons from melanoma. Nat Rev Cancer. 2003;3:411–21. doi: 10.1038/nrc1092. [DOI] [PubMed] [Google Scholar]
- 41.Xu Y, Li Q, Li XY, Yang QY, Xu WW, Liu GL. Short-term anti-vascular endothelial growth factor treatment elicits vasculogenic mimicry formation of tumors to accelerate metastasis. J Exp Clin Cancer Res. 2012;31:16. doi: 10.1186/1756-9966-31-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chang YS, di Tomaso E, McDonald DM, Jones R, Jain RK, Munn LL. Mosaic blood vessels in tumors: Frequency of cancer cells in contact with flowing blood. Proc Natl Acad Sci U S A. 2000;97:14608–13. doi: 10.1073/pnas.97.26.14608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jung HY, Fattet L, Yang J. Molecular pathways: Linking tumor microenvironment to epithelial-mesenchymal transition in metastasis. Clin Cancer Res. 2015;21:962–8. doi: 10.1158/1078-0432.CCR-13-3173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cooke VG, LeBleu VS, Keskin D, Khan Z, O’Connell JT, Teng Y, et al. Pericyte depletion results in hypoxia-associated epithelial-to-mesenchymal transition and metastasis mediated by met signaling pathway. Cancer Cell. 2012;21:66–81. doi: 10.1016/j.ccr.2011.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rubenstein JL, Kim J, Ozawa T, Zhang M, Westphal M, Deen DF, et al. Anti-VEGF antibody treatment of glioblastoma prolongs survival but results in increased vascular cooption. Neoplasia. 2000;2:306–14. doi: 10.1038/sj.neo.7900102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Blouw B, Song H, Tihan T, Bosze J, Ferrara N, Gerber HP, et al. The hypoxic response of tumors is dependent on their microenvironment. Cancer Cell. 2003;4:133–46. doi: 10.1016/s1535-6108(03)00194-6. [DOI] [PubMed] [Google Scholar]
- 47.Grepin R, Guyot M, Jacquin M, Durivault J, Chamorey E, Sudaka A, et al. Acceleration of clear cell renal cell carcinoma growth in mice following bevacizumab/Avastin treatment: The role of CXCL cytokines. Oncogene. 2012;31:1683–94. doi: 10.1038/onc.2011.360. [DOI] [PubMed] [Google Scholar]
- 48.Pàez-Ribes M, Allen E, Hudock J, Takeda T, Okuyama H, Viñals F, et al. Antiangiogenic therapy elicits malignant progression of tumors to increased local invasion and distant metastasis. Cancer Cell. 2009;15:220–31. doi: 10.1016/j.ccr.2009.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hu YL, DeLay M, Jahangiri A, Molinaro AM, Rose SD, Carbonell WS, et al. Hypoxia-induced autophagy promotes tumor cell survival and adaptation to antiangiogenic treatment in glioblastoma. Cancer Res. 2012;72:1773–83. doi: 10.1158/0008-5472.CAN-11-3831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Prieto-Domínguez N, Ordóñez R, Fernández A, García-Palomo A, Muntané J, González-Gallego J, et al. Modulation of autophagy by sorafenib: Effects on treatment response. Front Pharmacol. 2016;7:151. doi: 10.3389/fphar.2016.00151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gonzalez-Malerva L, Park J, Zou L, Hu Y, Moradpour Z, Pearlberg J, et al. High-throughput ectopic expression screen for tamoxifen resistance identifies an atypical kinase that blocks autophagy. Proc Natl Acad Sci U S A. 2011;108:2058–63. doi: 10.1073/pnas.1018157108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sui X, Chen R, Wang Z, Huang Z, Kong N, Zhang M, et al. Autophagy and chemotherapy resistance: A promising therapeutic target for cancer treatment. Cell Death Dis. 2013;4:e838. doi: 10.1038/cddis.2013.350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gotink KJ, Broxterman HJ, Labots M, de Haas RR, Dekker H, Honeywell RJ, et al. Lysosomal sequestration of sunitinib: A novel mechanism of drug resistance. Clin Cancer Res. 2011;17:7337–46. doi: 10.1158/1078-0432.CCR-11-1667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Giuliano S, Cormerais Y, Dufies M, Grépin R, Colosetti P, Belaid A, et al. Resistance to sunitinib in renal clear cell carcinoma results from sequestration in lysosomes and inhibition of the autophagic flux. Autophagy. 2015;11:1891–904. doi: 10.1080/15548627.2015.1085742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Schroeder M, Viezens L, Wellbrock J, Fiedler W, Rüther W, Algenstaedt P, et al. Sunitinib treatment reduces tumor growth and limits changes in microvascular properties after minor surgical intervention in an in vivo model of secondary breast cancer growth in bone. J Surg Oncol. 2016;113:515–21. doi: 10.1002/jso.24185. [DOI] [PubMed] [Google Scholar]
- 56.Boichuk S, Parry JA, Makielski KR, Litovchick L, Baron JL, Zewe JP, et al. The DREAM complex mediates GIST cell quiescence and is a novel therapeutic target to enhance imatinib-induced apoptosis. Cancer Res. 2013;73:5120–9. doi: 10.1158/0008-5472.CAN-13-0579. [DOI] [PubMed] [Google Scholar]
- 57.Patrikidou A, Chabaud S, Ray-Coquard I, Bui BN, Adenis A, Rios M, et al. Influence of imatinib interruption and rechallenge on the residual disease in patients with advanced GIST: Results of the BFR14 prospective French Sarcoma Group randomised, phase III trial. Ann Oncol. 2013;24:1087–93. doi: 10.1093/annonc/mds587. [DOI] [PubMed] [Google Scholar]
- 58.Naumov GN, Townson JL, MacDonald IC, Wilson SM, Bramwell VH, Groom AC, et al. Ineffectiveness of doxorubicin treatment on solitary dormant mammary carcinoma cells or late-developing metastases. Breast Cancer Res Treat. 2003;82:199–206. doi: 10.1023/B:BREA.0000004377.12288.3c. [DOI] [PubMed] [Google Scholar]
- 59.da Fonseca LM, da Silva VA, Freire-de-Lima L, Previato JO, Mendonça-Previato L, Capella MA. Glycosylation in cancer: Interplay between multidrug resistance and epithelial-to-mesenchymal transition? Front Oncol. 2016;6:158. doi: 10.3389/fonc.2016.00158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Croci DO, Cerliani JP, Dalotto-Moreno T, Méndez-Huergo SP, Mascanfroni ID, Dergan-Dylon S, et al. Glycosylation-dependent lectin-receptor interactions preserve angiogenesis in anti-VEGF refractory tumors. Cell. 2014;156:744–58. doi: 10.1016/j.cell.2014.01.043. [DOI] [PubMed] [Google Scholar]


