ABSTRACT
The small GTPase, Cdc42, is a key regulator of actin dynamics, functioning to connect multiple signals to actin polymerization through effector proteins of the Wiskott-Aldrich syndrome protein (WASP) and Transducer of Cdc42-dependent actin assembly (TOCA) families. WASP family members serve to couple Cdc42 with the actin nucleator, the Arp2/3 complex, via direct interactions. The regulation of these proteins in the context of actin dynamics has been extensively studied. Studies on the TOCA family, however, are more limited and relatively little is known about their roles and regulation. In this commentary we highlight new structural and biophysical insight into the involvement of TOCA proteins in the pathway of Cdc42-dependent actin dynamics. We discuss the biological implications of the low affinity interactions between the TOCA family and Cdc42, as well as probing the sequential binding of TOCA1 and the WASP homolog, N-WASP, to Cdc42. We place our current research in the context of the wealth of biophysical, structural and functional data from earlier studies pertaining to the Cdc42/N-WASP/Arp2/3 pathway of actin polymerization. Finally, we describe the molecular basis for a sequential G protein-effector handover from TOCA1 to N-WASP.
KEYWORDS: actin, Cdc42, filopodia, endocytosis, NMR, N-WASP, protein-protein interaction, TOCA1WASP
Dynamic regulation of the actin cytoskeleton underpins a multitude of cellular processes, from cell movement and polarization1,2 to cell division.3 The actin cytoskeleton and the proteins involved in its regulation are also fundamentally linked to endocytosis and membrane trafficking.4,5 Members of the Rho family of small GTPases have emerged as important overseers of the actin cytoskeleton and a number of Rho family members and their downstream effector proteins have been linked to specific actin pathways.6-10
Among the Rho family actin regulators is Cdc42,9 a small GTPase responsible for a large number of eukaryotic cell signaling pathways (for a review see ref. 11). The pronounced effect of Cdc42 upon actin networks is, in part, mediated by members of a relatively well-studied family of Cdc42 effector proteins, the Wiskott Aldrich Syndrome protein (WASP) family.12,13 WASP and its ubiquitously expressed homolog N-WASP are multi domain adaptor proteins that connect Cdc42 to an actin nucleator, the Arp2/3 complex.12-14
WASP and N-WASP comprise an N-terminal domain known as the WASP-homology region 1 (WH1) implicated in binding to many proteins including the WASP-interacting protein (WIP),15 a basic region implicated in specific lipid interactions16 and a G protein binding domain (GBD) involved in Cdc42-binding (Fig. 1A).12 The GBD is connected by a long linker to a C-terminal Verprolin-homology, Cofilin-homology, acidic (VCA) domain, which binds the Arp2/3 complex and monomeric actin, leading to actin polymerization.13,17 This linker contains a proline rich region implicated in interactions with SH3 domain-containing proteins such as Grb2.18 Their multi domain structure and many binding partners make WASP/N-WASP ideal signal integration proteins, integrating multiple upstream signals and relaying them to the Arp2/3 complex (reviewed in ref. 19).
Figure 1.
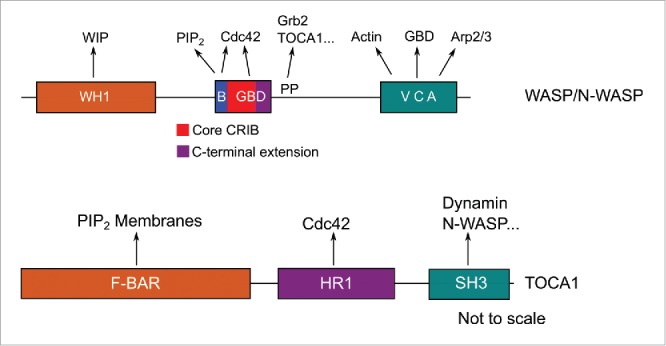
A representation of the domain structures of A) WASP and N-WASP and B) the TOCA family. WH1 = WASP-homology 1; B = basic region; GBD = G protein binding domain; PP = Polyproline region; VCA = Verprolin-homology, Cofilin-homology, acidic domain. F-BAR = Fes/CIP4-BAR; HR1 = Homology Region 1; SH3 = Src-homolgy 3. Arrows indicate interactions with other moieties.
Another family of multi domain Cdc42 effectors, the Transducer of Cdc42 dependent actin assembly (TOCA) family, have more recently emerged as important players in Cdc42-dependent actin pathways,20 which act upstream of the WASP family. The founding member of the TOCA family, TOCA1, mediates Cdc42-dependent activation of N-WASP in Xenopus cell lysates and its importance in a multitude of actin-related processes has been demonstrated. For example, TOCA1 has been implicated in membrane trafficking and endocytosis,4,21-24 filopodia formation,25 transcriptional reprogramming via nuclear actin,26 neurite elongation27 and cell motility and invasion.28,29
Much like WASP/N-WASP, the TOCA family feature protein-lipid and protein-protein interaction domains (Fig. 1B). An N-terminal, Fes/CIP4-BAR (F-BAR) domain mediates oligomerization and interactions with the membrane, a central homology region 1 (HR1) domain is implicated in Cdc42-binding and a C-terminal SH3 domain interacts with several proteins of actin pathways, including N-WASP.20 Little is known however, about the regulation of TOCA1 or about how TOCA1 and its multiple interactions fit into the pathway of Cdc42/TOCA1/N-WASP-dependent actin polymerization.
In contrast to TOCA1, a multitude of biophysical and structural studies have elucidated the complex regulation of the WASP family proteins (for a review see ref. 19). WASP and N-WASP are thought to exist in an equilibrium between folded (inactive) and unfolded (active) states.30 The folded form is dependent on the presence of intramolecular interactions. Full length, folded N-WASP shows low basal activity toward the Arp2/3 complex but this activity is synergistically increased by its allosteric activators, Cdc42 and phosphatidylinositol 4,5-bisphosphate [PI(4,5)P2].16,31 In the folded state, which has micromolar affinity for Cdc42,32 the G protein binding domain (GBD) forms a helical structure that contacts the VCA domain.33,34 This contact opposes the VCA-Arp2/3 and VCA-actin interactions. The isolated GBD of (N-)WASP, which exhibits much higher, nanomolar affinity for Cdc42,33 is largely unstructured. When bound to Cdc42, the GBD adopts a conformation that is incompatible with the helical conformation seen in the presence of the VCA domain, including a short antiparallel β-sheet.35 The high affinity of the interaction between Cdc42 and the unfolded WASP GBD drives the equilibrium in favor of the active state of the full-length WASP.
The multi-domain structure of TOCA1, as well as the responsiveness of TOCA1 to Cdc42 and PI(4,5)P2, raised the question of whether a similar allosteric activation mechanism exists for TOCA1 as it does for (N-)WASP. We carried out binding assays with a range of TOCA1 constructs and showed that all of the HR1 domain-containing constructs, including full length TOCA1, bound to activated Cdc42 with equivalent affinities, which were in the micromolar range.36 It therefore appears that the HR1 domain is sufficient for maximal binding. Moreover, the activation of TOCA1 by Cdc42 is not comparable to the situation seen for (N-)WASP because full length TOCA1 and the HR1 domain were equally competent for Cdc42 binding.
The affinity of full length TOCA1 for Cdc42 is similar to that of full length (N-)WASP. In contrast, the affinity of the TOCA1 HR1 domain alone is markedly lower than that of the isolated (N-)WASP GBD (∼5 μM compared to ∼30 nM). This affinity is also much lower than previously studied G protein-HR1 domain interactions. For example, the HR1a domain of PRK1 binds to RhoA with a Kd of 60 nM37 and the HR1b domain of PRK1 binds Rac1 with a Kd of 68 nM.38 Other members of the TOCA family, FBP17 and CIP4, also bind Cdc42 with micromolar affinities, similar to that of TOCA1,36 and so it appears that the Cdc42-binding HR1 domains make lower affinity complexes with their cognate small G proteins than other G protein-binding HR1 domains.
The significantly lower affinity raised the question of whether the TOCA1 HR1-Cdc42 interaction was markedly different from the previously studied G protein-HR1 interactions or that the structure of the HR1 domain itself was atypical. After all, the TOCA family HR1 domains share a high degree of identity with one another (60-70 %) but are more divergent from the previously studied PRK1 HR1 domains (∼20 %). The HR1 domains of PRK1 are also relatively divergent from each other, with their relatively low sequence identities (∼25 %) being reflected in subtle structural differences between the individual coiled-coil domains. For example, they differ in the relative lengths of the 2 helices and in the secondary structure of the region N-terminal to the coiled-coil.38,39
The NMR structure of the TOCA1 HR136 revealed the expected coiled-coil, reminiscent of the PRK1 HR1 domains,39,40 but there were notable differences in the N-terminal region and, perhaps more importantly, in the loop between the 2 α-helices. This loop is unstructured in the HR1a and HR1b domains of PRK1 but in TOCA1 it forms 2 stretches of 310-helix. Furthermore, the sidechains of the loop residues are occluded and make extensive contacts with both α-helices of the coiled-coil.
Analysis of the Cdc42-binding interface of TOCA1 using NMR spectroscopy revealed that the G protein binding region was in fact generally comparable to that of the PRK1 HR1 domains,38,39 despite the markedly different affinities. Furthermore, similarly to the PRK1 HR1b domain, the data indicated that there was no large scale conformational change upon binding and that the TOCA1 coiled-coil undergoes at most only minor, localized, conformational change upon Cdc42 binding. This is in stark contrast to the predominantly unstructured GBD of WASP, which becomes ordered upon Cdc42 binding and forms an intermolecular β-sheet.35 The Cdc42 binding interface of TOCA1, did however, include the occluded loop residues of the TOCA1 HR1 and so we postulate that these occluded side chains will reorient in the presence of Cdc42. The occluded conformation of these binding residues may be responsible for the lower affinity of the interaction when compared with other G protein-HR1 domain interactions.
Our model of the Cdc42-HR1TOCA1 complex, which is comparable to the G protein-PRK1 HR1 complexes, shows that Gln2 of Cdc42 may disrupt the loop-helix contact formed by Asn380TOCA1, Val376TOCA1 and Tyr 377TOCA1. We have also identified other specific contacts that may contribute to TOCA1-Cdc42 binding specificity, for example a potential salt bridge formed between Arg68Cdc42 and Glu395TOCA1. The HR1 domain contacts Cdc42 predominantly via its helices and the partially structured loop region, which is again very different to the binding mode of the WASP GBD. Importantly, our model showed that the TOCA1 and (N-)WASP binding sites partially overlap, indicating that TOCA1 and (N-)WASP are likely to compete for binding to Cdc42.
We carried out NMR experiments to determine whether a ternary complex could be observed between Cdc42, TOCA1 and N-WASP, as had previously been suggested.20,22 Our data showed that the TOCA1 HR1 domain was fully displaced by the N-WASP CRIB domain, but not vice versa. Pyrene actin experiments were in agreement with this unidirectional competition. Previous studies have shown that TOCA1 is recruited earlier than N-WASP during the formation of filopodia-like structures on supported lipid bilayers41 and so we postulate that this unidirectional competition represents an irreversible step in the pathway of Cdc42/TOCA1/N-WASP-dependent actin assembly.
Our model of the Cdc42-HR1TOCA1 complex, when compared with the Cdc42-WASP structure,35 allows us to speculate upon an effector handover mechanism by which this unidirectional step is driven. The (N-)WASP GBD can be compartmentalized into 3 regions based on binding studies, the basic region just upstream of the Cdc42- and Rac1-interactive binding (CRIB) region, the core CRIB and the C-terminal extension. The core CRIB is found to bind with a Kd of 470 nM, while the inclusion of a C-terminal extension to the core CRIB increases the affinity to 77 nM.33 Mutation of the basic region N-terminal to the CRIB affects binding, specifically by reducing the on-rate, and so it has been postulated that binding is initiated by an electrostatic steering mechanism involving this region.42 The basic region does not interact with Cdc42 in the resulting complex but instead binds to PI(4,5)P2 allowing cooperative activation of N-WASP.43
In our model, the basic region and core CRIB of WASP are able to interact with Cdc42 without steric hindrance from the HR1 domain. We therefore expect that these regions of (N-)WASP can form initial, low affinity contacts with Cdc42 in the presence of the TOCA1 HR1 domain. The region of (N-)WASP just C-terminal to the core CRIB does, however, sterically clash with the TOCA1 HR1 domain and so formation of the final, high affinity, Cdc42-WASP complex would be expected to cause displacement of the HR1 domain.
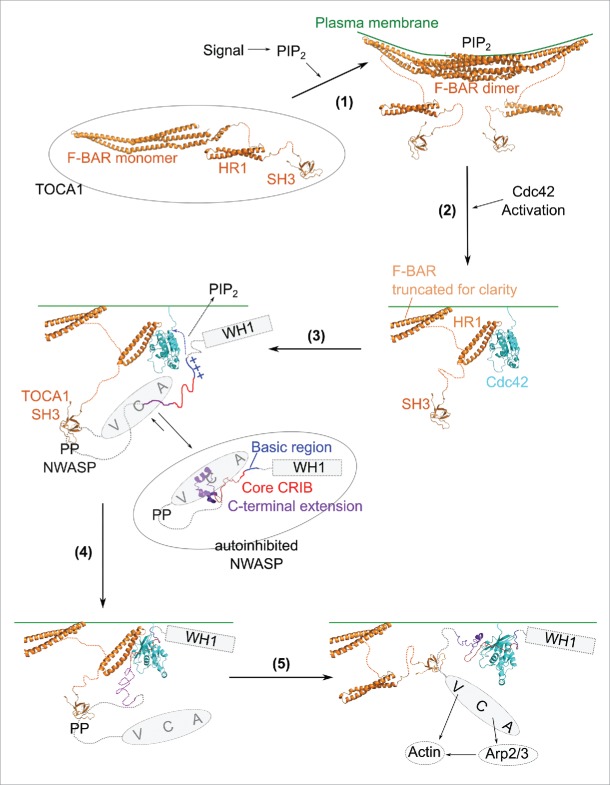
The pathway of Cdc42/(N-)WASP/Arp2/3-dependent actin assembly is evidently complex, with multidirectional, interlinked equilibria relying upon additive, synergistic and cooperative effects (for reviews see refs. 19, 31). Our data pertaining to TOCA1-Cdc42 binding is consistent with this current understanding. While this is difficult to portray in a simple schematic, by considering the model of the Cdc42-HR1TOCA1 complex and the NMR experiments alongside the available in vitro binding data, it is possible to construct a simplified scheme incorporating the wealth of available structural information. This describes how the early stages of Cdc42-dependent actin nucleation may proceed via N-WASP and TOCA1 (Fig. 2).
Figure 2.
Step 1: TOCA1 is represented by the structure of the CIP4 F-BAR domain,49 the TOCA1 HR1 domain36 and the CIP4 SH3 domain (PDB ID 2CT4, Miyamoto et al. unpublished), connected by flexible linkers. Cell signaling through PI(4,5)P2 pushes the equilibrium in favor of membrane-localized TOCA1 followed by dimerization via the F-BAR domain. Step 2: Cdc42·GTP (Mott and Owen, structure unpublished), activated in response to extracellular signals, binds to TOCA1. The model of the Cdc42-HR1 complex is shown.36 The majority of the F-BAR domain is omitted for clarity. Step 3: TOCA1 is now poised to activate N-WASP. Autoinhibited N-WASP shows the structure of the GBD with the cofilin homology region,34 with the WH1 (box) and VCA (oval) connected to the GBD via flexible linkers. The WH1 domain would be bound to WIP but WIP is omitted for clarity. Binding of the TOCA1 SH3 domain to the polyproline region (PP) of N-WASP positions N-WASP for Cdc42 and PI(4,5)P2 binding, allowing initial interactions of the basic region (blue crosses) and core CRIB (red, unstructured) with Cdc42. Step 4: The initial interactions between N-WASP and Cdc42 rapidly push the equilibrium in favor of the fully unfolded, high affinity conformation such that the C-terminal region of the GBD can now bind, displacing the TOCA1 HR1 domain (step 5). The Cdc42-GBD complex is represented by the Cdc42-WASP GBD structure.35 The VCA domains of the clustered N-WASP molecules are now free for robust activation of actin polymerization via the Arp2/3 complex.
Step 1: The low affinity of the Cdc42-TOCA1 interaction, together with estimates of their cellular concentrations, indicates that the 2 proteins are only likely to interact when they are co-localized. TOCA1 can be cytoplasmic or membrane bound and the membrane bound state is heavily favored by PI(4,5)P2 signaling.
Step 2: Homodimerization via the F-BAR domain, and subsequent oligomerization leads to clustering of TOCA1 at the membrane, which results in sufficient local concentrations of TOCA1 to achieve its interaction with Cdc42, providing Cdc42 activation has occurred coincidentally.
Step 3: Once membrane-localized and bound to Cdc42, TOCA1 is in a position to affect the WASP family by clustering mechanisms, and also potentially via allosteric effects, both of which appear to be important for full WASP activation.19 The TOCA1-Cdc42 interaction stabilizes TOCA1 at the membrane leading to clustering of TOCA1. The interaction of the TOCA1 SH3 domain with proline-rich sequences in (N-)WASP leads to (N-)WASP clustering. Such clustering has been observed on supported lipid bilayers following TOCA1 recruitment.41 The (N-)WASP-TOCA1 interaction may therefore serve to position (N-)WASP for Cdc42 and PI(4,5)P2 binding. The interaction with TOCA1 could also have an allosteric effect on (N-)WASP although this has not yet been demonstrated. TOCA1 may also affect (N-)WASP binding to WIP, although it is not yet clear whether TOCA1 binding destabilizes the (N-)WASP-WIP complex or activates the WIP-bound (N-)WASP directly.20
Step 4: The positioning of (N-)WASP by TOCA1, along with a potential allosteric effect, allows the (N-)WASP basic region and core CRIB to contact Cdc42. The intermediate states would be short-lived and sparsely populated, as the folded conformation and the unfolded, high affinity conformation of (N-)WASP would each be highly favored over this transient state. The high affinity of the unfolded GBD for Cdc42 would ensure that the unfolded state is ultimately favored and thus these initial contacts between the GBD and Cdc42 rapidly push the equilibrium in favor of unfolded (N-)WASP, allowing the complete GBD to contact Cdc42.
Step 5: The C-terminal region of the GBD now sterically clashes with the TOCA1 HR1 domain, promoting its displacement. The 100x higher affinity of (N-)WASP ensures unidirectional competition with the TOCA1 HR1 for Cdc42·GTP, as we have observed in NMR experiments with the free GBD (in its high affinity state). The VCA domains of the clustered (N-)WASP molecules can then elicit robust recruitment and activation of the Arp2/3 complex and thus actin nucleation.
The mechanism is depicted as stepwise in our simplified model, to illustrate the molecular mechanism of effector handover and the interactions that may influence the equilibria involved. We do not, however, expect a simple stepwise pathway but rather a complex network of interconnected equilibria, relying upon an intricate and layered series of low affinity interactions reminiscent of those described in relation to clathrin-dependent endocytosis.44,45 Much like aspects of the endocytic signaling pathways, this connected interplay of protein-protein and protein-lipid interactions serves to prevent misfiring of actin polymerization pathways. For example, the model described in Figure 2 would prevent Cdc42/WASP-dependent actin nucleation in the absence of membrane-bending F-BAR proteins, thus regulating the location and direction of membrane protrusions or invaginations and ensuring that all of the necessary machinery is in place before eliciting energy-demanding actin nucleation processes.
It is easy to envisage the presence of certain regulatory thresholds necessary for progression through the pathway, for example a critical level of clustered TOCA1 prior to robust N-WASP recruitment. Indeed such regulatory thresholds have been demonstrated previously in relation to (N-)WASP activation. For example, in vitro activation of (N-)WASP by PI(4,5)P2 occurs above a sharp threshold, arising from the cooperativity that is inherent to the multivalent interaction of the basic region of (N-)WASP with PI(4,5)P2 and the autoinhibitory interaction between the basic region and the VCA domain.46
An irreversible step is implicit in our model, ensuring that the pathway is unidirectional. The displacement of the TOCA1 (or indeed the FBP17 or CIP4) HR1 domains from Cdc42, mediated by N-WASP, could precede the displacement of these F-BAR proteins from the membrane. The negative feedback loop observed for FBP17, where the FBP17-induced membrane tension leads to displacement of FBP17 from the leading edge of the cell 47, could be dependent on the earlier displacement of the HR1-Cdc42 interaction by N-WASP.
Our comparison of the Cdc42-WASP GBD complex with the model of the Cdc42-TOCA1 complex provides the first molecular description of a G protein effector handover. We have described how such a handover relies upon specific membrane localization, directional recruitment of tightly regulated signaling proteins, interrelated binding equilibria and partially overlapping binding sites. Interactions with multiple effectors is a general feature across the different families of small G proteins (reviewed in ref. 48), and so directional effector handovers, such as the one described here, may also occur in other small G protein signaling pathways.
Abbreviations
- CIP4
Cdc42-interacting protein 4
- CRIB
Cdc42- and Rac-interactive binding
- F-BAR
Fes/CIP4 homology BAR
- GBD
G protein binding domain.
- HR1
homology region 1
- N-WASP
Neural Wiskott-Aldrich syndrome protein
- PI(4,5)P2
phosphatidylinositol 4,5-bisphosphate
- PRK
protein kinase C related kinase
- SH3
Src-homology 3
- TOCA
transducer of Cdc42-dependent actin assembly protein
- WASP
Wiskott-Aldrich syndrome protein
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Funding
JRW is supported by a Herchel Smith studentship.
References
- [1].Drubin DG, Nelson WJ. Origins of cell polarity. Cell [Internet] 1996. [cited 2016February4]; 84:335-44. Available from: http://linkinghub.elsevier.com/retrieve/pii/S0092867400812787; PMID:8608587; https://doi.org/ 10.1016/S0092-8674(00)81278-7 [DOI] [PubMed] [Google Scholar]
- [2].Mitchison T., Cramer L. Actin-based cell motility and cell locomotion. Cell [Internet] 1996; 84:371-9. Available from: http://linkinghub.elsevier.com/retrieve/pii/S0092867400812817; PMID:8608590; https://doi.org/ 10.1016/S0092-8674(00)81281-7 [DOI] [PubMed] [Google Scholar]
- [3].Field C, Li R, Oegema K. Cytokinesis in eukaryotes: a mechanistic comparison. Curr Opin Cell Biol [Internet] 1999; 11:68-80. Available from: http://linkinghub.elsevier.com/retrieve/pii/S095506749980009X; PMID:10047527; https://doi.org/ 10.1016/S0955-0674(99)80009-X [DOI] [PubMed] [Google Scholar]
- [4].Giuliani C, Troglio F, Bai Z, Patel FB, Zucconi A, Malabarba MG, Disanza A, Stradal TB, Cassata G, Confalonieri S, et al.. Requirements for F-BAR Proteins TOCA-1 and TOCA-2 in actin dynamics and membrane trafficking during caenorhabditis elegans oocyte growth and embryonic epidermal morphogenesis. PLoS Genet [Internet] 2009; [cited 2016March30]; 5:e1000675. Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=2744924&tool=pmcentrez&rendertype=abstract; PMID:19798448; https://doi.org/ 10.1371/journal.pgen.1000675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Kaksonen M, Toret CP, Drubin DG. Harnessing actin dynamics for clathrin-mediated endocytosis. Nat Rev Mol Cell Biol [Internet] 2006; 7:404-14. Available from: http://www.nature.com/doifinder/10.1038/nrm1940; PMID:16723976; https://doi.org/ 10.1038/nrm1940 [DOI] [PubMed] [Google Scholar]
- [6].Machesky LM, Hall A. Rho: a connection between membrane receptor signalling and the cytoskeleton. Trends Cell Biol [Internet] 1996; [cited 2016March30]; 6:304-10. Available from: http://linkinghub.elsevier.com/retrieve/pii/096289249610026X; PMID:15157438; https://doi.org/ 10.1016/0962-8924(96)10026-X [DOI] [PubMed] [Google Scholar]
- [7].Hall A. Small GTP-binding proteins and the regulation of the actin cytoskeleton. Annu Rev Cell Biol [Internet] 1994, [cited 2016March30]; 10:31-54. Available from: http://onlinelibrary.wiley.com/doi/10.1002/cbdv.200490137/abstract; PMID:7888179; https://doi.org/ 10.1146/annurev.cb.10.110194.000335 [DOI] [PubMed] [Google Scholar]
- [8].Hall A. Rho GTPases and the actin cytoskeleton. Science (80- ) [Internet] 1998, [cited 2015October30]; 279:509-14. Available from: http://www.sciencemag.org/cgi/doi/10.1126/science.279.5350.509; PMID:9438836; https://doi.org/ 10.1126/science.279.5350.509 [DOI] [PubMed] [Google Scholar]
- [9].Nobes CD, Hall A. Rho, Rac, and Cdc42 GTPases regulate the assembly of multimolecular focal complexes associated with actin stress fibers, lamellipodia, and filopodia. Cell [Internet] 1995, [cited 2016January19]; 81:53-62. Available from: http://linkinghub.elsevier.com/retrieve/pii/0092867495903704; PMID:7536630; https://doi.org/ 10.1016/0092-8674(95)90370-4 [DOI] [PubMed] [Google Scholar]
- [10].Ridley AJ, Hall A. The small GTP-binding protein rho regulates the assembly of focal adhesions and actin stress fibers in response to growth factors. Cell [Internet] 1992, [cited 2016February19]; 70:389-99. Available from: http://www.sciencedirect.com/science/article/pii/0092867492901637; PMID:1643657; https://doi.org/ 10.1016/0092-8674(92)90163-7 [DOI] [PubMed] [Google Scholar]
- [11].Etienne-Manneville S. Cdc42–the centre of polarity. J Cell Sci [Internet] 2004, [cited 2016February25]; 117:1291-300. Available from: http://www.ncbi.nlm.nih.gov/pubmed/15020669; PMID:15020669; https://doi.org/ 10.1242/jcs.01115 [DOI] [PubMed] [Google Scholar]
- [12].Symons M, Derry JM, Karlak B, Jiang S, Lemahieu V, Mccormick F, Francke U, Abo A. Wiskott-Aldrich syndrome protein, a novel effector for the GTPase CDC42Hs, is implicated in actin polymerization. Cell [Internet] 1996, [cited 2016March30]; 84:723-34. Available from: http://linkinghub.elsevier.com/retrieve/pii/S0092867400810508; PMID:8625410; https://doi.org/ 10.1016/S0092-8674(00)81050-8 [DOI] [PubMed] [Google Scholar]
- [13].Rohatgi R, Ma L, Miki H, Lopez M, Kirchhausen T, Takenawa T, Kirschner MW. The Interaction between N-WASP and the Arp2/3 Complex Links Cdc42-Dependent Signals to Actin Assembly. Cell [Internet] 1999, [cited 2016March28]; 97:221-31. Available from: http://www.ncbi.nlm.nih.gov/pubmed/10219243; PMID:10219243; https://doi.org/ 10.1016/S0092-8674(00)80732-1 [DOI] [PubMed] [Google Scholar]
- [14].Ma L, Rohatgi R, Kirschner MW. The Arp2/3 complex mediates actin polymerization induced by the small GTP-binding protein Cdc42. Proc Natl Acad Sci [Internet] 1998, [cited 2016March30]; 95:15362-7. Available from: http://www.pnas.org/cgi/doi/10.1073/pnas.95.26.15362; PMID:9860974; https://doi.org/ 10.1073/pnas.95.26.15362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Ramesh N, Antón IM, Hartwig JH, Geha RS. WIP, a protein associated with wiskott-aldrich syndrome protein, induces actin polymerization and redistribution in lymphoid cells. Proc Natl Acad Sci U S A [Internet] 1997; 94:14671-6. Available from: http://www.ncbi.nlm.nih.gov/pubmed/9405671; PMID:9405671; https://doi.org/ 10.1073/pnas.94.26.14671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Rohatgi R, Ho HY, Kirschner MW. Mechanism of N-WASP activation by CDC42 and phosphatidylinositol 4, 5-bisphosphate. J Cell Biol [Internet] 2000, [cited 2016March30]; 150:1299-310. Available from: http://www.jcb.org/lookup/doi/10.1083/jcb.150.6.1299; PMID:10995436; https://doi.org/ 10.1083/jcb.150.6.1299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Machesky LM, Insall RH. Signaling to Actin Dynamics. J Cell Biol [Internet] 1999, [cited 2016March30]; 146:267-72. Available from: http://jcb.rupress.org/content/146/2/267.short; PMID:10427083; https://doi.org/ 10.1083/jcb.146.2.267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Miki H, Miura K, Takenawa T. N-WASP, a novel actin-depolymerizing protein, regulates the cortical cytoskeletal rearrangement in a PIP2-dependent manner downstream of tyrosine kinases. EMBO J [Internet] 1996, [cited 2016March30]; 15:5326-35. Available from: http://www.ncbi.nlm.nih.gov/pubmed/24332715; PMID:8895577 [PMC free article] [PubMed] [Google Scholar]
- [19].Padrick SB, Rosen MK. Physical mechanisms of signal integration by WASP family proteins. Annu Rev Biochem [Internet] 2010, [cited 2016March30]; 79:707-35. Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3017724&tool=pmcentrez&rendertype=abstract; PMID:20533885; https://doi.org/ 10.1146/annurev.biochem.77.060407.135452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Ho HH, Rohatgi R, Lebensohn AM, Le Ma Li J, Gygi SP, Kirschner MW. Toca-1 mediates Cdc42-dependent actin nucleation by activating the N-WASP-WIP complex. Cell [Internet] 2004, [cited 2016March30]; 118:203-16. Available from: http://www.ncbi.nlm.nih.gov/pubmed/15260990; PMID:15260990; https://doi.org/ 10.1016/j.cell.2004.06.027 [DOI] [PubMed] [Google Scholar]
- [21].Fricke R, Gohl C, Dharmalingam E, Grevelhörster A, Zahedi B, Harden N, Kessels M, Qualmann B, Bogdan S. Drosophila Cip4/Toca-1 integrates membrane trafficking and actin dynamics through WASP and SCAR/WAVE. Curr Biol [Internet] 2009, [cited 2016March30]; 19:1429-37. Available from: http://www.ncbi.nlm.nih.gov/pubmed/19716703; PMID:19716703; https://doi.org/ 10.1016/j.cub.2009.07.058 [DOI] [PubMed] [Google Scholar]
- [22].Bu W, Lim KB, Yu YH, Chou AM, Sudhaharan T, Ahmed S. Cdc42 interaction with N-WASP and Toca-1 regulates membrane tubulation, vesicle formation and vesicle motility: implications for endocytosis. PLoS One [Internet] 2010, [cited 2016February23]; 5:1-16. Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=2921345&tool=pmcentrez&rendertype=abstract; PMID:NOT_FOUND; https://doi.org/ 10.1371/journal.pone.0012153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Tsujita K, Suetsugu S, Sasaki N, Furutani M, Oikawa T, Takenawa T. Coordination between the actin cytoskeleton and membrane deformation by a novel membrane tubulation domain of PCH proteins is involved in endocytosis. J Cell Biol [Internet] 2006, [cited 2016March30]; 172:269-79. Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=2063556&tool=pmcentrez&rendertype=abstract; PMID:16418535; https://doi.org/ 10.1083/jcb.200508091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Bai Z, Grant BD. A TOCA/CDC-42/PAR/WAVE functional module required for retrograde endocytic recycling. Proc Natl Acad Sci U S A [Internet] 2015, [cited 2016March30]; 112:E1443-52. Available from: http://www.ncbi.nlm.nih.gov/pubmed/25775511; PMID:25775511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Bu W, Chou AM, Lim KB, Sudhaharan T, Ahmed S. The Toca-1-N-WASP complex links filopodial formation to endocytosis. J Biol Chem [Internet] 2009, [cited 2016March30]; 284:11622-36. Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=2670167&tool=pmcentrez&rendertype=abstract; PMID:19213734; https://doi.org/ 10.1074/jbc.M805940200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Miyamoto K, Pasque V, Jullien J, Gurdon JB. Nuclear actin polymerization is required for transcriptional reprogramming of Oct4 by oocytes. Genes Dev [Internet] 2011, [cited 2016February4]; 25:946-58. Available from: http://genesdev.cshlp.org/content/25/9/946.short; PMID:21536734; https://doi.org/ 10.1101/gad.615211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Kakimoto T, Katoh H, Negishi M. Regulation of neuronal morphology by Toca-1, an F-BAR/EFC protein that induces plasma membrane invagination. J Biol Chem [Internet] 2006, [cited 2016March30]; 281:29042-53. Available from: http://www.ncbi.nlm.nih.gov/pubmed/16885158; PMID:16885158; https://doi.org/ 10.1074/jbc.M604025200 [DOI] [PubMed] [Google Scholar]
- [28].Hu J, Mukhopadhyay A, Craig AWB. Transducer of Cdc42-dependent actin assembly promotes epidermal growth factor-induced cell motility and invasiveness. J Biol Chem [Internet] 2011, [cited 2016March30]; 286:2261-72. Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3023521&tool=pmcentrez&rendertype=abstract; PMID:21062739; https://doi.org/ 10.1074/jbc.M110.157974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Chander H, Truesdell P, Meens J, Craig WB. Transducer of Cdc42-dependent actin assembly promotes breast cancer invasion and metastasis. Oncogene [Internet] 2013, [cited 2016March30]; 32:3080-90. Available from: http://www.ncbi.nlm.nih.gov/pubmed/22824798; PMID:22824798; https://doi.org/ 10.1038/onc.2012.317 [DOI] [PubMed] [Google Scholar]
- [30].Leung DW, Rosen MK. The nucleotide switch in Cdc42 modulates coupling between the GTPase-binding and allosteric equilibria of Wiskott-Aldrich syndrome protein. Proc Natl Acad Sci [Internet] 2005, [cited 2016March30]; 102:5685-90. Available from: http://www.pnas.org/cgi/doi/10.1073/pnas.0406472102; PMID:15821030; https://doi.org/ 10.1073/pnas.0406472102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Prehoda KE, Lim WA. How signaling proteins integrate multiple inputs: a comparison of N-WASP and Cdk2. Curr Opin Cell Biol [Internet] 2002; 14:149-54. Available from: http://linkinghub.elsevier.com/retrieve/pii/S0955067402003071; PMID:11891112; https://doi.org/ 10.1016/S0955-0674(02)00307-1 [DOI] [PubMed] [Google Scholar]
- [32].Buck M, Xu W, Rosen MK. A two-state allosteric model for autoinhibition rationalizes WASP signal integration and targeting. J Mol Biol [Internet] 2004, [cited 2016March30]; 338:271-85. Available from: http://www.ncbi.nlm.nih.gov/pubmed/15066431; PMID:15066431; https://doi.org/ 10.1016/j.jmb.2004.02.036 [DOI] [PubMed] [Google Scholar]
- [33].Rudolph MG, Bayer P, Abo A, Kuhlmann J, Vetter IR, Wittinghofer A. The Cdc42/Rac interactive binding region motif of the Wiskott Aldrich syndrome protein (WASP) is necessary but not sufficient for tight binding to Cdc42 and structure formation. J Biol Chem [Internet] 1998, [cited 2016March30]; 273:18067-76. Available from: http://www.jbc.org/cgi/doi/10.1074/jbc.273.29.18067; PMID:9660763; https://doi.org/ 10.1074/jbc.273.29.18067 [DOI] [PubMed] [Google Scholar]
- [34].Kim AS, Kakalis LT, Abdul-Manan N, Liu GA, Rosen MK. Autoinhibition and activation mechanisms of the Wiskott–Aldrich syndrome protein. Nature [Internet] 2000, [cited 2016March30]; 404:151-8. Available from: http://www.nature.com/doifinder/10.1038/35004513; PMID:10724160; https://doi.org/ 10.1038/35004513 [DOI] [PubMed] [Google Scholar]
- [35].Abdul-Manan N, Aghazadeh B, Liu GA, Majumdar A, Ouerfelli O, Siminovitch KA, Rosen MK. Structure of Cdc42 in complex with the GTPase-binding domain of the “Wiskott-Aldrich syndrome” protein. Nature [Internet] 1999, [cited 2016March30]; 399:379-83. Available from: http://www.nature.com/nature/journal/v399/n6734/abs/399379a0.html; PMID:10360578; https://doi.org/ 10.1038/20726 [DOI] [PubMed] [Google Scholar]
- [36].Watson JR, Fox HM, Nietlispach D, Gallop JL, Owen D, Mott HR. Investigation of the interaction between Cdc42 and its Effector TOCA1: Handover of Cdc42 to the actin regulator N-WASP is facilitated by differential binding affinities. J Biol Chem [Internet] 2016; 291:13875-90. Available from: http://www.jbc.org/lookup/doi/10.1074/jbc.M116.724294; PMID:27129201; https://doi.org/ 10.1074/jbc.M116.724294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Hutchinson CL, Lowe PN, McLaughlin SH, Mott HR, Owen D. Differential binding of RhoA, RhoB, and RhoC to protein kinase C-related kinase (PRK) isoforms PRK1, PRK2, and PRK3: PRKs have the highest affinity for RhoB. Biochemistry [Internet] 2013, [cited 2016March30]; 52:7999-8011. Available from: http://www.ncbi.nlm.nih.gov/pubmed/24128008; PMID:24128008; https://doi.org/ 10.1021/bi401216w [DOI] [PubMed] [Google Scholar]
- [38].Owen D, Lowe PN, Nietlispach D, Brosnan CE, Chirgadze DY, Parker PJ, Blundell TL, Mott HR. Molecular dissection of the interaction between the small G proteins Rac1 and RhoA and protein kinase C-related kinase 1 (PRK1). J Biol Chem [Internet] 2003, [cited 2016March30]; 278:50578-87. Available from: http://www.ncbi.nlm.nih.gov/pubmed/14514689; PMID:14514689; https://doi.org/ 10.1074/jbc.M304313200 [DOI] [PubMed] [Google Scholar]
- [39].Maesaki R, Ihara K, Shimizu T, Kuroda S, Kaibuchi K, Hakoshima T. The structural basis of Rho effector recognition revealed by the crystal structure of human RhoA complexed with the effector domain of PKN/PRK1. Mol Cell [Internet] 1999, [cited 2016March30]; 4:793-803. Available from: http://linkinghub.elsevier.com/retrieve/pii/S1097276500803895; PMID:10619026; https://doi.org/ 10.1016/S1097-2765(00)80389-5 [DOI] [PubMed] [Google Scholar]
- [40].Modha R, Campbell LJ, Nietlispach D, Buhecha HR, Owen D, Mott HR. The Rac1 polybasic region is required for interaction with its effector PRK1. J Biol Chem [Internet] 2008, [cited 2016March30]; 283:1492-500. Available from: http://www.ncbi.nlm.nih.gov/pubmed/18006505; PMID:18006505; https://doi.org/ 10.1074/jbc.M706760200 [DOI] [PubMed] [Google Scholar]
- [41].Lee K, Gallop JL, Rambani K, Kirschner MW. Self-Assembly of Filopodia-Like Structures on Supported Lipid Bilayers. Science (80- ) [Internet] 2010, [cited 2016March30]; 329:1341-5. Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=2982780&tool=pmcentrez&rendertype=abstract; PMID:20829485; https://doi.org/ 10.1126/science.1191710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Hemsath L, Dvorsky R, Fiegen D, Carlier M-F, Ahmadian MR. An electrostatic steering mechanism of Cdc42 recognition by Wiskott-Aldrich syndrome proteins. Mol Cell [Internet] 2005, [cited 2016March30]; 20:313-24. Available from: http://www.ncbi.nlm.nih.gov/pubmed/16246732; PMID:16246732; https://doi.org/ 10.1016/j.molcel.2005.08.036 [DOI] [PubMed] [Google Scholar]
- [43].Prehoda KE, Scott JA, Mullins RD, Lim WA. Integration of multiple signals through cooperative regulation of the N-WASP-Arp2/3 complex. Science [Internet] 2000; 290:801-6. Available from: http://www.ncbi.nlm.nih.gov/pubmed/11052943\nhttp://www.sciencemag.org/content/290/5492/801.full.pdf; PMID:NOT_FOUND; https://doi.org/ 10.1126/science.290.5492.801 [DOI] [PubMed] [Google Scholar]
- [44].Praefcke GJK, Ford MGJ, Schmid EM, Olesen LE, Gallop JL, Peak-Chew S-Y, Vallis Y, Babu MM, Mills IG, McMahon HT. Evolving nature of the AP2 alpha-appendage hub during clathrin-coated vesicle endocytosis. EMBO J [Internet] 2004, [cited 2016February28]; 23:4371-83. Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=526462&tool=pmcentrez&rendertype=abstract; PMID:15496985; https://doi.org/ 10.1038/sj.emboj.7600445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Höning S, Ricotta D, Krauss M, Späte K, Spolaore B, Motley A, Robinson M, Robinson C, Haucke V, Owen DJ. Phosphatidylinositol-(4,5)-bisphosphate regulates sorting signal recognition by the clathrin-associated adaptor complex AP2. Mol Cell [Internet] 2005, [cited 2016January25]; 18:519-31. Available from: http://www.ncbi.nlm.nih.gov/pubmed/15916959; PMID:15916959; https://doi.org/ 10.1016/j.molcel.2005.04.019 [DOI] [PubMed] [Google Scholar]
- [46].Papayannopoulos V, Co C, Prehoda KE, Snapper S, Taunton J, Lim WA. A polybasic motif allows N-WASP to act as a sensor of PIP2 density. Mol Cell 2005; 17:181-91; PMID:15664188; https://doi.org/ 10.1016/j.molcel.2004.11.054 [DOI] [PubMed] [Google Scholar]
- [47].Tsujita K, Takenawa T, Itoh T. Feedback regulation between plasma membrane tension and membrane-bending proteins organizes cell polarity during leading edge formation. Nat Cell Biol [Internet] 2015, [cited 2016March6]; 17:749-58. Available from: http://www.ncbi.nlm.nih.gov/pubmed/25938814; PMID:25938814; https://doi.org/ 10.1038/ncb3162 [DOI] [PubMed] [Google Scholar]
- [48].Mott HR, Owen D. Structures of Ras superfamily effector complexes: What have we learnt in two decades? Crit Rev Biochem Mol Biol [Internet] 2015, [cited 2016February16]; 50:85-133. Available from: http://www.tandfonline.com/doi/full/10.3109/10409238.2014.999191; PMID:25830673; https://doi.org/ 10.3109/10409238.2014.999191 [DOI] [PubMed] [Google Scholar]
- [49].Shimada A, Niwa H, Tsujita K, Suetsugu S, Nitta K, Hanawa-Suetsugu K, Akasaka R, Nishino Y, Toyama M, Chen L, et al.. Curved EFC/F-BAR-domain dimers are joined end to end into a filament for membrane invagination in endocytosis. Cell [Internet] 2007, [cited 2016March30]; 129:761-72. Available from: http://www.ncbi.nlm.nih.gov/pubmed/17512409; PMID:17512409 [DOI] [PubMed] [Google Scholar]