ABSTRACT
The p21-activated kinases (PAKs) are Cdc42/Rac–activated serine-threonine protein kinases that regulate several key cancer-relevant signaling pathways, such as the Mek/Erk, PI3K/Akt, and Wnt/β–catenin cascades. Pak1 is frequently overexpressed and/or hyperactivated in different human cancers, including breast, ovary, prostate, and brain cancer. PAK1 genomic amplification at 11q13 is the most common mechanism of Pak1 hyperactivation, though Pak1 mRNA and/or protein may be overexpressed in the absence of gene amplification. In previous in vitro and in vivo studies we have shown that ovarian cancer cells with amplified/overexpressed Pak1 were significantly more sensitive to pharmacologic inhibition of Pak1 compared to cells without 11q13 amplification. In the present study we examined if additional signaling pathways might be targeted in tandem with the Group I Pak inhibitor Frax-1036 in ovarian cancer cells. Using the ICCB Known Bioactives Library, we found that the cytotoxic effect of Frax-1036 was significantly higher in combination with the PKCδ inhibitor, Rottlerin, suggesting that Pak inhibitors might be combined with other agents to treat 11q13-amplified ovarian cancer.
KEYWORDS: chromosomal amplification, oncogene, ovarian cancer, p21 inactivated kinase, protein kinase, sensitized screen, signal transduction
Introduction
p21-activated kinases (Paks) are serine/threonine-specific protein kinases that are involved in a number of signaling pathways required for oncogenesis.1 By sequence and structure, the 6 mammalian Paks can be divided into 2 groups: group I (Pak1-3) and group II (Pak 4–6).2 Pak1 is hyperactivated in many human cancers and such activation is positively correlated with advanced grade and decreased survival.3
The most common mechanism of Pak1 hyperactivation in cancer cells is gene amplification of PAK1 on chromosome 11q13.1 Pak1 can be also hyperactivated by mutations in upstream regulators such as Rac1 or Cdc42.4 When activated, Pak1 has oncogenic signaling effects in cells, promoting cell proliferation, invasion, metastasis, and evasion of apoptosis. Our previous data5 and studies by other groups6 show that PAK1 amplified tumors are highly dependent on Pak1 signaling. For these reasons, it is important to understand the key downstream effectors of Pak1 and their potential value as drug targets for the treatment of cancer.
Several group I Pak inhibitors have been discovered over the past few years.7 These include the pan-Pak inhibitor PF-3578309,8 and the Group I-selective Pak inhibitors: Frax-597, −716, or −1036. Recently, another potent and selective Frax-based inhibitor of the group I Paks, G-5555 was described.9 In an array of 23 breast cancer cell lines, G-5555 showed significantly greater growth inhibitory activity in cell lines that were PAK1-amplified compared to non-amplified lines. However, the therapeutic window for group I Pak inhibitors may be narrow, as these compounds have cardiotoxic effects at higher doses.7 To avoid these effects, the combination of lower doses of a group I Pak inhibitor with a second compound targeting other pathways or processes might be useful. For example, the efficacy of Frax-1036 was potentiated in combination with the microtubule inhibitor docetaxel and led to increased apoptosis in 11q13 amplified breast cancer cells.10 These findings support Pak1 as a potential target in 11q13 amplified cancers and suggest combination therapy as an approach to increase anti-tumor efficacy without evoking unacceptable toxicities. To identify such compounds, we performed a sensitized chemical screen in 11q13-amplified ovarian cancer cells using the ICCB Known Bioactives Library. We found that Rottlerin significantly increased the efficacy of Frax-1036 in 11q13 amplified ovarian cancer cells in vitro. We explored the molecular mechanisms for this combination and showed increased antiproliferative effects in ovarian cancer cells in vitro and anti-tumor activity in an animal model.
Results
Synergistic effects of Frax-1036 and Rottlerin in vitro
In our previous studies we found that ovarian cancer cells with amplified 11q13 and/or overexpressed Pak1 (OVCAR-3 and OV-90) were more sensitive to pharmacologic Pak1 inhibition. To investigate the mechanisms of these effects and to find compounds that could potentially enhance Frax-1036 anticancer effects, we performed a sensitized screen using Frax-1036 plus the ICCB Known Bioactives Library in the 11q13 amplified OVCAR-3 ovarian cancer cell line. OVCAR-3 cells were treated with compounds alone or with the addition of 6 μM Frax-1036. Cell viability was assessed after 3 d using an Alamar Blue assay. We found that the cytotoxic effect of Frax-1036 was significantly higher in combination with a PKCδ inhibitor, Rottlerin. To verify these results, we examined the effect of Rottlerin on the proliferation of OVCAR-3 and OV-90 cell lines. Cells were cultured with increasing concentrations of Rottlerin for 3 days, and cell proliferation was assessed by Alamar Blue assay. We found that Rottlerin inhibited cell proliferation in a dose-dependent manner (Fig. 1A) with IC50 values determined as 3 μM in OVCAR-3 cells and 6 μM in OV-90 cells. To characterize potential interactions between Frax-1036 and Rottlerin, human ovarian cancer cell lines with hyperactivated Pak1 were exposed to varying concentrations of Rottlerin with or without Frax-1036 (3 μM and 1 μM for OVCAR3 and OV-90 respectively) (Fig. 1A), and cell viability was assessed after 3 d. These concentrations were selected since they had relatively modest independent effects on cell proliferation and survival in each of the ovarian cell lines tested. The combination of both inhibitors was substantially more effective (2.2-3-fold changes in Rottlerin IC50, 1 μM, and 2.7 μM for OVCAR-3 and OV-90) than either single agent alone and produced a significant decrease in ovarian cancer cell survival. In contrast, the combination of these concentrations of Rottlerin and Frax-1036 had no significant effect on human ovarian cancer cells without amplified 11q13 (data not shown).
Figure 1.
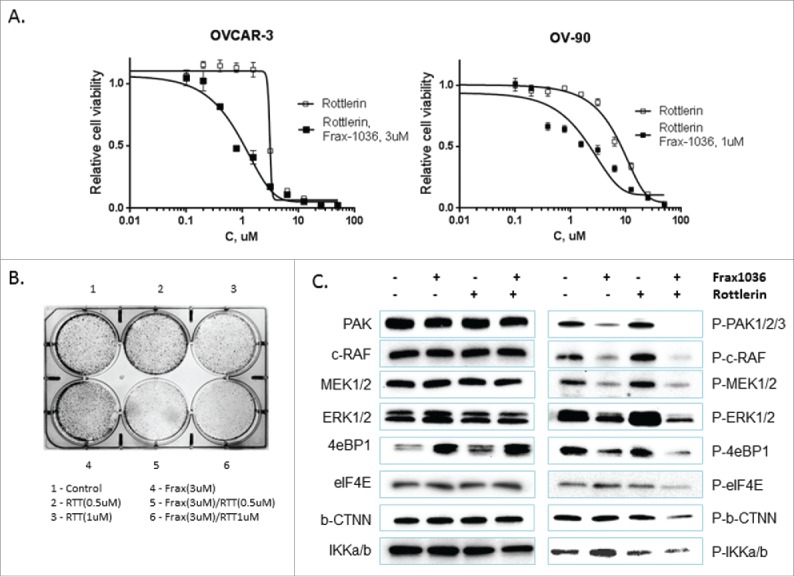
Frax-1036 and Rottlerin co-administration synergizes the inhibition of OVCAR-3 cell line growth (A) and colony formation (B). Frax-1036 and Rottlerin synergistically inhibit phosphorylation of critical signaling molecules such as Mek, Erk1/2, β-catenin, and 4EBP1 (C)
The cytotoxic effect of Frax-1036 and Rottlerin was further confirmed using a clonogenic assay in OVCAR-3 cells (Fig. 1B). Cells were treated for 1 day with or without compounds and allowed to grow for an additional 1-week period. Whereas only modest effects were seen at 0.5-1 μM concentrations of Rottlerin, addition of 3 μM Frax-1036 significantly increased the degree of inhibition, despite having no effect on clonogenicity when administered as a single agent at this concentration (Fig. 1B).
Effects of Frax-1036 and Rottlerin on signaling
To assess the mechanism by which Frax-1036 and Rottlerin affect cellular proliferation, human OVCAR-3 ovarian cancer cells were treated with each of these compounds alone or in combination for 24 h. Cell lysates were examined by Western blot for activation of downstream signaling pathway components. As expected, OVCAR-3 cells treated with Frax-1036 alone and in combination with Rottlerin showed significant decrease of phospho-Pak1 levels and decreased phosphorylation of c-raf, Mek and Erk. OVCAR-3 cells treated with both compounds showed significant decrease of phosphorylated forms of β-catenin and IKKα/β (Fig. 1C). In addition, combined exposure of Frax-1036 and Rottlerin resulted in decreased phosphorylation of the translation repression protein 4E-BP1 and the translation factor eIF4E. Taken together, these data demonstrate that Frax-1036 and Rottlerin co-administration inhibited phosphorylation of critical signaling molecules of different signaling pathways.
Frax-1036 and Rottlerin in vivo
In our previous studies we showed that administration of Frax-1036 at a dose of 30 mg/kg significantly decreased tumor growth in 11q13 amplified ovarian cancer xenograft models. Because we observed a strong interaction between Frax-1036 and Rottlerin in vitro we decreased to dose of Frax-1036 to 20 mg/kg for our mouse studies. To determine the effect of Frax-1036 and Rottlerin on tumor growth in vivo, we used an OVCAR-3 xenograft model. Tumor progression was monitored twice a week for each animal. When tumors had reached a volume of 100–200 mm3, treatment with Frax-1036 (20 mg/kg, oral, once daily), Rottlerin (20 mg/kg, oral, once daily), a combination of both, or vehicle control was initiated for a period of 22 d.
Analysis of the tumor growth for the animals in 4 groups demonstrated that mice receiving the combination of Frax-1036 and Rottlerin showed a significantly reduced mean tumor volume (364 ± 98 mm3) compared with mice receiving Frax-1036 alone (636 ± 250 mm3), Rottlerin alone (832 ± 135 mm3), or control treatment (1121 ± 350 mm3) by day 1 (P < 0.01 for all 3 comparisons) (Fig. 2).
Figure 2.

Frax-1036 and Rottlerin co-administration significantly slowed tumor growth in OVCAR-3 xenograft tumors compared to control.
Taken together, these data demonstrate that combination of Frax-1036 and Rottlerin has a significant anti-proliferative activity against ovarian cancer cells with elevated Pak1 level in vitro and anti-tumor activity in vivo.
Discussion
About 25% of ovarian cancers are characterized genetically by amplification of chromosomal region 11q13. It has been shown that a number of the genes in this amplicon, including CCND1, RSF1, EMSY, and GAB2, are commonly overexpressed in 11q13 amplified cancer cells and have also been suggested to play roles in tumorigenesis.11,12
The p21-activated kinase 1 (PAK1) gene resides at 11q13.5 is frequently overexpressed in a number of human cancers, including breast, prostate, and ovarian cancer and correlated with poor overall survival.13 Knockdown of Pak1 has been shown to inhibit the growth of breast cancer cells,6,14 the proliferation of NSCLC cancers in vitro and in vivo,6 colon cancer cell growth in vitro and in vivo,15,16 and the growth of gastric cancer cells.17 In our previous studies we have shown that that ovarian cancer cell lines with 11q13 amplification and elevated Pak1 levels are highly sensitive to Pak1 genetic and pharmacologic inhibition in vitro and in vivo.5 In an array of 23 breast cancer cell lines, another group I Pak inhibitor G-5555 showed significantly greater growth inhibitory activity in cell lines that were PAK1-amplified compared to non-amplified lines.7
These studies have provided a conceptual basis for the development of anti-Pak drugs as potential cancer therapeutics. However, recent animal studies with selective group I Pak inhibitors G-555, G-9791 and other members of this series, showed that group I Pak inhibitors might have unacceptable cardiotoxic effects most likely mediated by Pak2.7 While there is one example of a selective Pak1 inhibitor that might avoid these issues,18 another approach might be to combine lower doses of a group I Pak inhibitor with a second compound targeting other pathways.
In the present study we focused on finding the compound that could enhance the antiproliferative effect of Pak inhibitors used at lower doses. Using ICCB Known Bioactives Library we found that combination of Frax-1036 and Rottlerin significantly increased the cytotoxic effect in PAK1-amplified ovarian cancer cells in vitro. Rottlerin is a natural plant polyphenol, and is known to affect several pathways that regulate cell survival, apoptosis, autophagy, and invasion.19 Rottlerin was initially reported as inhibitor of PKCs with selectivity for PKCδ.20 In addition to PKCs, Rottlerin was later shown to inhibit the activity of a number of unrelated kinases, such as Akt/PKB and CaMKs.21 CaMKI/II and CaMKIII activity is suppressed by Rottlerin with potency similar to that of PKCδ.22,23 Moreover, Rottlerin has mitochondrial uncoupling properties that cause ATP depletion and inhibition of cellular processes controlled by phosphorylated molecules.24 Rottlerin has also been reported to potentiate the effects of antineoplastic drugs through inhibition of Erk and Akt phosphorylation and down-regulation of crucial cell-cycle proteins, such as cyclins and CDKs.25
We found that Frax-1036 and Rottlerin, and particularly the combination of both, inhibited ovarian cancer cells proliferation and colony formation in vitro and tumor growth in vivo. This inhibition was accompanied by a decreased phosphorylation of critical signaling molecules such as Mek, Erk1/2, β-Catenin, and 4EBP1 shown by Western blot analysis. Reductions in Erk1/2 and β-Catenin signaling is consistent with the observed lower level of cell proliferation in treated 11q13 cells and, in the case of 4EBP1, the observed loss of phosphorylation is expected to reduce cap-dependent translation by stabilizing the binding of 4EBP1 to the translational factor eIF4E.26 We speculate that Rottlerin enhances Pak1 antiproliferative effects in amplified 11q13 ovarian cancer cells by combined inhibition of several key signaling pathways.
In previous studies, we observed that doses of Frax-1036 greater than 45 mg/kg were not tolerated in our animal model.5 These observations are in keeping with recent studies showing that Pak1 inhibitor G-5555, in an H292 non-small cell lung cancer (NSCLC) xenograft study in mice, imparted 60% tumor growth inhibition when administered at an oral dose of 25 mg/kg, as well as in a PAK1 amplified breast cancer xenograft model, MDA-MB-175 cells.7 Doses greater than 25 mg/kg were not well tolerated and doses of 40 mg/kg were associated with death of the majority of study animals within 2–4 h after dosing. In the present study we investigated the effect of a lower dose (20 mg/kg) of Frax-1036 in combination with Rottlerin on tumor growth in vivo. We found that Rottlerin potentiated the antiproliferative effects of Frax-1036 with lower systemic toxicity in vivo. These findings suggest that targeted agents might be combined with Pak inhibitors in the treatment of 11q13 amplified ovarian cancer, via combined inhibition of cell proliferative and protein synthesis pathways.
Materials and methods
Animal experiments
All animal experiments were approved by the Fox Chase Cancer Center Institutional Animal Care and Use Committee (IACUC) and carried out according to NIH-approved protocols in compliance with the guide for the Care and Use of Laboratory Animals.
Statistical analysis
Statistical analysis was conducted using the unpaired Student t test. Values of P < 0.05 were considered significant.
Cell lines, cell culture - OVCAR-3 and OV-90 cell lines were acquired from the American Type Culture Collection (ATCC), authenticated, tested for mycoplasma contamination, and maintained in early passages, no more than 6 months after receipt from the ATCC. Cells were grown in RPMI-1640 medium supplemented with 10% FBS, 100 U/mL penicillin, and 100 μg/mL streptomycin at 37°C in 95% air/5% CO2.
Western blot analysis - Following the experimental treatment, Western blot analysis were performed as previously described.27 Immunoblot analyses were carried out on lysates extracted from cells or tumors. Protein concentration was determined, and equal amounts of total proteins were separated on SDS-PAGE. Antibodies used included total (SCT #2602) and phospho-Pak1 (SC #2606), Mek (SCT#9121), phospho-Mek pSer298 (SCT#9128), Erk (SCT#9102), phospho-Erk1/2 (pThr202/pTyr204) (SCT#9101), β-catenin (#9562), phospho-β-catenin (#9567) were from Cell Signaling Technology.
Retroviral transductions
An inducible shRNA-bearing retrovirus against Pak1 was previously described27 and oligonucleotide used in this study are as follows: Pak1 shRNA-1 5′-GAT CCCCGA AGA GAG GTT CAG CTA AAT TCA AGA GAT TTA GCT GAA CCT CTCTTC TTT TTT GGA AA-3′; the ΦNX packaging cell line (Orbigen) was transfected using Lipofectamine 2000 (Invitrogen). Viral supernatants were harvested 48 hr post-transfection and filtered. Ovarian cancer cells were incubated with retroviral supernatant supplemented with 4 μg/ml polybrene for 4 h at 37°C, and then were cultured in growth media for 48 h for viral integration. Green fluorescent protein (GFP)-positive infected cells were selected by flow cytometry (GFP).
Cell viability assay
Cells were plated at 4 × 103 cells per well in 96-well plates overnight and treated with various concentrations of Rottlerin and Frax-1036. Cell viability was measured by AlamarBlue assay and the half maximal inhibitory concentration (IC50) was calculated.
Tumor xenografts in SCID mice: FRAX-1036 treatment - Six-week-old female SCID mice were injected with 5 × 106 OVCAR-3 cells into the flank, and tumors were allowed to develop. Upon identification of a palpable tumor (minimal volume of 150-200 mm3), mice were randomly divided into 4 groups (10 mice in each group). Vehicle, Rottlerin (20 mg/kg), Frax-1036 (20 μg/kg body weight) or combination of drugs was administered via oral gavage every day for 22 d. Tumor length (L) and width (W) were measured with a caliper and tumor volumes were calculated with the formula (L × W2)/2. At the end of the treatment period, the animals were euthanized and the tumors were used for biochemical studies.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Funding
We thank D. Connolly for ovarian cancer cell lines and for insightful comments on the manuscript. This work was supported by grants from the NIH to J.C. (R01-CA142928 and R01-CA148805), Ovarian SPORE P50 CA083638 (Pilot Award to J.C.), Fox Chase Cancer Center (P30-CA006927), as well as by an appropriation from the State of Pennsylvania.
References
- [1].Radu M, Semenova G, Kosoff R, Chernoff J. PAK signalling during the development and progression of cancer. Nat Rev Cancer 2014; 14:13-25; PMID:24505617; https://doi.org/ 10.1038/nrc3645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Arias-Romero LE, Chernoff J. A tale of two Paks. Biol Cell 2008; 100:97-108; PMID:18199048; https://doi.org/ 10.1042/BC20070109 [DOI] [PubMed] [Google Scholar]
- [3].Holm C, Rayala S, Jirstrom K, Stal O, Kumar R, Landberg G. Association between Pak1 expression and subcellular localization and tamoxifen resistance in breast cancer patients. J Natl Cancer Inst 2006; 98:671-80; PMID:16705121; https://doi.org/ 10.1093/jnci/djj185 [DOI] [PubMed] [Google Scholar]
- [4].Prudnikova TY, Rawat SJ, Chernoff J. Molecular pathways: targeting the kinase effectors of RHO-family GTPases. Clin Cancer Res 2015; 21:24-9; PMID:25336694; https://doi.org/ 10.1158/1078-0432.CCR-14-0827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Prudnikova TY, Villamar-Cruz O, Rawat SJ, Cai KQ, Chernoff J. Effects of p21-activated kinase 1 inhibition on 11q13-amplified ovarian cancer cells. Oncogene 2016; 35:2178-85; PMID:26257058; https://doi.org/ 10.1038/onc.2015.278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Ong CC, Jubb AM, Haverty PM, Zhou W, Tran V, Truong T, Turley H, O'Brien T, Vucic D, Harris AL, et al.. Targeting p21-activated kinase 1 (PAK1) to induce apoptosis of tumor cells. Proc Natl Acad Sci U S A 2011; 108:7177-82; PMID:21482786; https://doi.org/ 10.1073/pnas.1103350108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Rudolph J, Murray LJ, Ndubaku CO, O'Brien T, Blackwood E, Wang W, Aliagas I, Gazzard L, Crawford JJ, Drobnick J, et al.. Chemically Diverse Group I p21-Activated Kinase (PAK) Inhibitors Impart Acute Cardiovascular Toxicity with a Narrow Therapeutic Window. J Med Chem 2016; 59:5520-41; PMID:27167326; https://doi.org/ 10.1021/acs.jmedchem.6b00638 [DOI] [PubMed] [Google Scholar]
- [8].Murray BW, Guo C, Piraino J, Westwick JK, Zhang C, Lamerdin J, Dagostino E, Knighton D, Loi CM, Zager M, et al.. Small-molecule p21-activated kinase inhibitor PF-3758309 is a potent inhibitor of oncogenic signaling and tumor growth. Proc Natl Acad Sci U S A 2010; 107:9446-51; PMID:20439741; https://doi.org/ 10.1073/pnas.0911863107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Ndubaku CO, Crawford JJ, Drobnick J, Aliagas I, Campbell D, Dong P, Dornan LM, Duron S, Epler J, Gazzard L, et al.. Design of selective PAK1 inhibitor G-5555: improving properties by employing an unorthodox low-pK a polar moiety. ACS Med Chem Lett 2015; 6:1241-6; PMID:26713112; https://doi.org/ 10.1021/acsmedchemlett.5b00398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Ong CC, Gierke S, Pitt C, Sagolla M, Cheng CK, Zhou W, Jubb AM, Strickland L, Schmidt M, Duron SG, et al.. Small molecule inhibition of group I p21-activated kinases in breast cancer induces apoptosis and potentiates the activity of microtubule stabilizing agents. Breast Cancer Res 2015; 17:59; PMID:25902869; https://doi.org/ 10.1186/s13058-015-0564-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Brown LA, Kalloger SE, Miller MA, Shih Ie M, McKinney SE, Santos JL, Swenerton K, Spellman PT, Gray J, Gilks CB, et al.. Amplification of 11q13 in ovarian carcinoma. Genes Chromosomes Cancer 2008; 47:481-9; PMID:18314909; https://doi.org/ 10.1002/gcc.20549 [DOI] [PubMed] [Google Scholar]
- [12].Choi JH, Sheu JJ, Guan B, Jinawath N, Markowski P, Wang TL, Shih Ie M. Functional analysis of 11q13.5 amplicon identifies Rsf-1 (HBXAP) as a gene involved in paclitaxel resistance in ovarian cancer. Cancer Res 2009; 69:1407-15; PMID:19190325; https://doi.org/ 10.1158/0008-5472.CAN-08-3602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Davidson B, Shih Ie M, Wang TL. Different clinical roles for p21-activated kinase-1 in primary and recurrent ovarian carcinoma. Hum Pathol 2008; 39:1630-6; PMID:18656238; https://doi.org/ 10.1016/j.humpath.2008.03.009 [DOI] [PubMed] [Google Scholar]
- [14].Shrestha Y, Schafer EJ, Boehm JS, Thomas SR, He F, Du J, Wang S, Barretina J, Weir BA, Zhao JJ, et al.. PAK1 is a breast cancer oncogene that coordinately activates MAPK and MET signaling. Oncogene 2012; 31:3397-408; PMID:22105362; https://doi.org/ 10.1038/onc.2011.515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Huynh N, Liu KH, Baldwin GS, He H. P21-activated kinase 1 stimulates colon cancer cell growth and migration/invasion via ERK- and AKT-dependent pathways. Biochim Biophys Acta 2010; 1803:1106-13; PMID:20595063; https://doi.org/ 10.1016/j.bbamcr.2010.05.007 [DOI] [PubMed] [Google Scholar]
- [16].He H, Huynh N, Liu KH, Malcontenti-Wilson C, Zhu J, Christophi C, Shulkes A, Baldwin GS. P-21 activated kinase 1 knockdown inhibits beta-catenin signalling and blocks colorectal cancer growth. Cancer Lett 2012; 317:65-71; PMID:22100495; https://doi.org/ 10.1016/j.canlet.2011.11.014 [DOI] [PubMed] [Google Scholar]
- [17].Liu S, Goldstein RH, Scepansky EM, Rosenblatt M. Inhibition of rho-associated kinase signaling prevents breast cancer metastasis to human bone. Cancer Res 2009; 69:8742-51; PMID:19887617; https://doi.org/ 10.1158/0008-5472.CAN-09-1541 [DOI] [PubMed] [Google Scholar]
- [18].Karpov AS, Amiri P, Bellamacina C, Bellance MH, Breitenstein W, Daniel D, Denay R, Fabbro D, Fernandez C, Galuba I, et al.. Optimization of a Dibenzodiazepine Hit to a Potent and Selective Allosteric PAK1 Inhibitor. ACS Med Chem Lett 2015; 6:776-81; PMID:26191365; https://doi.org/ 10.1021/acsmedchemlett.5b00102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Maioli E, Torricelli C, Valacchi G. Rottlerin and cancer: novel evidence and mechanisms. ScientificWorldJournal 2012; 2012:350826; PMID:22272173; https://doi.org/ 10.1100/2012/350826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Gschwendt M, Muller HJ, Kielbassa K, Zang R, Kittstein W, Rincke G, Marks F. Rottlerin, a novel protein kinase inhibitor. Biochem Biophys Res Commun 1994; 199:93-8; PMID:8123051; https://doi.org/ 10.1006/bbrc.1994.1199 [DOI] [PubMed] [Google Scholar]
- [21].Davies SP, Reddy H, Caivano M, Cohen P. Specificity and mechanism of action of some commonly used protein kinase inhibitors. Biochem J 2000; 351:95-105; PMID:10998351; https://doi.org/ 10.1042/bj3510095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Gschwendt M, Kittstein W, Marks F. Elongation factor-2 kinase: effective inhibition by the novel protein kinase inhibitor rottlerin and relative insensitivity towards staurosporine. FEBS Lett 1994; 338:85-8; PMID:8307162; https://doi.org/ 10.1016/0014-5793(94)80121-5 [DOI] [PubMed] [Google Scholar]
- [23].Cho SI, Koketsu M, Ishihara H, Matsushita M, Nairn AC, Fukazawa H, Uehara Y. Novel compounds, ‘1,3-selenazine derivatives’ as specific inhibitors of eukaryotic elongation factor-2 kinase. Biochim Biophys Acta 2000; 1475:207-15; PMID:10913818; https://doi.org/ 10.1016/S0304-4165(00)00061-1 [DOI] [PubMed] [Google Scholar]
- [24].Soltoff SP. Rottlerin is a mitochondrial uncoupler that decreases cellular ATP levels and indirectly blocks protein kinase Cdelta tyrosine phosphorylation. J Biol Chem 2001; 276:37986-92; PMID:11498535 [DOI] [PubMed] [Google Scholar]
- [25].Jane EP, Premkumar DR, Pollack IF. Coadministration of sorafenib with rottlerin potently inhibits cell proliferation and migration in human malignant glioma cells. J Pharmacol Exp Ther 2006; 319:1070-80; PMID:16959960; https://doi.org/ 10.1124/jpet.106.108621 [DOI] [PubMed] [Google Scholar]
- [26].Pause A, Belsham GJ, Gingras AC, Donze O, Lin TA, Lawrence JC Jr., Sonenberg N. Insulin-dependent stimulation of protein synthesis by phosphorylation of a regulator of 5′-cap function. Nature 1994; 371:762-7; PMID:7935836; https://doi.org/ 10.1038/371762a0 [DOI] [PubMed] [Google Scholar]
- [27].Arias-Romero LE, Villamar-Cruz O, Huang M, Hoeflich KP, Chernoff J. Pak1 kinase links ErbB2 to beta-catenin in transformation of breast epithelial cells. Cancer Res 2013; 73:3671-82; PMID:23576562; https://doi.org/ 10.1158/0008-5472.CAN-12-4453 [DOI] [PMC free article] [PubMed] [Google Scholar]


