Abstract
The aim of this study was to evaluate the effects of probiotic VSL#3 on glomerular filtration rate (GFR) in dogs affected by chronic kidney disease (CKD). The treatment group (n = 30) received prescription renal diet and probiotic VSL#3 (112 to 225 × 109 lyophilized bacteria per 10 kg body weight, PO, q24h for 2 months); the control group (n = 30) received prescription renal diet and standard therapy. All dogs underwent GFR measurement at the beginning of the study (T0) and were re-evaluated by GFR measurement after 2 months (T1). The GFR was significantly higher (P = 0.0001) in the treatment group compared to the control group at T1. In the treatment group, the GFR was significantly higher (P = 0.0008) at T1 compared to T0. In the control group, the GFR was significantly lower (P = 0.001) at T1 compared to T0. VSL#3 supplementation seemed to be efficient in reducing deterioration of GFR over time in dogs affected by CKD.
Résumé
Effets du probiotique VSL no 3 sur le taux de filtration glomérulaire chez les chiens affectés par la maladie rénale chronique : étude pilote. Le but de la présente étude consistait à évaluer les effets du probiotique VSL no 3 sur le taux de filtration glomérulaire (TFG) chez des chiens affectés de maladie rénale chronique (MRC). Le groupe de traitement (n = 30) a reçu une diète de prescription rénale et le probiotique VSL no 3 (112 à 225 × 109 de bactéries lyophilisées par 10 kg de poids corporel), PO, q24h pendant 2 mois; le groupe témoin (n = 30) a reçu une diète de prescription rénale et une thérapie standard. Tous les chiens ont subi une mesure du TFG au début de l’étude (T0) et ont été réévalués par la mesure du TFG après 2 mois (T1). Le TFG était significativement supérieur (P = 0,0001) dans le groupe de traitement comparativement au groupe témoin à T1. Dans le groupe de traitement, le TFG était significativement supérieur (P = 0,0008) à T1 comparativement à T0. Dans le groupe témoin, le TFG était significativement inférieur (P = 0,001) à T1 comparativement à T0. La supplémentation au VSL no 3 semblait être efficace pour la réduction de la détérioration du TFG au fil du temps chez les chiens atteints de MRC.
(Traduit par Isabelle Vallières)
Introduction
Uremic retention solutes are generated along the gastrointestinal tract and mostly cleared by the kidneys. Their accumulation in serum is negatively correlated with the level of renal function and glomerular sclerosis (1,2). According to recent studies in human medicine (3), the gastrointestinal tract seems to be involved in the pathophysiology of uremic syndrome and contributes to its clinical signs. The ability of probiotics to modulate the intestinal microbiota and to reduce the progression of chronic kidney disease (CKD) has been investigated in in vitro and in vivo studies in animals and humans (4).
VSL#3 is a high-dose, multi-strain probiotic product containing viable lyophilized bacteria consisting of 4 strains of Lactobacillus (L. casei, L. plantarum, L. acidophilus, and L. delbrueckii subsp. bulgaricus), 3 strains of Bifidobacterium (B. longum, B. breve, and B. infantis), and 1 strain of Streptococcus salivarius subsp. thermophilus. The VSL#3 strains have shown efficacy in humans for the prevention, treatment, and maintenance of remission of pouchitis and ulcerative colitis (5); it also seems to accelerate healing of gastric ulcers (6) and reduce portal pressure in patients with cirrhosis (7). Recently, VSL#3 has also been used in dogs with idiopathic inflammatory bowel disease (IBD) with promising results (5).
The aim of this study was to investigate the effects of the administration of VSL#3 on GFR in dogs affected by spontaneous CKD.
Materials and methods
Sixty client-owned dogs affected by CKD were recruited for this study. Sample size was calculated based on a power analysis with an alpha of 0.05 and power of 0.80. There were no restrictions on breed or gender of the dogs. Dogs in International Renal Interest Society (IRIS) stages 2 and 3 were persistently azotemic, had ultrasound findings consistent with CKD (decreased cortico-medullary distinction) and glomerular filtration rate (GFR) < 60 mL/min/m2; dogs in IRIS stage 1 had ultrasound findings consistent with CKD (decreased cortico-medullary distinction) and GFR < 60 mL/min/m2 (8). All patients were classified according to the plasma concentration of creatinine based on IRIS guidelines. Stage 1 (IRIS) included non-azotemic dogs (creatinine < 123.8 μmol/L), with ultrasound findings consistent with CKD, inadequate urinary concentrating ability (USG < 1.030), and GFR < 60 mL/min/m2. Patients were considered proteinuric if they were found repeatedly with a urine protein: creatinine (UPC) ratio ≥ 0.5 in 3 or more specimens, obtained ≥ 2 wk apart. IRIS 1 dogs, which met the inclusion criteria for CKD IRIS stage 1, but with a USG > 1.030, were considered eligible for the study if they had protein-losing nephropathy. For ethical reasons, animals were excluded from the study if they were in IRIS stage 4 (creatinine > 442 μmol/L). Dogs with evidence of acute kidney injury (AKI) or other significant systemic or organ-related disease, such as, neoplastic, cardiovascular, liver, or gastrointestinal disease, assessed by clinical and ultrasound evaluation and serum biochemistry were not included in the study. Dogs with CKD with evidence of positive urine culture were excluded from the study. After the full workup 24 dogs in IRIS stage 1, 16 dogs in IRIS stage 2, and 20 dogs in IRIS stage 3 were considered eligible for the study.
Dogs with persistent proteinuria (n = 32) were treated with benazepril (Fortekor; Novartis Animal Health, Varese, Italy), 0.25 to 0.5 mg/kg body weight (BW), PO, once to twice daily. Dogs with vomiting and/or poor appetite (n = 9) were treated with maropitant (Cerenia; Pfizer Italia, Latina, Italy), 1 mg/kg BW, PO, once daily and ranitidine (Zantadine; CEVA Salute Animale, Agrate Brianza, Italy), 2 mg/kg BW, PO, twice daily. Dogs showing hypo-proliferative anemia (n = 4) with hematocrit (HCT) < 20% were treated with darbopoetin-alpha (Aranesp; AMGEN, Milano, Italy), 0.5 to 1 μg/kg BW, SC, once weekly. Dogs (n = 20) with a history of hypertension [blood pressure (BP) > 160 mmHg] were maintained on a combination of benazepril and amlodipine (Amodip; CEVA Salute Animale), 0.25 to 0.5 mg/kg BW, PO, once daily. Dogs with serum phosphate > 1.6 mmol/L (n = 10) were treated with aluminium hydroxide, 50 to 100 mg/kg BW, PO, daily. Dogs in IRIS stages 2 and 3 with clinical signs (vomiting, poor appetite) and/or proteinuria, anemia, hypertension, and hyperphosphatemia were started on appropriate treatment weeks to months prior to T0. These drugs were continued during the study period.
On the day of enrolment (T0) 12 of the 24 dogs with IRIS stage 1, 8 of the 16 dogs with IRIS stage 2, and 10 of 20 the dogs with IRIS stage 3 were randomized into 2 groups (control group and treatment group) using a computer-generated randomization list. The control group (CG) consisted of 30 dogs (IRIS stage 1, n = 12; IRIS stage 2, n = 8; IRIS stage 3, n = 10). The treatment group (TG) consisted of 30 dogs (IRIS stage 1, n = 12; IRIS stage 2, n = 8; IRIS stage 3, n = 10). Dogs in the TG received VSL#3 at the dose of 112 to 225 × 109 lyophilized bacteria per 10 kg BW, PO, q24h for 60 d (5), in addition to the ongoing therapy. After randomization, patients of both groups were submitted to GFR evaluation through the plasma clearance of iohexol (8), evaluation of serum creatinine, urea, phosphate, complete urinalysis and UPC, urine culture, and blood pressure monitoring (PetMAP-Ramsey Medical, Tampa, Florida, USA). For blood pressure a mean of 5 consecutive measurements was considered. Hydration status of patients was assessed before sampling of blood and evaluation of GFR, in order to be sure they were not dehydrated. None of the dogs was dehydrated at the time sampling of blood and determination of GFR. Data were recorded as T0. For both groups, GFR, serum creatinine, urea, phosphate, complete urinalysis and UPC, blood pressure, and urine culture were reassessed at T1. This study was conducted in a single-blinded manner. To keep the investigator blinded to the study, a dispenser was used to supply VSL#3, according to a predetermined randomization code. Each owner was instructed not to mention VSL#3 at the time of the recheck. The study was approved by the University of Pisa animal care committee.
Statistical analysis was conducted with commercial software (GraphPad Prism-Software; La Jolla, California, USA). Data were tested for normality with D’Agostino and Pearson test. Data were non-normally distributed and were presented as median (range). Differences among groups were assessed using a Wilcoxon signed-rank test. A level of P ≤ 0.05 was considered significant for all tests.
Results
At baseline (T0) among the 30 dogs in the CG, 14 were proteinuric (UPC > 0.5) and 4 were borderline proteinuric (UPC > 0.2 and < 0.5); 1 dog was severely hypertensive (BP > 180 mmHg) and 3 dogs were moderately hypertensive (BP > 160 mmHg and < 180 mmHg). Among the 30 dogs in the TG, 18 were proteinuric (UPC > 0.5) and 3 were borderline proteinuric (UPC > 0.2 and < 0.5); 4 dogs were moderately hypertensive (BP > 160 mmHg and < 180 mmHg). At T0 there were no significant differences in age, weight, GFR, serum creatinine, urea, phosphate, blood pressure, UPC, and USG between dogs of the CG and TG (Table 1). A combination of benazepril and amlodipine was used to control blood pressure in 11/30 dogs in the CG and in 9/30 dogs in the TG. In dogs of the CG, GFR was lower (P = 0.0002), and creatinine and USG were higher (P = 0.001 and P = 0.04 respectively) at T1 compared with T0. There was no significant difference in urea, phosphate, blood pressure, and UPC between T0 and T1. At T1, 24/30 dogs of the CG were proteinuric and 2/30 were borderline proteinuric; while 15/30 dogs of the TG were proteinuric and 3/30 were borderline proteinuric. There was no significant difference in the number of proteinuric and non-proteinuric dogs between the CG and the TG (P = 0.18). When only proteinuric dogs (n = 14) of the CG were considered, no significant difference in UPC was found between T0 and T1. In dogs of the TG, GFR, and USG were higher (P = 0.001 and P = 0.0001, respectively), and UPC was lower (P = 0.006) at T1 compared with T0. When only proteinuric dogs (n = 18) of the TG were considered, a significant reduction (P = 0.006) in UPC was found at T1. At T1, 4/30 dogs of the CG were moderately hypertensive; while 2/30 dogs of the TG were moderately hypertensive and 1 dog was severely hypertensive. No significant difference was observed in creatinine, urea, phosphate, and blood pressure between T0 and T1. Values of GFR, creatinine, urea, phosphate, blood pressure, UPC, and USG for the CG and the TG at T0 and T1 are reported in Table 2.
Table 1.
Signalment and baseline values (T0) of GFR, serum creatinine, urea, phosphate, blood pressure, UPC, and USG of dogs in the control group (CG) and the treatment group (TG).
| CG (n = 30) | TG (n = 30) | P-value | |
|---|---|---|---|
| Age (years) | 5.2 (1 to 12) | 6.8 (1 to 13) | 0.32 |
| Body weight (kg) | 28.5 (7.4 to 72) | 29.3 (8 to 72) | 0.51 |
| GFR (mL/min/m2) | 37 (12 to 59) | 40 (15 to 56) | 0.23 |
| Creatinine (μmol/L) | 159.1 (79.5 to 212.1) | 123.7 (79.5 to 265.2) | 0.63 |
| Urea (mmol/L) | 22.8 (9.6 to 56) | 20.7 (6.4 to 39.9) | 0.47 |
| Phosphate (mmol/L) | 1.3 (0.9 to 2.1) | 1.3 (0.9 to 3.1) | 0.78 |
| BP (beats/min) | 128 (115 to 189) | 130 (115 to 176) | 0.98 |
| UPC | 0.9 (0.08 to 7.5) | 1.29 (0.13 to 4.94) | 0.89 |
| USG | 1.016 (1.005 to 1.046) | 1.015 (1.002 to 1.038) | 0.31 |
Data were non-normally distributed and presented as median (range). P ≤ 0.05 was considered significant. BP — blood pressure; UPC — urine protein to creatinine ratio; USG — urine specific gravity.
Table 2.
Values of GFR, serum creatinine, urea, phosphate, blood pressure, UPC, and USG of dogs in the control group (CG) and treatment group (TG) at T0 and T1.
| CG (n = 30) | TG (n = 30) | |||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| T0 | T1 | P-value | T0 | T1 | P-value | |
| GFR (mL/min/m2) | 37 (12 to 59) | 30 (10 to 47) | 0.0002 | 40 (15 to 56) | 48 (11 to 75) | 0.001 |
| Creatinine (μmol/L) | 150.2 (79.5 to 521.5) | 159.1 (79.5 to 212.1) | 0.001 | 123.7 (79.5 to 265.2) | 123.7 (79.5 to 415.4) | 0.87 |
| Urea (mmol/L) | 22.8 (9.6 to 56) | 22.1 (8.2 to 89.2) | 0.28 | 20.7 (6.4 to 39.9) | 18.2 (6.4 to 54.6) | 0.10 |
| Phosphate (mmol/L) | 1.3 (0.9 to 2.1) | 1.7 (0.8 to 4.7) | 0.27 | 1.3 (0.9 to 3.1) | 1.2 (1 to 3.6) | 0.85 |
| BP (beats/min) | 128 (115 to 189) | 127 (115 to 170) | 0.58 | 130 (115 to 176) | 130 (115 to 185) | 0.68 |
| UPC | 0.9 (0.08 to 7.5) | 1.2 (0.1 to 9.3) | 0.04 | 1.29 (0.13 to 4.94) | 0.77 (0.09 to 3.76) | 0.006 |
| USG | 1.016 (1.005 to 1.046) | 1.012 (1.005 to 1.048) | 0.04 | 1.015 (1.002 to 1038) | 1.018 (1.010 to 1.047) | 0.0001 |
Data were non-normally distributed and presented as median (range). P ≤ 0.05 was considered significant. BP — blood pressure; GFR — glomerular filtration rate; UPC — urine protein to creatinine ratio; USG — urine specific gravity.
Discussion
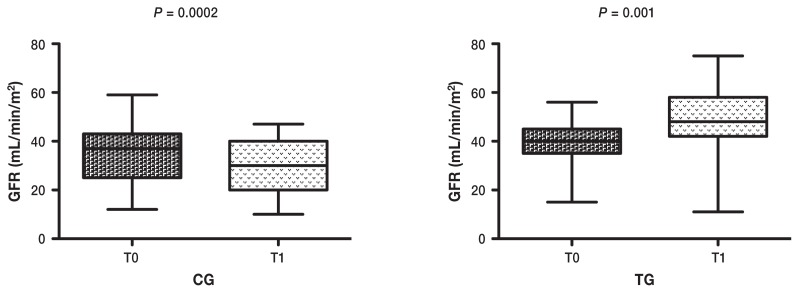
In this study we determined that GFR, measured through the plasma clearance of iohexol, is increased in dogs treated with VSL#3, compared with dogs treated with standard therapy (Figure 1). Our findings are in agreement with previous results (9), in which the group of patients on probiotic and prebiotic supplementation showed a significantly reduced decline of GFR over time, compared with the group of patients on a protein-restricted diet only (9). In our study, patients on prescription renal diet only showed a significant reduction in GFR over time. This finding may be due to an incomplete ability of prescription renal diet to block the production of uremic retention solutes. Koppe et al (4) postulated that the production of uremic retention solutes, mainly generated by protein degradation, cannot be completely blocked by a low-protein diet, and modelling intestinal microbiota can be considered as an additional beneficial intervention (4). The reason for using probiotics during CKD is to enhance the intestinal removal of uremic retention solutes. A food-grade, Gram-positive bacterium, in a probiotic formulation, was previously found to be beneficial to rodents (10), miniature pigs (11), and cats (12) with renal failure. Ranganathan et al (13) reported that probiotic dietary supplements facilitated the reduction of blood concentrations of uremic toxins, reduced the progression of renal impairment, and prolonged survival in rats with CKD. In 1 report (14) the use of probiotics (in particular Kibow biotics) in 2 uremic dogs showed favorable and encouraging results, while Polzin (15) did not find any significant difference between 32 CKD dogs treated with Azodyl (Vétoquinol, Paris, France) versus placebo. In human medicine, CKD has been associated with alterations of the gastrointestinal mucosa and disequilibrium in the intestinal flora. This condition is responsible for an increased transformation of amino acids into uremic retention solutes (16). Elevated serum concentrations of indoxyl-sulfate, p-cresyl sulfate, and trimethylamine n-oxide were negatively correlated with the level of kidney function and were predictors of CKD progression (1). These uremic toxins would be responsible for a worsening of renal function by different mechanisms. One study in experimental rats suggested that elevated serum concentration of uremic toxins may accelerate the onset of kidney tubular damage. In nephrectomized rats, GFR was significantly lower in rats treated with uremic toxins compared with controls. The reduction in GFR correlated with a higher glomerular sclerosis, which was promoted by the elevated levels of uremic toxins (17). In a previous study by Miyazaki et al (2), the administration of indoxyl-sulfate to uremic rats mediated the kidney expression of genes related to tubule-interstitial fibrosis and was associated with significant decline in renal function and worsening of glomerular function (2). Elevated levels of indoxyl sulfate were also associated with vascular stiffness, aortic calcifications and high cardio-vascular mortality in humans affected by CKD (18).
Figure 1.
Wilcoxon rank-sum test (P < 0.05) between GFR values at T0 (baseline) and T1 (after 2 months) in dogs of control group (n = 30) and in dogs of treatment group (n = 30). In dogs of control group GFR was lower (P = 0.0002) at T1 compared with T0. In dogs of treatment group GFR was higher at T1 (P = 0.001) compared with T0.
If we compare the serum values of creatinine and urea in the 2 groups of patients, we notice that, for the treatment group, there was no significant difference between T0 and T1, while for the control group there was a significant increase in serum creatinine at T1. The increase in serum creatinine in CG seems to reflect a worsening of renal function; in TG both creatinine and urea showed only a non-significant trend to reduction at T1, compared with T0, despite a significant improvement in GFR. This finding was not unexpected. Serum creatinine and urea are generally used as indirect markers of renal function, but they may be affected by extra-renal factors. We opted to measure GFR, as it is universally considered the gold standard test to assess overall renal function (19). It is also possible that the trend to reduction in creatinine and urea in the TG may be due to a direct degradation by VSL#3. VSL#3 contains, among others, Lactobacillus delbrueckii, which has been reported to hydrolyze urea in vitro (13). Therefore, reduction in serum levels of urea and creatinine in patients treated with probiotics should be evaluated carefully, as it may not reflect an actual improvement of kidney function (4).
The improvement of GFR in the TG was also accompanied by a significant increase in USG and reduction of UPC at T1. In dogs in the CG, USG was significantly reduced at T1, compared with T0, while UPC showed only a non-significant trend towards reduction. These findings may reflect an overall improvement of kidney function in patients treated with VSL#3. A recent study (20) reported that supplementation with Lactobacillus species in rats with CKD reduced systemic inflammation and proteinuria, playing a protective role in reducing the progression of CKD (20).
The present study has a few limitations. As we had no clear evidence of potential benefits of VSL#3 in controlling clinical signs of CKD and reducing the progression of the disease, we did not consider it ethical to enrol dogs with IRIS stage 4 and/or end-stage renal disease. As a consequence, we have no data regarding the effects of VSL#3 on GFR in these 2 populations. Because of the relatively low number of patients enrolled in the study, we opted to consider all CKD patients together. A larger study is recommended to compare the effects of VSL#3 on GFR in dogs at different stages of CKD, in order to determine whether the severity of CKD may or may not affect the efficacy of VSL#3. Another limitation of the present study is that during the study period 20/60 dogs were on a combination of benazepril and amlodipine to control blood pressure. Although no randomization for blood pressure was done prior to T0, the number of dogs on benazepril and amlodipine was almost equal in both CG (n = 11) and TG (n = 9). However, the authors cannot exclude that the concomitant use of benazepril and amlodipine in association with VSL#3 might contribute to improve UPC at T1 in this group of patients. It should also be noted that the slightly higher number of hypertensive dogs in CG might affect the progression of CKD and contribute to the worsening of GFR and UPC at T1.
In conclusion, the administration of VSL#3 at the dose of 112 to 225 × 109 lyophilized bacteria per 10 kg BW, PO, q24h for 60 d seemed to affect significantly GFR, USG, and UPC in dogs with CKD. After 2 months of VSL#3 supplementation, treated dogs showed a significant improvement in GFR and USG and a significant reduction of UPC compared to control dogs. Our findings seem to support a potential role of VSL#3 in reducing the progression of CKD in dogs. Results from this pilot study should encourage a larger study. CVJ
Footnotes
Use of this article is limited to a single copy for personal study. Anyone interested in obtaining reprints should contact the CVMA office (hbroughton@cvma-acmv.org) for additional copies or permission to use this material elsewhere.
References
- 1.Ramezani A, Raj DS. The gut microbioma, kidney disease, and targeted interventions. J Am Soc Nephrol. 2014;25:657–670. doi: 10.1681/ASN.2013080905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Miyazaki T, Ise M, Seo H, Niwa T. Indoxyl sulphate increases the gene expressions of TGF-beta 1, TIMP-1 and pro-alpha 1(I) collagen in uremic rat kidneys. Kidney Int Suppl. 1997;62:S15–S22. [PubMed] [Google Scholar]
- 3.Vitetta L, Gobe G. Uremia and chronic kidney disease: The role of the gut microflora and therapies with pro- and prebiotics. Mol Nutr Food Res. 2013;57:824–832. doi: 10.1002/mnfr.201200714. [DOI] [PubMed] [Google Scholar]
- 4.Koppe L, Mafra D, Fouque D. Probiotics and chronic kidney disease. Kidney Int. 2015;88:958–966. doi: 10.1038/ki.2015.255. [DOI] [PubMed] [Google Scholar]
- 5.Rossi G, Pengo G, Caldin M, et al. Comparison of microbiological, histological, and immunomodulatory parameters in response to treatment with either combination therapy with prednisone and metronidazole or probiotic VSL#3 strains in dogs with idiopathic inflammatory bowel disease. PLoS ONE. 2014;9:e94699. doi: 10.1371/journal.pone.0094699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dharmani P, De Simone C, Chadee K. The probiotic mixture VSL#3 accelerates gastric ulcer healing by stimulating vascular endothelial growth factor. PLoS ONE. 2013;8:e58671. doi: 10.1371/journal.pone.0058671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tandon P, Moncrief K, Madsen K, et al. Effects of probiotic therapy on portal pressure in patients with cirrhosis: A pilot study. Liver Int. 2009;29:1110–1115. doi: 10.1111/j.1478-3231.2009.02020.x. [DOI] [PubMed] [Google Scholar]
- 8.Lippi I, Meucci V, Guidi G, Soldani G. Glomerular filtration rate evaluation in the dog throughout the plasmatic clearance of iohexol: Simplified methods. Veterinaria. 2008;22:53–60. [Google Scholar]
- 9.Pavan M. Influence of prebiotic and probiotic supplementation on the progression of chronic kidney disease. Minerva Urol Nefrol. 2016;68:222–226. [PubMed] [Google Scholar]
- 10.Ranganathan N, Patel B, Ranganathan P, et al. Probiotic amelioration of azotemia in 5/6th nephrectomized Sprague-Dawley rats. Scientific World Journal. 2005;5:652–660. doi: 10.1100/tsw.2005.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Patel B, Zelenaia O, Dheer R, et al. Gut-based uremia therapy: Oral bacteriotherapy effectively reduces severity of azotemia in 5/6th nephrectomized mini pigs. Proc of the International Society of Nephrology Conference on Prevention of Progression of Renal Disease; International Society of Nephrology, Hong Kong, China; June 2004. [Google Scholar]
- 12.Palmquist R. A preliminary clinical evaluation of Kibow Biotics, a probiotic agent, on feline azotemia. J Am Holistic Vet Med Assoc. 2006;24:23–27. [Google Scholar]
- 13.Ranganathan N, Patel G, Ranganathan P, Dheer R, Pamquist R, Van Engelenberg G. Effect of feeding Kibow Biotics® to cats and dogs in kidney failure. Proceedings of the 39th Annual Meeting of the American Society of Nephrology; American Society of Nephrology, San Diego, California. November 2006. [Google Scholar]
- 14.Ranganathan P, Marczely J, Dheer J, et al. Initial trial of probiotic bacteria as therapy for uremia in dogs. Proc of the American Society of Nephrology; American Society of Nephrology, St. Louis, Missouri. October 2004; p. 768A. [Google Scholar]
- 15.Polzin D. Probiotic therapy of chronic kidney disease. Proc of the Annual Meeting of the American College of Veterinary Internal Medicine; American College of Veterinary Internal Medicine, Denver, Colorado. June 2011. [Google Scholar]
- 16.Evenepoel P, Meijers BK, Bammens BR, Verbeke K. Uremic toxins originating from colonic microbial metabolism. Kidney Int Suppl. 2009;114:S12–S19. doi: 10.1038/ki.2009.402. [DOI] [PubMed] [Google Scholar]
- 17.Satoh M, Hayashi H, Watanabe M, et al. Uremic toxins overload accelerates renal damage in a rat model of chronic renal failure. Nephron Exp Nephrol. 2003;95:e111–118. doi: 10.1159/000074327. [DOI] [PubMed] [Google Scholar]
- 18.Barreto FC, Barreto DV, Liabeuf S, et al. Serum indoxyl sulphate is associated with vascular disease and mortality in chronic kidney disease patients. Clin J Am Soc Nephrol. 2009;4:1551–1558. doi: 10.2215/CJN.03980609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lefbvre HP. Renal function testing. In: Bartges J, Polzin DJ, editors. Nephrology and Urology of Small Animals. Chichester, UK: Wiley-Blackwell; 2011. pp. 91–96. [Google Scholar]
- 20.Yoshifuji A, Wakino S, Irie J, et al. Gut lactobacillus protects against the progression of renal damage by modulating the gut environment in rats. Nephrol Dial Transplant. 2016;31:401–412. doi: 10.1093/ndt/gfv353. [DOI] [PubMed] [Google Scholar]