Abstract
Background
Obesity is part of the established risk factors for breast cancer (BC) in postmenopausal females. Circulating leptin increases in parallel with the increase of body weight and fat reservoir.
Methods
This research investigated the link between leptin phenotype and the clinicopathological factors in BC. A large set of breast cancer cases (449), and 27 non-cancerous tissue samples of breast were employed for leptin expression recognition using immunohistochemistry staining.
Results
Cytoplasmic immunohistochemical staining of leptin was recognized in 376 (83.7%) and 25 (92.6%) of BC and control cases respectively. Leptin immunostaining were significantly associated with age, histotypes, grade, stage, lymph node involvement, tumor recurrence, hormone receptor phenotypes, ER and HER2 expressions, and p-values were (P = 0.0233), (P = 0.0001), (P = 0.050), (P = 0.0291), (P = 0.0300), (P = 0.0023), (P = 0.0021), (P = 0.0279) respectively. Reasonable proportion of cases with low staining score was more prevalent in all subgroups of clinicopathological parameters except ER- PR+ HER2- hormone receptor phenotype and mucinous carcinoma which showed high level of leptin immunoreactivity. Tumor recurrence is less prevailing in high score leptin immunostaining cases. Furthermore, Log Rank (Mantel-Cox) test findings revealed considerably different survival distributions were observed for the different categories of leptin immunostaining scores (P = 0.032). Negative leptin immunostaining is related to poor survival.
Conclusions
Our preliminary findings support leptin clinical value in confirming BC diagnosis as well as prognosis. These results suggest that leptin molecule is an important biomarker that could identify type, grade, stage, lymph node involvement, relapse and prognosis in breast cancer.
Keywords: Leptin, Breast cancer, Immunohistochemistry
Background
Breast cancer (BC) is a shattering tumor and an important cause of worldwide death [1]. Recently published data stated that breast neoplasms are the most frequent malignancy among females with approximately 1,700,000 new registered cases and around 580,000 demises of BC in the United States of America in 2015 according to the American Cancer Society [2]. In Saudi Arabia, BC has a comparable rank among cancers and neoplasms accounting for (25.8%) of all registered neoplasms in females in 2012 as stated by the Saudi Cancer Registry [3].
BC has been distinguished as a high complex heterogeneous tumor with distinctive cellular origin and various histotypes, progression and metastatic potential [4]. Irrespective of noteworthy improvements in the diagnosis and treatment of BC, the tumor is still considered a big challenge to clinicians due to bad prognosis and big recurrence proportion in some histotypes of BC particularly triple negative, for instance up to 40% of newly registered cases relapse in 5 years [5]. The management of BC is subject to the clinicopathological parameters of patients, such as grade and stage of cancer as measures of pleasant or bad prognosis. Nevertheless, these factors are not enough to guess the clinical consequences and worse yet, may produce variations in a cluster of neoplasms with the same grade or stage. This is essentially due to heterogeneity of BC cells [6]. Therefore, it is necessary to find novel diagnostic markers and medicinal modalities which help in the diagnosis and prognosis of BC, enhance the stratification of high risk patients and improve clinical outcomes [7].
Leptin is one hundred and sixty seven amino acid residues molecule that is encrypted by the Obese gene (Ob) [8]. It was expressed firstly in white adipose tissue; however, later it was found that other tissues express leptin such as the liver, ovaries, placenta, stomach, pituitary gland and skeletal muscles [9]. It is now established that leptin has several roles and counted a member of adipokines [10]. Many investigations have illustrated the function of leptin in tumor cells proliferation, movement, invasion and apoptosis inhibition [11–13]. Some other reports have examined leptin function in several tumor development risks, but the results are controversial [14, 15]. Unquestionably definite proof is needed to elucidate leptin’s exact function in the growth and progress of breast tumors, as perception of leptin correlation with breast cancer can improve our awareness of breast carcinogenesis and support improving management and preventive plans. Thus, the current study describes leptin immunoexpression in BC and evaluates the association between leptin phenotype and the clinical factors as well as follow-up data of breast cancer.
Methods
Four hundred fourty nine cases of BC and 27 control cases, which include fibroadenomas and normal breast tissue, were taken from the archive of pathological sciences department at King Abdulaziz University Hospital in Saudi Arabia. Sections from tumor paraffin blocks were hematoxylin and eosin stained and histologically evaluated. The unit of medical records provided us with patients’ clinicopathological data (age, size, type, grade and stage of tumors) (Table 1). WHO recommendation regarding grade and stage of BC was applied. All tumors and control cases blocks were utilized in the production of tissue microarray. This study has met all the instruction and requirement of the ethical committee approval.
Table 1.
Describe the distribution of various clinicopathological variables with leptin immunostaining in breast cancer
| Leptin immunostaining | ||||||||
|---|---|---|---|---|---|---|---|---|
| Negative | Low | High | ||||||
| Count | Row N % | Count | Row N % | Count | Row N % | P-Value | ||
| Type of tissue | Leptin in breast cancer | 73 | 16.3% | 274 | 61.0% | 102 | 22.7% | 0.0778 |
| Leptin in control group | 2 | 7.4% | 14 | 51.9% | 11 | 40.7% | ||
| Age in Years | <40 | 16 | 23.2% | 30 | 43.5% | 23 | 33.3% | 0.0233 |
| 40–49 | 16 | 14.0% | 67 | 58.8% | 31 | 27.2% | ||
| 50–59 | 24 | 17.9% | 89 | 66.4% | 21 | 15.7% | ||
| 60–69 | 11 | 16.2% | 46 | 67.6% | 11 | 16.2% | ||
| > = 70 | 6 | 11.8% | 31 | 60.8% | 14 | 27.5% | ||
| NA | 0 | 0.0% | 11 | 84.6% | 2 | 15.4% | ||
| Hormone receptor phenotype | ER- PR- HER2- | 16 | 23.2% | 36 | 52.2% | 17 | 24.6% | 0.0021 |
| ER- PR- HER2+ | 6 | 8.8% | 40 | 58.8% | 22 | 32.4% | ||
| ER- PR+ HER2- | 0 | 0.0% | 2 | 28.6% | 5 | 71.4% | ||
| ER- PR+ HER2+ | 0 | 0.0% | 7 | 77.8% | 2 | 22.2% | ||
| ER+ PR- HER2- | 7 | 14.0% | 33 | 66.0% | 10 | 20.0% | ||
| ER+ PR- HER2+ | 3 | 17.6% | 11 | 64.7% | 3 | 17.6% | ||
| ER+ PR+ HER2- | 34 | 23.6% | 81 | 56.3% | 29 | 20.1% | ||
| ER+ PR+ HER2+ | 7 | 8.2% | 64 | 75.3% | 14 | 16.5% | ||
| ER | ER- | 22 | 14.4% | 85 | 55.6% | 46 | 30.1% | 0.0279 |
| ER+ | 51 | 17.2% | 189 | 63.9% | 56 | 18.9% | ||
| PR | PR- | 32 | 15.7% | 120 | 58.8% | 52 | 25.5% | 0.4410 |
| PR+ | 41 | 16.7% | 154 | 62.9% | 50 | 20.4% | ||
| HER | HER2- | 57 | 21.1% | 152 | 56.3% | 61 | 22.6% | 0.0021 |
| HER2+ | 16 | 8.9% | 122 | 68.2% | 41 | 22.9% | ||
| Lymph node involvement | NEGATIVE | 33 | 17.8% | 100 | 54.1% | 52 | 28.1% | 0.0300 |
| POSITIVE | 40 | 15.2% | 174 | 65.9% | 50 | 18.9% | ||
| Size of tumor | <2 | 10 | 16.7% | 34 | 56.7% | 16 | 26.7% | 0.5784 |
| >5 | 13 | 12.0% | 68 | 63.0% | 27 | 25.0% | ||
| 2–5 | 50 | 17.8% | 172 | 61.2% | 59 | 21.0% | ||
| Grade | I | 13 | 17.8% | 42 | 57.5% | 18 | 24.7% | 0.0500 |
| II | 45 | 18.8% | 151 | 63.2% | 43 | 18.0% | ||
| III | 15 | 10.9% | 81 | 59.1% | 41 | 29.9% | ||
| Histotype | DCIS | 4 | 23.5% | 9 | 52.9% | 4 | 23.5% | 0.0001 |
| Ductal | 65 | 15.9% | 255 | 62.2% | 90 | 22.0% | ||
| Mucinous carcinoma | 0 | 0.0% | 1 | 11.1% | 8 | 88.9% | ||
| Lobular | 4 | 30.8% | 9 | 69.2% | 0 | 0.0% | ||
| Stage | I | 8 | 16.0% | 27 | 54.0% | 15 | 30.0% | 0.0291 |
| II(a) | 24 | 18.3% | 69 | 52.7% | 38 | 29.0% | ||
| II(b) | 22 | 15.8% | 92 | 66.2% | 25 | 18.0% | ||
| III | 4 | 6.1% | 49 | 74.2% | 13 | 19.7% | ||
| IV | 15 | 23.8% | 37 | 58.7% | 11 | 17.5% | ||
| Vascular Invasion | Negative | 55 | 17.8% | 184 | 59.5% | 70 | 22.7% | 0.4058 |
| Positive | 18 | 12.9% | 90 | 64.3% | 32 | 22.9% | ||
| Recurrence | No | 57 | 14.3% | 253 | 63.4% | 89 | 22.3% | 0.0023 |
| Yes | 16 | 32.0% | 21 | 42.0% | 13 | 26.0% | ||
Tissue microarray production (TMA)
Four hundred fourty nine cases of BC and 27 control cases were used to assemble tissue microarray [16]. TMA blocks have been cut and placed on coated slides, then they have been immunohistochemically stained.
Immunohistochemistry staining protocol
Multimer molecule based scientific knowledge were employed in the immunohistochemistry staining of BC sections to apply anti-leptin rabbit polyclonal antibody with dilution ratio of 1 to 100 [catalog code: sc-842, Santa Cruz Biotechnology, USA), and ULTRAVIEW TM DAB visualizing protocol. Immunohistochemistry autostainer (BenchMark ULTRA, Ventana, Arizona, USA) was used for immunohistochemistry staining. Every staining run contained a slide treated with tris buffer in place of the Ob antibody as a negative control. Slide section of placenta tissue was employed as positive control. Cases with brown granular cytoplasmic stain in more than 5% of tumor cells were counted positive.
Leptin immunoreactivity has been scored, by two pathologists, for staining intensity and positively stained cells percentage. The frequency of positive cells was evaluated applying semiquantitative method in 3 fields with lenses of 40 amplification power. Leptin staining intensity has been given scores 0, 1, 2, 3 and 4 representing negative, weak, moderate and strong staining respectively. Scores of staining intensity has been presented as negative staining (0), low level immunoreactivity (1) and high level (2 and 3). When a disparity between the two pathologists’ staining scores has happened, the lowest score value was reported.
Statistical analysis
All data were assessed statistically by IBM-SPSS software (version 21). All data values were presented as percentages and incidences. The association between clinicopathological factors of BC and leptin expression was explored statistically by chi-square test. Comparison of survival distributions for various leptin immunohistochemistry staining intensity levels was assessed applying Log Rank (Mantel-Cox) test in addition to Kaplan Meier survival curves. The level of significance was counted when P < 0.05.
Results
All BC cases were reviewed and their clinicopathological factors have been presented in Table 1. The histotypes of breast cancer cases of the current study, in descending order, were infiltrating ductal carcinoma, ductal carcinoma in situ, infiltrating lobular carcinoma and mucinous carcinoma which counted 91.3%, 3.8%, 2.9 and 2% respectively (Table 1). The mean age of patients was 50.7 years varying from 24 to 94 years.
Brown granular cytoplasmic leptin immunoexpression was detected in the transformed epithelium of 376 (83.7%) BC cases and 25 (92.6) cases of control group (Fig. 1).
Fig. 1.
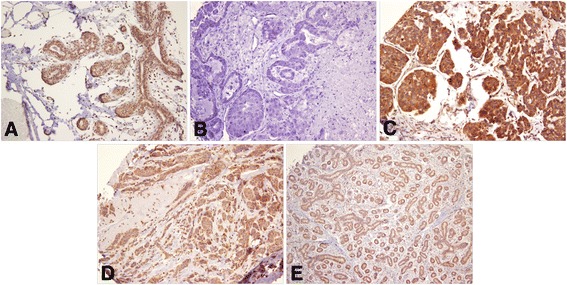
Granular cytoplasmic expression of leptin in breast cancer. a strong positive staining in normal breast tissue (20 X); b negative stained breast cancer (20 X); c strong positive staining in epithelial cells of breast cancer (20 X); d weak positive staining in epithelial cells of breast cancer (20 X); e weak positive staining in fibroadenoma (10 X)
Leptin expression did not show any statistical significant difference between BC and control cases. The distribution of leptin phenotypes which identified in BC transformed epithelial cells and its association with different clinicopathological variables were reported in Table 1. Percentage of positively stained cells ranged from 5% to 100% in breast tumors of the present study. About 40% of breast cancer cases showed leptin immunoreactivity in more than 50% of their transformed epithelial cells. Small fraction of cases (<10%) showed moderate to strong leptin immunoreactivity in stromal cells; however, these cases were of no statistical significance.
Leptin immunostaining is significantly related with age (P = 0.0233), reasonable proportion of low scores staining is observed in all age groups. Breast cancer histotypes showed significant association with leptin immunostaining (P = 0.0001). DCIS, invasive ductal carcinoma and invasive lobular carcinoma histotypes showed more frequently low scores of leptin immunostaining while the vast majority of mucinous carcinomas were of high immunostaining scores. Grade of breast tumors is marginally significant with leptin immunostaining (P = 0.050). Grade II is more frequent with low leptin immunoreactivity. Breast carcinoma stage was also significantly associated with leptin expression (P = 0.0291). A considerable fraction of stage II (b) and stage III were found to be common with low leptin immunostaining. Significantly, more cases with metastases in lymph nodes were observed in low score staining (P = 0.0300). Tumor recurrence was significantly associated with cases of low leptin immunostaining scores (P = 0.0023). Recurrence is less prevailing in cases with high score of leptin immunostaining. Furthermore, hormone receptor phenotypes were significantly associated with leptin expression (P = 0.0021). All hormone receptor phenotypes were significantly more prevalent in cases with low staining scores except “ER- PR+ HER2- “which was more common in cases with high leptin scores. Distributions of ER and HER2 expression were significantly different by leptin immunostaining (P = 0.0279 and P = 0.0021 respectively), while PR expression was not. Log Rank (Mantel-Cox) test outcomes revealed that significant different survival distributions were observed for different categories of leptin immunostaining scores (P = 0.032). Negative leptin immunostaining is related to poor survival significantly (Fig. 2). No significant associations of leptin immunostaining in transformed epithelium with tumor size, vascular invasion and type of tissue (malignant vs control) were observed.
Fig. 2.
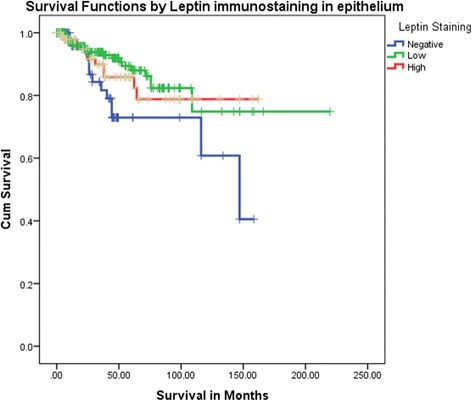
Kaplan Meier survival curves by pattern of leptin immunostaining shows significantly poor survival behavior associated with negative leptin immunostaining in breast cancer
Discussion
Several serological studies stated evidences that elevated leptin concentration in serum is correlated with breast cancer risk and counted it as an independent risk factor, in addition to its involvement in many malignancy stages including as cell growth, invasion, migration, metastases, recurrence and therapy response in some organs such as liver [17], lung [18], stomach [19], thyroid [20], uterus [12], colon [21]. A number of investigations were launched to identify the mechanisms which link leptin with tumor growth and progression of breast cancer [22, 23]. Some studies reported a direct role of leptin in BC development and aggression, and others showed that serum adipocytokines apply their biological roles on recipient tissues and cells not just by typical endocrinological mechanisms but additionally via autocrine or paracrine systems [11, 22–29]. However, leptin expression in mammary tumor tissue is not characteristic of blood leptin levels, but could be a result of the paracrine mechanism [22]. Furthermore, leptin intervenes estrogen effects on malignant tissue via a paracrine pathway, as well as enhances other influences that participate in cell growth and angiogenesis during breast cancer development [23, 30]. Moreover, leptin autoregulation enhances its signal through motivating its expression and its receptor, thus supports an autocrine mechanism [29]. To the best of our knowledge, few studies evaluated leptin expression in breast cancer tissues (Table 2) [31–40] of which the outcomes failed to confirm the results of leptin serological studies and the correlation of leptin immunoexpression with clinicopathological findings of breast carcinoma patients.
Table 2.
Correlation between high level of leptin immunoreactivity and clinicopathological parameters in the current study compared to previous studies
| Previous studies | Leptin immunostaining prevalence in breast cancer cases | Leptin immunostaining prevalence in noncancerous breast tissue | Age | Size of tumor | Histotype | Grade | Stage | Recurrence | Lymph Node involvement | Hormone receptor phenotype (ER, PR, HER2) | ER expression | PR expression | HER2 expression | Vascular Invasion | Alive/Deceased status | Survival |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| The current study | 83.7% (61% weak & 22.7% strong) | 92.6% (51.9% weak & 40.7% strong) | P = 0.0233 | NS | P = 0.0001 | All grades low scores P = 0.0500 | P = 0.0291 | P = 0.0023 | P = 0.0300 | ER-, PR+, HER2-P = 0.0021 | ER+ P = 0.0279 | NS | P = 0.0021 | NS | NS | Absent or Weak staining -Poor survival |
| [1] | 100% (7.9% weak & 92.1% strong) | 100% (100% weak) | NS | NS | NS | NS | NS | NS | NS | Overexpression – poor survival | ||||||
| [2] | 60% | 0% | ||||||||||||||
| [3] | 86.4% (30.4% weak & 56% strong) | 43.3% (43.3% weak) | NS | NS | High grade high scores P = 0.031 | NS | NS | |||||||||
| [4] | NS | NS | NS | NS | NS | |||||||||||
| [5] | 79.6% (52.5% weak & 27.1& strong) | 77.5% (50% weak & 27.5% strong) | ||||||||||||||
| [6] | 79.6% | NS | NS | NS | NS | NS | NS | NS | NS | NS | ||||||
| [7] | 85% | 76.5% | NS | NS | NS | |||||||||||
| [8] | 39% | NS | NS | NS | ER-, PR-, HER2-P = 0.022 | NS | NS | NS | NS | |||||||
| [9] | 83% | NS | P < 0.001 | NS | NS | NS | NS | NS | NS | |||||||
| [10] | 61% | 40% | NS | NS | NS |
NS not significant
In our report, the incidence of leptin immunostaining (92.6%) in the 27 control cases, which was seen only in the cytoplasmic space of glandular epithelial cells, is almost similar to the results of Ishikawa, Kitayama and Nagawa [31] who described positive leptin immunohistochemistry staining in 100% of noncancerous breast tissue, and higher than those of Caldefie-Chezet and associates [32], Garofalo et al. [33], Jarde and coworkers [35], and Colbert and colleagues [40]. In respect of the percentage of positive breast carcinoma cases for leptin immunoexpression, our results are in line with those of Garofalo and associates [33], Fiorio and coworkers [36], Jarde and associates [37] and Jeong team [39] who detected leptin immunoexpression in 86.4%, 79.6%, 79.6% and 83% of breast carcinomas respectively, but with different immunoreactivity levels; and varied from those of Ishikawa, Kitayama and Nagawa [31], Caldefie-Chezet and associates [32], Kim [38] and Colbert and colleagues [40].
Our investigation is pioneer to report immunohistochemical staining of leptin is considerably correlated with patients’ clinicopathological findings such as age, histotype, grade, stage, recurrence, lymph node involvement, hormone receptor phenotype, ER expression, HER2 expression and survival of patients with breast carcinoma. Whereas, all the previous studies (Table 2) did not detect similar correlation except Ishikawa, Kitayama and Nagawa [31] who reported that strong leptin immunostaining is only associated with poor survival; Garofalo and associates [33] associated leptin immunostaining only with high grade tumors; Jeong and colleagues [39] linked leptin expression with histotype of breast cancer; and Colbert and coworkers [40] stated significant relationship with triple negative breast carcinoma.
Nevertheless, our results are in agreement with several other reports which have documented that immunoexpression of leptin is linked with one or more of the clinical factors such as tumor stage, infiltration, metastasis, relapse, therapy resistance and bad prognostic outcomes of several tumors including laryngeal cancer [41], esophageal cancer [42], stomach cancer [43], lung cancer [44], and thyroid cancer [45].
Main differences between our report and previous ones can be justified by techniques sensitivity, the diversity of populations, variations in sample size and the semi-quantitative reading of immunostaining. Still, studies with broader panel of cases are certainly of great value for assessing the value of leptin immunostaining in diagnoses and prognoses of breast malignancies.
Conclusions
Leptin immunostaining is a useful method in supporting the diagnoses and prognoses of breast carcinoma. Our findings proposes that leptin could be a helpful biomarker in identifying the histotype, stage, grade, relapse and prognosis in BC. The association of leptin immunostaining with many clinicopathological factors proposes a role of leptin in BC progression.
Acknowledgements
This project was funded by the National Plan for Science, Technology and Innovation (MAARIFAH) – King Abdulaziz City for Science and Technology - the Kingdom of Saudi Arabia – award number (10-BIO-1255-03). The authors also acknowledge with thanks, Science and Technology Unit, King Abdulaziz University for technical support.
Funding
This project was funded by the National Plan for Science, Technology and Innovation (MAARIFAH) – King Abdulaziz City for Science and Technology - the Kingdom of Saudi Arabia – award number (10-BIO-1255-03).
Availability of data and materials
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- BC
Breast cancer
- DCIS
Invasive ductal carcinoma
- ER
Estrogen receptor
- HER2
Human epidermal growth factor receptor 2
- Ob
Obese gene
- PR
Progesterone receptor
- TMA
Tissue microarray
- WHO
World Health Organization
Authors’ contributions
JAl-M, ME and AAl-Dconceived of the study and designed the experiments. LD, KAl-S, and BAl-M performed the technical conduction of the experiments. JAl-M, MNK, AA, NB, SA, PD, BB and MAl-Q analyzed and discussed the results, and drafted the manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to participate
This study was approved by the Unit of Biomedical Ethics, Research Committee. Document number: 582/34/D. The Unit of Biomedical Ethics did not request consent to participate because this study is a retrospective study which is using archival material.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Mohamad Nidal Khabaz, Email: mnkhabaz@kau.edu.sa.
Amer Abdelrahman, Email: aaaali@kau.edu.sa.
Nadeem Butt, Email: nshafique@kau.edu.sa.
Lila Damnhory, Email: lailahhd71@hotmail.com.
Mohamed Elshal, Email: melshal2002@yahoo.com.
Alia M. Aldahlawi, Email: aaldahlawi@kau.edu.sa, Email: alia008@hotmail.com
Swsan Ashoor, Email: sawsan_ashoor@hotmail.com.
Basim Al-Maghrabi, Email: Bjalmaghrabi@gmail.com.
Pauline Dobson, Email: pauladobro1911@gmail.com.
Barry Brown, Email: barleybro41@outlook.com.
Kaltoom Al-Sakkaf, Email: kalsakkaf@kau.edu.sa.
Mohmmad Al-Qahtani, Email: mhalqahtani@kau.edu.sa.
Jaudah Al-Maghrabi, Phone: +9662 6401000, Email: jalmaghrabi@hotmail.com, Email: jalmgrabi@kau.edu.sa.
References
- 1.Jemal A, Siegel R, Ward E, Hao Y, Xu J, Murray T, Thun MJ. Cancer statistics, 2008. CA Cancer J Clin. 2008;58(2):71–96. doi: 10.3322/CA.2007.0010. [DOI] [PubMed] [Google Scholar]
- 2.American Cancer Society. Cancer Facts & Figures 2015. Atlanta: American Cancer Society; 2015. https://www.cancer.org/content/dam/cancer-org/research/cancer-facts-andstatistics/annual-cancer-facts-and-figures/2015/cancer-facts-and-figures-2015.pdf.
- 3.Saudi Cancer Registry. Cancer Registry report 2013. http://www.chs.gov.sa/Ar/HealthCenters/NCC/CancerRegistry/CancerRegistryReports/2013.pdf.
- 4.Di Cosimo S, Baselga J. Management of breast cancer with targeted agents: importance of heterogeneity. [corrected] Nat Rev Clin Oncol. 2010;7(3):139–147. doi: 10.1038/nrclinonc.2009.234. [DOI] [PubMed] [Google Scholar]
- 5.Dent R, Trudeau M, Pritchard KI, Hanna WM, Kahn HK, Sawka CA, Lickley LA, Rawlinson E, Sun P, Narod SA. Triple-negative breast cancer: clinical features and patterns of recurrence. Clin Cancer Res. 2007;13(15 Pt 1):4429–4434. doi: 10.1158/1078-0432.CCR-06-3045. [DOI] [PubMed] [Google Scholar]
- 6.Brooks MD, Burness ML, Wicha MS. Therapeutic implications of cellular heterogeneity and plasticity in breast cancer. Cell Stem Cell. 2015;17(3):260–271. doi: 10.1016/j.stem.2015.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Inoue K, Fry EA. Novel molecular markers for breast cancer. Biomark Cancer. 2016;8:25–42. doi: 10.4137/BIC.S38394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang Y, Proenca R, Maffei M, Barone M, Leopold L, Friedman JM. Positional cloning of the mouse obese gene and its human homologue. Nature. 1994;372(6505):425–432. doi: 10.1038/372425a0. [DOI] [PubMed] [Google Scholar]
- 9.Baratta M. Leptin--from a signal of adiposity to a hormonal mediator in peripheral tissues. Med Sci Monit. 2002;8(12):RA282–RA292. [PubMed] [Google Scholar]
- 10.Allison MB, Myers MG., Jr 20 years of leptin: connecting leptin signaling to biological function. J Endocrinol. 2014;223(1):T25–T35. doi: 10.1530/JOE-14-0404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dubois V, Jarde T, Delort L, Billard H, Bernard-Gallon D, Berger E, Geloen A, Vasson MP, Caldefie-Chezet F. Leptin induces a proliferative response in breast cancer cells but not in normal breast cells. Nutr Cancer. 2014;66(4):645–655. doi: 10.1080/01635581.2014.894104. [DOI] [PubMed] [Google Scholar]
- 12.Zhou X, Li H, Chai Y, Liu Z. Leptin inhibits the apoptosis of endometrial carcinoma cells through activation of the nuclear factor kappaB-inducing Kinase/IkappaB Kinase pathway. Int J Gynecol Cancer. 2015;25(5):770–778. doi: 10.1097/IGC.0000000000000440. [DOI] [PubMed] [Google Scholar]
- 13.Lipsey CC, Harbuzariu A, Daley-Brown D, Gonzalez-Perez RR. Oncogenic role of leptin and notch interleukin-1 leptin crosstalk outcome in cancer. World J Methodol. 2016;6(1):43–55. doi: 10.5662/wjm.v6.i1.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mantzoros CS, Magkos F, Brinkoetter M, Sienkiewicz E, Dardeno TA, Kim SY, Hamnvik OP, Koniaris A. Leptin in human physiology and pathophysiology. Am J Physiol Endocrinol Metab. 2011;301(4):E567–E584. doi: 10.1152/ajpendo.00315.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Luhn P, Dallal CM, Weiss JM, Black A, Huang WY, Lacey JV, Jr, Hayes RB, Stanczyk FZ, Wentzensen N, Brinton LA. Circulating adipokine levels and endometrial cancer risk in the prostate, lung, colorectal, and ovarian cancer screening trial. Cancer Epidemiol Biomark Prev. 2013;22(7):1304–1312. doi: 10.1158/1055-9965.EPI-13-0258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Al-Maghrabi J, Emam E, Gomaa W, Saggaf M, Buhmeida A, Al-Qahtani M, Al-Ahwal M: C-MET immunostaining in colorectal carcinoma is associated with local disease recurrence. BMC Cancer 2015, 15:676. [DOI] [PMC free article] [PubMed]
- 17.Saxena NK, Sharma D, Ding X, Lin S, Marra F, Merlin D, Anania FA. Concomitant activation of the JAK/STAT, PI3K/AKT, and ERK signaling is involved in leptin-mediated promotion of invasion and migration of hepatocellular carcinoma cells. Cancer Res. 2007;67(6):2497–2507. doi: 10.1158/0008-5472.CAN-06-3075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zheng XJ, Yang ZX, Dong YJ, Zhang GY, Sun MF, An XK, Pan LH, Zhang SL. Downregulation of leptin inhibits growth and induces apoptosis of lung cancer cells via the notch and JAK/STAT3 signaling pathways. Biol Open. 2016;5(6):794–800. doi: 10.1242/bio.017798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee KN, Choi HS, Yang SY, Park HK, Lee YY, Lee OY, Yoon BC, Hahm JS, Paik SS. The role of leptin in gastric cancer: clinicopathologic features and molecular mechanisms. Biochem Biophys Res Commun. 2014;446(4):822–829. doi: 10.1016/j.bbrc.2014.02.072. [DOI] [PubMed] [Google Scholar]
- 20.Yang YC, Chin YT, Hsieh MT, Lai HY, Ke CC, Crawford DR, Lee OK, Fu E, Mousa SA, Grasso P, et al. Novel leptin OB3 peptide-induced signaling and progression in thyroid cancers: comparison with leptin. Oncotarget. 2016;7(19):27641–27654. doi: 10.18632/oncotarget.8505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ogunwobi OO, Beales IL. Cyclo-oxygenase-independent inhibition of apoptosis and stimulation of proliferation by leptin in human colon cancer cells. Dig Dis Sci. 2007;52(8):1934–1945. doi: 10.1007/s10620-007-9784-6. [DOI] [PubMed] [Google Scholar]
- 22.Vona-Davis L, Rose DP. Adipokines as endocrine, paracrine, and autocrine factors in breast cancer risk and progression. Endocr Relat Cancer. 2007;14(2):189–206. doi: 10.1677/ERC-06-0068. [DOI] [PubMed] [Google Scholar]
- 23.Gonzalez RR, Cherfils S, Escobar M, Yoo JH, Carino C, Styer AK, Sullivan BT, Sakamoto H, Olawaiye A, Serikawa T, et al. Leptin signaling promotes the growth of mammary tumors and increases the expression of vascular endothelial growth factor (VEGF) and its receptor type two (VEGF-R2) J Biol Chem. 2006;281(36):26320–26328. doi: 10.1074/jbc.M601991200. [DOI] [PubMed] [Google Scholar]
- 24.Li K, Wei L, Huang Y, Wu Y, Su M, Pang X, Wang N, Ji F, Zhong C, Chen T. Leptin promotes breast cancer cell migration and invasion via IL-18 expression and secretion. Int J Oncol. 2016;48(6):2479–2487. doi: 10.3892/ijo.2016.3483. [DOI] [PubMed] [Google Scholar]
- 25.Assiri AM, Kamel HF. Evaluation of diagnostic and predictive value of serum adipokines: Leptin, resistin and visfatin in postmenopausal breast cancer. Obes Res Clin Pract. 2016;10(4):442–453. doi: 10.1016/j.orcp.2015.08.017. [DOI] [PubMed] [Google Scholar]
- 26.Strong AL, Ohlstein JF, Biagas BA, Rhodes LV, Pei DT, Tucker HA, Llamas C, Bowles AC, Dutreil MF, Zhang S, et al. Leptin produced by obese adipose stromal/stem cells enhances proliferation and metastasis of estrogen receptor positive breast cancers. Breast Cancer Res. 2015;17:112. doi: 10.1186/s13058-015-0622-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ando S, Barone I, Giordano C, Bonofiglio D, Catalano S. The multifaceted mechanism of Leptin Signaling within tumor microenvironment in driving breast cancer growth and progression. Front Oncol. 2014;4:340. doi: 10.3389/fonc.2014.00340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mohammadzadeh G, Ghaffari MA, Bafandeh A, Hosseini SM. Association of serum soluble leptin receptor and leptin levels with breast cancer. J Res Med Sci. 2014;19(5):433–438. [PMC free article] [PubMed] [Google Scholar]
- 29.Chen C, Chang YC, Liu CL, Chang KJ, Guo IC. Leptin-induced growth of human ZR-75-1 breast cancer cells is associated with up-regulation of cyclin D1 and c-Myc and down-regulation of tumor suppressor p53 and p21WAF1/CIP1. Breast Cancer Res Treat. 2006;98(2):121–132. doi: 10.1007/s10549-005-9139-y. [DOI] [PubMed] [Google Scholar]
- 30.Schmidt S, Monk JM, Robinson LE, Mourtzakis M. The integrative role of leptin, oestrogen and the insulin family in obesity-associated breast cancer: potential effects of exercise. Obes Rev. 2015;16(6):473–487. doi: 10.1111/obr.12281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ishikawa M, Kitayama J, Nagawa H. Enhanced expression of leptin and leptin receptor (OB-R) in human breast cancer. Clin Cancer Res. 2004;10(13):4325–4331. doi: 10.1158/1078-0432.CCR-03-0749. [DOI] [PubMed] [Google Scholar]
- 32.Caldefie-Chezet F, Damez M, de Latour M, Konska G, Mishellani F, Fusillier C, Guerry M, Penault-Llorca F, Guillot J, Vasson MP. Leptin: a proliferative factor for breast cancer? Study on human ductal carcinoma. Biochem Biophys Res Commun. 2005;334(3):737–741. doi: 10.1016/j.bbrc.2005.06.077. [DOI] [PubMed] [Google Scholar]
- 33.Garofalo C, Koda M, Cascio S, Sulkowska M, Kanczuga-Koda L, Golaszewska J, Russo A, Sulkowski S, Surmacz E. Increased expression of leptin and the leptin receptor as a marker of breast cancer progression: possible role of obesity-related stimuli. Clin Cancer Res. 2006;12(5):1447–1453. doi: 10.1158/1078-0432.CCR-05-1913. [DOI] [PubMed] [Google Scholar]
- 34.Kim Y, Kim SY, Lee JJ, Seo J, Kim YW, Koh SH, Yoon HJ, Cho KS. Effects of the expression of leptin and leptin receptor (OBR) on the prognosis of early-stage breast cancers. Cancer Res Treat. 2006;38(3):126–132. doi: 10.4143/crt.2006.38.3.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jarde T, Caldefie-Chezet F, Damez M, Mishellany F, Penault-Llorca F, Guillot J, Vasson MP. Leptin and leptin receptor involvement in cancer development: a study on human primary breast carcinoma. Oncol Rep. 2008;19(4):905–911. [PubMed] [Google Scholar]
- 36.Fiorio E, Mercanti A, Terrasi M, Micciolo R, Remo A, Auriemma A, Molino A, Parolin V, Di Stefano B, Bonetti F, et al. Leptin/HER2 crosstalk in breast cancer: in vitro study and preliminary in vivo analysis. BMC Cancer. 2008;8:305. doi: 10.1186/1471-2407-8-305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jarde T, Caldefie-Chezet F, Damez M, Mishellany F, Perrone D, Penault-Llorca F, Guillot J, Vasson MP. Adiponectin and leptin expression in primary ductal breast cancer and in adjacent healthy epithelial and myoepithelial tissue. Histopathology. 2008;53(4):484–487. doi: 10.1111/j.1365-2559.2008.03121.x. [DOI] [PubMed] [Google Scholar]
- 38.Kim HS. Leptin and leptin receptor expression in breast cancer. Cancer Res Treat. 2009;41(3):155–163. doi: 10.4143/crt.2009.41.3.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jeong YJ, Bong JG, Park SH, Choi JH, Oh HK. Expression of leptin, leptin receptor, adiponectin, and adiponectin receptor in ductal carcinoma in situ and invasive breast cancer. J Breast Cancer. 2011;14(2):96–103. doi: 10.4048/jbc.2011.14.2.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Colbert LS, Wilson K, Kim S, Liu Y, Oprea-Ilies G, Gillespie C, Dickson T, Newman G, Gonzalez-Perez RR. NILCO biomarkers in breast cancer from Chinese patients. BMC Cancer. 2014;14:249. doi: 10.1186/1471-2407-14-249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gallina S, Sireci F, Lorusso F, Dib DV, Speciale R, Marchese D, Costantino C, Napoli G, Tessitore V, Cucco D, et al. The immunohistochemical peptidergic expression of leptin is associated with recurrence of malignancy in laryngeal squamous cell carcinoma. Acta Otorhinolaryngol Ital. 2015;35(1):15–22. [PMC free article] [PubMed] [Google Scholar]
- 42.Duan X, Tang P, Zhang H, Yu Z. Expression of leptin and adiponectin in esophageal squamous cell carcinoma and their clinical significance. Zhonghua Zhong Liu Za Zhi. 2014;36(11):839–843. [PubMed] [Google Scholar]
- 43.Bain GH, Collie-Duguid E, Murray GI, Gilbert FJ, Denison A, McKiddie F, Ahearn T, Fleming I, Leeds J, Phull P, et al. Tumour expression of leptin is associated with chemotherapy resistance and therapy-independent prognosis in gastro-oesophageal adenocarcinomas. Br J Cancer. 2014;110(6):1525–1534. doi: 10.1038/bjc.2014.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Xu YJ, Shao YF, Zhao X, Geng YT, Wang K, Yin YM. Expression and clinical significance of leptin, the functional receptor of leptin (OB-Rb) and HER-2 in non-small-cell lung cancer: a retrospective analysis. J Cancer Res Clin Oncol. 2011;137(12):1841–1848. doi: 10.1007/s00432-011-1054-5. [DOI] [PubMed] [Google Scholar]
- 45.Fan YL, Li XQ. Expression of leptin and its receptor in thyroid carcinoma: distinctive prognostic significance in different subtypes. Clin Endocrinol. 2015;83(2):261–267. doi: 10.1111/cen.12598. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.


