Abstract
Objectives
Annual screening for gonorrhea (NG) and chlamydia (CT) is recommended for all sexually-active persons living with HIV (PLWH) but is poorly implemented. Studies demonstrating no increases in NG and/or CT (NG/CT) case detection in clinics that successfully expanded NG/CT screening raise questions about this broad screening approach. We evaluated NG/CT case detection in the HIV Research Network during 2004–2014, a period of expanding testing.
Methods
We analyzed linear time trends in annual testing (patients tested divided by all patients in care), test positivity (patients positive divided by all tested), and case detection (the number of patients with a positive result divided by all patients in care) using multivariate repeated measures logistic regression. We determined trends overall and stratified by men who have sex with men (MSM), men who have sex exclusively with women (MSW), and women.
Results
Among 15,614 patients (50% MSM, 26% MSW, 24% women), annual NG/CT testing increased from 22% in 2004 to 60% in 2014 (adjusted odds ratio [AOR] per year 1.22 [1.21, 1.22]). Despite the increase in testing, test positivity also increased (AOR per year 1.10 [1.07, 1.12]), and overall case detection increased from 0.8% in 2004 to 3.9% in 2014 (AOR per year 1.20 [1.17, 1.22]). Case detection was highest among MSM but increased over time among all three groups.
Conclusions
NG/CT case detection increased as testing expanded in the population. This supports a broad approach to NG/CT screening among PLWH in order to decrease transmission and complications of NG/CT and of HIV.
INTRODUCTION
Approximately 5–10% of persons receiving care at HIV clinics are infected with Neisseria gonorrhoeae (NG) and/or Chlamydia trachomatis (CT) at any given time1–7 Detecting and treating these generally-asymptomatic infections improves health by preventing their spread and downstream complications and may also reduce HIV transmission through 1) identifying opportunities for counseling about risky sexual behavior, and 2) decreasing mucosal inflammation and HIV RNA levels at the site of infection.8–10 Starting in 2003, the United States (US) and other countries have recommended at least annual NG/CT screening among the relatively-broad group of all sexually-active persons living with HIV (PLWH).11
Since 2003, a number of clinical cohorts have reported increases in NG/CT testing.12–14 These increases notwithstanding, overall NG/CT testing has remained low. In seven geographically-diverse HIV clinics within the HIV Research Network (HIVRN) cohort, NG/CT testing increased each year starting in 2004, but as of 2010 the annual testing rate was still only 39% of patients engaged in care.14 Data from the nationally-representative Medical Monitoring Project and from other large cohorts have shown similarly low NG/CT screening rates as recently as 2011.12,13,15,16
Since publication of screening recommendations, HIV clinics in Maryland, US, and Ontario, Canada, separately reported that several-year increases in annual NG/CT testing were countered by declines in NG/CT test positivity such that overall NG/CT case detection remained constant.12,17 The failure of increased testing to increase case detection leads to questions about the utility of the broad approach to screening all sexually active PLWH as compared to a more targeted approach.12,18 In this study, we determined trends in NG/CT test positivity and case detection in the HIVRN during 2004–2014, to assess whether case detection increased with the known rise in testing over this time period. Based on known variation in testing rates and test positivity by sexual risk group,12,14,19 we stratified analyses by men who have sex with men (MSM), men who have sex exclusively with women (MSW), and women.
METHODS
Sample
The HIVRN includes 12 adult HIV clinical care sites that are widely-distributed across the US.20 Sites prospectively collect demographic, laboratory, and visit data from electronic medical records and structured chart reviews. Submission of NG/CT laboratory data is optional for HIVRN sites, and we restricted this analysis to 4 sites (from 4 different states including 2 in the West, 1 in the South, and 1 in the Northeast) that submitted complete NG/CT testing and result data from January 1, 2004 through December 31, 2014. Three additional HIVRN sites previously examined for testing trends,14 were excluded because they were unable to submit comprehensive result data prior to 2010 or testing or result data subsequent to 2010. We did not include the few years of data available from these sites because of risk of introducing bias in multi-year trends. We included all patients aged 18 years or older during calendar years of active follow-up, defined as having at least one outpatient visit and one CD4 count within the calendar year. Patients were allowed to return to observation after one or more years out of active follow-up. Institutional review boards at the individual sites and the data coordinating center at Johns Hopkins University School of Medicine approved the collection and use of these data.
Outcomes
The primary outcomes were 1) testing, defined as having at least one NG and/or CT test at any body site (genitalia, rectum, or mouth) during a year of active follow-up in care; 2) test positivity, defined as having at least one positive NG and/or CT result during any year when testing occurred, regardless of the number of tests performed; and 3) case detection, defined as having at least one positive NG and/or CT result during a year of active follow-up in care (with untested persons being included in the denominator). Data distinguishing the body sites of tests were unavailable. For each HIVRN site, data were retrieved from institutional electronic health record databases. These databases contain data on assays ordered at the institution. However, in no case, do the databases contain laboratory data performed outside the institution (for example at a public sexually transmitted infection clinic.)
For the purpose of exploring how many distinct testing episodes occurred for an individual patient within a year, we grouped tests into episodes based on occurring within 30 days of one another. By this we hoped to eliminate instances of counting tests of cure or of patients providing simultaneously ordered samples from different body sites on different days as separate testing episodes.
Exposures
The exposure of primary interest was time in calendar years. We defined MSM as persons who self-identified as men and reported ever having male sexual contact at the time of enrolment into the cohort. Additional time-fixed exposures included HIVRN clinical site, race/ethnicity, and injection drug use (IDU) as an HIV risk factor. Time-variable exposures included age in years, the annual number of HIV provider visits, CD4 count, and HIV RNA, with the latter two variables defined as the first recorded value of each calendar year.
Analysis
We created binary outcome variables for testing, test positivity, and case detection within each patient year (PY), and conducted analyses at the PY level. We performed logistic regression to assess separate time trends in testing, test positivity, and case detection using generalized estimating equations to adjust variance for within-person correlation. Based on previous testing results for 2004–2010 and upon initial, graphical inspection of the data, we parameterized time linearly. Our overall population model included sexual risk group (MSM, MSW, or women) as an exposure. We then created separate models within each group. All multivariate models included HIVRN clinical site as a covariate, results of which are suppressed. We used Stata 14.0 (StataCorp LP, College Station, TX, USA) to conduct all analyses.
RESULTS
A total of 15,614 patients contributed 69,694 PY during the 2004–2014 study period. The median number of years in care was 3 (interquartile range [IQR], 1–6). The sample was 50% MSM, 26% MSW, and 24% women (Table 1). Overall, the median age in the first year of observation was 44 years-old; for MSM it was 42, for MSW 47, and for women 42. The full cohort was 44% non-Hispanic Black, including 26% of MSM, 58% of MSW, and 65% of women. During the first year of observation, the median number of HIV provider visits for the full sample and for each subgroup was 3 (IQR 2–5). Eleven percent of all 69,694 PY involved only a single HIV provider visit within the calendar year. During the first year of observation, 42% of the overall cohort had an HIV RNA level >400 copies/milliliter on the first measurement of the year, with little variation among MSM, MSW, and women. Among patients observed during 2014, this percentage decreased to 19%, again with little variation by sexual risk group.
Table 1.
Participant characteristics during first year of observation
| Total (N=15,614) | MSM (N=7,762) | MSW (N=4,099) | Women (N=3,753) | |||||
|---|---|---|---|---|---|---|---|---|
| N | % | N | % | N | % | N | % | |
| Age (years) | ||||||||
| Under 30 | 1,908 | 12 | 1,154 | 15 | 263 | 6 | 491 | 13 |
| 30–39 | 3,623 | 23 | 1,949 | 25 | 683 | 17 | 991 | 26 |
| 40–49 | 5,742 | 37 | 2,785 | 36 | 1,609 | 39 | 1,348 | 36 |
| 50 or older | 4,341 | 28 | 1,874 | 24 | 1,544 | 38 | 923 | 25 |
| Race/ethnicity | ||||||||
| Non-Hispanic White | 5,850 | 37 | 4,043 | 52 | 1,030 | 25 | 777 | 21 |
| Non-Hispanic Black | 6,894 | 44 | 2,055 | 26 | 2,383 | 58 | 2,456 | 65 |
| Hispanic | 2,286 | 15 | 1,282 | 17 | 571 | 14 | 433 | 12 |
| Other | 382 | 5 | 382 | 5 | 115 | 3 | 87 | 2 |
| History of IDU | ||||||||
| No | 12,114 | 78 | 6,954 | 90 | 2,364 | 58 | 2,796 | 75 |
| Yes | 3,500 | 22 | 808 | 10 | 1,735 | 42 | 957 | 25 |
| Annual HIV care visits | ||||||||
| ≤ 3 | 8,296 | 53 | 4,042 | 52 | 2,266 | 55 | 1,988 | 53 |
| 4 to 6 | 4,725 | 30 | 2,381 | 31 | 1,187 | 29 | 1,157 | 31 |
| ≥ 7 | 2,593 | 17 | 1,339 | 17 | 646 | 16 | 608 | 16 |
| CD4 count (cells/μl)* | ||||||||
| ≤ 200 | 3,185 | 20 | 1,316 | 17 | 1,126 | 27 | 743 | 20 |
| 201 to 350 | 2,986 | 19 | 1,438 | 19 | 849 | 21 | 699 | 19 |
| ≥ 351 | 9,443 | 60 | 5,008 | 65 | 2,124 | 52 | 2,311 | 62 |
| HIV RNA (copies/ml)* | ||||||||
| ≤ 400 | 9,079 | 58 | 4,725 | 61 | 2,335 | 57 | 2,019 | 54 |
| > 400 | 6,535 | 42 | 3,037 | 39 | 1,764 | 43 | 1,734 | 46 |
MSM, men who have sex with men; MSW, men who have sex exclusively with women; IDU, injection drug use
First measurement of the calendar year
Annual NG/CT Testing
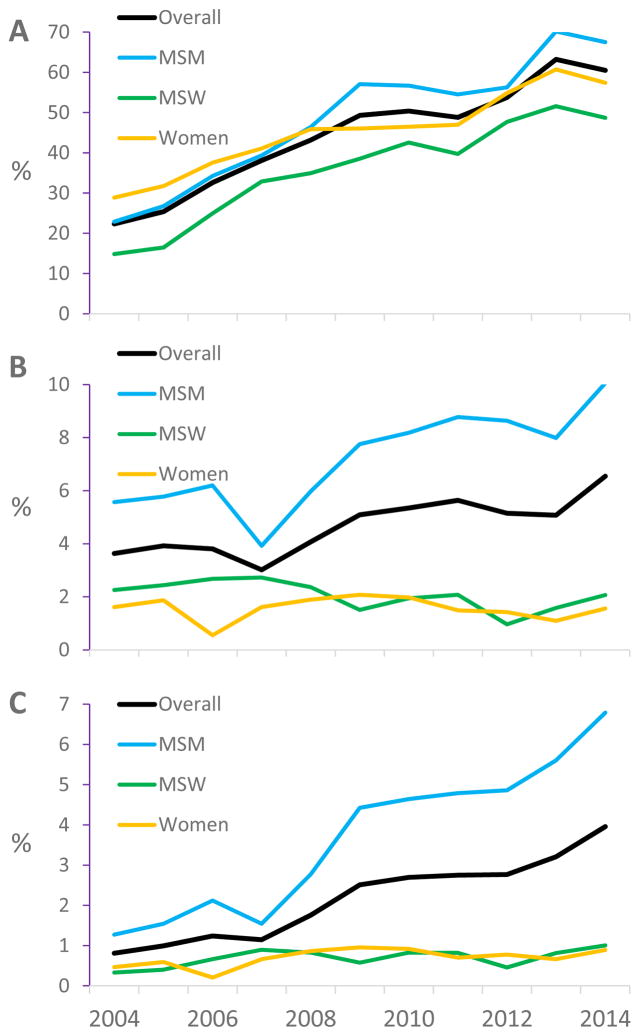
Overall, 11,184 patients (72%) underwent NG/CT testing during at least one of the years of study observation. During 2004, 22% of the overall cohort had NG/CT testing performed at least once, including 23% of MSM, 15% of MSW, and 29% of women (Figure 1A). In general, the proportion of patients tested for NG/CT increased year over year among the full cohort and among each subgroup. In 2014, 60% of the overall cohort was tested for NG/CT, unadjusted odds ratio (OR) per year over the study interval 1.16 (95% confidence interval 1.15, 1.16). In 2014, 67% of MSM were tested (unadjusted OR per year 1.18 [1.17, 1.19]), 49% of MSW were tested (1.16 [1.15, 1.17]), and 57% of women were tested (1.12 [1.11, 1.13]).
Figure 1.
Annual gonorrhea/chlamydia Testing (A), Test Positivity (B), and Case Detection (C).
Seventy-four percent of 31,419 PY with any NG/CT testing included just one testing episode, 19% two episodes, and 7% three or more episodes. Eighty-two percent of testing episodes included just one sample (99% of which were tested for both NG and CT), 10% of episodes included two samples (drawn at different times and/or body sites), 5% included three samples, and 3% more than 3 samples.
In multivariate analysis (Table 2), annual testing increased over time among the full group, adjusted OR (AOR) per year 1.22 (1.21, 1.22). MSM (AOR 0.99 [0.93, 1.05]) were equally likely and MSW (0.72 [0.68, 0.76]) were less likely to be tested than women. Age, race/ethnicity, number of visits, CD4 count >200 cells/microliter, and HIV RNA >400 copies/milliliter were also associated with testing. In addition to calendar time, factors with the largest effect size in the full group model included younger age (AOR for <30 years-old, 2.60 [2.40, 2.82] and for 30–39 years-old, 1.96 [1.85, 2.08], both compared to ≥50 years-old) and number of visits (2.03 [1.93, 2.13] for ≥7 visits compared to ≤3 visits). Calendar time was strongly associated with testing in each subgroup, MSM AOR 1.25 (1.24, 1.26), MSW 1.20 (1.19, 1.22), and women 1.17 (1.16, 1.19). The pattern of factors associated with testing among each of the subgroups generally resembled the pattern in the full group.
Table 2.
Factors Associated with Gonorrhea/Chlamydia Testing
| Total (N=15,614) | MSM (N=7,762) | MSW (N=4,099) | Women (N=3,753) | |
|---|---|---|---|---|
| Calendar time | ||||
| Linear parameter 2004–2014, per year | 1.22 (1.21, 1.22) | 1.25 (1.24, 1.26) | 1.20 (1.19, 1.22) | 1.17 (1.16, 1.19) |
| Sexual risk group | ||||
| MSM | 0.99 (0.93, 1.05) | |||
| MSW | 0.72 (0.68, 0.76) | |||
| Women | 1.00 (Ref) | |||
| Age (years) | ||||
| Under 30 | 2.60 (2.40, 2.82) | 2.90 (2.60, 3.24) | 2.02 (1.65, 2.46) | 2.63 (2.26, 3.06) |
| 30–39 | 1.96 (1.85, 2.08) | 2.04 (1.87, 2.22) | 1.62 (1.43, 1.84) | 2.20 (1.97, 2.46) |
| 40–49 | 1.43 (1.36, 1.49) | 1.44 (1.35, 1.55) | 1.28 (1.18, 1.40) | 1.62 (1.48, 1.78) |
| 50 or older | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) |
| Race/ethnicity | ||||
| Non-Hispanic White | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) |
| Non-Hispanic Black | 1.33 (1.25, 1.41) | 1.23 (1.13, 1.35) | 1.31 (1.16, 1.48) | 1.45 (1.29, 1.63) |
| Hispanic | 1.11 (1.03, 1.19) | 1.12 (1.02, 1.22) | 1.01 (0.87, 1.17) | 1.23 (1.05, 1.44) |
| Other | 1.13 (0.99, 1.29) | 1.13 (0.96, 1.33) | 1.05 (0.77, 1.43) | 1.32 (0.96, 1.81) |
| History of IDU | ||||
| No | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) |
| Yes | 1.03 (0.97, 1.08) | 0.92 (0.83, 1.02) | 1.00 (0.91, 1.09) | 1.10 (0.99, 1.21) |
| Annual HIV care visits | ||||
| ≤ 3 | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) |
| 4 to 6 | 1.59 (1.53, 1.65) | 1.59 (1.51, 1.68) | 1.58 (1.47, 1.70) | 1.61 (1.50, 1.73) |
| ≥ 7 | 2.03 (1.93, 2.13) | 2.10 (1.96, 2.25) | 1.94 (1.77, 2.14) | 2.02 (1.84, 2.22) |
| CD4 count (cells/μl)* | ||||
| ≤ 200 | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) |
| 201 – 350 | 1.12 (1.05, 1.18) | 1.16 (1.06, 1.26) | 1.08 (0.97, 1.20) | 1.08 (0.97, 1.21) |
| ≥ 351 | 1.23 (1.16, 1.30) | 1.28 (1.18, 1.39) | 1.21 (1.10, 1.34) | 1.18 (1.07, 1.32) |
| HIV RNA (copies/ml)* | ||||
| ≤ 400 | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) |
| > 400 | 1.15 (1.11, 1.20) | 1.20 (1.13, 1.27) | 1.10 (1.02, 1.19) | 1.14 (1.06, 1.23) |
Values are Adjusted Odds Ratio (95% Confidence Interval). All models included an indicator variable to adjust for HIV Research Network site. MSM, men who have sex with men; MSW, men who have sex exclusively with women; IDU, injection drug use
First measurement of the calendar year
Test positivity
Among 11,184 patients tested at least once over the full study period, 1,176 (10.5%) had at least one positive result for NG/CT or both. Within the full cohort, test positivity increased from 3.6% in 2004 to 6.5% in 2014, unadjusted OR per year 1.05 (1.03, 1.07) (Figure 1B). This increasing trend was driven by the increase among MSM from 5.6% in 2004 to 10.1% in 2014, unadjusted OR per year 1.07 (1.05, 1.09). Crude test positivity was stable among both MSW and women, unadjusted ORs per year 0.95 (0.88, 1.02) and 1.01 (0.94, 1.09), respectively. The mean over time was 1.9% among MSW and 1.6% among women.
In multivariate analysis (Table 3), test positivity increased within the full cohort (AOR per year 1.10 (1.07, 1.12). MSM (AOR 4.09 [3.31, 5.05]) and MSW (1.57 [1.18, 2.08]) were more likely than women to test positive. In stratified analyses, test positivity increased among MSM (1.12 (1.09, 1.15), was stable among MSW 0.96 (0.89, 1.04), and increased among women 1.09 (1.02, 1.16). Younger age was strongly associated with test positivity within the full group (AOR for <30 years-old, 5.33 [4.32, 6.59] and for 30–39 years-old, 3.16 [2.59, 3.85]) and in each subgroup. Race/ethnicity was not associated with test positivity in the full group nor in any subgroup. Among MSM, having >3 visits, CD4 >200 cells/microliter, and RNA >400 copies/milliliter were associated with having a positive test result.
Table 3.
Factors Associated with Gonorrhea/Chlamydia Test Positivity
| Total (N=11,184) | MSM (N=5,855) | MSW (N=2,614) | Women (N=2,715) | |
|---|---|---|---|---|
| Calendar time | ||||
| Linear parameter 2004–2014, per year | 1.10 (1.07, 1.12) | 1.12 (1.09, 1.15) | 0.96 (0.89, 1.04) | 1.09 (1.02, 1.16) |
| Sexual risk group | ||||
| MSM | 4.09 (3.31, 5.05) | |||
| MSW | 1.57 (1.18, 2.08) | |||
| Women | 1.00 (Ref) | |||
| Age (years) | ||||
| Under 30 | 5.33 (4.32, 6.59) | 5.11 (4.05, 6.45) | 6.83 (3.26, 14.34) | 16.37 (7.26, 36.89) |
| 30–39 | 3.16 (2.59, 3.85) | 3.28 (2.65, 4.07) | 2.34 (1.10, 4.94) | 5.58 (2.44, 12.78) |
| 40–49 | 2.02 (1.67, 2.46) | 1.92 (1.55, 2.36) | 2.34 (1.12, 4.91) | 4.06 (1.79, 9.24) |
| 50 or older | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) |
| Race/ethnicity | ||||
| Non-Hispanic White | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) |
| Non-Hispanic Black | 0.92 (0.77, 1.09) | 0.92 (0.75, 1.11) | 1.63 (0.93, 2.88) | 1.37 (0.80, 2.34) |
| Hispanic | 0.86 (0.73, 1.02) | 0.85 (0.72, 1.01) | 0.95 (0.46, 1.99) | 1.13 (0.54, 2.38) |
| Other | 0.75 (0.54, 1.04) | 0.70 (0.49, 0.99) | 1.16 (0.34, 4.01) | 2.88 (0.82, 10.10) |
| History of IDU | ||||
| No | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) |
| Yes | 0.72 (0.59, 0.89) | 0.73 (0.57, 0.94) | 0.38 (0.21, 0.68) | 1.24 (0.78, 1.98) |
| Annual HIV care visits | ||||
| ≤ 3 | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) |
| 4 to 6 | 1.39 (1.22, 1.59) | 1.54 (1.33, 1.78) | 0.80 (0.44, 1.46) | 1.19 (0.76, 1.86) |
| ≥ 7 | 1.60 (1.35, 1.88) | 1.73 (1.44, 2.09) | 1.12 (0.60, 2.11) | 1.48 (0.93, 2.36) |
| CD4 count (cells/μl)* | ||||
| ≤ 200 | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) |
| 201 – 350 | 1.84 (1.45, 2.33) | 1.92 (1.47, 2.51) | 2.11 (0.99, 4.49) | 1.40 (0.69, 2.85) |
| ≥ 351 | 2.01 (1.63, 2.48) | 2.19 (1.72, 2.79) | 2.00 (0.97, 4.11) | 1.34 (0.71, 2.51) |
| HIV RNA (copies/ml)* | ||||
| ≤ 400 | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) |
| > 400 | 1.39 (1.22, 1.58) | 1.32 (1.15, 1.53) | 1.73 (1.14, 2.62) | 1.38 (0.89, 2.13) |
Values are Adjusted Odds Ratio (95% Confidence Interval). All models included an indicator variable to adjust for HIV Research Network site. MSM, men who have sex with men; MSW, men who have sex exclusively with women; IDU, injection drug use
First measurement of the calendar year
Case detection
Among the full cohort, case detection increased from 0.8% of patients engaged in care in 2004 (whether tested or not) being found to have NG/CT or both at least once to 3.9% in 2014 (unadjusted OR per year 1.15 (1.13, 1.17) (Figure 1C). Case detection increased among each subgroup: among MSM from 1.3% to 6.8% (unadjusted OR per year 1.16 [1.14, 1.18]), among MSW from 0.3% to 1.0% (1.06 [1.00, 1.13]), and among women from 0.5% to 0.9% (1.05 [1.00, 1.11]). In multivariate analyses (Table 4), case detection increased over time in the full group and in each subgroup. MSM had nearly 4 times the odds (AOR 3.94 [3.16, 4.91]) and MSW approximately the same odds (1.31 [0.99, 1.74]) as women for being diagnosed with NG/CT. Younger age was associated with case detection in all subgroups. Non-IDU status, higher CD4 count, and HIV RNA >400 copies/milliliter were associated with case detection among both MSM and MSW. Race/ethnicity was not associated with case detection.
Table 4.
Factors Associated with Gonorrhea/Chlamydia Case Detection
| Total (N=15,614) | MSM (N=7,762) | MSW (N=4,099) | Women (N=3,753) | |
|---|---|---|---|---|
| Calendar time | ||||
| Linear parameter 2004–2014, per year | 1.20 (1.17, 1.22) | 1.22 (1.19, 1.25) | 1.09 (1.02, 1.16) | 1.12 (1.06, 1.19) |
| Sexual risk group | ||||
| MSM | 3.94 (3.16, 4.91) | |||
| MSW | 1.31 (0.99, 1.74) | |||
| Women | 1.00 (Ref) | |||
| Age (years) | ||||
| Under 30 | 7.59 (6.19, 9.29) | 7.28 (5.81, 9.14) | 7.94 (4.11, 15.32) | 17.45 (7.63, 39.89) |
| 30–39 | 4.09 (3.38, 4.96) | 4.14 (3.35, 5.12) | 2.53 (1.33, 4.83) | 6.13 (2.70, 13.95) |
| 40–49 | 2.27 (1.89, 2.73) | 2.11 (1.72, 2.59) | 2.32 (1.35, 3.98) | 3.54 (1.59, 7.88) |
| 50 or older | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) |
| Race/ethnicity | ||||
| Non-Hispanic White | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) |
| Non-Hispanic Black | 0.98 (0.82, 1.17) | 0.97 (0.79, 1.18) | 1.75 (0.96, 3.18) | 1.15 (0.63, 2.10) |
| Hispanic | 0.88 (0.75, 1.03) | 0.87 (0.73, 1.03) | 1.10 (0.55, 2.22) | 1.09 (0.50, 2.41) |
| Other | 0.76 (0.55, 1.04) | 0.70 (0.50, 0.99) | 1.21 (0.35, 4.19) | 3.04 (0.92, 9.99) |
| History of IDU | ||||
| No | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) |
| Yes | 0.72 (0.59, 0.88) | 0.72 (0.56, 0.93) | 0.42 (0.24, 0.71) | 1.15 (0.70, 1.91) |
| Annual HIV care visits | ||||
| ≤ 3 | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) |
| 4 to 6 | 1.70 (1.51, 1.92) | 1.83 (1.60, 2.09) | 1.03 (0.69, 1.54) | 1.46 (0.97, 2.19) |
| ≥ 7 | 2.19 (1.90, 2.53) | 2.36 (2.01, 2.77) | 1.35 (0.82, 2.21) | 1.74 (1.07, 2.83) |
| CD4 count (cells/μl)* | ||||
| ≤ 200 | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) |
| 201 – 350 | 1.96 (1.58, 2.44) | 2.01 (1.56, 2.59) | 2.22 (1.23, 4.02) | 1.12 (0.62, 2.04) |
| ≥ 351 | 2.22 (1.81, 2.71) | 2.40 (1.90, 3.04) | 2.10 (1.19, 3.69) | 1.05 (0.61, 1.80) |
| HIV RNA (copies/ml)* | ||||
| ≤ 400 | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) |
| > 400 | 1.46 (1.29, 1.65) | 1.38 (1.20, 1.58) | 1.86 (1.26, 2.75) | 1.60 (1.07, 2.40) |
Values are Adjusted Odds Ratio (95% Confidence Interval). All models included an indicator variable to adjust for HIV Research Network site. MSM, men who have sex with men; MSW, men who have sex exclusively with women; IDU, injection drug use
First measurement of the calendar year
As a sensitivity analysis, we examined test positivity and case detection trends separately for NG and CT. (Testing trends were identical as 99% of testing episodes included assays for both bacteria). Inferences in the sensitivity analysis were unchanged. Among the full group, univariate test positivity time trends were 1.07 (1.04, 1.10) per year for NG and 1.06 per year (1.03, 1.08) for CT (multivariate test positivity models did not converge). Multivariate case detection time trends were 1.21 (1.18, 1.25) per year for NG and 1.20 (1.17, 1.22) per year for CT.
Finally, we examined combined NG/CT outcomes within each HIVRN site to assess whether trends seen in the full study population were evident in the individual sites. Within each site, NG/CT testing increased over time within the full clinic population (OR’s per year ranged from 1.11 [1.09, 1.13] to 1.17 [1.15, 1.18]) and AOR’s per year from 1.14 [1.06, 1.23] to 1.23 [1.22, 1.25]). Test positivity was stable or increasing in univariate analysis in three HIVRN sites; the statistical model for the fourth site did not converge. Test positivity increased in multivariate analysis in two sites, and models in the other two sites did not converge. Case detection increased in every HIVRN site in univariate analysis (OR’s per year ranged from 1.07 [1.02, 1.13] to 1.15 [1.13, 1.18]) and in multivariate analysis in two sites for which models converged.
DISCUSSION
Over 11 years in our 4-site sample, NG/CT testing expanded steadily overall and within each subgroup, reaching 60% within the full population (67% of MSM, 49% of MSW, and 57% of women) in 2014. Contrary to previous studies, test positivity also increased overall and was either increasing or stable within each subgroup. This resulted in steady increases in case detection. In other words, as a greater percentage of patients was tested each year, a greater percentage of patients infected with NG/CT was identified, overall and within each subgroup.
These results generally support the broad screening approach (that all sexually-active PLWH be considered for testing at least annually) that has been recommended in the US since 2003.11 A more targeted approach, for example limiting screening to MSM <30 years-old, might be more appropriate if broad screening did not yield increased case detection.18 The increase in case detection contrasts the findings of two previous studies (one of which occurred at one HIVRN site with that site’s 2004–2007 data included in the present study).12,17 However, increased case detection with increased screening has been reported in a cohort in Washington State and in a US military cohort in the past decade.21,22 The present study occurred over a longer time period since guideline publication and in a larger sample than the previous studies.
While supportive, the present study does not provide conclusive evidence for a broad approach to NG/CT screening. Data from other centers and disease modeling studies comparing various targets would provide more evidence. Also, the current screening guidelines include the proviso that patients at particularly high risk (for example, those recently positive for NG/CT) should be screened multiple times per year. Our study focused only on breadth – who should be screened annually – not the frequency of screening among those screened. Future studies might determine the extent to which more frequent testing within a year tends to yield more frequent case detection.
The increase in testing we observed demonstrates the feasibility of implementing NG/CT screening above rates of 20–40% that were reported by many centers in the past decade.13,15,16,21,23,24 These relatively low rates stood in contrast to syphilis testing rates that have consistently been much higher in other cohorts13,15,21 and averaged 66% in the HIVRN during 2004–2010.14 At least one other multisite HIV cohort has reported achieving 50–60% NG/CT testing rates as of 2009.25 Unique barriers to NG/CT screening include time to obtain urine and mucosal swab samples, difficulty engaging in sexual history discussions, and the lack of Food and Drug Administration approval for rectal and oral site nucleic acid test kits. 18,26 Each of the 4 clinics in our sample undertook one or more systematic efforts to increase NG/CT screening during 2004–2014. Interventions included provider education, electronic health record reminders, clinic-wide performance reports, individual provider report cards, and coupling rectal NG/CT screening with rectal cancer screening. Assessing the individual effectiveness of each intervention is an area for future work.
Despite the increase, there is still room to improve testing according to the current guidelines. While the ceiling level of “sexually-active” persons is not known, it is probably at least as high as the 77% of patients who were screened for syphilis in 2010 in the HIVRN.14 Self-collection of rectal and vaginal samples is accurate, may be preferable to provider collection, and may be one way to further increase screening.27–29
Increasing rates of NG and CT within the HIVRN sites’ metropolitan areas may have contributed to the increasing test positivity among MSM and to the stable test positivity among MSW and women. Nationally, the combined reported rates of NG and CT in the general population increased approximately 30% from 2004 to 2014 with parallel increases among men and women.19 Similar increases were seen in the four metropolitan areas hosting the HIVRN sites.19,30 Although there are no comprehensive surveillance data regarding NG and CT specifically among MSM, there are indications through the Gonococcal Isolate Surveillance Project that increases among MSM may have exceeded increases among MSW and women.19 However, even if expanding community-level NG and CT epidemics contributed to the test positivity trends we observed, this does not change the inference that a broad screening strategy may currently be appropriate.
Another possible contributor to the increase in NG/CT test positivity among MSM is expanded screening at the rectal and oral sites. Historically, screening at these sites has been much lower (<10% annually) than urine screening.3,12,13,17 However, the rectal and oral sites are much more likely to harbor asymptomatic disease with 50–85% of infections existing at the extragenital compartment and not having concurrent genital tract infection. Increasing screening at extragenital sites is frequently described as a priority.2,3,12,13,31,32 One HIVRN clinic undertook an intervention to specifically increase rectal NG/CT screening (coupling rectal NG/CT screening with rectal cancer screening). Unfortunately, we do not have data on body site of testing and cannot assess the extent to which extragenital testing might be contributing to the increased test positivity. This again does not affect support for a broad screening strategy. As a greater percentage of patients were tested (regardless of body site), a greater percentage were identified as having NG/CT.
As expected from previous studies12,14,19 MSM were the most likely group to be tested and to test positive. Younger age is also a well-established risk factor for NG/CT.12,19,22 African-American race is associated with increased NG/CT prevalence in the general US population.19 However, this association has not consistently been seen among HIV cohorts.3,12,22,33 Among MSM, the association of test positivity (and case detection) with more annual visits may be at least partly due to reverse causation, with positive test results prompting provider visits for treatment and/or further testing.
HIV transmission risk is greatly reduced when HIV RNA is suppressed through antiretroviral therapy.34 While non-suppressed HIV RNA at the first visit of the year was associated with a slightly increased likelihood of NG/CT test positivity and case detection, the majority of our cohort (81% in 2014) had suppressed HIV RNA at the first visit (though not necessarily consistent suppression year-long), and most NG/CT case detection occurred among these persons. This finding is relevant to future studies that would attempt to determine the utility of NG/CT screening for reducing HIV transmission. It is not clear why having unsuppressed HIV RNA was independently associated with NG/CT case detection. It is possible that psychosocial characteristics such as depression and/or active drug use could be underlying factors leading to both non-adherence to antiretroviral therapy and to risky sexual behavior.
This study has several limitations. First, as above, we cannot distinguish body sites of tests. Second, we cannot distinguish screening tests from diagnostic tests. Some of the increase in testing we observed may have resulted from an increase in symptomatic cases, reported exposures, or reported risky behaviors. However, since community NG/CT epidemics expanded by only 30% over the study interval, we would not expect increased diagnostic testing to have contributed the majority of the nearly 300% increase in testing during the study interval. Third, because our laboratory data collection does not capture assays ordered outside the institution, our results likely underestimate of the breadth of testing and case detection. However, while there may have been some exceptions (patients verbally reporting outside testing or providers receiving outside test results), we expect the data we capture closely resembles the data that would have been available to clinicians. Finally, while our sample is a relatively large HIV cohort from geographically-distributed sites, it includes only 4 distinct sites, and these sites cannot be assumed to be representative of either their local communities or the nation.
In summary, NG/CT testing increased steadily for 11 years following the publication of screening guidelines. NG/CT test positivity did not fall over this interval, and case detection therefore increased steadily along with testing. These findings support the current broad approach to screening. Despite progress, our sites can continue to expand the percent of patients screened annually. That expansion may be expected to yield further NG/CT case detection, and thus to help reduce overall NG/CT and HIV transmission and complications.
Acknowledgments
Sources of Funding: This work was supported by contracts from AHRQ (HHSA290201100007C) and HRSA (HHSH250201600009C) to R.D.M. and K.A.G., by NIH grants U01 DA036935 and P30 AI094189 to R.D.M., by K23 AI084854 to S.A.B., K 23 AI112477 to A.E.N., K23 MH105284 to A.K.M., P30 AI036214 to W.C.M., and T32 AI102623 to J.R.R.
Participating Sites
Alameda County Medical Center, Oakland, California (Howard Edelstein, M.D.)
Children’s Hospital of Philadelphia, Philadelphia, Pennsylvania (Richard Rutstein, M.D.)
Drexel University, Philadelphia, Pennsylvania (Amy Baranoski, M.D., Sara Allen, C.R.N.P.)
Fenway Health, Boston, Massachusetts (Stephen Boswell, M.D.)
Johns Hopkins University, Baltimore, Maryland (Kelly Gebo, M.D., Richard Moore, M.D., Allison Agwu M.D.)
Montefiore Medical Group, Bronx, New York (Robert Beil, M.D.)
Montefiore Medical Center, Bronx, New York (Uriel Felsen, M.D.)
Oregon Health and Science University, Portland, Oregon (P. Todd Korthuis, M.D.)
Parkland Health and Hospital System, Dallas, Texas (Ank Nijhawan, M.D., Muhammad Akbar, M.D.)
St. Jude’s Children’s Research Hospital and University of Tennessee, Memphis, Tennessee (Aditya Gaur, M.D.)
Mount Sinai St. Luke’s and Mount Sinai West, New York, New York (Judith Aberg, M.D., Antonio Urbina, M.D.)
Tampa General Health Care, Tampa, Florida (Charurut Somboonwit, M.D.)
Trillium Health, Rochester, New York (William Valenti, M.D.)
University of California, San Diego, California (W. Christopher Mathews, M.D.)
Sponsoring Agencies
Agency for Healthcare Research and Quality, Rockville, Maryland (Fred Hellinger, Ph.D., John Fleishman, Ph.D., Irene Fraser, Ph.D.)
Health Resources and Services Administration, Rockville, Maryland (Robert Mills, Ph.D., Faye Malitz, M.S.)
Data Coordinating Center
Johns Hopkins University (Richard Moore, M.D., Jeanne Keruly, C.R.N.P., Kelly Gebo, M.D., Cindy Voss, M.A., Charles Collins, M.P.H., Rebeca Diaz-Reyes)
Footnotes
Conference Presentation: The findings herein were presented, in part, at the Conference on Retroviruses and Opportunistic Infections, February 2016, Boston MA
Conflicts of Interest: K.A.G. has been a consultant for Bristol-Myers Squibb and Tibotec and has been an expert witness for the US government. J.A.A. has been on Scientific Advisory Boards for Janssen, Merck, and Viiv and has received funds to her institution for multicenter trials from Bristol-Myers Squibb, Gilead, and Glaxo SmithKline. R.D.M. has consulted for Medscape. A.E.N. has received research funding from Gilead. S.A.B. has been a consultant for Bristol-Myers Squibb. There were no potential conflicts for the remaining authors.
Works Cited
- 1.Kalichman SC, Pellowski J, Turner C. Prevalence of sexually transmitted co-infections in people living with HIV/AIDS: systematic review with implications for using HIV treatments for prevention. Sex Transm Infect. 2011;87(3):183–190. doi: 10.1136/sti.2010.047514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Soni S, White JA. Self-screening for Neisseria gonorrhoeae and Chlamydia trachomatis in the human immunodeficiency virus clinic—high yields and high acceptability. Sex Transm Dis. 2011;38(12):1107–1109. doi: 10.1097/OLQ.0b013e31822e6136. [DOI] [PubMed] [Google Scholar]
- 3.Rieg G, Lewis RJ, Miller LG, Witt MD, Guerrero M, Daar ES. Asymptomatic sexually transmitted infections in HIV-infected men who have sex with men: prevalence, incidence, predictors, and screening strategies. AIDS Patient Care STDs. 2008;22(12):947–954. doi: 10.1089/apc.2007.0240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Phipps W, Stanley H, Kohn R, Stansell J, Klausner JD. Syphilis, chlamydia, and gonorrhea screening in HIV-infected patients in primary care, San Francisco, California, 2003. AIDS Patient Care STDs. 2005;19(8):495–498. doi: 10.1089/apc.2005.19.495. [DOI] [PubMed] [Google Scholar]
- 5.Heiligenberg M, van der Loeff MFS, de Vries HJ, et al. Low prevalence of asymptomatic sexually transmitted infections in HIV-infected heterosexuals visiting an HIV clinic in the Netherlands. AIDS. 2012;26(5):646–649. doi: 10.1097/QAD.0b013e3283504bbf. [DOI] [PubMed] [Google Scholar]
- 6.Heiligenberg M, Wermeling PR, van Rooijen MS, et al. Recreational drug use during sex and sexually transmitted infections among clients of a city sexually transmitted infections clinic in Amsterdam, the Netherlands. Sex Transm Dis. 2012;39(7):518–527. doi: 10.1097/OLQ.0b013e3182515601. [DOI] [PubMed] [Google Scholar]
- 7.Carpenter RJ, Refugio ON, Adams N, et al. Prevalence and factors associated with asymptomatic gonococcal and chlamydial infection among US Navy and Marine Corps men infected with the HIV: a cohort study. BMJ Open. 2013;3(5):e002775. doi: 10.1136/bmjopen-2013-002775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Johnson LF, Lewis DA. The effect of genital tract infections on HIV-1 shedding in the genital tract: a systematic review and meta-analysis. Sex Transm Dis. 2008;35(11):946–959. doi: 10.1097/OLQ.0b013e3181812d15. [DOI] [PubMed] [Google Scholar]
- 9.Fisher JD, Fisher WA, Cornman DH, Amico RK, Bryan A, Friedland GH. Clinician-delivered intervention during routine clinical care reduces unprotected sexual behavior among HIV-infected patients. JAIDS J Acquir Immune Defic Syndr. 2006;41(1):44–52. doi: 10.1097/01.qai.0000192000.15777.5c. [DOI] [PubMed] [Google Scholar]
- 10.Cohen MS, Hoffman IF, Royce RA, et al. Reduction of concentration of HIV-1 in semen after treatment of urethritis: implications for prevention of sexual transmission of HIV-1. The Lancet. 1997;349(9069):1868–1873. doi: 10.1016/s0140-6736(97)02190-9. [DOI] [PubMed] [Google Scholar]
- 11.Health Resources and Services Administration, Administration Services, HIV Medicine Association of the Infectious Diseases Society of America others. Incorporating HIV prevention into the medical care of persons living with HIV. Recommendations of CDC, the Health Resources and Services Administration, the National Institutes of Health, and the HIV Medicine Association of the Infectious Diseases Society of America. MMWR Recomm Rep Morb Mortal Wkly Rep Recomm ReportsCenters Dis Control. 2003;52(RR-12):1. [PubMed] [Google Scholar]
- 12.Burchell AN, Grewal R, Allen VG, et al. Modest rise in chlamydia and gonorrhoea testing did not increase case detection in a clinical HIV cohort in Ontario, Canada. Sex Transm Infect. 2014;90:608–614. doi: 10.1136/sextrans-2014-051647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hoover KW, Butler M, Workowski K, et al. STD screening of HIV-infected MSM in HIV clinics. Sex Transm Dis. 2010;37(12):771–776. doi: 10.1097/OLQ.0b013e3181e50058. [DOI] [PubMed] [Google Scholar]
- 14.Berry SA, Ghanem KG, Mathews WC, et al. Brief Report: Gonorrhea and Chlamydia Testing Increasing but Still Lagging in HIV Clinics in the United States. JAIDS J Acquir Immune Defic Syndr. 2015;70(3):275–279. doi: 10.1097/QAI.0000000000000711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Blair JM, Fagan JL, Frazier EL, et al. Behavioral and clinical characteristics of persons receiving medical care for HIV infection—Medical Monitoring Project, United States, 2009. MMWR Surveill Summ. 2014;63(Suppl 5):1–22. [PubMed] [Google Scholar]
- 16.Grewal R, Allen VG, Gardner S, et al. Serosorting and recreational drug use are risk factors for diagnosis of genital infection with chlamydia and gonorrhoea among HIV-positive men who have sex with men: results from a clinical cohort in Ontario, Canada. Sex Transm Infect. 2017;93:71–75. doi: 10.1136/sextrans-2015-052500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Berry SA, Ghanem KG, Page KR, et al. Increased gonorrhoea and chlamydia testing did not increase case detection in an HIV clinical cohort 1999–2007. Sex Transm Infect. 2011;87(6):469–475. doi: 10.1136/sextrans-2011-050051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Berry SA. Gonorrhoea and chlamydia screening in HIV clinics: time for new tools and targets. Sex Transm Infect. 2014;90(8):574–575. doi: 10.1136/sextrans-2014-051700. [DOI] [PubMed] [Google Scholar]
- 19.Centers for Disease Control and Prevention. Sexually Transmitted Disease Surveillance 2015. Atlanta: U.S. Department of Health and Human Services; 2016. https://www.cdc.gov/std/stats15/std-surveillance-2015-print.pdf. [Google Scholar]
- 20.Yehia BR, Gebo KA, Hicks PB, et al. Structures of care in the clinics of the HIV Research Network. AIDS Patient Care STDs. 2008;22(12):1007–1013. doi: 10.1089/apc.2008.0093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kahle E, Zhang Q, Golden M, Goldbaum G, Buskin S. Trends in evaluation for sexually transmitted infections among HIV-infected people, King County, Washington. Sex Transm Dis. 2007;34(12):940–946. doi: 10.1097/olq.0b013e31813e0a48. [DOI] [PubMed] [Google Scholar]
- 22.Spaulding AB, Lifson AR, Iverson ER, et al. Gonorrhoea or chlamydia in a US military HIV-positive cohort. Sex Transm Infect. 2012;88(4):266–271. doi: 10.1136/sextrans-2011-050173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Teague R, Mijch A, Fairley CK, et al. Testing rates for sexually transmitted infections among HIV-infected men who have sex with men attending two different HIV services. Int J STD AIDS. 2008;19(3):200–202. doi: 10.1258/ijsa.2007.007131. [DOI] [PubMed] [Google Scholar]
- 24.Hutchinson J, Goold P, Wilson H, Jones K, Estcourt C. Sexual health care of HIV-positive patients: an audit of a local service. Int J STD AIDS. 2003;14(7):493–496. doi: 10.1258/095646203322025821. [DOI] [PubMed] [Google Scholar]
- 25.John Snow International. HIVQUAL-US Performance Data Report: Ryan White Part C and Part D Funded Programs. 2011 http://nationalqualitycenter.org/files/hivqual-us-performance-data-report-2009-march-2-2012/
- 26.Carter JW, Jr, Hart-Cooper GD, Butler MO, Workowski KA, Hoover KW. Provider barriers prevent recommended sexually transmitted disease screening of HIV-infected men who have sex with men. Sex Transm Dis. 2014;41(2):137–142. doi: 10.1097/OLQ.0000000000000067. [DOI] [PubMed] [Google Scholar]
- 27.Dukers-Muijrers NH, Schachter J, van Liere GA, Wolffs PF, Hoebe CJ. What is needed to guide testing for anorectal and pharyngeal Chlamydia trachomatis and Neisseria gonorrhoeae in women and men? Evidence and opinion. BMC Infect Dis. 2015;15(1):533. doi: 10.1186/s12879-015-1280-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.van der Helm JJ, Hoebe CJ, van Rooijen MS, et al. High performance and acceptability of self-collected rectal swabs for diagnosis of Chlamydia trachomatis and Neisseria gonorrhoeae in men who have sex with men and women. Sex Transm Dis. 2009;36(8):493–497. doi: 10.1097/OLQ.0b013e3181a44b8c. [DOI] [PubMed] [Google Scholar]
- 29.Arias M, Jang D, Gilchrist J, et al. Ease, Comfort, and Performance of the HerSwab Vaginal Self-Sampling Device for the Detection of Chlamydia trachomatis and Neisseria gonorrhoeae. Sex Transm Dis. 2016;43(2):125–129. doi: 10.1097/OLQ.0000000000000406. [DOI] [PubMed] [Google Scholar]
- 30.Centers for Disease Control and Prevention. Sexually Transmitted Disease Surveillance 2008. Atlanta, GA: U.S. Department of Health and Human Services; Nov, 2009. [Google Scholar]
- 31.Short R, Gardner E, Blum J, Vaughn S, Rowan S. Open Forum Infectious Diseases. Vol. 2. Oxford University Press; 2015. [Accessed March 17, 2016]. Prevalence of Gonorrhea and Chlamydia Infections Among Human Immunodeficiency Virus-Infected Men by Anatomic Site and Presence or Absence of Symptoms; pp. S14–S15. https://ofid-oxfordjournals-org.ezp-prod1.hul.harvard.edu/content/2/suppl_1/S14.3.full. [Google Scholar]
- 32.Lutz AR. Screening for asymptomatic extragenital gonorrhea and chlamydia in men who have sex with men: significance, recommendations, and options for overcoming barriers to testing. LGBT Health. 2015;2(1):27–34. doi: 10.1089/lgbt.2014.0056. [DOI] [PubMed] [Google Scholar]
- 33.Mayer KH, O’Cleirigh C, Skeer M, et al. Which HIV-infected men who have sex with men in care are engaging in risky sex and acquiring sexually transmitted infections: findings from a Boston community health centre. Sex Transm Infect. 2010;86(1):66–70. doi: 10.1136/sti.2009.036608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cohen MS, Chen YQ, McCauley M, et al. Prevention of HIV-1 infection with early antiretroviral therapy. N Engl J Med. 2011;365(6):493–505. doi: 10.1056/NEJMoa1105243. [DOI] [PMC free article] [PubMed] [Google Scholar]