Abstract
Context
Monoclonal antibodies are being investigated for chronic pain to overcome the shortcomings of current treatment options.
Objective
To provide a practical overview of monoclonal antibodies in clinical development for use in chronic pain conditions, with a focus on mechanisms of action and relevance to specific classes.
Methods
Qualitative review using a systematic strategy to search for randomized controlled trials, systematic and nonsystematic (narrative) reviews, observational studies, nonclinical studies, and case reports for inclusion. Studies were identified via relevant search terms using an electronic search of MEDLINE via PubMed (1990 to June 2017) in addition to hand-searching reference lists of retrieved systematic and nonsystematic reviews.
Results
Monoclonal antibodies targeting nerve growth factor, calcitonin gene-related peptide pathways, various ion channels, tumor necrosis factor-α, and epidermal growth factor receptor are in different stages of development. Mechanisms of action are dependent on specific signaling pathways, which commonly involve those related to peripheral neurogenic inflammation. In clinical studies, there has been a mixed response to different monoclonal antibodies in several chronic pain conditions, including migraine, neuropathic pain conditions (e.g., diabetic peripheral neuropathy), osteoarthritis, chronic back pain, ankylosing spondylitis, and cancer. Adverse events observed to date have generally been mild, although further studies are needed to ensure safety of monoclonal antibodies in early stages of development, especially where there is an overlap with non-pain-related pathways. High acquisition cost remains another treatment limitation.
Conclusion
Monoclonal antibodies for chronic pain have the potential to overcome the limitations of current treatment options, but strategies to ensure their appropriate use need to be determined.
Keywords: Antibody therapy, biologics, central sensitization, chronic pain, monoclonal antibodies, peripheral sensitization
Introduction
According to the current International Association for the Study of Pain taxonomy, pain is an “unpleasant sensory and emotional experience associated with actual or potential tissue damage, or described in terms of such damage.”1 This definition emphasizes the effects of pain, regardless of the source of pain perception, but provides no details on the types or causes of pain.
Several concepts are relevant to understanding the types and causes of pain. Temporally, pain is divided into acute and chronic (persisting beyond the normal time expected for healing) types, with three months generally used to delineate chronic nonmalignant pain.2 Both acute and chronic pain can be divided into nociceptive and neuropathic pain types, although acute pain tends to be predominantly nociceptive. Nociceptive pain signifies neuronal activation of pain pathways secondary to actual or potential tissue damage. In contrast, chronic neuropathic pain “is caused by a lesion or disease of the somatosensory nervous system.”2 However, as with many classifications and concepts applied to biological systems, there is an overlap between nociceptive and neuropathic pain. Transition from acute nociceptive to chronic neuropathic pain can be observed clinically and involves multiple peripheral and central mechanisms, including increased membrane excitability of peripheral nerves and dorsal root ganglia, spinal cord synaptic plasticity, changes in inhibitory control and descending modulation, central sensitization, and even immune to nervous system interactions.3,4 In such individuals, nociceptive and neuropathic pain types may coexist. In chronic neuropathic pain, several other mechanistic and clinical concepts are also important. Clinically, neuropathic pain is characterized by (1) hyperalgesia, or increased sensitivity to pain, and (2) allodynia, where pain or an increase in pain can be stimulated by normally nonpainful stimuli.2
Central and peripheral sensitization are characterized by a distorted or amplified response to pain, out of proportion to the noxious stimuli.5 These phenomena can occur to varying degrees in nociceptive, neuropathic, and inflammatory types of pain. Central sensitization is an amplified pain response involving an increased state of excitability of central neurons that can be detected by long-term changes in nociceptive withdrawal reflexes and increases in cortical event-related potential amplitudes.5 With peripheral sensitization, pain can be abnormally propagated by changes in the neuropeptide signaling that forms the basis of neurogenic inflammation, involving processes such as vasodilatation, plasma extravasation, infiltration of cytokines, and attraction of macrophages.6 During peripheral sensitization, the excitation threshold of nociceptors decreases so that nonpainful stimuli activate painful responses and noxious stimuli evoke even stronger responses than in the nonsensitized state.7 A variety of proinflammatory mediators, especially eicosanoids, bradykinin, neurotrophins, and cytokines, have been implicated in neuropathic pain and reveal the close link between inflammation and neural hypersensitivity.6,8 Visceral pain represents another basis of chronic pain conditions commonly seen in clinical practice and comprises visceral and somatic afferent inputs, which may also be affected by cognitive, emotional, and autonomic brain centres (the so-called “brain–gut axis”).9 Visceral pain may be associated with both peripheral and central sensitization, which involve inflammatory mediators and increased excitability of the spinal cord and higher center neurons, respectively.9
Numerous therapeutic options are currently available for chronic pain conditions. Nonpharmacological options (e.g., pain education, exercise therapy) are often used as an initial treatment step before introducing pharmacological and other treatment strategies. Nonpharmacological options can also help reduce the required dose of pharmacological treatments. However, the usefulness of nonpharmacological options extends beyond this initial period to help control chronic pain, usually in combination with drug and other therapies, in individual chronic pain patients. Regarding pharmacological treatments, small molecule agents, including acetaminophen, nonsteroidal anti-inflammatory drugs (NSAIDs), antidepressants such as serotonin and norepinephrine-reuptake inhibitor drugs (SNRIs), calcium-channel alpha(2)delta ligands, opioids, tramadol, and tapentadol, have been the traditional mainstay of chronic pain management. A detailed description of the mechanisms by which these agents treat pain is beyond the scope of this review. However, in brief, opioids mimic the actions of endogenous opioid peptides by interacting with presynaptic mu, delta, or kappa opioid receptors to reduce neuronal excitability and inhibit the release of nociceptive neurotransmitters. Acetaminophen and NSAIDs inhibit cyclooxygenase to reduce prostaglandin synthesis, which produces analgesic and anti-inflammatory actions. Calcium-channel alpha(2)delta ligands, such as gabapentin and pregabalin, bind to the alpha(2)delta subunit of voltage-dependant calcium channels to reduce calcium influx into neurons and thereby decrease the release of pain neurotransmitters such as glutamate, norepinephrine, and substance P. The analgesic action of antidepressants is complex but often involves reuptake inhibition of neurotransmitters including noradrenaline and serotonin involved in descending inhibition of pain transmission. Tramadol and tapentadol combine these noradrenergic and serotonergic effects with mu opioid receptor activity. Although these agents remain the most widely used for chronic pain, a variety of approaches such as reformulations to provide alternative delivery methods have been investigated and implemented to enhance the utility and overcome limitations of currently available therapeutic options.10 Further, it has become clear that there is a need for new therapeutic options based on recognized limitations of existing therapies. Such limitations mainly include lack of efficacy, either as acute or prophylactic treatment, or undesirable adverse effects. For example, in the case of migraine therapy, most triptans were introduced in the early 1990s but remain underutilized, whereas prophylactic agents have the potential for serious and/or bothersome adverse events.11
In this setting, biologic therapies, particularly monoclonal antibodies (mAbs), have been increasingly investigated for a variety of chronic pain conditions.12–14 Based on these investigations, several mAbs have emerged as attractive alternatives to small molecule therapies.13,15 The fundamental nature of mAbs provides several potential benefits for the treatment of chronic pain states. Firstly, the high affinity and specificity of mAbs for predetermined ligands involved in pain transmission allows for specific and effective targeting of molecules involved in pain transmission, especially those related to neurogenic inflammation. This high specificity may also lead to the relative absence of unwanted adverse effects, which are a common and sometimes treatment-limiting issue with other pharmacological treatments. Further, the long elimination half-life of mAbs allows for less frequent dosing, which may improve patient acceptability and adherence. Conversely, a number of potential disadvantages of mAbs when used for chronic pain have been identified. Firstly, the molecular size, hydrophilicity, and gastric degradation of mAbs preclude oral administration and necessitate parenteral administration by intravenous, intramuscular, or subcutaneous routes. As a result of hydrophilicity and large molecular size, mAbs are highly limited in their ability to cross the blood–brain barrier. Finally, from a practical perspective, the high cost of developing, manufacturing, and purifying mAbs for commercial use remains a major challenge to their widespread application.16
Although there are a large number of clinical trials of mAbs for chronic pain conditions and reviews describing these studies, literature describing the broad treatment landscape to help practicing physicians is limited. Therefore, this qualitative review designed for physicians aims to (1) provide a practical overview of mAbs in clinical development for chronic pain in relation to their mechanisms of action in common clinical target conditions, (2) lay a foundation to help pain specialists and physicians treating patients with chronic pain to make informed treatment decisions based on these mechanisms of chronic pain, and (3) provide our clinical opinion on the major changes envisioned in this therapeutic area. As use of mAbs as a therapeutic option for chronic pain increases, it is expected that there will be an increasing need for education of both physicians and patients on mAbs.
Material and methods
This qualitative literature review on mAbs for chronic pain used a systematic, preplanned search strategy to locate potentially relevant human clinical studies in English for inclusion. In terms of publication types, evidence from randomized controlled trials, systematic and nonsystematic (narrative) reviews, observational studies, nonclinical studies, and case reports were included. As this review focused primarily on pathophysiological mechanisms in a diverse range of chronic and intermittent (e.g., migraine) pain conditions, no specific restrictions based on outcome measures were included. Search terms for relevant pain conditions were combined using Boolean operators with “monoclonal antibodies” as the intervention search term (Table 1). Studies were identified using electronic searches of MEDLINE via PubMed (1 January 1990 to 9 June 2017) in addition to hand-searching reference lists of relevant systematic and nonsystematic reviews retrieved during the electronic searches.
Table 1.
Included search terms for pain conditions and interventions.
| Subject | Search terms |
|---|---|
| Pain conditions | Acute pain, Chronic pain, Cancer pain, Migraine, Postherpetic neuralgia, Diabetic neuropathy, CLBP, Neuropathic pain, Central sensitization, Peripheral sensitization |
| Interventions | Monoclonal antibodies |
CLBP, chronic low back pain.
Monoclonal antibodies: Mechanisms of action and clinical applications
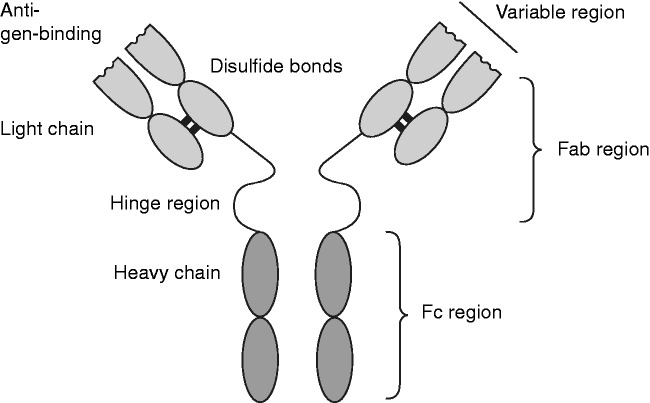
mAbs are artificially produced antibodies developed from single animal or human cell lines and consist of large B-cell-derived glycoproteins made up of two heavy and two light chains held together by disulfide bonds to form a Y-shaped protein (Figure 1). Variable regions within the mAb chains confer antigen specificity that provide different therapeutic actions at different ligands or receptor sites. During the development of mAbs, immunoglobulin isotypes with long elimination half-lives are usually chosen, as these provide an extended pharmacological response that allows for reduced administration frequency.17 As such, half-lives of mAbs under development for chronic pain conditions allow administration at greater intervals between doses than conventional agents. In contrast to small molecule agents, toxicity of mAbs is generally related to ligand- or receptor-mediated interactions rather than dose accumulation. Therefore, the maximal tolerated dose may be difficult to define for mAbs, as adverse reactions may occur even at low doses in susceptible individuals.18 Several distinct pathways and mechanisms have been identified by which specific mAbs can alter pain transmission and perception.
Figure 1.
Schematic representation of monoclonal antibody structure showing functional regions.
Fab: fragment antigen-binding; Fc: fragment crystallizable.
Nerve growth factor
Pain-related mechanisms
Nerve growth factor (NGF) is a pleiotropic neurotrophin that plays a major role in the generation and maintenance of both nociceptive and neuropathic pain.10,12,13 Several sources of evidence support the role of NGF in chronic pain states.12,19,20 Hypoalgesia has been observed in knockout mice lacking NGF as well as in patients with genetic polymorphisms that affect NGF expression.20 In contrast, hyperalgesia has been associated with experimental and clinical administration of NGF in animals and humans, respectively.20 NGF can produce pain perception and hyperalgesia via a number of mechanisms.10,12,13,19,21 Firstly, expression of NGF has been found to occur early in response to inflammatory mediators such as interleukin-1 and tumor necrosis factor-α (TNF-α) that are involved in neurogenic pain transmission.12 In this setting, NGF pain signaling predominantly acts by binding to the tropomyosin receptor kinase A (TrkA) to form an extremely stable complex with an estimated half-life greater than 100 h. This, in turn, facilitates the autophosphorylation of the TrkA intracellular domain and subsequent downstream pain signaling cascades, including the mitogen-activated protein kinase (MAPK) pathway on nociceptive terminals.12,20,22 Secondly, NGF sensitizes nociceptive neurons to painful stimuli through upregulation of ion channels and receptors present on primary afferent nerve fibers and increases the release of pain mediators (e.g., substance P) that potentiate the pain response.12,20 In neuropathic pain, NGF may sensitize nociceptors leading to hyperalgesia.7 In an animal study of neuropathic pain, MNAC13, an anti-TrkA mAb, induced analgesia in both inflammatory and neuropathic pain models in CD1 mice.23 Similarly, AF-556-NA, a polyclonal anti-NGF antibody, reversed tactile allodynia in established models of neuropathic and inflammatory pain in rats and mice.24 In preclinical studies, anti-NGF αD11 mAb effectively antagonized rodent NGF to produce significant and longstanding analgesic effects of persistent pain in mice models.25
Clinical applications
Several mAbs that target NGF, including tanezumab, fulranumab, and fasinumab are in clinical development for various chronic pain conditions, particularly osteoarthritis (OA; Table 2),13 with tanezumab being the most advanced. Phase 3 trials of tanezumab resumed in 2015, following a partial clinical hold on the development program by the US Food and Drug Administration (FDA) in 2012 due to adverse changes noted in the sympathetic nervous system of animals.42 Evidence supports the role of neuropathic pain mechanisms in OA that may be addressed by anti-NGF mAbs.43,44 To date, tanezumab has been shown effective for reducing pain and other symptoms associated with OA of the knee or hip in seven placebo-controlled phase 2/3 clinical trials and in open-label and active-control studies.26–29,45–49 In one study, tanezumab produced reductions in pain scores and also provided improvements in physical function and stiffness scores, with a lower adverse event frequency than oxycodone.45 When added to sustained-release diclofenac, tanezumab resulted in significant improvements in pain, function, and global assessments in a randomized, placebo-controlled trial of 604 patients with moderate-to-severe knee or hip OA.26 Similarly, evidence supports the involvement of neuropathic pain mechanisms in chronic low back pain (CLBP), and NGF has specifically been implicated in pain transmission in patients with CLBP.30,50 In one randomized controlled trial, tanezumab provided statistically and clinically superior analgesic efficacy to placebo and naproxen in patients with CLBP without radiculopathy.30 A larger randomized controlled trial of tanezumab 10 mg (n = 321) or 20 mg (n = 527) found that both doses provided similar and sustained improvements in a number of effectiveness outcome measures, including the Brief Pain Inventory Short Form, Roland Morris Disability Questionnaire, and Patient’s Global Assessment of CLBP.31 These improvements were sustained in a long-term extension study.32 Further, tanezumab provided effective pain reduction in 38 patients with diabetic peripheral neuropathy in a randomized controlled trial,51 although similar results were not shown in a trial of patients with postherpetic or posttraumatic neuralgia.33 In patients with metastatic bone pain, agents that target NGF appear to be a promising strategy.52 In a phase 2 placebo-controlled study, tanezumab was associated with a nonstatistically significant greater reduction in average pain compared with placebo in 59 patients (placebo, n = 30; tanezumab, n = 29) with painful bone metastases.34 In an open-label extension, mean pain scores were reduced compared with baseline through to 40 weeks.34
Table 2.
| Generic name (Sponsor) | Pain conditions under investigation | Study phase | Demonstrated efficacya to date |
|---|---|---|---|
| Tanezumab (Pfizer) | OA of knee/hip | 2–3 | Yes |
| CLBP | 2 | Yes | |
| DPN | 2 | Yes | |
| Interstitial cystitis | 2 | Yes | |
| CP/CPPS | 2 | No | |
| Cancer-related pain | 2 | Yes | |
| Fulranumab (Janssen Research & Development) | OA of knee/hip | 1–2 | Yes |
| CLBP | 2 | No | |
| DPN | 2 | Yes | |
| PHN/PTN | 2 | No | |
| Interstitial cystitis | 2 | No | |
| Cancer-related pain | 2 | No | |
| Fasinumab (Regeneron Pharmaceuticals, Sanofi) | OA of knee/hip | 2–3 | Yes |
| CLBP, including acute sciatic pain | 2–3 | No | |
| ABT-110 (previously PG110) (AbbVie) | CLBP | 2 | Nob |
Compared with placebo.
Study prematurely ended.
CP, chronic prostatitis; CLBP, chronic low back pain; CP/CPPS, chronic prostatitis/chronic pelvic pain syndrome; DPN, diabetic peripheral neuropathy; OA, osteoarthritis; PHN, postherpetic neuralgia; PTN, posttraumatic neuralgia.
Fulranumab has been investigated in patients with chronic OA of the knee or hip,35,53,54 CLBP,35 diabetic peripheral neuropathy,36 postherpetic/posttraumatic neuralgia,33 interstitial cystitis,37 and cancer-related pain.38 In a phase 2 study, fulranumab improved pain, stiffness, and physical function among patients with moderate to severe knee or hip OA pain inadequately controlled by a stable analgesic regimen of NSAIDs and/or opioids (≤200 mg of oral morphine equivalents per day).35 For all efficacy parameters, these improvements were sustained over a 92-week double-blind extension phase.54 In a phase 2, placebo-controlled study of patients with moderate-to-severe painful diabetic peripheral neuropathy, fulranumab demonstrated dose-related reductions in daily pain.36
Fasinumab is under investigation in phase 2/3 studies of patients with OA of either the knee or the hip. In one completed placebo-controlled study, fasinumab was associated with significant improvements in walking knee pain, stiffness, and function.55 Fasinumab has also been investigated for the treatment of acute sciatica but provided no significant clinical benefit compared with placebo for either pain or functional limitations.56 Further trials in back pain have not yet reported results.
Safety and tolerability
Anti-NGFs are well tolerated with a low rate of treatment discontinuation noted in clinical trials (generally <10%).13,26,30–37,45,47,48,51,53,54 Peripheral edema, arthralgia/myalgia, headache, burning sensation, paresthesia/hypoesthesia, and pain in the extremities are among the most commonly observed treatment-emergent adverse events.13,51 Joint failure requiring total joint replacement is an unpredicted safety signal that led the US FDA to place pain-related trials of NGF antagonists on clinical hold. However, in the case of tanezumab, this partial clinical hold was subsequently lifted after a review of a large body of nonclinical data was submitted, leading to the resumption of the phase 3 clinical program. A dose-dependent increase in the risk of rapidly destructive arthropathies, which is greater with a longer duration of anti-NGF exposure and with NSAID coadministration, has also been noted.13 Cutaneous sensory symptoms consistent with peripheral neuropathies are uncommon but may be longstanding and represent unmasking or worsening of existing neuropathies.13
Calcitonin gene-related peptide
Pain-related mechanisms
Calcitonin gene-related peptide (CGRP) is a well characterized neuropeptide occurring in two isoforms (α- and β-). The α-isoform is the most prevalent and is distributed widely throughout the central and peripheral nervous systems.57 The CGRP peptide acts on receptors consisting of a calcitonin receptor-like receptor linked to receptor activity modifying protein 1 (RAMP1), a small transmembrane-spanning essential for full functionality. CGRP has been shown to be involved in both pain transmission and inflammation.57 Importantly, CGRP affects nociceptive transmission by modulating the function of other neurotransmitters and, in the trigeminal ganglion, is often co-expressed with substance P and serotonin receptors.18,57 Immunohistochemistry and radioimmunology studies have demonstrated that CGRP is mainly produced in the cell bodies of both ventral and dorsal root neurons, and is especially common in the trigeminal system, including trigeminal nerve endings, the trigeminal ganglion, and higher order neurons.57
CGRP is considered to have a major role in migraine pathogenesis, as confirmed by studies showing that intravenous CGRP infusion triggers migraine-like attacks in patients with migraine.58 The release of CGRP at trigeminal nerve endings induces vasodilation, edema, and dural mast cell degranulation, leading to neurogenic inflammation, secondary to sensory nerve activation (Figure 2).18,57,59 CGRP also acts on second-order neurons in the trigeminal ganglion that project to the trigeminal nucleus caudalis and C1-C2, which transmit pain signals from the brainstem to the thalamus.18 Satellite glia in the trigeminal ganglion may also respond in a similar way to mast cells in response to CGRP, leading to proinflammatory cytokine release and sensitization of sensory neurons.59 However, central effects of CGRP are also thought to be involved in the development of migraine. In particular, CGRP may contribute to photophobia and the exacerbation of migraine by light, which are common features of clinical migraine attacks (Figure 2).59 Potentiation of migraine pain by light may involve a significant contribution from the posterior thalamus, which contains CGRP-immunoreactive cell bodies (Figure 2).59
Figure 2.
The spectrum of activity of calcitonin gene-related peptide in migraine pain transmission.
CGRP: calcitonin gene-related peptide.
Clinical applications
mAbs may target either the CGRP peptide or the CGRP receptor. In theory, targeting the receptor may result in complete blockade of signaling, compared with targeting the peptide. Arguably, this could lead to increased efficacy but also greater safety concerns. However, neither greater efficacy nor poorer tolerability profiles have been demonstrated in clinical studies.
Anti-CGRP mAbs have predominantly been investigated in migraine and related headache conditions. A recent systematic review revealed that studies have also investigated the role of CGRP in the development of somatic, visceral, inflammatory, and neuropathic pain.60 These studies frequently found correlations between CGRP levels and pain, especially for somatic pain conditions such as OA and CLBP, although no consensus was found for neuropathic pain conditions. To date, there have been no clinical studies to assess the effectiveness of treatments targeting CGRP for pain conditions other than migraine.
For prophylaxis of migraine and related headache conditions, four anti-CGRP mAbs (galcanezumab, eptinezumab, erenumab, and fremanezumab) are currently in clinical development (Table 3).61–63 Most of these mAbs target the CGRP peptide whereas erenumab (AMG-334) targets the CGRP receptor. The clinical spectrum of migraine includes episodic and chronic migraine attacks. Episodic migraine, the most common clinical presentation, is defined as up to 14 headache days (with or without aura) per month.69 However, episodic migraine may transition to chronic migraine, defined as the occurrence of headaches on at least 15 days per month for at least three months, with headaches having features of migraine on at least eight days per month.70 All four anti-CGRP mAbs investigated to date have led to significant reductions from baseline in either episodic and/or chronic migraine days per month compared with placebo.64–67,71,72 A subsequent analysis of phase 2 placebo-controlled trials concluded that anti-CGRP mAbs in clinical development led to similar changes from baseline in migraine days compared with placebo (range: −1.0 to −2.6 days) and numbers needed to treat for responders (range: 4.0–6.2).73 Therefore, targeting the CGRP peptide itself or its receptor does not appear to lead to relative differences in efficacy. In patients with episodic migraine, benefits of anti-CGRP mAbs have been validated in a recent meta-analysis of these placebo-controlled trials.74 Additional phase 2 and 3 studies to further determine the efficacy of these anti-CGRP mAbs in episodic and chronic migraine as well as cluster headache are underway or have recently completed with fully published results anticipated (Table 3).
Table 3.
| Generic name | Sponsor | Mechanism of action | Published studies (migraine type) | Studies underwaya (headache type) |
|---|---|---|---|---|
| Galcanezumab (LY-2951742) | Eli Lilly, Indianapolis, IN, USA | Potent, selective, long-term CGRP inhibition | 1 × Phase 2 (EM) | 2 × Phase 2 (EM) 8 × Phase 3 (EM, CM, CH) |
| Eptinezumab (ALD-403) | Alder Biopharmaceutical, Bothell, WA, USA | Long-term CGRP inhibition | 1 × Phase 2 (EM) | 1 × Phase 2 (CM) 3 × Phase 3 (EM, CM) |
| Erenumab (AMG-334) | Amgen, Cambridge, UK | Potent, selective CGRP receptor antagonism | 2 × Phase 2 (EM, CM) | 3 × Phase 2 (EM, CM) 1 × Phase 3 (EM) |
| Fremanezumab (TEV-48125) | Teva, Petah Tikva, Israel | Long-term CGRP inhibition | 2 × Phase 2 (EM, CM) | 6 × Phase 3 (EM, CM, CH) |
Based on clinicaltrials.gov records; some trials are complete but are awaiting full publication of results.
CGRP, calcitonin gene-related peptide; CH, cluster headache; CM, chronic migraine; EM, episodic migraine.
Safety and tolerability
For mAbs directed against CGRP, adverse events noted in phase 2 clinical studies were generally similar to those noted with placebo and included injection-site reactions, infections, abdominal and back pain, and fatigue.64–67,71 Discontinuation due to adverse events from anti-CGRP mAbs was very low.65–67,71,72 The physiological vasodilatory role of CGRP raises the possibility of vasoconstriction and cardiovascular adverse events as a possible risk.18 However, repeated in vitro studies and in vivo studies in animals and humans have demonstrated that both CGRP receptor antagonists and mAbs directed against CGRP lack such vasoconstrictive activity in coronary and cerebral arteries.18 Indeed, a lack of major cardiovascular effects is the major advantage of inhibition of CGRP over triptans in terms of tolerability profile.75 Inhibition of CGRP via mAbs is also less likely to cause other serious adverse events, including liver toxicity observed with some small molecule CGRP receptor antagonists.76 However, the possible long-term effects of depleting CGRP need to be assessed further in clinical studies.77
Ion channels
Pain-related mechanisms
Ion channels are transmembrane proteins that allow ions to pass into and out of cells to mediate cell excitability and permit cellular signaling.78 A number of ion channels, including sodium, calcium, potassium, gamma aminobutyric acid, and transient receptor potential channels (TRPs) have been identified as being involved in pain transmission.78–80 Experimental studies in animals and humans suggest that certain voltage-gated sodium channels (NaVs), especially NaV1.7 and NaV1.8, are highly expressed in nociceptive neurons of the dorsal root ganglia as well as in small diameter sensory C and Aδ neurons involved in neuropathic and inflammatory pain nociception.79,81 The action of inflammatory mediators, such as serotonin, may also be mediated by increasing the excitability of sodium channels in sensory neurons.79 N-type voltage-gated calcium channels are also highly expressed in dorsal root ganglion cell bodies and at the presynaptic terminals where afferent sensory fibers form synapses with postsynaptic dorsal horn neurons.80 Blockade of N-type voltage-gated calcium channels has been shown to inhibit release of neurotransmitters from central terminals of primary afferent neurons, thereby reducing pain.82
Clinical applications
Ion channels have been identified as having a role in mediating pain transmission in primary afferents in response to sensitizing factors such as inflammatory mediators (e.g., serotonin, prostaglandin).78 In particular, TRPs are a broad group of cation channels involved in transduction and transmission mechanisms.10 Activation of TRPs allows the influx of sodium and calcium and cellular depolarization in response to a wide range of irritants involved in migraine.78 The fact that TRP receptors are expressed on peripheral sensory neurons, and even nonneuronal cells, and are activated by a wide range of noxious stimuli (e.g., heat, pH, irritants) make them desirable potential targets for pain relief. Indeed, TRP receptors have been implicated in a wide range of pain states, including dental pain, migraine, visceral, neuropathic, and cancer-related pain.83 Several non-mAb agents that target TRP receptors have been investigated, including multiple TRP cation channel V1 inhibitors (TRPV1)-targeted therapies to potentially treat migraine. However, to date, there are no mAbs targeting TRP receptors in clinical development. Similarly, voltage-gated calcium channels are thought to be causally involved in neuropathic pain and blockers of different receptor types (e.g., N-, T-, L-) have been developed or under investigation. These include the cone snail peptides ω-conotoxin-GVIA and ω-conotoxin-MVIIA (Ziconotide, Prialt®), as well as better known treatments such as gabapentin and pregabalin, calcium-channel alpha(2)delta ligands that reduce the calcium-dependent release of multiple neurotransmitters.80 However, once again, mAbs targeting calcium channels involved in pain transmission are not in clinical development. An experimental mAb that targeted a NaV1.7 channel reduced pain and itch in mice when this mAb was administered by the systemic and intrathecal routes.81 This confirmed that NaV1.7 controls pain via both peripheral and central mechanisms and also the feasibility of developing a therapeutic mAb to target pain via these ion channels. However, despite the great potential of mAbs targeting sodium or other ion channels based on preclinical studies, there are no agents in clinical development for the treatment of pain.
Safety and tolerability
Regarding mAb ion channel antagonists, a key challenge in developing clinically useful agents is the potential for interrupting transmission of a wide range of unrelated neural and nonneural pathways.10 For example, non-mAbs that target sodium channels lack specificity for pain-related transmission, leading to the potential for serious effects such as cardiotoxicity.81 Another example is the case of TRPV1 inhibitors, which can lead to significant hypothermia and loss of heat perception, resulting in burns.10 Evolving research into the mechanisms by which ion channel antagonists may interrupt pain transmission will need to conduct careful investigation into the effects on other essential pathways. Further, it is yet to be determined if selective mAbs targeting these mechanisms of action will have the same shortcomings.
Tumor necrosis factor-α
Pain-related mechanisms
TNF-α is a pleiotropic cytokine with a well-characterized role in inflammation that has been shown to be effective at reducing symptoms and markers of inflammation in conditions such as rheumatoid arthritis and complex regional pain syndrome.8,84–86 However, TNF-α is also involved at multiple levels in both peripheral and central mechanisms of pain transmission.8,86 TNF-α is secreted by immune cells including T-cells, macrophages, and neutrophils, and is integral to inflammatory response. However, in both mice and human studies, high TNF-α expression has been detected at sites of nerve injury, consistent with it being a neuropathic pain-related cytokine.8 Further, peripheral sensitization of injured nerves may be mediated by neuronal apoptosis in the dorsal root ganglion via caspase-3 cell death pathways stimulated by TNF-α.87 Regarding central pain transmission, there is good support for a key role of TNF-α in a so-called “immune-to-brain” model of pain transmission.8 For example, evidence suggests that TNF-α mediates central mechanisms of neuropathic pain through microglial systems, in which spinal astrocytes may lead to chronic pain sensitization via TNF-α-induced activation of the MAPK system, which is also involved in NGF-mediated pain transmission.8,88 Microglial release of TNF-α also increases neurotransmission in the dorsal horn via presynaptic increase in glutamate release (via TRPV1 activation) and postsynaptic increase in N-methyl-D-aspartate and other receptor activity. In a study of mice and humans, neutralization of TNF-α has been shown to block nociceptive CNS activity in the thalamus and somatosensory cortex, as well as activating the limbic system.84 These effects were noted as early as 24 h after infusion, whereas clinical and laboratory markers of inflammation (e.g., joint swelling, acute phase reactants) were not affected by the anti-TNF-α inhibitors at these early time points. Finally, in terms of mechanisms specifically related to back pain, TNF-α-induced release of inflammatory mediators, including IL-6 and nitric oxide, has been noted in pathologic intervertebral disc cells.89
Clinical applications
Currently available mAbs that target TNF-α, including adalimumab, etanercept, and infliximab, have been assessed in a range of chronic autoimmune inflammatory conditions that include pain as a major symptom, particularly rheumatoid arthritis. The anti-inflammatory effects of TNF-α inhibitors that lead to pain relief can be difficult to differentiate from effects produced by pain-specific pathways.
Regarding specific pain conditions, mAb TNF-α antagonists have been assessed in ankylosing spondylitis, CLBP with or without radiculopathy, and sciatica. Ankylosing spondylitis is a chronic autoimmune inflammatory disease and a prototypical spondyloarthritis that mainly affects the axial skeleton with possible peripheral joint and nonarticular involvement. Evidence from a study combining a questionnaire, mechanical and thermal thresholds, and brain imaging has indicated that ankylosing spondylitis is a mixed pain condition with a neuropathic component.90 In a study of 10 patients with ankylosing spondylitis, anti-TNF-α treatment led to a reduction in Bath Ankylosing Spondyloarthritis Disease Activity Index, pain intensity (as assessed by a visual analogue scale), and analgesic consumption.91 A more recent retrospective chart review of 129 ankylosing spondylitis patients found that, after ≥10 weeks of TNF-α inhibitor treatment, approximately 60% of patients had clinically significant improvements (>30%) in pain, including neuropathic pain.92 Regarding CLBP with or without radiculopathy, or sciatica, recent systematic reviews have found that there is currently insufficient evidence to recommend these agents apart from a reduction in the risk of discectomy or radicular block.93–95 However, given that significant reductions in pain intensity were noted in several individual trials, there is sufficient evidence to suggest that further trials are needed, and that results from these could change clinical recommendations in the future.
Safety and tolerability
The tolerability of available mAb TNF-α antagonists has been well characterized in studies of conventional indications, including cancer therapy, psoriasis, inflammatory arthritis, and seronegative spondyloarthritis. A review of the safety and tolerability of mAb TNF-α antagonists in psoriasis concluded that adverse events with relevant agents were not common and the risk of severe adverse events was low.96 In a meta-analysis of trials in CLBP with radiculopathy, a pooled analysis found no difference in the incidence of adverse events between mAb TNF-α antagonists and placebo (risk ratio = 0.93).95 Among the serious adverse events that may occur with TNF-α inhibitors are infections (including reactivation of tuberculosis), malignancies (e.g., lymphoma), and worsening of congestive heart failure.97
Epidermal growth factor receptor
Pain-related mechanisms
The epidermal growth factor receptor (EGFR) is a member of the ErbB family of tyrosine kinase receptors involved in the pathogenesis and progression of different carcinoma types.98 Hence, EGFR inhibitors have been mainly investigated as potential cancer treatments. For example, cetuximab, in combination with irinotecan, is approved for use for the treatment of EGFR-expressing, metastatic, colorectal cancer following failure of irinotecan, or as a single agent in patients with EGFR-expressing, metastatic, colorectal cancer who are intolerant to irinotecan.99 However, the observation of rapid pain reduction with cetuximab in the absence of tumor shrinkage has raised the possibility that this agent may specifically disrupt pain transmission via mechanism-specific pathways.100 Specifically, inhibition of EGFR can interfere with MAPK signaling, which is a key driver of neuropathic pain via multiple inflammatory mediators.100,101
Clinical applications
The notion that anti-EGFR mAbs may be an effective strategy for pain treatment was first suggested in a 62-year-old male patient with neuropathic pain associated with a recurrence of metastatic rectal cancer.100 This patient experienced rapid pain relief following initiation of the anti-EGFR mAb cetuximab in the presence of pain relief despite radiological progression, suggesting the direct involvement of the EGFR pathway as the mechanism of pain relief.100 The possibility suggested by this case led to an open-label study of 20 patients with neuropathic pain who were treated with one of four anti-EGFR mAbs (cetuximab, panitumumab, gefitinib, and erlotinib).101 Almost all patients experienced rapid (<24 h) clinically significant pain relief (≥2 point decrease on a 0–10 numerical rating scale for worst pain in the last 24 h).101 Despite these positive results, controlled clinical trials are still awaited, and the use of anti-EGFR mAbs for pain relief is not currently an approved indication.
Safety and tolerability
Anti-EGFR molecules represent a relatively new form of potential treatment for chronic pain. In cancer patients treated with cetuximab, common adverse events include skin problems (acne-like rash, skin drying and cracking, infections, and abnormal hair growth) and hypersensitivity reactions.99 In the open-label study of patients with neuropathic pain treated with anti-EGFR mAbs, mild skin changes were the most commonly noted adverse events and were noted in 80% of patients.101
Anti-drug antibodies and the importance of assay sensitivity
The pharmacokinetics of mAbs are complex and greatly affected by variability in clearance and absorption, which can lead to wide interindividual differences in exposure.102 Anti-drug antibodies (ADAs) may also affect mAb pharmacokinetics and hence exposure, leading to loss of efficacy or response. Repeated episodic exposure is a key factor that exacerbates the development of ADAs. To reduce the possibility of such exposure, periods during which mAbs are extremely low (i.e., nonmeasurable) should be avoided.102
From a practical perspective, direct measurement of mAb levels may help avoid low levels that may contribute to ADA development. As a result, the various platforms for detecting mAbs and their relative sensitivity and other features should be carefully considered. In addition to the traditional enzyme-linked immunosorbent assay (ELISA), several alternative assay platforms, including the Meso Scale Discovery® (MSD), Gyros®, AlphaLISA®, and LC-MS/MS technologies with improved sensitivity, dynamic range, and other advantages have been developed.103 Optimal assay sensitivity, which has been noted with MSD and Gyros®, is particularly important when mAb levels have a short half-life related to factors such as body weight and disease severity.103
The future of monoclonal antibodies in chronic pain
Many patients with chronic pain remain a challenge to treat and respond only partially to currently available treatment options, which are often poor at targeting pain specifically, leading to a range of adverse effects. For patients with chronic pain that is unresponsive or poorly tolerant to conventional forms of treatment, mAbs may address an unmet need. For example, current prophylactic treatments for migraine are often ineffective and can also lead to adverse events that can contribute to poor treatment adherence.76 In this setting, certain mAbs have emerged as a possible option.12
The development of mAbs for chronic pain faces several obstacles before widespread clinical application can be considered. Firstly, with the exception of tanezumab, results of some clinical trials have been less than remarkable.13 Secondly, the tolerability and safety of many mAbs in development for these indications is not entirely clear. Thirdly, high costs remain an important practical issue when considering the use of mAbs for chronic pain and may limit widespread application of these agents. Based on these limitations, some have questioned whether mAbs will adequately meet expectations for chronic pain therapy.13
The future of mAbs for chronic pain will likely depend on (1) additional research to fully characterize the therapeutic potential of agents in terms of efficacy and safety and (2) the ability to match patients in terms of their likelihood to respond to mAb therapy to help ensure agents are maximally effective in the chosen population, while minimizing overall harm and direct treatment costs. Additional research includes carefully conducted clinical studies to determine the safety of mAbs and to subsequently characterize their efficacy, including comparison with accepted treatment options. In addition to efficacy, which generally relates to pain reduction, improvements in function and quality of life as well as effects on pharmacoeconomic parameters also need to be assessed. Regarding patient matching, strategies related to clinical factors, biomarkers, or genetic markers may need to be developed to identify individual patients with the greatest need and likelihood of treatment success from mAb therapy. Assessment of need may be based on demonstrating that patients have exhausted optimized treatment options and combinations, or that there are serious tolerability or safety issues with less expensive therapies. Determining likelihood of treatment success is potentially more difficult to assess. However, phenotyping may present one possible solution, although the actual prognostic value of this needs to be investigated further.
To reduce safety concerns, more data on condition-specific risks and commitments by sponsor companies to submit additional nonclinical data before initiating further clinical trials have been suggested. These strategies may facilitate clinical trial approvals from regulatory bodies.12 Despite the apparent effectiveness of certain mAbs in certain chronic pain conditions, similar results are not always replicated in other chronic pain conditions.12 Further, the design and conduct of clinical trials, including issues related to, for example, patient inclusion/exclusion criteria and disease severity, can greatly affect clinical outcomes and the ability to replicate findings among different trials. Hence, no conclusions should be made regarding replication of study findings unless almost identical clinical trial protocols have been used. Future long-term studies should also provide more information about the incidence and clinical consequences of anti-mAbs antibodies. Studies should also assess the potential for loss of response with continuous mAb therapy, and which forms of combination treatment with other pharmacological and nonpharmacological options are suitable for each kind of chronic pain.
Conclusion
For chronic pain inadequately treated by current treatments, mAbs represent a potential new option. Major classes of mAbs in clinical development for chronic pain target NGF, CGRP, TNF-α, ion channels, and EGFR, each of which has specific mechanisms of action related to pain transmission as well as central and peripheral sensitization. Specific mAbs have been shown to be effective for a variety of chronic pain states, including migraine, neuropathic pain, OA, CLBP, ankylosing spondylitis, and pain associated with cancer (e.g., bone metastases). However, the ongoing clinical development and application of these agents in clinical practice depends on their ability to demonstrate efficacy while balancing these benefits against potential tolerability issues. In addition to tolerability concerns, cost considerations represent a key potential limitation of mAbs for chronic pain. For these reasons, patients with unmet needs in terms of pain relief, prevention of pain, or tolerability issues from currently available treatments are mostly likely to benefit from mAbs targeting pain pathways. Clinicians who treat chronic pain should begin to become acquainted with information regarding these treatment options in development, as they are likely to change the therapeutic landscape of chronic pain management. In doing so, clinicians should become better equipped at characterizing suitable patients for these new options according to disease state, mechanism of action, comorbidity, safety, and tolerability profile.
Acknowledgments
Medical writing assistance was provided by Mark Snape, MBBS, CMPP and Serina Stretton, PhD, CMPP of ProScribe—Envision Pharma Group. ProScribe’s services complied with international guidelines for Good Publication Practice (GPP3).
Author Contributions
All authors participated in the interpretation of collected literature, and in the drafting, critical revision, and approval of the final version of the manuscript.
Declaration of conflicting interests
The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: HJD, KWJ, and VK are current employees of and own shares in Eli Lilly and Company. JY is a former employee of Eli Lilly and Company. RG has received speaker’s honoraria from Eli Lilly and Company, Grunenthal, Mundipharma, and Pfizer and has been a board member for Grunenthal. AA has received speaker’s honoraria from Abbvie and Pfizer.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This narrative review was sponsored by Eli Lilly and Company which was involved in the preparation of the manuscript.
References
- 1.International Association for the Study of Pain. IASP taxonomy, https://www.iasp-pain.org/Taxonomy (accessed 19 June 2016).
- 2.Treede RD, Rief W, Barke A, et al. A classification of chronic pain for ICD-11. Pain 2015; 156: 1003–1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Apkarian AV, Baliki MN, Farmer MA. Predicting transition to chronic pain. Curr Opin Neurol 2013; 26: 360–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kyranou M, Puntillo K. The transition from acute to chronic pain: might intensive care unit patients be at risk? Ann Intensive Care 2012; 2: 36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Woolf CJ. Central sensitization: implications for the diagnosis and treatment of pain. Pain 2011; 152: S2–S15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Clark AK, Old EA, Malcangio M. Neuropathic pain and cytokines: current perspectives. J Pain Res 2013; 6: 803–814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Campbell JN, Meyer RA. Mechanisms of neuropathic pain. Neuron 2006; 52: 77–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Leung L, Cahill CM. TNF-alpha and neuropathic pain – a review. J Neuroinflammation 2010; 7: 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sikandar S, Dickenson AH. Visceral pain: the ins and outs, the ups and downs. Curr Opin Support Palliat Care 2012; 6: 17–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Burgess G, Williams D. The discovery and development of analgesics: new mechanisms, new modalities. J Clin Invest 2010; 120: 3753–3759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Martelletti P. The therapeutic armamentarium in migraine is quite elderly. Expert Opin Drug Metab Toxicol 2015; 11: 175–177. [DOI] [PubMed] [Google Scholar]
- 12.Bannwarth B, Kostine M. Targeting nerve growth factor (NGF) for pain management: what does the future hold for NGF antagonists? Drugs 2014; 74: 619–626. [DOI] [PubMed] [Google Scholar]
- 13.Bannwarth B, Kostine M. Biologics in the treatment of chronic pain: a new era of therapy? Clin Pharmacol Ther 2015; 97: 122–124. [DOI] [PubMed] [Google Scholar]
- 14.Chessell IP, Dudley A, Billinton A. Biologics: the next generation of analgesic drugs? Drug Discov Today 2012; 17: 875–879. [DOI] [PubMed] [Google Scholar]
- 15.Knezevic NN, Mandalia S, Raasch J, et al. Treatment of chronic low back pain – new approaches on the horizon. J Pain Res 2017; 10: 1111–1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Samaranayake H, Wirth T, Schenkwein D, et al. Challenges in monoclonal antibody-based therapies. Ann Med 2009; 41: 322–331. [DOI] [PubMed] [Google Scholar]
- 17.Keizer RJ, Huitema AD, Schellens JH, et al. Clinical pharmacokinetics of therapeutic monoclonal antibodies. Clin Pharmacokinet 2010; 49: 493–507. [DOI] [PubMed] [Google Scholar]
- 18.Bigal ME, Walter S, Rapoport AM. Calcitonin gene-related peptide (CGRP) and migraine current understanding and state of development. Headache 2013; 53: 1230–1244. [DOI] [PubMed] [Google Scholar]
- 19.McKelvey L, Shorten GD, O’Keeffe GW. Nerve growth factor-mediated regulation of pain signalling and proposed new intervention strategies in clinical pain management. J Neurochem 2013; 124: 276–289. [DOI] [PubMed] [Google Scholar]
- 20.Watson JJ, Allen SJ, Dawbarn D. Targeting nerve growth factor in pain: what is the therapeutic potential? BioDrugs 2008; 22: 349–359. [DOI] [PubMed] [Google Scholar]
- 21.Eibl JK, Strasser BC, Ross GM. Structural, biological, and pharmacological strategies for the inhibition of nerve growth factor. Neurochem Int 2012; 61: 1266–1275. [DOI] [PubMed] [Google Scholar]
- 22.Abdiche YN, Malashock DS, Pons J. Probing the binding mechanism and affinity of tanezumab, a recombinant humanized anti-NGF monoclonal antibody, using a repertoire of biosensors. Protein Sci 2008; 17: 1326–1335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ugolini G, Marinelli S, Covaceuszach S, et al. The function neutralizing anti-TrkA antibody MNAC13 reduces inflammatory and neuropathic pain. Proc Natl Acad Sci U S A 2007; 104: 2985–2990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wild KD, Bian D, Zhu D, et al. Antibodies to nerve growth factor reverse established tactile allodynia in rodent models of neuropathic pain without tolerance. J Pharmacol Exp Ther 2007; 322: 282–287. [DOI] [PubMed] [Google Scholar]
- 25.Covaceuszach S, Marinelli S, Krastanova I, et al. Single cycle structure-based humanization of an anti-nerve growth factor therapeutic antibody. PLoS One 2012; 7: e32212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Balanescu AR, Feist E, Wolfram G, et al. Efficacy and safety of tanezumab added on to diclofenac sustained release in patients with knee or hip osteoarthritis: a double-blind, placebo-controlled, parallel-group, multicentre phase III randomised clinical trial. Ann Rheum Dis 2014; 73: 1665–1672. [DOI] [PubMed] [Google Scholar]
- 27.Brown MT, Murphy FT, Radin DM, et al. Tanezumab reduces osteoarthritic hip pain: results of a randomized, double-blind, placebo-controlled phase III trial. Arthritis Rheum 2013; 65: 1795–1803. [DOI] [PubMed] [Google Scholar]
- 28.Lane NE, Schnitzer TJ, Birbara CA, et al. Tanezumab for the treatment of pain from osteoarthritis of the knee. N Engl J Med 2010; 363: 1521–1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schnitzer TJ, Ekman EF, Spierings EL, et al. Efficacy and safety of tanezumab monotherapy or combined with non-steroidal anti-inflammatory drugs in the treatment of knee or hip osteoarthritis pain. Ann Rheum Dis 2015; 74: 1202–1211. [DOI] [PubMed] [Google Scholar]
- 30.Katz N, Borenstein DG, Birbara C, et al. Efficacy and safety of tanezumab in the treatment of chronic low back pain. Pain 2011; 152: 2248–2258. [DOI] [PubMed] [Google Scholar]
- 31.Kivitz AJ, Gimbel JS, Bramson C, et al. Efficacy and safety of tanezumab versus naproxen in the treatment of chronic low back pain. Pain 2013; 154: 1009–1021. [DOI] [PubMed] [Google Scholar]
- 32.Gimbel JS, Kivitz AJ, Bramson C, et al. Long-term safety and effectiveness of tanezumab as treatment for chronic low back pain. Pain 2014; 155: 1793–1801. [DOI] [PubMed] [Google Scholar]
- 33.Wang H, Romano G, Fedgchin M, et al. Fulranumab in patients with pain associated with postherpetic neuralgia and postraumatic neuropathy: efficacy, safety, and tolerability results from a randomized, double-blind, placebo-controlled, phase-2 study. Clin J Pain 2017; 33: 99–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sopata M, Katz N, Carey W, et al. Efficacy and safety of tanezumab in the treatment of pain from bone metastases. Pain 2015; 156: 1703–1713. [DOI] [PubMed] [Google Scholar]
- 35.Sanga P, Katz N, Polverejan E, et al. Efficacy, safety, and tolerability of fulranumab, an anti-nerve growth factor antibody, in the treatment of patients with moderate to severe osteoarthritis pain. Pain 2013; 154: 1910–1919. [DOI] [PubMed] [Google Scholar]
- 36.Wang H, Romano G, Frustaci ME, et al. Fulranumab for treatment of diabetic peripheral neuropathic pain: a randomized controlled trial. Neurology 2014; 83: 628–637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang H, Russell LJ, Kelly KM, et al. Fulranumab in patients with interstitial cystitis/bladder pain syndrome: observations from a randomized, double-blind, placebo-controlled study. BMC Urol 2017; 17: 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Slatkin N, Zaki N, Sanga P, et al. 382) Efficacy, safety, and tolerability of fFulranumab as adjunctive therapy for cancer-related pain: a randomized, double-blind, placebo-controlled, multicenter study. J Pain 2016; 17: S70–S71. [DOI] [PubMed] [Google Scholar]
- 39.Jayabalan P, Schnitzer TJ. Tanezumab in the treatment of chronic musculoskeletal conditions. Expert Opin Biol Ther 2017; 17: 245–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nickel JC, Mills IW, Crook TJ, et al. Tanezumab reduces pain in women with interstitial cystitis/bladder pain syndrome and patients with nonurological associated somatic syndromes. J Urol 2016; 195: 942–948. [DOI] [PubMed] [Google Scholar]
- 41.Zheng S, Hunter DJ, Xu J, et al. Monoclonal antibodies for the treatment of osteoarthritis. Expert Opin Biol Ther 2016; 16: 1529–1540. [DOI] [PubMed] [Google Scholar]
- 42.Pfizer Press Release. Pfizer and Lilly preparing to resume phase 3 chronic pain program for tanezumab, http://www.pfizer.com/news/press-release/press-release-detail/pfizer_and_lilly_preparing_to_resume_phase_3_chronic_pain_program_for_tanezumab (accessed 28 July 2016).
- 43.Duarte RV, Raphael JH, Dimitroulas T, et al. Osteoarthritis pain has a significant neuropathic component: an exploratory in vivo patient model. Rheumatol Int 2014; 34: 315–320. [DOI] [PubMed] [Google Scholar]
- 44.Oteo-Alvaro A, Ruiz-Iban MA, Miguens X, et al. High prevalence of neuropathic pain features in patients with knee osteoarthritis: a cross-sectional study. Pain Pract 2014; 15: 618–626. [DOI] [PubMed] [Google Scholar]
- 45.Spierings EL, Fidelholtz J, Wolfram G, et al. A phase III placebo- and oxycodone-controlled study of tanezumab in adults with osteoarthritis pain of the hip or knee. Pain 2013; 154: 1603–1612. [DOI] [PubMed] [Google Scholar]
- 46.Brown MT, Murphy FT, Radin DM, et al. Tanezumab reduces osteoarthritic knee pain: results of a randomized, double-blind, placebo-controlled phase III trial. J Pain 2012; 13: 790–798. [DOI] [PubMed] [Google Scholar]
- 47.Ekman EF, Gimbel JS, Bello AE, et al. Efficacy and safety of intravenous tanezumab for the symptomatic treatment of osteoarthritis: 2 randomized controlled trials versus naproxen. J Rheumatol 2014; 41: 2249–2259. [DOI] [PubMed] [Google Scholar]
- 48.Nagashima H, Suzuki M, Araki S, et al. Preliminary assessment of the safety and efficacy of tanezumab in Japanese patients with moderate to severe osteoarthritis of the knee: a randomized, double-blind, dose-escalation, placebo-controlled study. Osteoarthritis Cartilage 2011; 19: 1405–1412. [DOI] [PubMed] [Google Scholar]
- 49.Schnitzer TJ, Lane NE, Birbara C, et al. Long-term open-label study of tanezumab for moderate to severe osteoarthritic knee pain. Osteoarthritis Cartilage 2011; 19: 639–646. [DOI] [PubMed] [Google Scholar]
- 50.Fishbain DA, Cole B, Lewis JE, et al. What is the evidence that neuropathic pain is present in chronic low back pain and soft tissue syndromes? An evidence-based structured review. Pain Med 2014; 15: 4–15. [DOI] [PubMed] [Google Scholar]
- 51.Bramson C, Herrmann DN, Carey W, et al. Exploring the role of tanezumab as a novel treatment for the relief of neuropathic pain. Pain Med 2015; 16: 1163–1176. [DOI] [PubMed] [Google Scholar]
- 52.Pantano F, Zoccoli A, Iuliani M, et al. New targets, new drugs for metastatic bone pain: a new philosophy. Expert Opin Emerg Drugs 2011; 16: 403–405. [DOI] [PubMed] [Google Scholar]
- 53.Gow JM, Tsuji WH, Williams GJ, et al. Safety, tolerability, pharmacokinetics, and efficacy of AMG 403, a human anti-nerve growth factor monoclonal antibody, in two phase I studies with healthy volunteers and knee osteoarthritis subjects. Arthritis Res Ther 2015; 17: 282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sanga P, Katz N, Polverejan E, et al. Long-term safety and efficacy of fulranumab in patients with moderate-to-severe osteoarthritis pain: a phase II randomized, double-blind, placebo-controlled extension study. Arthritis Rheumatol 2017; 69: 763–773. [DOI] [PubMed] [Google Scholar]
- 55.Tiseo PJ, Kivitz AJ, Ervin JE, et al. Fasinumab (REGN475), an antibody against nerve growth factor for the treatment of pain: results from a double-blind, placebo-controlled exploratory study in osteoarthritis of the knee. Pain 2014; 155: 1245–1252. [DOI] [PubMed] [Google Scholar]
- 56.Tiseo PJ, Ren H, Mellis S. Fasinumab (REGN475), an antinerve growth factor monoclonal antibody, for the treatment of acute sciatic pain: results of a proof-of-concept study. J Pain Res 2014; 7: 523–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bigal ME, Walter S. Monoclonal antibodies for migraine: preventing calcitonin gene-related peptide activity. CNS Drugs 2014; 28: 389–399. [DOI] [PubMed] [Google Scholar]
- 58.Hansen JM, Hauge AW, Olesen J, et al. Calcitonin gene-related peptide triggers migraine-like attacks in patients with migraine with aura. Cephalalgia 2010; 30: 1179–1186. [DOI] [PubMed] [Google Scholar]
- 59.Raddant AC, Russo AF. Calcitonin gene-related peptide in migraine: intersection of peripheral inflammation and central modulation. Expert Rev Mol Med 2011; 13: e36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Schou WS, Ashina S, Amin FM, et al. Calcitonin gene-related peptide and pain: a systematic review. J Headache Pain 2017; 18: 34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Shi L, Lehto SG, Zhu DX, et al. Pharmacologic characterization of AMG 334, a potent and selective human monoclonal antibody against the calcitonin gene-related peptide receptor. J Pharmacol Exp Ther 2016; 356: 223–231. [DOI] [PubMed] [Google Scholar]
- 62.Schuster NM, Vollbracht S, Rapoport AM. Emerging treatments for the primary headache disorders. Neurol Sci 2015; 36 Suppl 1: 109–113. [DOI] [PubMed] [Google Scholar]
- 63.Walter S, Bigal ME. TEV-48125: a review of a monoclonal CGRP antibody in development for the preventive treatment of migraine. Curr Pain Headache Rep 2015; 19: 6. [DOI] [PubMed] [Google Scholar]
- 64.Bigal ME, Edvinsson L, Rapoport AM, et al. Safety, tolerability, and efficacy of TEV-48125 for preventive treatment of chronic migraine: a multicentre, randomised, double-blind, placebo-controlled, phase 2b study. Lancet Neurol 2015; 14: 1091–1100. [DOI] [PubMed] [Google Scholar]
- 65.Bigal ME, Dodick DW, Rapoport AM, et al. Safety, tolerability, and efficacy of TEV-48125 for preventive treatment of high-frequency episodic migraine: a multicentre, randomised, double-blind, placebo-controlled, phase 2b study. Lancet Neurol 2015; 14: 1081–1090. [DOI] [PubMed] [Google Scholar]
- 66.Dodick DW, Goadsby PJ, Silberstein SD, et al. Safety and efficacy of ALD403, an antibody to calcitonin gene-related peptide, for the prevention of frequent episodic migraine: a randomised, double-blind, placebo-controlled, exploratory phase 2 trial. Lancet Neurol 2014; 13: 1100–1107. [DOI] [PubMed] [Google Scholar]
- 67.Sun H, Dodick DW, Silberstein S, et al. Safety and efficacy of AMG 334 for prevention of episodic migraine: a randomised, double-blind, placebo-controlled, phase 2 trial. Lancet Neurol 2016; 15: 382–390. [DOI] [PubMed] [Google Scholar]
- 68.Barbanti P, Aurilia C, Fofi L, et al. The role of anti-CGRP antibodies in the pathophysiology of primary headaches. Neurol Sci 2017; 38: 31–35. [DOI] [PubMed] [Google Scholar]
- 69.Katsarava Z, Buse DC, Manack AN, et al. Defining the differences between episodic migraine and chronic migraine. Curr Pain Headache Rep 2012; 16: 86–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Headache Classification Committee of the International Headache Society (IHS). The International Classification of Headache Disorders, 3rd edition (beta version). Cephalalgia 2013; 33: 629–808. [DOI] [PubMed] [Google Scholar]
- 71.Dodick DW, Goadsby PJ, Spierings EL, et al. Safety and efficacy of LY2951742, a monoclonal antibody to calcitonin gene-related peptide, for the prevention of migraine: a phase 2, randomised, double-blind, placebo-controlled study. Lancet Neurol 2014; 13: 885–892. [DOI] [PubMed] [Google Scholar]
- 72.Tepper S, Ashina M, Reuter U, et al. Safety and efficacy of erenumab for preventive treatment of chronic migraine: a randomised, double-blind, placebo-controlled phase 2 trial. Lancet Neurol 2017; 16: 425–434. [DOI] [PubMed] [Google Scholar]
- 73.Mitsikostas DD, Reuter U. Calcitonin gene-related peptide monoclonal antibodies for migraine prevention: comparisons across randomized controlled studies. Curr Opin Neurol 2017; 30: 272–280. [DOI] [PubMed] [Google Scholar]
- 74.Hong P, Wu X, Liu Y. Calcitonin gene-related peptide monoclonal antibody for preventive treatment of episodic migraine: a meta analysis. Clin Neurol Neurosurg 2017; 154: 74–78. [DOI] [PubMed] [Google Scholar]
- 75.de Prado BM, Russo AF. CGRP receptor antagonists: a new frontier of anti-migraine medications. Drug Discov Today Ther Strateg 2006; 3: 593–597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Mitsikostas DD, Rapoport AM. New players in the preventive treatment of migraine. BMC Med 2015; 13: 279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Giamberardino MA, Affaitati G, Curto M, et al. Anti-CGRP monoclonal antibodies in migraine: current perspectives. Intern Emerg Med 2016; 11: 1045–1057. [DOI] [PubMed] [Google Scholar]
- 78.Yan J, Dussor G. Ion channels and migraine. Headache 2014; 54: 619–639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Christidis N, Kang I, Cairns BE, et al. Expression of 5-HT3 receptors and TTX resistant sodium channels (Na(V)1.8) on muscle nerve fibers in pain-free humans and patients with chronic myofascial temporomandibular disorders. J Headache Pain 2014; 15: 63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Perret D, Luo ZD. Targeting voltage-gated calcium channels for neuropathic pain management. Neurotherapeutics 2009; 6: 679–692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Lee JH, Park CK, Chen G, et al. A monoclonal antibody that targets a NaV1.7 channel voltage sensor for pain and itch relief. Cell 2014; 157: 1393–1404. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 82.Schmidtko A, Lotsch J, Freynhagen R, et al. Ziconotide for treatment of severe chronic pain. Lancet 2010; 375: 1569–1577. [DOI] [PubMed] [Google Scholar]
- 83.Nilius B, Szallasi A. Transient receptor potential channels as drug targets: from the science of basic research to the art of medicine. Pharmacol Rev 2014; 66: 676–814. [DOI] [PubMed] [Google Scholar]
- 84.Hess A, Axmann R, Rech J, et al. Blockade of TNF-alpha rapidly inhibits pain responses in the central nervous system. Proc Natl Acad Sci U S A 2011; 108: 3731–3736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Maini RN, Breedveld FC, Kalden JR, et al. Therapeutic efficacy of multiple intravenous infusions of anti-tumor necrosis factor alpha monoclonal antibody combined with low-dose weekly methotrexate in rheumatoid arthritis. Arthritis Rheum 1998; 41: 1552–1563. [DOI] [PubMed] [Google Scholar]
- 86.Dirckx M, Groeneweg G, Wesseldijk F, et al. Report of a preliminary discontinued double-blind, randomized, placebo-controlled trial of the anti-TNF-alpha chimeric monoclonal antibody infliximab in complex regional pain syndrome. Pain Pract 2013; 13: 633–640. [DOI] [PubMed] [Google Scholar]
- 87.Kim SH, Nam JS, Choi DK, et al. Tumor necrosis factor-alpha and apoptosis following spinal nerve ligation injury in rats. Korean J Pain 2011; 24: 185–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Ji RR, Gereau RW, 4th, Malcangio M, et al. MAP kinase and pain. Brain Res Rev 2009; 60: 135–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Sinclair SM, Shamji MF, Chen J, et al. Attenuation of inflammatory events in human intervertebral disc cells with a tumor necrosis factor antagonist. Spine (Phila Pa 1976) 2011; 36: 1190–1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Wu Q, Inman RD, Davis KD. Neuropathic pain in ankylosing spondylitis: a psychophysics and brain imaging study. Arthritis Rheum 2013; 65: 1494–1503. [DOI] [PubMed] [Google Scholar]
- 91.Morovic-Vergles J, Gamulin S. Anti-TNFalpha therapy and control of chronic pain in ankylosing spondylitis. J Pain Symptom Manage 2010; 40: e9–e11. [DOI] [PubMed] [Google Scholar]
- 92.Wu Q, Inman RD, Davis KD. Tumor necrosis factor inhibitor therapy in ankylosing spondylitis: differential effects on pain and fatigue and brain correlates. Pain 2015; 156: 297–304. [DOI] [PubMed] [Google Scholar]
- 93.Wang YF, Chen PY, Chang W, et al. Clinical significance of tumor necrosis factor-alpha inhibitors in the treatment of sciatica: a systematic review and meta-analysis. PLoS One 2014; 9: e103147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Williams NH, Lewis R, Din NU, et al. A systematic review and meta-analysis of biological treatments targeting tumour necrosis factor alpha for sciatica. Eur Spine J 2013; 22: 1921–1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Pimentel DC, El Abd O, Benyamin RM, et al. Anti-tumor necrosis factor antagonists in the treatment of low back pain and radiculopathy: a systematic review and meta-analysis. Pain Physician 2014; 17: E27–E44. [PubMed] [Google Scholar]
- 96.Semble AL, Davis SA, Feldman SR. Safety and tolerability of tumor necrosis factor-alpha inhibitors in psoriasis: a narrative review. Am J Clin Dermatol 2014; 15: 37–43. [DOI] [PubMed] [Google Scholar]
- 97.Singh JA, Wells GA, Christensen R, et al. Adverse effects of biologics: a network meta-analysis and Cochrane overview. Cochrane Database Syst Rev 2011. DOI: 10.1002/14651858.CD008794.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Normanno N, De Luca A, Bianco C, et al. Epidermal growth factor receptor (EGFR) signaling in cancer. Gene 2006; 366: 2–16. [DOI] [PubMed] [Google Scholar]
- 99.Wong SF. Cetuximab: an epidermal growth factor receptor monoclonal antibody for the treatment of colorectal cancer. Clin Ther 2005; 27: 684–694. [DOI] [PubMed] [Google Scholar]
- 100.Kersten C and Cameron MG. Cetuximab alleviates neuropathic pain despite tumour progression. BMJ Case Rep 2012; 2012. [DOI] [PMC free article] [PubMed]
- 101.Kersten C, Cameron MG, Laird B, et al. Epidermal growth factor receptor-inhibition (EGFR-I) in the treatment of neuropathic pain. Br J Anaesth 2015; 115: 761–767. [DOI] [PubMed] [Google Scholar]
- 102.Mould DR. The pharmacokinetics of biologics: a primer. Dig Dis 2015; 33(Suppl 1): 61–69. [DOI] [PubMed] [Google Scholar]
- 103.Leary BA, Lawrence-Henderson R, Mallozzi C, et al. Bioanalytical platform comparison using a generic human IgG PK assay format. J Immunol Methods 2013; 397: 28–36. [DOI] [PubMed] [Google Scholar]