Abstract
Transplantation of fetal ventral mesencephalic (VM) neurons for Parkinson’s disease (PD) is limited by poor survival and suboptimal integration of grafted tissue into the host brain. In a 6-hydroxydopamine rat model of PD, we investigated the feasibility of simultaneous transplantation of rat fetal VM tissue and polymer-encapsulated C2C12 myoblasts genetically modified to produce glial cell line–derived neurotrophic factor (GDNF) or mock-transfected myoblasts on graft function. Amphetamine-induced rotations were assessed prior to transplantation and 2, 4, 6 and 9 wk posttransplantation. We found that rats grafted with VM transplants and GDNF capsules showed a significant functional recovery 4 wk after implantation. In contrast, rats from the VM transplant and mock-capsule group did not improve at any time point analyzed. Moreover, we detected a significantly higher number of tyrosine hydroxylase immunoreactive (TH-ir) cells per graft (2-fold), a tendency for a larger graft volume and an overall higher TH-ir fiber outgrowth into the host brain (1.7-fold) in the group with VM transplants and GDNF capsules as compared to the VM transplant and mock-capsule group. Most prominent was the TH-ir fiber outgrowth toward the capsule (9-fold). Grafting of GDNF-pretreated VM transplants in combination with the implantation of GDNF capsules resulted in a tendency for a higher TH-ir fiber outgrowth into the host brain (1.7-fold) as compared to the group transplanted with untreated VM transplants and GDNF capsules. No differences between groups were observed for the number of surviving TH-ir neurons or graft volume. In conclusion, our findings demonstrate that simultaneous transplantation of fetal VM tissue and encapsulated GDNF-releasing cells is feasible and support the graft survival and function. Pretreatment of donor tissue with GDNF may offer a way to further improve cell transplantation approaches for PD.
Keywords: Parkinson disease, transplantation, GDNF, rat, encapsulated cells
Introduction
Parkinson’s disease (PD) is mainly characterized by the progressive loss of dopaminergic (DAergic) neurons in the nigrostriatal system leading to a depletion of dopamine in the striatum, which in turn is responsible for severe motor disturbances. Current pharmacological treatments can alleviate these motor symptoms1 but have limitations as they become less effective with time and are associated with side effects .2 So far, strategies based on the application of neurotrophic factors and the transplantation of DAergic neurons have displayed promising results to tackle the disease progression or restore sufficient dopamine supply to the striatum (for review, see the study by Athauda and Foltynie, Kalia et al., Li et al.3–5). Fetal nigral tissue can be transplanted bilaterally into the caudate and putamen with few postoperative complications.6 This procedure has been shown to be safe in the long term and to confer clinical benefits in PD patients.7,8 Indeed, follow-up assessment of 2 cases with bilateral intrastriatal transplantation proved that this intervention can substantially improve the quality of life of PD patients.9 Nevertheless, it became clear from clinical trials that patient selection and handling of the fetal donor tissue need to be optimized.7,10–12 In the pregrafting phase, the organotypic ventral mesencephalic (VM) cultures offer the possibility of effective in vitro storage and treatment of the cells prior to transplantation.13 Of particular importance is the still suboptimal survival and poor innervation of the host brain by grafted DAergic neurons.14 Moreover, Collier et al. reported that survival, growth, and function of transplanted DAergic neurons are reduced in aged rats and they suggested that this is due to less trophic support from the host brain.15 Among these trophic factors, glial cell line–derived neurotrophic factor (GDNF) has gained most attention due to its compelling neuroprotective actions and promotion of survival and morphological differentiation of DAergic neurons.12,16,17 Hence, the combination of neurotrophic factors and cell transplantation may offer ways to improve graft function.18–20 However, some studies reported that genetically modified cells releasing the neurotrophic factor fibroblast growth factor 2 (FGF2) need to be in direct contact with dopaminergic transplanted cells in order to improve graft function.21 It has been shown that treating the cells with GDNF prior to transplantation improved engraftment of DAergic neurons in animal models of PD22,23 and in a pilot human clinical trial.24 Clinical trials investigating delivery of GDNF to treat PD patients, however, showed so far an equivocal outcome (for review see Lindholm et al., Domanskyi et al.12,25), possibly due to a number of technical as well as disease-related aspects, for example, the activity of neurotrophic factors in pathological settings.4 Accordingly, the inability of neurotrophic factors to cross the blood–brain barrier (BBB) and the potential induction of side effects due to the widespread distribution of their cognate receptors throughout the brain17 are challenging hurdles. Thus, selective targeting of the transplanted cells without affecting larger parts of the host brain is crucial. In this respect, cell bioengineering offers the possibility of delivering specific neurotrophic factors into the brain parenchyma. It has been reported that co-transplantation of DAergic grafts with engineered cells continuously releasing GDNF led to significantly increased survival and sprouting of grafted DAergic neurons and to functional recovery in an animal model of PD.26–28 Such approaches, however, are still associated with the potential risk of rejection of transplanted cells and tumor formation.27 In contrast, the use of cell lines engineered to produce neurotrophic factors and encapsulated in a porous polymer membrane is immunocompatible and can be withdrawn.29 Furthermore, polymer capsules were well tolerated after intraventricular implantation in human subjects for up to 2 y, supporting the safety and feasibility of this therapeutic intervention.30
Although we have previously shown that implanted GDNF-releasing capsules 1 wk prior to transplantation of VM tissue demonstrated a significantly improved graft function as assessed over a period of 6-wk posttransplantation, several critical aspects remained unsolved.27 Hence, in the present study, we first investigated the feasibility of a simultaneous transplantation of rat fetal nigral tissue and polymer-encapsulated myoblasts genetically modified to produce GDNF on graft function in the time frame of 9 wk posttransplantation. Secondly, we assessed whether a further improved survival and function of transplants can be achieved with the combination of GDNF-releasing implants and GDNF-pretreated VM donor tissue.
Materials and Methods
Animals
Female Sprague-Dawley rats (Janvier Labs, Le Genest-Saint-Isle, France) were housed at 12-h light–dark cycle with food and water ad libitum. For the preparation of the transplants, pregnant Sprague-Dawley rats were purchased from Janvier Labs. All experiments were carried out in the light phase and in accordance with the guidelines of the Animal Research Ethics Committee of the Canton Berne, Switzerland, and the University of Bern Animal Care and Use Committee, Switzerland.
Experimental Design
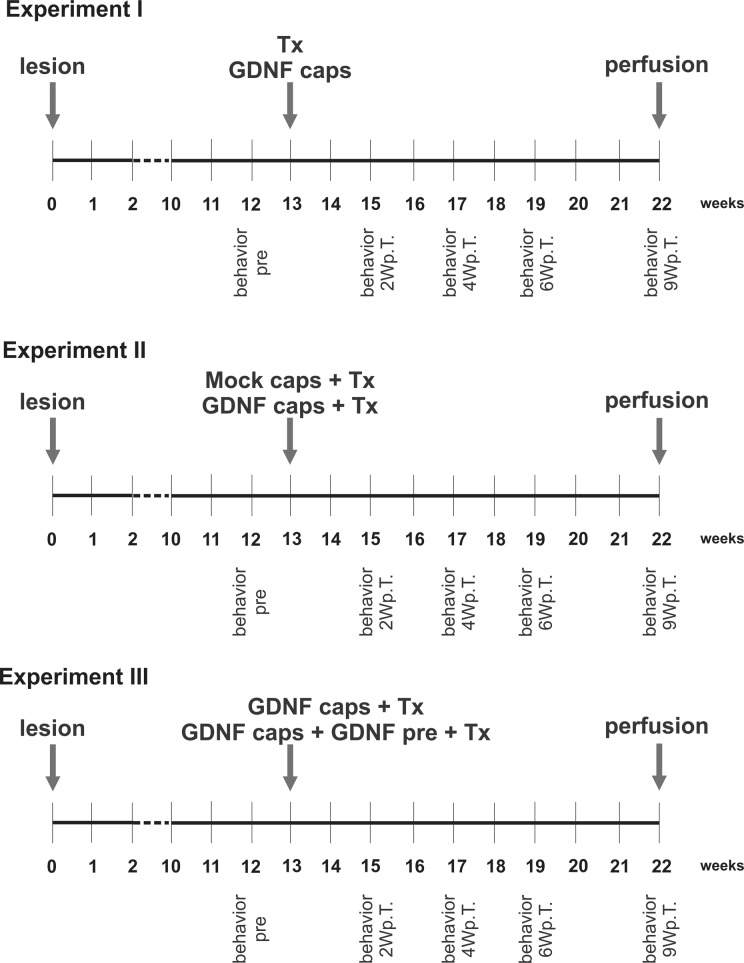
The present work was split into 3 experimental parts: experiment I, II, and III (Fig. 1). Initially, we explored the asymmetrical rotation behavior of hemi-parkinsonian rats in response to amphetamine and treatment with either half a fetal VM tissue alone or GDNF-releasing capsules alone (experiment I). Thereafter, we analyzed the asymmetrical rotation behavior in response to amphetamine of hemi-parkinsonian rats treated with either half a fetal VM tissue and a mock capsule or half a fetal VM tissue and GDNF-releasing capsule (experiment II). Moreover, histological analyses were conducted for TH-ir cell number per graft, TH-ir fiber outgrowth into the host striatum and for graft volume. In experiment III, we analyzed the same parameters as in experiment II but in hemi-parkinsonian rats implanted with a GDNF-releasing capsule with either half a VM tissue or half a VM tissue-pretreated with GDNF.
Fig. 1.
Schematic diagram of the experimental setup. A lesion induced by injection of 6-hydroxydopamine into the right striatum produced a hemi-parkinsonian rat model. Thirteen weeks after the lesion, the rats received either a transplant of fetal rat ventral mesencephalic tissue (Tx) or a glial cell line–derived neurotrophic factor (GDNF)-releasing capsule (GDNF caps; experiment I), a ventral mesencephalic (VM) transplant and a GDNF-releasing capsule or a mock capsule (Mock caps; experiment II), a GDNF-releasing capsule with a control VM transplant or with a GDNF-pretreated VM transplant (pre-Tx; experiment III) into the striatum. Nine weeks after the transplantation or capsule implantation, the rats were perfused and their brains used for histological analyses. The rats turning response to amphetamine was assessed 12 wk after the lesion (behavior pre) and 2, 4, 6 and 9 wk after the transplantation (behavior 2, 4, 6, 9 Wp.T.).
Hemi-Parkinsonian Rat Model
Sprague Dawley rats weighing 220 to 250 g were anesthetized (nembutal, 40 mg/kg, intraperitoneal [IP]) and mounted on a stereoscopic frame (Kopf Instruments, Tujunga, CA, USA). 6-Hydroxydopamine (6-OHDA) lesions were performed as described earlier.23 Briefly, animals received an injection of 4 μL of 32 mM 6-OHDA (Sigma-Aldrich, St Louis, MO, USA) into the right ascending mesotelencephalic pathway through a small burr hole created in the skull. The injection was performed over 6 min using a 10-μL Hamilton syringe. The following coordinates in relation to bregma were used: posterior 2.8 mm, lateral 2.0 mm, and 8.4 mm ventral to the dura, and the incisor bar was set at −3.9 mm. Thereafter, the rats were allowed to recover for 12 wk. The following numbers of animals were included for the 3 experimental groups: experiment I: GDNF-capsule group, n = 4 and VM transplant group, n = 4; experiment II: VM transplant and mock-capsule group, n = 11 and VM transplant and GDNF-capsule group, n = 11 (1 animal died during the experimental period and was therefore not included for the final analyses; hence, this group consisted of 10 rats); experiment III: GDNF-capsule and VM transplant group, n = 6 and GDNF-capsule and GDNF-pretreated VM transplant group, n = 6.
Capsule Preparation and Enzyme-Linked Immunosorbent Assay (ELISA) Measurements
C2C12 mouse myoblasts were genetically modified with the pP1-DNT-hGDNF plasmid as previously described.31 Nontransfected C2C12 control cells and transfected C2C12 cells were filled into 5-mm long polymer fibers (150,000 cells per capsule) and were heat sealed.31 The amount of GDNF released from the capsules was determined using an ELISA prior to implantation and after explantation of the capsules.27,31,32 GDNF release 2 d prior to transplantation was 9.8 ± 4.5 ng/mL/24 h and 10.8 ± 2.3 ng/mL/24 h in experiment I and II, respectively. In all capsules, surviving cells were detected at the time of capsule retrieval at the end of the experiments. Likewise, all capsules were found to produce GDNF at the time of sacrifice with 9.5 ± 2.5 ng/mL/24 h and 28.7 ± 9.3 ng/mL/24 h in experiment I and II, respectively. In experiment III, GDNF release 2 d prior to transplantation was 20.3 ± 1.4 ng/mL/24 h and 71.0 ± 3.9 ng/mL/24 h at the end of the experiments. In all mock capsules, GDNF release was below the detection level.
Preparation of Transplants
Cultures of fetal rat VM were prepared using the free-floating roller-tube culture technique described by Spenger et al. with minor modifications.33 In brief, time-pregnant Sprague-Dawley rats (Janvier Labs) were anesthetized (nembutal, 40 mg/kg, IP) and their fetuses removed by cesarean section. Thereafter, the VM was dissected from the fetuses aged E14 embryonic day 14 ([E14], E0=day of vaginal plug), was cut in the midline, and was divided into 4 equally sized pieces. Each piece was transferred into a conical 15-mL plastic tube containing 1 mL of culture medium (55% Dulbecco’s modified Eagle medium [DMEM Gibco, Reinach, Switzerland], 32.5% Hank’s balanced salt solution [HBSS Gibco, Reinach, Switzerland], 1.5% glucose, 10% fetal calf serum [FCS Gibco, Reinach, Switzerland], 1% 0.01 M 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid [HEPES Merck KGaA, Darmstadt, Germany]) and was placed in a roller drum (60 revolutions/h) in an incubator (37° C) with 5% CO2. Cultures were grown for 7 d in vitro (DIV), and the medium was changed after DIV 3 and DIV 6. For experiment III, cultures were randomly assigned to the control or to the GDNF group. GDNF (10 ng/mL; Promega was added at DIV 0 and then at every medium change [Dübendorf, Switzerland]). Control cultures were grown in medium with no trophic factor added.23
Transplantation Surgery
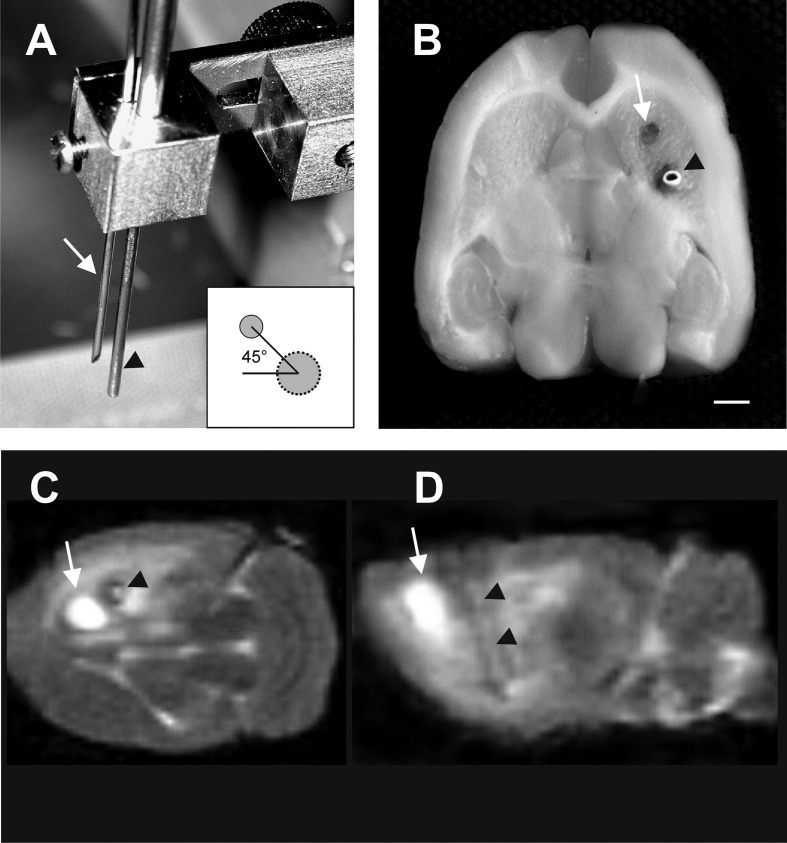
Thirteen weeks after the 6-OHDA, lesioned rats were anesthetized (nembutal, 40 mg/kg, IP) and mounted on a stereoscopic frame. For transplantation, 2 VM cultures, corresponding to half of a VM (1 rostral and 1 caudal part),27,34 were loaded into a 20-gauge spinal needle (Unisys Corp., Tokyo, Japan) and the capsule loaded into a specially designed cannula with an inserter, as depicted in Fig. 2A. The cultures and the capsule were stereotactically injected into the right caudate putamen slowly over 10 min. The following coordinates in relation to bregma were used for placement of the needle: posterior 1.0 mm, lateral 2.7 mm, and 4.5 mm ventral to the dura, and the incisor bar was set at −2.5 mm. After injection, the needle and cannula were slowly retracted (1 mm/min). The capsule was placed laterally in the striatum at an angle of 45° in respect to the tissue grafts. This was achieved by fixing in the same holder at the selected alignment and distance (45° angle; 1.5 mm distance) the specially designed cannula, with the inserter for the capsules and the needle used for grafting (Fig. 2A, B).
Fig. 2.
Illustration of simultaneous capsule and tissue transplantation. Photographs depicting the specially designed cannula with the needles used for simultaneous placement of ventral mesencephalic (VM) cultures (white arrow) and capsules (black arrowhead; A) and the position after transplantation in the right striatum (B). In the T2-weighted magnetic resonance scans, the site of injection of VM cultures could be noticed as a hyperintense area (white arrow) and the capsule as a hypointense area (black arrowheads) 2 d after simultaneous implantation of the transplants and capsules (coronal level, C; sagittal level, D). Scale bar: 2 mm.
Magnetic Resonance Imaging
Magnetic resonance (MR) images were used to verify the placement of tissue grafts and capsules (Fig. 2C, D). MR scanning was performed on a Siemens Magnetom Vision at 1.5 T (Siemens, Erlangen, Germany) using a flexible surface coil, as previously described.35,36 In brief, rats were anesthetized (nembutal, 40 mg/kg IP) and placed into a polyvinyl chloride rat holder. Coronal and sagittal T2-weighted images were obtained as previously described.35,36 In brief, the field of view was set to 80 mm (8/8). The T2-weighted images were recorded with time to repetition = 3,300 ms and time to echo = 119 ms at a slice gap of 0.2 mm. Twenty radiofrequency excitations were employed and summed for signal averaging to increase signal to noise ratio. Acquisition time was 16:03 min.
Behavioral Testing
Amphetamine-induced rotational behavior was tested in all rats before the transplantation (pre) and 2, 4, 6, and 9 wk after the transplantation (Fig. 1). Immediately after injection of d-amphetamine sulfate (2.5 mg/kg IP), rats were placed in automated rotameter cylinders and monitored for 90 min. Only rats exhibiting a net rotational asymmetry of at least 5 full ipsilateral body turns/min were selected for the experiments and were assigned to the groups in order to have a balanced pretransplantation rotation score.13,27
Perfusion and Tissue Processing
Two days after the last rotation behavior test, rats were deeply anesthetized (nembutal, 40 mg/kg, IP) and mounted in the stereoscopic frame. The capsules were carefully removed from the brains and placed in maintenance medium.27,31 Thereafter, the rats were perfused through the ascending aorta first with a prewash solution of 200 mL 0.1 M phosphate-buffered saline (PBS), pH 7.4 containing heparin (1,000 IE/100 mL; NOVO Nordisk), followed by 250 mL fixative (4% paraformaldehyde [PFA] and 0.16% picric acid in PBS). Immediately thereafter, the brains were removed from the skull, postfixed overnight in 4% PFA, and cryoprotected in 20% sucrose in PBS solution. Horizontal sections were cut at 30 µm on a freezing microtome (Frigocut; Reichert-Jung) and the sections mounted onto gelatin chrome–alum-precoated glass slides.
Immunohistochemistry and Analysis of Histological Sections
Every third slice containing a graft was selected for tyrosine hydroxylase (TH) immunohistochemistry. After 3 rinses in PBS, tissue sections were preincubated in 0.3% Triton X-100 in PBS plus 10% horse serum (HS) for 60 min, washed, and incubated overnight with the rabbit polyclonal anti-TH antibody (1:500; Pel Freez) for 48 h at 4 °C in 0.1% Triton X-100 in PBS plus 2.5% HS. Following 3 washes, sections were incubated with a biotinylated secondary antibody (horse antirabbit 1:200; Vector Labs) in 0.1% Triton X-100 in PBS plus 2.5% HS for 90 min. Endogenous peroxidase was blocked by 3% H2O2 and 10% methanol in PBS for 10 min. Following incubation with an avidin–peroxidase complex (1:150; Vector Labs) for 45 min, specifically bound antibody was visualized with a metal-enhanced 3,3′-diaminobenzidine (DAB) substrate kit (Pierce). Sections were dehydrated in alcohol, cleared in xylene, and mounted in Eukitt.
Histological Analysis
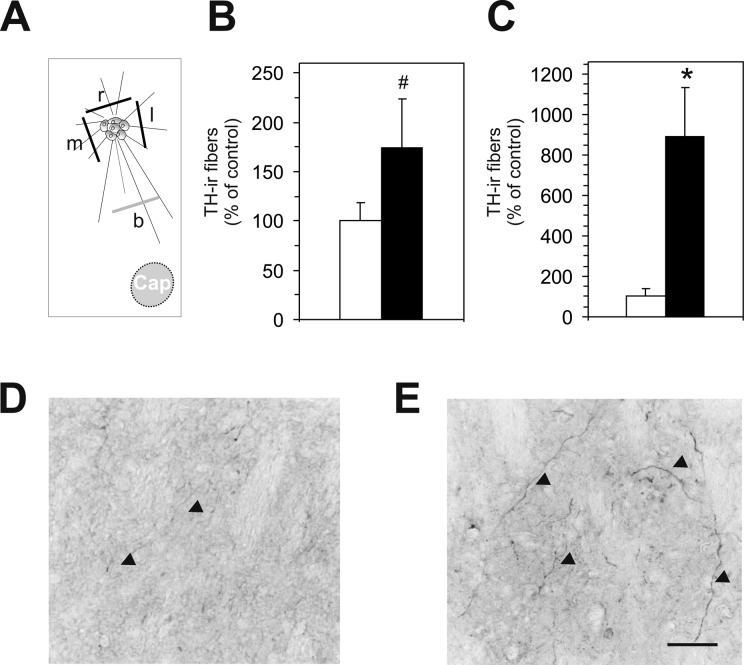
Histological analyses were conducted by a researcher blinded to the treatments, as described previously.23,37 In brief, for the estimation of the graft volume, every third section containing a graft was used to determine the graft boundaries using an Olympus microscope (Olympus DP72) equipped with a digital camera and connected to a PC with a calibrated neuron tracing software (Cellsens Dimension; Olympus). Thereafter, an automated computation integrated the areas to yield the graft volume. TH-ir-positive cell numbers were counted in the same sections at 40× magnifications on an Olympus light microscope equipped with a motorized stage and a digital camera connected to a PC. To correct 4 double counting, the Abercrombie’s formula was applied.13,38 Graft-derived TH-ir fibers were determined at 3 sites, that is, medial, rostral, and lateral of the graft host interface (distance form border: 100 µm), and at the site in the middle between graft and capsule. All TH-ir fibers crossing a virtual line of 300-µm length were counted using an Olympus microscope equipped with a digital camera and connected to a PC with a calibrated neuron tracing software (Cellsens Dimension; Olympus). For the analyses, we have chosen a 300-μm line as we did in our previous studies,23,27 based on the observation that this length is feasible for assessing the TH-ir fiber outgrowth from different graft sizes as well as providing a reasonable means of overall TH-ir fiber outgrowth from the grafts. The mean numbers of TH-ir fibers of the medial, rostral, and lateral sites were summarized as fiber growth from the graft (Fig. 3A).
Fig. 3.
Histological assessment of fiber outgrowth in experiment II. Schematic drawing illustrating the assessment of the tyrosine hydroxylase immunoreactive (TH-ir) fiber outgrowth medial (m), rostral (r), and lateral (l) from the graft as well as at the site in the middle (b) between graft and capsule (Cap; A). Quantitative analysis of TH-ir fibers crossing a virtual line of 300 µm determined at the 3 sites, that is, medial, rostral, and lateral of the graft host interface (B) and of TH-ir fibers crossing a virtual line between tissue graft and capsules in the dopamine-depleted host striatum (C). Note the significantly higher TH-ir fiber growth between the ventral mesencephalic (VM) transplant and glial cell line–derived neurotrophic factor (GDNF)-capsule compared to the VM transplant and mock-capsule group. Values are expressed as mean ± standard error of the mean (SEM) and presented as percentage of the mock-capsule groups. *P < 0.05 versus the corresponding mock-capsule group; #P < 0.1 versus the corresponding mock-capsule group. Representative photomicrographs illustrating the higher number of TH-ir fibers (arrow heads) between the graft and the capsule in the VM transplant and GDNF-capsule group (E) as compared to the VM transplant and mock-capsule group (D). Scale bar: 50 µm.
Statistical Analysis
Statistical comparisons were performed by means of commercially available software package (GraphPad Prism 4, CA, USA). Analysis of variance (ANOVA) followed by Student–Newman–Keuls post hoc test was used to compare treatment groups in the behavioral assessments. For the comparison between 2 groups, the Welch t test for unpaired samples or the Mann-Whitney rank sum test was used. Differences were considered statistically significant at P ≤ 0.05. Values are presented as mean ± standard error of the mean (SEM).
Results
Assessment of the 6-OHDA Lesions
Analysis of 6-OHDA-lesioned animals showed a nearly complete loss of TH-ir neurons in the right substantia nigra which was in accordance with the outcome seen with the pregrafting amphetamine-induced rotational asymmetry and our previous study.13 We observed no loss of the rat’s body weight due to the simultaneous transplantation of capsules and tissue grafts as assessed over the experimental period of 9 wk posttransplantation (data not shown).
Experiment I
Amphetamine-induced rotational behavior
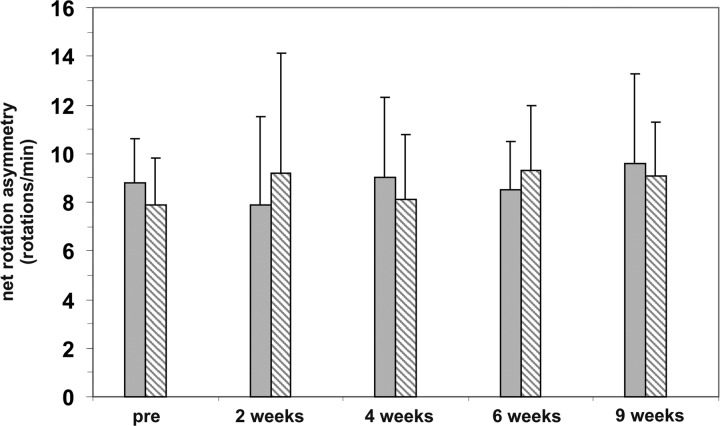
As expected, we found that 6-OHDA-lesioned rats that were transplanted with VM cultures only and 6-OHDA-lesioned rats receiving GDNF capsules only did not show altered amphetamine-induced rotational asymmetry34 (Fig. 4).
Fig. 4.
Behavioral assessment in experiment I. Amphetamine-induced ipsilateral net rotations of hemi-parkinsonian 6-hydroxydopamine (6-OHDA)-lesioned rats implanted with glial cell line–derived neurotrophic factor (GDNF) capsules alone (hatched bars) or VM transplants alone (gray bars). Behavior was assessed before grafting (pre) and 2, 4, 6, and 9 wk after transplantation surgery. No change in the number of ipsilateral turns could be observed. Values are expressed as mean ± standard error of the mean (SEM) and presented as ipsilateral rotation per minute.
Experiment II
Amphetamine-induced rotational behavior
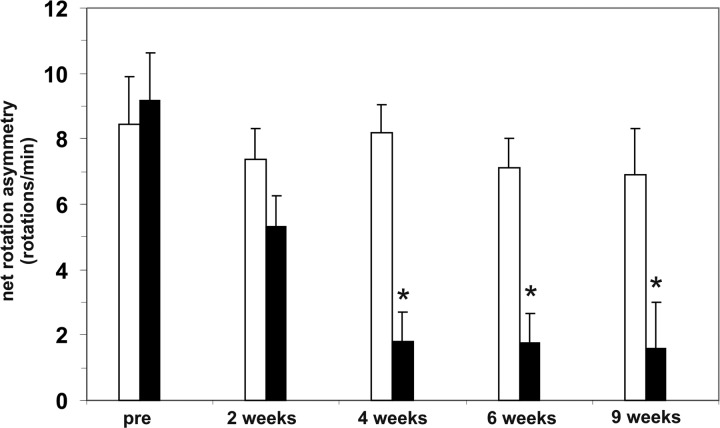
Similar to the outcome in experiment I, no reduction in the rotational asymmetry was observed in the group of rats transplanted with VM and mock capsules over the experimental period (Fig. 5). Most importantly, however, a near complete recovery was observed in the rats simultaneously transplanted with VM and GDNF capsules 4 wk after transplantation compared to pregrafting values. No further improvement was observed at later time points, that is, 6 and 9 wk after transplantation (Fig. 5).
Fig. 5.
Behavioral assessment in experiment II. Amphetamine-induced ipsilateral net rotations of hemi-parkinsonian 6-hydroxydopamine (6-OHDA)-lesioned rats implanted with ventral mesencephalic (VM) transplant and mock capsules (open bars) and VM transplant and glial cell line–derived neurotrophic factor (GDNF) capsules (filled bars) before grafting (pre) and 2, 4, 6, and 9 wk after transplantation surgery. Rats that received VM transplants and GDNF capsules demonstrated a significant reduction in rotational asymmetry. Values are expressed as mean ± standard error of the mean (SEM) and presented as ipsilateral rotations per minute. *P < 0.05 versus the corresponding rotations before grafting (pre).
Histological Analyses
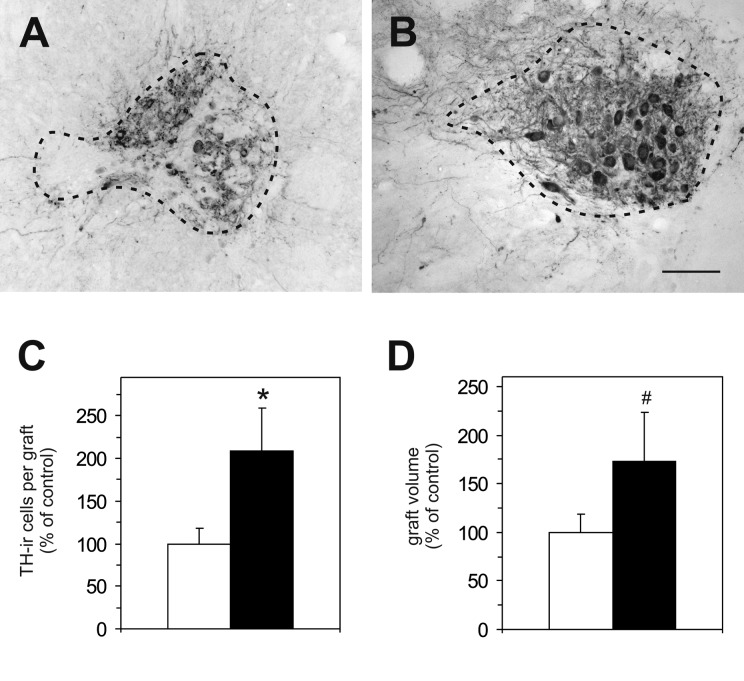
Analysis of the brains revealed that in all rats surviving TH-ir neurons were present. The number of TH-ir neurons per graft was found to be significantly (2.1-fold) higher in the VM transplant and GDNF-capsule group (Fig. 6B, C) as compared to rats with VM transplant and mock-capsules (Fig. 6A, C). Similarly, graft volume was larger (1.7-fold) in the VM transplant and GDNF-capsule group as compared to the VM transplant and mock-capsule group, however, did not reach statistical significance (Fig. 6D). The number of TH-ir fibers growing around the graft showed an overall tendency to be higher (1.7-fold) in the VM transplant and GDNF-capsule group as compared to rats in the VM transplant and mock-capsule group (Fig. 3B). Notably, number of TH-ir fibers between transplant and capsule was significantly higher (8.9-fold) in the VM transplant and GDNF-capsule group (Fig. 3C, E) as compared to the VM transplant and mock-capsule group (Fig. 3C, D).
Fig. 6.
Histological assessments in experiment II. Representative photomicrographs of tyrosine hydroxylase immunoreactive (TH-ir) neurons in the dopamine-depleted host striatum 9 wk after transplantation from rats transplanted with ventral mesencephalic (VM) transplant and mock capsules (A) and rats implanted with VM transplants and glial cell line–derived neurotrophic factor (GDNF) capsules (B). Scale bar = 40 µm. Quantification of the number of TH-ir cells in the grafts (C) and graft volume (D) of rats transplanted with VM transplants and mock capsules (white bars) and VM transplants and GDNF capsules (black bars). Note the significant increase in TH-ir cells per graft in the VM transplant and GDNF-capsule group compared to the VM transplant and mock-capsule group. Moreover, a tendency toward a larger graft volume was detected in the VM transplant and GDNF-capsule group as compared to the VM transplant and mock-capsule group. Values are expressed as mean ± standard error of the mean (SEM) and presented as percentage of mock-capsule group. *P < 0.05 versus the corresponding mock-capsule group. #P < 0.1 versus the corresponding mock-capsule group.
Experiment III
Amphetamine-induced rotational behavior
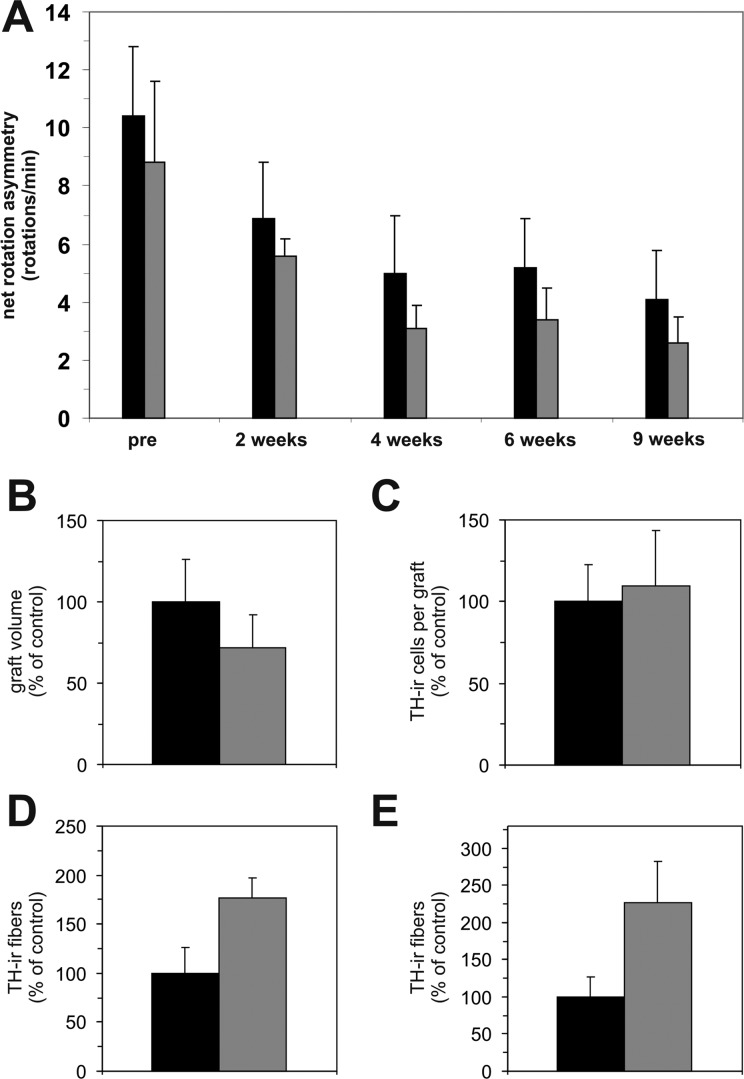
Given the observation that the GDNF released by the capsule exerted a remarkable effect on rat behavior and graft fiber outgrowth, we next reasoned whether these benefits might be further enhanced by concomitantly transplanting VM cultures that have been treated with GDNF prior to transplantation. Accordingly, we found that the amphetamine-induced rotational behavior improved over the 9-wk posttransplantation period but did not differ between the 2 experimental groups (Fig. 7A).
Fig. 7.
Behavioral and histological assessments in experiment III: Amphetamine-induced ipsilateral net rotations of hemi-parkinsonian 6-hydroxydopamine (6-OHDA)-lesioned rats implanted with glial cell line–derived neurotrophic factor (GDNF) capsules and control ventral mesencephalic (VM) transplant (black bars) or with GDNF capsules and GDNF-pretreated VM transplant (gray bars) before (pre) and 2, 4, 6, and 9 wk after transplantation surgery (A). Both groups improved asymmetrical turning behavior over time; however, no significant differences could be observed between groups. In line with this observation, no significant differences in graft volume (B) and TH-ir cell numbers per graft (C) could be found between the 2 treatment groups. A modestly higher tyrosine hydroxylase immunoreactive (TH-ir) fiber outgrowth from the graft (D) and between the graft and capsule (E) was detected in the GDNF-capsule and GDNF-pretreated VM transplant group as compared to the GDNF-capsule and VM transplant group. Values are expressed as mean ± standard error of the mean (SEM) and presented as percentage of the GDNF-capsule and VM transplant group.
Histological Analyses
Immunohistochemical analysis of the grafts demonstrated that the pretreatment with GDNF did not significantly influence the graft volume (Fig. 7B) or the number of TH-ir neurons in the transplants (Fig. 7C) compared to the GDNF-capsule and VM transplant group. The number of TH-ir fibers around the graft (Fig. 7D) and between transplant and capsule (Fig. 7E) was augmented in the GDNF-capsule and GDNF-pretreated VM transplant group as compared to the GDNF-capsule and VM transplant group (by 1.7-fold and 2.3-fold, respectively), but the increase was not statistically significant.
Discussion
The present study shows that GDNF released from engineered encapsulated cells promotes the functional recovery in hemi-parkinsonian rats when co-transplanted with fetal rat VM tissue. It is important to note that in our experiments these 2 treatment regimens were individually necessary but not sufficient to reach therapeutic efficacy. The subtherapeutic amount of VM tissue transplanted (corresponding to half a VM) was deliberately chosen in order to uncover the effect of other treatments37 and is in agreement with our previous studies.27,34 Similarly, no behavioral recovery was found in the GDNF-capsule-only group. Moreover, the body weight of the animal was not affected by the treatment, indicating that the capsules were well tolerated. In contrast to our observations, others reported that capsules releasing GDNF implanted 2 wk after a 6-OHDA lesion resulted in reduced rotations as compared to mock-treated animals.39 This different outcome is likely due to the animal models employed. In fact Date et al. used intrastriatal 6-ODHA injections39 typically leading to only partial denervation of the striatum,40 whereas we used animals with medial forebrain bundle lesions. In addition, in our experimental setting, the implantation of GDNF capsules was done at a progressed stage of the disease, that is, 13 wk after the 6-OHDA lesions. We cannot exclude that the GDNF capsules might have exerted effects in the host tissue by, for example, promoting the sprouting of the remaining dopaminergic striatal fibers, but not enough to induce functional recovery.
Our results from experiment II are consistent with the hypothesis that the improved functional recovery is a consequence of better dopaminergic cell survival and integration into the host tissue.6,41 Accordingly, we found a 2.1-fold higher number of surviving TH-ir cells with GDNF treatment, which is in agreement with the 1.9-fold increase reported by Rosenblad et al.42 and the 2.6-fold increase observed in our previous study.27 Other reports, however, have described that the cell survival induced by GDNF is not necessarily associated with increased fiber outgrowth.28 In our work, the effect of the fiber outgrowth was particularly evident between the graft and the capsule. This preferential growth of the fibers in close proximity to the capsule is suggestive of a presence of a GDNF gradient into the host brain. How far GDNF can diffuse from the implantation sites was not investigated in the present study, but our data provide the functional evidence that GDNF can diffuse for at least 1.5 mm from the implantation site and this did not alter behavior. An earlier report demonstrated that the radius of GDNF-ir was at 11 mm from the infusion site43 in the monkey brain, while Ahn et al. demonstrated that GDNF-ir reached as far as 2 to 3 mm from the implanted GDNF-releasing capsules.32,44 The importance of determined levels of GDNF for therapeutic purposes has been highlighted by several studies describing severe side effects when bolus GDNF infusions were made into the striatum or into the ventricles.8,45–47 This notion would call for a wider distribution of capsules, particularly in the proximity of the grafts, for clinical applications in humans. The period of 4 wk after the transplantation in which the behavioral recovery was detected in the group of rats receiving concomitant VM transplant and GDNF capsules is shorter than the 12 wk period described in the xenograft study by Ahn et al.32 One reason for this difference might be the slower development of human compared to rat fetal tissue.
In the framework of the supportive actions of GDNF on the grafts, the duration of the exposure is an important aspect that should be considered. Sajadi et al. showed that a temporary delivery of GDNF is sufficient to induce long-lasting functional and morphological improvement.48 Hence, they concluded that GDNF needs to be present during the establishment of DAergic fibers and the source can be removed thereafter. In agreement, Winkler et al. postulated that GDNF needs to be present at the time of transplantation or shortly thereafter in order to be beneficial. In their study using a regime of delayed GDNF application by means of lentiviral transduction, no effects were observed.10 In the present work, presumably a shorter exposure time of VM tissue to GDNF would have been sufficient as indicated by the unchanged rotational asymmetry after 4 wk. Moreover, one can speculate that genetically modified encapsulated cells releasing growth factor(s) might support maintenance of a neuronal phenotype and/or maturation of transplanted neural stem cell–derived cells.49,50
Our results demonstrated that the pretreatment of VM tissue prior to transplantation did not exert significant differences in volume and number of TH-ir cells in the transplant as compared to the grafts cultured under standard conditions. These results are thus in agreement with the similar pattern of behavioral recovery displayed by the 2 groups. Moreover, the GDNF pretreatment of VM tissues moderately increased DAergic fiber outgrowth, but did not reach statistical significance. Overall, these observations are in line with our previous findings with regard to the increased fiber outgrowth but not with the enhanced survival of grafted DAergic cells.23 This divergence might be attributable to the extent of the pretreatment of the VM tissue with GDNF (which was longer in the present study). In fact, in our earlier report, the peak of behavioral improvement was observed in the VM tissues preincubated with GDNF for 4 d.23 It should be noted that our experimental design does not allow the monitoring of dynamic changes of the graft at the histological level upon transplantation. In this respect, the intrastriatal levels of GDNF in the long postgrafting period might level off the effect of the pretreatment on TH-ir cell number. Moreover, we speculate that an optimal combination of neurotrophic factors for pretreatment, that is, GDNF and NT-4/5,51 would result in an improved outcome.
In summary, our study provides evidence that an optimal pretreatment of graft tissue in combination with creating the best possible environment of the host tissue might improve transplantation approaches for PD.
Acknowledgments
The authors wish to express their gratitude to Tanja Bosnjak, Angélique D. Ducray, Sandra H. Krebs, and Janine-Ai Schlaeppi for excellent technical assistance. The authors thank Anne Zurn for many helpful discussions and for her interest in the study. Particularly, the authors like to thank Patrick Aebischer for providing the encapsulated cells and many helpful discussions and Vivianne Padrun for preparation of polymer preparation and the ELISA measurements.
Footnotes
Ethical Approval: This study was approved by the Animal Research Ethics Committee of the Canton Berne, Switzerland, and the University of Bern Animal Care and Use Committee, Switzerland.
Statement of Human and Animal Rights: All experiments were in accordance with the guidelines of the Animal Research Ethics Committee of the Canton Berne, Switzerland, and the University of Bern Animal Care and Use Committee, Switzerland.
Statement of Informed Consent: There are no human subjects in this article and informed consent is not applicable.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research and/or authorship of this article: This research was supported by the HANELA Foundation Switzerland and the Swiss National Science Foundation.
References
- 1. Dunning CJ, Reyes JF, Steiner JA, Brundin P. Can Parkinson’s disease pathology be propagated from one neuron to another? Prog Neurobiol. 2012;97(2):205–219. [DOI] [PubMed] [Google Scholar]
- 2. Cenci MA. Presynaptic mechanisms of l-DOPA-induced dyskinesia: the findings, the debate, and the therapeutic implications. Front Neurol. 2014;5:242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Athauda D, Foltynie T. The ongoing pursuit of neuroprotective therapies in Parkinson disease. Nat Rev Neurol. 2015;11(1):25–40. [DOI] [PubMed] [Google Scholar]
- 4. Kalia LV, Kalia SK, Lang AE. Disease-modifying strategies for Parkinson’s disease. Mov Disord. 2015;30(11):1442–1450. [DOI] [PubMed] [Google Scholar]
- 5. Li Z, Wang P, Yu Z, Cong Y, Sun H, Zhang J, Zhang J, Sun C, Zhang Y, Ju X. The effect of creatine and coenzyme q10 combination therapy on mild cognitive impairment in Parkinson’s disease. Eur Neurol. 2015;73(3–4):205–211. [DOI] [PubMed] [Google Scholar]
- 6. Brundin P, Karlsson J, Emgard M, Schierle GS, Hansson O, Petersen A, Castilho RF. Improving the survival of grafted dopaminergic neurons: a review over current approaches. Cell Transplant. 2000;9(2):179–195. [DOI] [PubMed] [Google Scholar]
- 7. Barker RA, Barrett J, Mason SL, Bjorklund A. Fetal dopaminergic transplantation trials and the future of neural grafting in Parkinson’s disease. Lancet Neurol. 2013;12(1):84–91. [DOI] [PubMed] [Google Scholar]
- 8. Kordower JH, Freeman TB, Chen EY, Mufson EJ, Sanberg PR, Hauser RA, Snow B, Olanow CW. Fetal nigral grafts survive and mediate clinical benefit in a patient with Parkinson’s disease. Mov Disord. 1998;13(3):383–393. [DOI] [PubMed] [Google Scholar]
- 9. Kefalopoulou Z, Politis M, Piccini P, Mencacci N, Bhatia K, Jahanshahi M, Widner H, Rehncrona S, Brundin P, Bjorklund A, et al. Long-term clinical outcome of fetal cell transplantation for Parkinson disease: two case reports. JAMA Neurol. 2014;71(1):83–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Winkler C, Georgievska B, Carlsson T, Lacar B, Kirik D. Continuous exposure to glial cell line-derived neurotrophic factor to mature dopaminergic transplants impairs the graft’s ability to improve spontaneous motor behavior in parkinsonian rats. Neuroscience. 2006;141(1):521–531. [DOI] [PubMed] [Google Scholar]
- 11. Kelly MJ, O‘Keeffe GW, Sullivan AM. Viral vector delivery of neurotrophic factors for Parkinson’s disease therapy. Expert Rev Mol Med. 2015;17:e8. [DOI] [PubMed] [Google Scholar]
- 12. Lindholm D, Makela J, Di Liberto V, Mudo G, Belluardo N, Eriksson O, Saarma M. Current disease modifying approaches to treat Parkinson’s disease. Cell Mol Life Sci. 2016;73(7):1365–1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Meyer M, Widmer HR, Wagner B, Guzman R, Evtouchenko L, Seiler RW, Spenger C. Comparison of mesencephalic free-floating tissue culture grafts and cell suspension grafts in the 6-hydroxydopamine-lesioned rat. Exp Brain Res. 1998;119(3):345–355. [DOI] [PubMed] [Google Scholar]
- 14. Petit GH, Olsson TT, Brundin P. The future of cell therapies and brain repair: Parkinson’s disease leads the way. Neuropathol Appl Neurobiol. 2014;40(1):60–70. [DOI] [PubMed] [Google Scholar]
- 15. Collier TJ, Sortwell CE, Daley BF. Diminished viability, growth, and behavioral efficacy of fetal dopamine neuron grafts in aging rats with long-term dopamine depletion: an argument for neurotrophic supplementation. J Neurosci. 1999;19(13):5563–5573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Schaller B, Andres RH, Huber AW, Meyer M, Perez-Bouza A, Ducray AD, Seiler RW, Widmer HR. Effect of GDNF on differentiation of cultured ventral mesencephalic dopaminergic and non-dopaminergic calretinin-expressing neurons. Brain Res. 2005;1036(1–2):163–172. [DOI] [PubMed] [Google Scholar]
- 17. Kirik D, Georgievska B, Bjorklund A. Localized striatal delivery of GDNF as a treatment for Parkinson disease. Nat Neurosci. 2004;7(2):105–110. [DOI] [PubMed] [Google Scholar]
- 18. Herran E, Ruiz-Ortega JA, Aristieta A, Igartua M, Requejo C, Lafuente JV, Ugedo L, Pedraz JL, Hernandez RM. In vivo administration of VEGF- and GDNF-releasing biodegradable polymeric microspheres in a severe lesion model of Parkinson’s disease. Eur J Pharm Biopharm. 2013;85(3 Pt B):1183–1190. [DOI] [PubMed] [Google Scholar]
- 19. Yasuhara T, Shingo T, Muraoka K, Kobayashi K, Takeuchi A, Yano A, Wenji Y, Kameda M, Matsui T, Miyoshi Y, et al. Early transplantation of an encapsulated glial cell line-derived neurotrophic factor-producing cell demonstrating strong neuroprotective effects in a rat model of Parkinson disease. J Neurosurg. 2005;102(1):80–89. [DOI] [PubMed] [Google Scholar]
- 20. Hoban DB, Howard L, Dowd E. GDNF-secreting mesenchymal stem cells provide localized neuroprotection in an inflammation-driven rat model of Parkinson’s disease. Neuroscience. 2015;303:402–411. [DOI] [PubMed] [Google Scholar]
- 21. Timmer M, Muller-Ostermeyer F, Kloth V, Winkler C, Grothe C, Nikkhah G. Enhanced survival, reinnervation, and functional recovery of intrastriatal dopamine grafts co-transplanted with Schwann cells overexpressing high molecular weight FGF-2 isoforms. Exp Neurol. 2004;187(1):118–136. [DOI] [PubMed] [Google Scholar]
- 22. Apostolides C, Sanford E, Hong M, Mendez I. Glial cell line-derived neurotrophic factor improves intrastriatal graft survival of stored dopaminergic cells. Neuroscience. 1998;83(2):363–372. [DOI] [PubMed] [Google Scholar]
- 23. Andereggen L, Meyer M, Guzman R, Ducray AD, Widmer HR. Effects of GDNF pretreatment on function and survival of transplanted fetal ventral mesencephalic cells in the 6-OHDA rat model of Parkinson’s disease. Brain Res. 2009;1276:39–49. [DOI] [PubMed] [Google Scholar]
- 24. Mendez I, Dagher A, Hong M, Hebb A, Gaudet P, Law A, Weerasinghe S, King D, Desrosiers J, Darvesh S, et al. Enhancement of survival of stored dopaminergic cells and promotion of graft survival by exposure of human fetal nigral tissue to glial cell line–derived neurotrophic factor in patients with Parkinson’s disease. report of two cases and technical considerations. J Neurosurg. 2000;92(5):863–869. [DOI] [PubMed] [Google Scholar]
- 25. Domanskyi A, Saarma M, Airavaara M. Prospects of neurotrophic factors for Parkinson’s disease: comparison of protein and gene therapy. Hum Gene Ther. 2015;26(8):550–559. [DOI] [PubMed] [Google Scholar]
- 26. Espejo M, Cutillas B, Arenas TE, Ambrosio S. Increased survival of dopaminergic neurons in striatal grafts of fetal ventral mesencephalic cells exposed to neurotrophin-3 or glial cell line-derived neurotrophic factor. Cell Transplant. 2000;9(1):45–53. [DOI] [PubMed] [Google Scholar]
- 27. Sautter J, Tseng JL, Braguglia D, Aebischer P, Spenger C, Seiler RW, Widmer HR, Zurn AD. Implants of polymer-encapsulated genetically modified cells releasing glial cell line-derived neurotrophic factor improve survival, growth, and function of fetal dopaminergic grafts. Exp Neurol. 1998;149(1):230–236. [DOI] [PubMed] [Google Scholar]
- 28. Clavreul A, Sindji L, Aubert-Pouessel A, Benoit JP, Menei P, Montero-Menei CN. Effect of GDNF-releasing biodegradable microspheres on the function and the survival of intrastriatal fetal ventral mesencephalic cell grafts. Eur J Pharm Biopharm. 2006;63(2):221–228. [DOI] [PubMed] [Google Scholar]
- 29. Zurn AD, Widmer HR, Aebischer P. Sustained delivery of GDNF: towards a treatment for Parkinson’s disease. Brain Res Brain Res Rev. 2001;36(2–3):222–229. [DOI] [PubMed] [Google Scholar]
- 30. Bloch J, Bachoud-Levi AC, Deglon N, Lefaucheur JP, Winkel L, Palfi S, Nguyen JP, Bourdet C, Gaura V, Remy P, Brugieres P, et al. Neuroprotective gene therapy for Huntington’s disease, using polymer-encapsulated cells engineered to secrete human ciliary neurotrophic factor: results of a phase I study. Hum Gene Ther. 2004;15:968–975. [DOI] [PubMed] [Google Scholar]
- 31. Tseng JL, Baetge EE, Zurn AD, Aebischer P. GDNF reduces drug-induced rotational behavior after medial forebrain bundle transection by a mechanism not involving striatal dopamine. J Neurosci. 1997;17(1):325–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ahn YH, Bensadoun JC, Aebischer P, Zurn AD, Seiger A, Bjorklund A, Lindvall O, Wahlberg L, Brundin P, Kaminski Schierle GS. Increased fiber outgrowth from xeno-transplanted human embryonic dopaminergic neurons with co-implants of polymer-encapsulated genetically modified cells releasing glial cell line-derived neurotrophic factor. Brain Res Bull. 2005;66(2):135–142. [DOI] [PubMed] [Google Scholar]
- 33. Spenger C, Studer L, Evtouchenko L, Egli M, Burgunder JM, Markwalder R, Seiler RW. Long-term survival of dopaminergic neurones in free-floating roller tube cultures of human fetal ventral mesencephalon. J Neurosci Methods. 1994;54(1):63–73. [DOI] [PubMed] [Google Scholar]
- 34. Matarredona ER, Meyer M, Seiler RW, Widmer HR. CGP 3466 increases survival of cultured fetal dopaminergic neurons. Restor Neurol Neurosci. 2003;21(1–2):29–37. [PubMed] [Google Scholar]
- 35. Guzman R, Lovblad KO, Meyer M, Spenger C, Schroth G, Widmer HR. Imaging the rat brain on a 1.5 T clinical MR-scanner. J Neurosci Methods. 2000;97(1):77–85. [DOI] [PubMed] [Google Scholar]
- 36. Guzman R, Meyer M, Lovblad KO, Ozdoba C, Schroth G, Seiler RW, Widmer HR. Striatal grafts in a rat model of Huntington’s disease: time course comparison of MRI and histology. Exp Neurol. 1999;156(1):180–190. [DOI] [PubMed] [Google Scholar]
- 37. Seiler S, Di Santo S, Widmer HR. Nogo-A neutralization improves graft function in a rat model of Parkinson’s disease. Front Cell Neurosci. 2016;10:87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Abercrombie M. Estimation of nuclear population from microtome sections. Anat Rec. 1946;94:239–247. [DOI] [PubMed] [Google Scholar]
- 39. Date I, Shingo T, Yoshida H, Fujiwara K, Kobayashi K, Takeuchi A, Ohmoto T. Grafting of encapsulated genetically modified cells secreting GDNF into the striatum of parkinsonian model rats. Cell Transplant. 2001;10(4–5):397–401. [PubMed] [Google Scholar]
- 40. Blandini F, Armentero MT, Martignoni E. The 6-hydroxydopamine model: news from the past. Parkinsonism Relat Disord. 2008;14(suppl 2):S124–S129. [DOI] [PubMed] [Google Scholar]
- 41. Karlsson J, Emgard M, Gido G, Wieloch T, Brundin P. Increased survival of embryonic nigral neurons when grafted to hypothermic rats. Neuroreport. 2000;11(8):1665–1668. [DOI] [PubMed] [Google Scholar]
- 42. Rosenblad C, Martinez-Serrano A, Bjorklund A. Glial cell line-derived neurotrophic factor increases survival, growth and function of intrastriatal fetal nigral dopaminergic grafts. Neuroscience. 1996;75(4):979–985. [DOI] [PubMed] [Google Scholar]
- 43. Ai Y, Markesbery W, Zhang Z, Grondin R, Elseberry D, Gerhardt GA, Gash DM. Intraputamenal infusion of GDNF in aged rhesus monkeys: distribution and dopaminergic effects. J Comp Neurol. 2003;461(2):250–261. [DOI] [PubMed] [Google Scholar]
- 44. Bensadoun JC, Pereira de Almeida L, Fine EG, Tseng JL, Deglon N, Aebischer P. Comparative study of GDNF delivery systems for the CNS: polymer rods, encapsulated cells, and lentiviral vectors. J Control Release. 2003;87(1–3):107–115. [DOI] [PubMed] [Google Scholar]
- 45. Zhang Z, Miyoshi Y, Lapchak PA, Collins F, Hilt D, Lebel C, Kryscio R, Gash DM. Dose response to intraventricular glial cell line-derived neurotrophic factor administration in parkinsonian monkeys. J Pharmacol Exp Ther. 1997;282(3):1396–1401. [PubMed] [Google Scholar]
- 46. Nutt JG, Burchiel KJ, Comella CL, Jankovic J, Lang AE, Laws ER, Jr, Lozano AM, Penn RD, Simpson RK, Jr, Stacy M, et al. Randomized, double-blind trial of glial cell line-derived neurotrophic factor (GDNF) in PD. Neurology. 2003;60(1):69–73. [DOI] [PubMed] [Google Scholar]
- 47. Giehl KM, Schacht CM, Yan Q, Mestres P. Infusion of GDNF into the cerebral spinal fluid through two different routes: effects on body weight and corticospinal neuron survival. Neuroreport. 1998;9(12):2809–2813. [DOI] [PubMed] [Google Scholar]
- 48. Sajadi A, Bensadoun JC, Schneider BL, Lo Bianco C, Aebischer P. Transient striatal delivery of GDNF via encapsulated cells leads to sustained behavioral improvement in a bilateral model of Parkinson disease. Neurobiol Dis. 2006;22(1):119–129. [DOI] [PubMed] [Google Scholar]
- 49. Li X, Tzeng SY, Liu X, Tammia M, Cheng YH, Rolfe A, Sun D, Zhang N, Green JJ, Wen X, et al. Nanoparticle-mediated transcriptional modification enhances neuronal differentiation of human neural stem cells following transplantation in rat brain. Biomaterials. 2016;84:157–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Cortes D, Robledo-Arratia Y, Hernandez-Martinez R, Escobedo-Avila I, Bargas J, Velasco I. Transgenic GDNF positively influences proliferation, differentiation, maturation and survival of motor neurons produced from mouse embryonic stem cells. Front Cell Neurosci. 2016;10:217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Meyer M, Matarredona ER, Seiler RW, Zimmer J, Widmer HR. Additive effect of glial cell line-derived neurotrophic factor and neurotrophin-4/5 on rat fetal nigral explant cultures. Neuroscience. 2001;108(2):273–284. [DOI] [PubMed] [Google Scholar]