Abstract
Bone nonunion is a pathological condition in which all bone healing processes have stopped, resulting in abnormal mobility between 2 bone segments. The incidence of bone-related injuries will increase in an aging population, leading to such injuries reaching epidemic proportions. Tissue engineering and cell therapy using mesenchymal stem cells (MSCs) have raised the possibility of implanting living tissue for bone reconstruction. Bone marrow was first proposed as the source of stem cells for bone regeneration. However, as the quantity of MSCs in the bone marrow decreases, the capacity of osteogenic differentiation of bone marrow stem cells is also impaired by the donor’s age in terms of reduced MSC replicative capacity; an increased number of apoptotic cells; formation of colonies positive for alkaline phosphatase; and decreases in the availability, growth potential, and temporal mobilization of MSCs for bone formation in case of fracture. Adipose-derived stem cells (ASCs) demonstrate several advantages over those from bone marrow, including a less invasive harvesting procedure, a higher number of stem cell progenitors from an equivalent amount of tissue harvested, increased proliferation and differentiation capacities, and better angiogenic and osteogenic properties in vivo. Subcutaneous native adipose tissue was not affected by the donor’s age in terms of cellular senescence and yield of ASC isolation. In addition, a constant mRNA level of osteocalcin and alkaline phosphatase with a similar level of matrix mineralization of ASCs remained unaffected by donor age after osteogenic differentiation. The secretome of ASCs was also unaffected by age when aiming to promote angiogenesis by vascular endothelial growth factor (VEGF) release in hypoxic conditions. Therefore, the use of adipose cells for bone tissue engineering is not limited by the donor’s age from the isolation of stem cells up to the manufacturing of a complex osteogenic graft.
Keywords: bone, aging, mesenchymal stem cells, adipose stem cells, functionality
Introduction
Bone is a highly specialized organ that serves many purposes. First and foremost, bone plays a structural role, but it serves other functions such as mineral storage (calcium, sodium, phosphate, and magnesium) and hematopoiesis as it stores marrow. Bone’s structural role includes protection for the brain, spinal cord, and chest organs, as well as rigid internal support for the limbs. The internal architecture of bone accounts for its lightness and high tensile strength, which result from its hollow and tubular shape composed of a collagen matrix with mineral deposits.1
A fracture is defined as a loss of continuity in the bone architecture. It can be the result of a direct trauma, repeated solicitations (fatigue/stress fractures), or other pathological situations such as cysts or tumors. A bone fracture is a pathological situation characterized by pain, swelling/hematoma, abnormal mobility/deformity of a bone segment, and functional impairment. Whereas soft-tissue healing of a wound creates a fibrous scar, bone tissue is unique in its scarless regenerative properties.
Two different types of bone repair exist: direct and indirect bone healing also called intramembranous and endochondral ossification, respectively.2–9 Direct or primary bone healing can occur in long bone only if there is a perfect reduction and stabilization of the bone fragments. It is a direct attempt made by the bone cortex to reestablish new haversian systems through the use of remodeling units known as cutting cones. A cutting cone unit is comprised of a cutting head of osteoclasts, which penetrate parallel to the axis of the bone and bridge the fracture, followed by osteoblasts that line the wall of the cutting cone. A blood vessel grows with the cutting head at the center of the unit. In this form of ossification, little or no callus is observed upon radiographic examination.
In contrast, the vast majority of long-bone fractures heal by indirect ossification, which is a combination of intramembranous and endochondral bone formation. Intramembranous ossification is the direct formation of bone from osteoprogenitors and mesenchymal stem cells (MSCs) residing in the periosteum along with the cutting cones remodeling the fracture, whereas endochondral bone healing involves the recruitment, proliferation, and differentiation of MSCs into cartilage that will secondarily calcify and eventually be replaced by bone.
At the end of primary repair, a phase of bone remodeling occurs. This phenomenon involves bone resorption by osteoclasts and a secondary new bone deposition by osteoblasts that will be enclosed in the new matrix in the form of osteocytes. Bone remodeling occurs constantly in the entire skeleton. Following a fracture, the normal shape of the bone will be reconstructed according to the load repartition on the structure. It is responsible for the scarless healing capacity of bone tissue.
Adequate bone healing thus requires a careful combination of physiological and biomechanical factors. The coordination and interaction between these factors leads to an uneventful restoration ad integrum. In contrast, any disturbance in the sequence of the healing cycle will end in complications such as delayed union or nonunion. Research on the key elements determining the pathway to bone regeneration has led to various models, one of which is referred to as the diamond concept.10
According to the diamond concept, there are 4 key factors in bone regeneration: (1) mechanical stability: the bridging fracture callus allows load transmission and restoration of the architecture of the bone according to Wolff’s law; (2) osteogenic cells: local multipotent MSCs are recruited and migrate to the fracture callus, and these cells differentiate into the osteoblastic and chondroblastic phenotype during callus maturation; (3) growth factors: cytokines such as interleukin-1 (IL-1) and -6 (IL-6), tumor necrosis factor-α, platelet-derived growth factor (PDGF), vascular endothelial growth factor (VEGF), insulin-like growth factor (IGF), and transforming growth factor-β (TGF-β) superfamily members initiate a cascade of cellular events starting the bone healing process; and (4) adequate scaffold: the extracellular matrix provides a natural support for cellular interaction.
Any disturbance in the delicate balance between these key factors will lead to complications such as nonunion. A bone nonunion is a pathological condition in which all bone healing processes have stopped, resulting in an abnormal mobility between 2 bone segments. This condition is also known as a pseudoarticulation or pseudarthrosis. An unhealed bone after 8 to 12 weeks of treatment is considered to be in delayed consolidation; after more than 6 mo, it is defined as a nonunion.11
The prevalence of nonunions varies with each bone and is considered to be around 10% for all long-bone fractures combined.11,12 This incidence can increase up to 30% when associated with comorbidities such as diabetes or a smoking habit.12,13 Identified risk factors can be systemic (e.g., systemic diseases, gender, age, and hormonal milieu) or local (infection, high-energy trauma, extended soft-tissue damage, loss of blood supply, irradiation, bone loss, extended periosteum damage, and bone denervation and instability).14,15
With the rise in life expectancy, the incidence of bone-related injuries in an aging population is on the rise and reaching epidemic proportions.16 A significant increase in the number of persons over 65 y of age is predicted to occur, with a rise from today’s 13% of the American population to 16.9% and 25.9% by 2030 and 2060, respectively.17 Consequently, the cost associated with bone nonunion has a major risk to increase drastically in the next few decades. Indeed, the total cost of these complications is currently estimated between 10,000 and 100,000 euros per patient in Europe.18,19
Therefore, other therapeutic approaches for the treatment of nonunions have been proposed, including the injection of concentrated autologous bone marrow or ex vivo culture expanded MSCs. These new cell therapy treatments are currently being investigated to facilitate bone healing. At clinicaltrials.gov, 15 clinical studies (4 completed, 4 recruiting, 4 not yet recruiting, and 3 of unknown status) are currently investigating the potential of MSCs from bone marrow, fat tissue, or umbilical cord. Nine of the studies include patients between 18 and 65 y, 3 studies have no age limitations, and 1 study extends to 75 y old.
In the context of population aging and the therapeutic use of MSCs to promote bone healing, this review investigated the impact of donor age on (1) the native cellular mechanisms of MSCs implied in the physiology and pathophysiology of bone healing and (2) the heterogeneity introduced by “macrodifferences” (starting material, isolating methods, and cell production processes) and “microdifferences” (donor-to-donor variability, donor conditions at the time of sampling) that could also significantly modify the cell manufacturing processes and quality of the final cell therapy product for bone healing.
Using autologous or allogeneic MSCs is a key parameter to develop a cell-based therapy for bone nonunion. Although autologous MSCs lead to no immunologic rejection, the process is economically difficult to implement and cellular autograft manufacturing is time-consuming. In contrast, the use of allogeneic MSCs allows for large-scale expansion of MSCs, and isolation from selected donors coupled with cryopreservation provides a readily available source of stem cells.
Impact of Age on Bone Marrow MSC Cellular Mechanism for Bone Healing
Bone tissue demonstrates a remarkable ability to remodel in association with its capacity to heal/regenerate following fracture as supported by the presence of a stem cell population in the bone marrow. Bone marrow comprises the stromal and hematopoietic compartments. The stromal tissue functions as a scaffold of cells that provide physical and functional support to the hematopoietic cells. The stromal fraction is able to adhere to tissue culture plastic, whereas the nonadherent hematopoietic cells can be readily removed from the adherent stromal cell cultures by a simple wash step.20 Under specific conditions, multipotent precursor cells in bone marrow stroma are able to differentiate into bone, cartilage, and adipose tissue.21,22 MSCs are a source of progenitors for osteoblasts, which are responsible for osteogenesis and also regulate osteoclastogenesis.
The molecular events that govern fracture healing are a complex network of signals of tissue damage, cell death, cell recruitment, cell proliferation, cell differentiation, and tissue formation.23 Different sets of effector molecules such as cyclooxygenase-2 (COX-2), IL-1, IL-6 (effectors of inflammation), transforming growth factor-β (TGF-β) (as a mitogen agent), fibroblast growth factor (FGF), insulin-like growth factor (IGF), platelet-derived growth factor (PDGF), bone morphogenetic proteins (BMPs, osteogenic agents), and VEGF and angiopoietins (angiogenic factors) demonstrated a direct impact on the proliferation and differentiation of MSCs.23
As bone density declines with age, the quantity of MSCs in the bone marrow decreases. Preclinical models in rats and mice demonstrated a discrepancy between experimental models. A significant reduction of bone progenitors in the bone marrow with age was found in one study,24 whereas another demonstrated a significantly higher number of progenitor cells in older mice (by 20%).25 In a study of humans, a significant reduction of the precursor cells was found in the second and third decades among iliac crest samples obtained from healthy patients aged 5 to 70 y.26 Muschler et al. also confirmed that the total number of nucleated cells in bone marrow aspirates decreased with donor age.27 In addition to the decrease of MSC number in the bone marrow, others reported a decrease of the replicative capacity of MSCs from human donors between 59 and 75 y of age.28,29 The decrease of MSCs from patients in this age range could be explained by a significantly shorter size of the mean telomere restriction fragment in comparison to patients younger than 18 y.28 On the other hand, Zhou et al. reported an increase with donor age of apoptotic cells with positive staining for senescence-associated β-galactosidase.29 Therefore, most of the research on human specimens indicates that aging decreases the availability, growth potential, and temporal mobilization of MSCs for bone formation in case of fracture.30
Age negatively affects the capacity of MSC harvesting and proliferation from bone marrow, and the capacity for osteogenic differentiation of bone marrow stem cells was also impaired by the donor’s age. Two studies found that the formation of colonies positive for alkaline phosphatase decreased with donor age over 50 y.28,31 Zhou et al. also confirmed the reduction of alkaline phosphatase expression in MSCs from bone marrow of older donors.29
In order to overcome the influence of age on the quality of stem cell products, the use of allogeneic bone marrow MSCs seems to be more appropriate for developing a cell therapy for bone healing. Most clinical studies reported at www.clinicaltrials.gov (with search items “bone fracture” and “bone marrow MSCs in bone healing for autologous and allogeneic MSC”) focused on the autologous approach for patients aged 18 to 75 y (12 vs. 2 trials, respectively). Two clinical phase 3 studies using the autologous bone marrow approach did not demonstrate efficacy of bone marrow stem cells in a large cohort of patients.32,33 Thus, it is crucial to consider the impact of age in inclusion criteria to homogenize the selection of patients for bone marrow stem cell transplantation.
To overcome the effect of age on the autologous source of stem cells, a new source of MSCs with a lower impact of donor age has been proposed. Adipose-derived stem cells (ASCs) were recently demonstrated to have several advantages over those from bone marrow, including a less invasive harvesting procedure, a higher number of stem cell progenitors from an equivalent amount of tissue harvested, increased proliferation and differentiation capacities, and better angiogenic and osteogenic properties in vivo.34–42 Although the MSCs from bone marrow and adipose tissue are quite similar, there are some innate differences in their biology (bone marrow MSCs and ASCs can be differentiated with regard to functional gene expression) and release of growth factors in a hypoxic environment (as found in the bone nonunion tissue with <1% oxygen).43,44 In a pig preclinical model, Schubert et al. confirmed the superiority of ASCs in comparison to bone marrow MSCs. Indeed, they demonstrated the importance of osteogenic differentiation before implantation to promote better in vitro and in vivo angiogenesis (by VEGF release) and osteogenesis of the ASCs.43 Schubert et al. also investigated the potential of osteogenic ASCs with juvenile pig.43–45
As with bone marrow-derived stem cells, questions remain about the impact of donor age in terms of ASC content, isolation, and efficacy (growth factor release by ASCs) to cure bone nonunion. Aside from financial considerations, the impact of age is the most important factor when determining if an autologous or allogeneic approach is better in clinical terms. Because preclinical animal models are not able to mimic the influence of the long-term course of age on ASCs, this review will focus on the relationship between human life span and ASC properties in terms of safety, proliferation, and osteogenicity for bone repair.
Impact of Age on the Adipose Tissue for ASC Isolation and Safety
Adipose tissue is a highly complex tissue composed of mature adipocytes (more than 90%) and a stromal vascular fraction, which includes fibroblasts, preadipocytes, vascular smooth muscle cells, endothelial cells, resident immune cells (monocytes/macrophages, lymphocytes), and ASCs.46–48 Although adipose tissue is a more reproducible site from which to isolate stem cells than bone marrow, the density of ASCs can be significantly impacted by the location and composition of the native adipose tissue. The main reservoir of ASCs is found in the subcutaneous adipose tissue of the human body rather than in the visceral fat tissue.49 Although there is a high density of stem cells per volume of tissue (±1 × 106 per g of adipose tissue), the cellular composition of the native fat tissue can be significantly influenced by a systemic condition as found in type 2 diabetic patients.50 Indeed, 2 studies demonstrated that the cellular composition of adipose tissue is very sensitive to chronic hyperglycemia (in type 2 diabetes), as evidenced by adipose tissue inflammation, which is characterized by infiltration of inflammatory cells, increased production of cytokines, and induced systemic insulin resistance.51,52 They found that excessive caloric intake led to increased oxidative stress in the adipose tissue of mice with type 2 diabetes and promoted senescence-like changes, such as an increase of senescence-associated galactosidase activity, p53 expression, and production of proinflammatory cytokines. Recently, Wu et al. demonstrated that the yield of ASCs was affected by donor age by comparing adipose tissue from infants (0.5 ± 0.3 y) versus adults (34 ± 11 y) and older patients (59 ± 11 y).53 Kornicka et al. and Choudhery et al. confirmed that ASC expansion was affected by a donor’s age >50 y old in terms of colony forming unit and doubling time of stem cell proliferation.54,55 In contrast, Dufrane et al. confirmed the similarity of ASC isolation in 8 human donors (19–62 y old) in terms of ASC expansion up to passage 4. This was confirmed in a larger series of patients (6–72 y old) transplanted with autologous ASCs for bone regeneration and wound healing.56,57 This difference between studies could be explained by the fact that older patients were characterized by different clinical histories in terms of diabetes, obesity,…(not reported in Kornicka et al. and Choudhery et al., see above).54,55
The risk of oncogenicity is another important issue that can be associated with the age of ASC donors. To date, no tumors were diagnosed in patients transplanted with ASCs since ASCs were reported with rare chromosomal abnormalities.56,58 Meza-Zepeda et al. also demonstrated no cytogenetic abnormalities after long-term culture of human MSCs for several months.60 At present, there are no data on the impact of donor age on human ASCs. In comparison to bone marrow MSCs, however, Chen et al. demonstrated that ASCs had a better resistance to senescence with lower levels of biomarkers related to senescence, while Choudhery et al. reported a higher cellular senescence (with a higher expression of p16 and p21 genes and a higher activity of senescence-associated β-galactosidase).55,61 Since MSCs in culture are in a highly proliferative state under nonphysiologic conditions (which may support the accumulation of DNA damage, resulting in loss of cell cycle regulation and eventually malignant transformation after long-term cell culture), the number of cellular passages of ASCs could explain the difference between experimental studies performed on human cells. In vitro, Dufrane et al. confirmed the stability of the ASC genome (from young patients aged between 6 and 13 up to patients older than 47 y old) up to passage 16.56,57,62 All genetic analyses revealed minor rates (near the detection threshold) of chromosomal aneuploidy, mainly tetrasomies, suggesting tetraploidy as classically observed in cultured cells (cutoff: ∼4.5%). Minor trisomy 7 was also detected in passages 1, 4, and 10 (cutoff: ∼2%) as previously reported.63 Cells could exhibit recurring chromosomal alterations without involving a selective growth advantage in vitro, and MSCs with or without chromosomal alterations did not induce tumor formation 8 weeks after injection in immunocompromised mice. A higher rate of monosomy 7 (15%) was detected in the sample at passage 16. Monosomy 7 is mainly involved in myeloid malignancies and is not described in mesenchymal tumors.64 These aneuploid cells do not have a proliferative advantage because they are not detected on the karyotype of metaphase cells. Some studies described karyotype changes in MSCs after 11 to 14 passages (1.5–5.95% of cells).65,66 In vivo oncologic safety (with young and old patients as donors of ASCs) was confirmed by the absence of adverse events in immunodeficient animal recipients 1 or 3 mo postimplantation and in patients up to 54 mo after implantation; ASC implantation after shorter in vitro culture (passage 4) and osteogenic differentiation before implantation (thus reducing the chromosomal abnormalities by genetic stability) avoided the selection of tumoral cell clones.56,57,62
The development of cellular therapies with the greatest clinical potential is fraught with safety concerns related to product purity.67 Indeed, unspecific isolation methods are commonly used that are based on plastic adherent growth with removal of nonadherent cells, which results in heterogeneous cell preparations (composed of ASCs and mainly fibroblasts), which can induce differences upon long-term culture in terms of more quickly proliferating cell types overgrowing the slowly dividing cells.68–71 This change of cellular growth can induce heterogeneity of the selection of the cell clone, highlighting the concerns of regulatory authorities about the negative impact of fibroblasts in stem cell therapy products and thus can influence the capacity of cellular expansion without genetic instability.72 ASCs and dermal fibroblasts from the same donors demonstrated a similar pattern of cellular proliferation up to passage 16 with no influence of donor age (19–62 y). A major difference between both cell types was found in terms of VEGF and stromal cell-derived factor 1 (SDF1)α secretions in low oxygen tension, which also validated the concept that ASCs secrete a lower amount of SDF-1α in comparison to dermal fibroblasts (in each oxygen and glucose condition). This tool can specifically discriminate fibroblasts and ASCs and can be rapidly implemented and performed before the release of ASC-based therapy (providing a response within 24 h) for young and older human donors of ASCs.73
Impact of Age on the Osteogenic Properties of ASCs
Although the human donor’s age seems to have no major deleterious effects on ASC isolation, expansion, purity of the cell therapy product, and genetic stability, questions remain regarding the capacity to induce new bone tissue formation for the treatment of bone nonunion in older patients.
The beneficial effects of MSCs on bone remodeling are mainly provided by a paracrine effect.74–76 The secretome of the ASCs contains various endocrine factors involved in the bone activity. In bone regeneration, implanted ASCs secrete various osteoblast-activating factors (macrophage colony- stimulating factor [M-CSF], receptor activator of nuclear factor kappa-B ligand [RANKL], BMP-2, BMP-4, and hepatocyte growth factor [HGF]) and bone-related extracellular matrix proteins, including HGF and extracellular matrix proteins, corroborating their paracrine role in osteogenesis.77 Wu et al. demonstrated that human ASCs, from infant to older ages, exhibit a similar pattern of expression of osteogenic genes such as RUNX-2 and osteocalcin, while Kornicka et al. and Choudhery et al. reported a lower in vitro osteogenicity by older ASCs (>50 y old).53–55 Although a small advantage was found in vitro when using ASCs obtained from infants, they conclude that elderly ASCs still represent a valuable stem cell source for osteogenesis (similar to adult cells) for autologous stem cell transplantation. These results were confirmed by Chen et al., who demonstrated a constant mRNA level of osteocalcin and alkaline phosphatase with an in vitro level of matrix mineralization in ASCs regardless of donor age.61 However, for in vivo bone reconstruction, the impact of age on ASCs properties can be overcome by growth factor release and osteogenic differentiation of ASCs (before transplantation).43 ASCs are angiogenic, as they express VEGF, FGF-2, and IL-6.75 Vériter et al. recently demonstrated that ASCs mostly secreted VEGF (to promote angiogenesis) in the hypoxic conditions found in a bone nonunion in contrast to a lack of stimulation for insulin-like growth factor-1 (IGF-1) and FGF-2.57 They also noted that the differentiation of ASCs did not induce a significantly greater release of BMP-2.57
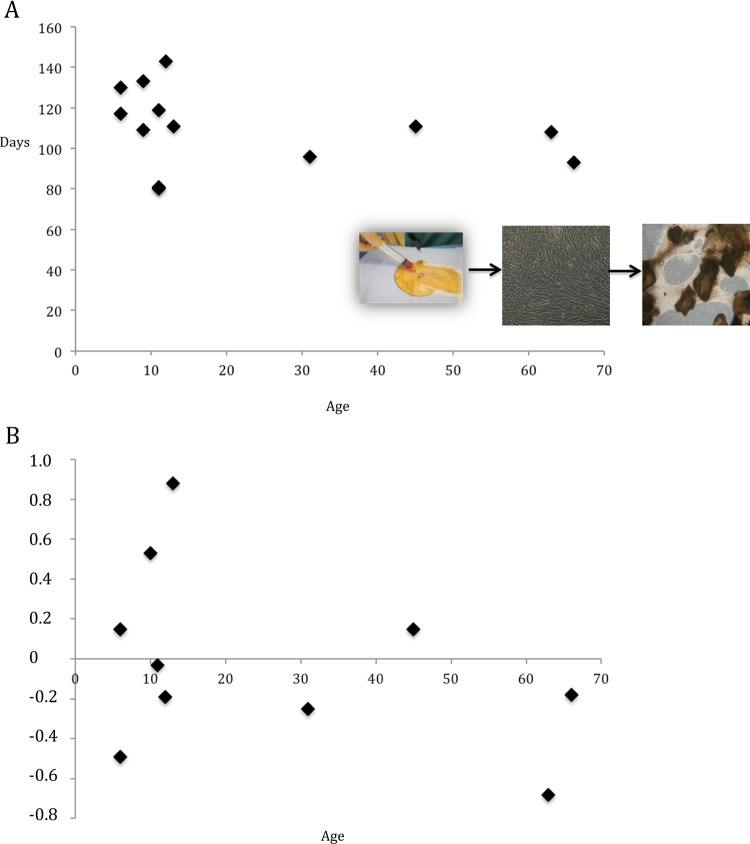
Critical size bone reconstruction (as found in bone nonunion) using stem cells also remains limited by the large size of bone defects and consequently the size of the engineered implant required for a 3-dimensional (3D) graft. Several scaffold-free systems have been investigated, but creating sufficient thickness to fill a critical size bone defect is difficult.78 Dufrane et al. developed a graft made of scaffold-free autologous ASCs differentiated in a 3D osteogenic structure with demineralized bone matrix [DBM] (Dufrane et al. patent: Multidimensional biomaterial and method for producing the same World Intellectual Property Organization (WIPO) 2010139792 A2; Fig. 1). Studies have demonstrated the safety and efficacy of this graft to cure a femoral critical size bone defect in a pig preclinical nonunion model at 6 mo postimplantation.44 Complete stem cell differentiation in an osteogenic 3D structure significantly improved the efficacy of bone reconstitution by promoting angiogenesis and osteogenesis and the safety by lowering the risk of growth factor release.43 After osteogenic differentiation, human and pig ASCs demonstrated similar in vitro (VEGF release and viability in hypoxic conditions) and in vivo (angiogenicity and osteogenicity with cellular engraftment and graft mineralization, respectively) properties.43,44 Subsequent to the preclinical experiments, these products were developed to treat specific patients with end-stage “untreatable” pathologies and in the case of conventional treatment failure. The capacity of human ASCs to produce a scaffold-free osteogenic 3D graft, clinical safety, and surgical feasibility were confirmed. The most important outcome was the proof of concept in terms of feasibility for manufacturing a scaffold-free 3D implant from human autologous ASCs differentiated into an osteogenic phenotype with demineralized bone matrix (DBM). For clinical application of this advanced therapy, all procedures were validated using human ASCs (following good manufacturing practices) and DBM with the goal of being able to uniformly reproduce the manufacture of a structural and stable 3D implant in all patients despite clinical constraints such as interdonor variability in terms of age. A mean of 105 d (without any impact of donor age) for graft manufacture was compatible with clinical implantation (Fig. 1A). The size of generated 3D bone-like tissue (a mean of 12.6 cm3 for the 3 grafts) was significantly increased by nearly 6 times (compared to 2 cm3 of native adipose tissue for each patient from 6 to 66 y old), and it was always sufficient to fill the bone defect. However, when donors of adipose tissue were classified by the age in groups of <18, 18 to 60, and >60 y old, the quantity of native adipose tissue required to generate an implant large enough to fill the bone defect was less for young donors in comparison to those over 18 y (3 g vs. 8 g, respectively). Finally, the quality (assessed by the potency histomorphological score in terms of cell/tissue composition and 3D characterization) of the final tissue-engineered product was not affected by the age of the donor of native tissue.44,56 A normal score for an optimal graft was established between −1 and +1 to obtain the in vivo efficacy for osteogenicity and bone tissue remodeling. In this series, we recorded scores of 0.14 ± 0.49, −0.05 ± 0.28, and −0.43 ± 0.35, respectively, for patients <18 y (mean, 10 y), 18 to 60 y old (mean, 41 y), and >60 y old (mean, 65 y), without any significant impact of donor age on the final structure of the 3D scaffold-free grafts (Figs 1B and 2). Therefore, the use of adipose cells for bone tissue engineering is not limited by donor age from the isolation of stem cells up to the manufacturing of a complex 3D scaffold-free graft.
Fig. 1.
(A, B) Impact of age on adipose stem cells differentiation to obtain a 3-dimensional (3D) scaffold-free and oteogenic graft. (A) No impact of the donor’s age was found on the time required to isolate and expand adipose stem cells (from native adipose tissue) and to obtain finally differentiated to the scaffold-free 3D osteogenic graft. The integrity of the 3D graft was histomorphologically assessed at the end of manufacturing by the potency score (before implantation). A score of the graft integrity (for the optimal 3D graft) between −1 and +1 in terms of cell viability counting, interconnective tissue integrity, and demineralized bone matrix content. Note that a final 3D graft was always obtained (individual donors without any impact of donor age, which ranged from 5 and 70 y) for adipose-derived stem cells incubated with demineralized bone matrix, demonstrating the reproducibility of the manufacturing procedures.
Fig. 2.

Impact of donor’s age on the integrity of the scaffold-free 3-dimensional (3D) graft. Macroscopically, the 3D osteogenic graft was obtained for donors aged below 18 y old and older 60 y old (upper level). Microscopically, the collagen matrix, the cellularity with 4′,6-diamidino-2-phenylindole (DAPI) staining (middle level, original magnification: ×10), and the osteocalcin expression (lower level, original magnification: ×25) were maintained in the 3D graft for young and old donors of adipose tissue for adipose-derived stem cell isolation, proliferation, and differentiation.
Conclusions
The impact of aging on ASC functions for bone tissue engineering is not a limiting factor for an autologous approach of cell therapy in terms of effectiveness of the regenerative capability and interaction with environmental extrinsic signals. Indeed, for clinical autologous cell transplantations, old and young ASCs are expected to produce similar effects. However, the role of exosomes secreted by human ASCs needs to be investigated effectively, both in laboratory and clinical settings, to assess the real impact of age on ASC properties, including intrinsic secretion and extrinsic interaction with the implanted host tissue. Finally, it is clear that a prospective controlled trial is needed to clinically assess the impact of age on a specific indication of bone regeneration, while determining the multiple factors that affect the ASC properties of patients, such as diabetes and obesity alone. These questions need to be answered before ASCs can be proposed as a standard autologous or allogeneic cell source for the treatment of bone defects.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
References
- 1. Rubin E. Essential pathology, 3rd ed Philadelphia (PA): Lippincott Williams and Wilkins; 2001. [Google Scholar]
- 2. Einhorn TA. The science of fracture healing. J Orthop Trauma. 2005;19(suppl 10):S4–S6. [DOI] [PubMed] [Google Scholar]
- 3. Gerstenfeld LC, Cullinane DM, Barnes GL, Graves DT, Einhorn TA. Fracture healing as a post-natal developmental process: molecular, spatial, and temporal aspects of its regulation. J Cell Biochem. 2003;88(5):873–884. [DOI] [PubMed] [Google Scholar]
- 4. Kalfas IH. Principles of bone healing. Neurosurg Focus. 2001;10(4):E1. [DOI] [PubMed] [Google Scholar]
- 5. Meyer U, Weismann HP. Bone and cartilage In: Schroeder G, editor. Bone and cartilage engineering. New York (NY): Springer; 2006. p. 7–46. [Google Scholar]
- 6. Miclau T, Schneider RA, Eames BF, Helms JA. Common molecular mechanisms regulating fetal bone formation and adult fracture repair In: Lieberman JR, Friedlaender GE, editors. Bone regeneration and repair: biology and clinical application. Totowa (NJ): Humana Press; 2005. p. 45–55. [Google Scholar]
- 7. Sfeir C, Ho L, Doll BA, Azari K, Hollinger JO. Fracture repair In: Lieberman JR, Friedlaender GE, editors. Bone regeneration and repair: biology and clinical applications. Totowa (NJ): Humana Press; 2005. p. 21–44. [Google Scholar]
- 8. Schindele A, McDonald MM, Bokko P, Little DG. Bone remodeling during fracture repair: the cellular picture. Semin Cell Dev Biol. 2008;19(5):459–466. [DOI] [PubMed] [Google Scholar]
- 9. Mackie EJ, Ahmed YA, Tatarczuch L, Chen KS, Mirams M. Endochondral ossification: how cartilage is converted into bone in the developing skeleton. Int J Biochem Cell Biol. 2008;40(1):46–62. [DOI] [PubMed] [Google Scholar]
- 10. Giannoudis PV, Einhorn TA, Marsh D. Fracture healing: the diamond concept. Injury. 2007;38(suppl 4):S3–S6. [DOI] [PubMed] [Google Scholar]
- 11. Megas P, Panagiotis M. Classification of non-union. Injury. 2005;36(suppl 4):S30–S37. [DOI] [PubMed] [Google Scholar]
- 12. Tzioupis C, Giannoudis PV. Prevalence of long-bone non-unions. Injury. 2007;38(suppl 2):S3–S9. [DOI] [PubMed] [Google Scholar]
- 13. Adams CI, Keating JF, Court-Brown CM. Cigarette smoking and open tibial fractures. Injury. 2001;32(1):61–65. [DOI] [PubMed] [Google Scholar]
- 14. Konttinen Y, Imai S, Suda A. Neuropeptides and the puzzle of bone remodeling: state of the art. Acta Orthop Scand. 1996;67(6):632–639. [DOI] [PubMed] [Google Scholar]
- 15. Rodriguez-Merchan EC, Forriol F. Nonunion: general principles and experimental data. Clin Orthop Relat Res. 2004;(419):4–12. [PubMed] [Google Scholar]
- 16. Rosenwasser MP, Cuellar D. Medical management of osteoporosis and the surgeons’ role. Injury. 2016;47(suppl 1):S62–S64. [DOI] [PubMed] [Google Scholar]
- 17. Gibon E, Lu L, Goodman SB. Aging, inflammation, stem cells, and bone healing. Stem Cell Res Ther. 2016;7:44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Mills LA, Simpson AHRW. The relative incidence of fracture non-union in the Scottish population (5.17 million): a 5-year epidemiological study. BMJ Open. 2013;3(2):pii:e002276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Dahabreh Z, Calori GM, Kanakaris NK, Nikolaou VS, Giannoudis PV. A cost analysis of treatment of tibial fracture nonunion by bone grafting or bone morphogenetic protein-7. Int Orthop. 2009;33(5):1407–1414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Black CR, Goriainov V, Gibbs D, Kanczler J, Tare RS, Oreffo RO. Bone tissue engineering. Curr Mol Biol Rep. 2015;1(3):132–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Friedenstein AY, Chailakhyan RK, Gerasimov UV. Bone marrow osteogenic stem cells: in vitro cultivation and transplantation in diffusion chambers. Cell Tissue Kinetics. 1987;20(3):263−272. [DOI] [PubMed] [Google Scholar]
- 22. Friedenstein AY, Chailakhyan RK, Lalykina KS. The development of fibroblast colonies in monolayer cultures of guinea-pig bone marrow and spleen cells. Cell Tissue Kinetics. 1970;3(4):393−403. [DOI] [PubMed] [Google Scholar]
- 23. Dimitriou R, Eleftherios T, Giannoudis P. Current concepts of molecular aspects of bone healing. Injury. 2005;36(12):1392–1404. [DOI] [PubMed] [Google Scholar]
- 24. Quarto R, Thomas D, Liang CT. Bone progenitor cell deficits and the age-associated decline in bone repair capacity. Calcif Tissue Intl. 1995;56(2):123–129. [DOI] [PubMed] [Google Scholar]
- 25. Chen TL. Inhibition of growth and differentiation of osteoprogenitors in mouse bone marrow stromal cell cultures by increased donor age and glucocorticoid treatment. Bone. 2004;35(1):83–95. [DOI] [PubMed] [Google Scholar]
- 26. Shigeno Y, Ashton BA. Human bone-cell proliferation in vitro decreases with human donor age. J Bone Joint Surg (Br). 1995;77(1):139–142. [PubMed] [Google Scholar]
- 27. Muschler GF, Nitto H, Boehm CA, Easley KA. Age- and gender-related changes in the cellularity of human bone marrow and the prevalence of osteoblastic progenitors. J Orthop Res. 2001;19(1):117–125. [DOI] [PubMed] [Google Scholar]
- 28. Baxter MA, Wynn RF, Jowitt SN, Wraith JE, Fairbairn LJ, Bellantuono I. Study of telomere length reveals rapid aging of human marrow stromal cells following in vitro expansion. Stem Cells. 2004;22(5):675–682. [DOI] [PubMed] [Google Scholar]
- 29. Zhou S, Greenberger JS, Epperly MW, Goff JP, Adler C, Leboff MS, Glowacki J. Age-related intrinsic changes in human bone-marrow-derived mesenchymal stem cells and their differentiation to osteoblasts. Aging Cell. 2008;7(3):335–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Stolzing A, Jones E, McGonagle D, Scutt A. Age-related changes in human bone marrow-derived mesenchymal stem cells: consequences for cell therapies. Mech Ageing Dev. 2008;129(3):163–173. [DOI] [PubMed] [Google Scholar]
- 31. Schecroun N, Delloye C. In vitro growth and osteoblastic differentiation of human bone marrow stromal cells supported by autologous plasma. Bone. 2004;35(2):517–524. [DOI] [PubMed] [Google Scholar]
- 32. Liebergall M, Schroeder J, Mosheiff R, Gazit Z, Yoram Z, Rasooly L, Daskal A, Khoury A, Weil Y, Beyth S. Stem cell-based therapy for prevention of delayed fracture union: a randomized and prospective preliminary study. Mol Ther. 2013;21(8):1631–1638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Weel H, Mallee WH, van Dijk CN, Blankevoort L, Goedegebuure S, Goslings JC, Kennedy JG, Kerkhoffs GM. The effect of concentrated bone marrow aspirate in operative treatment of fifth metatarsal stress fractures; a double-blind randomized controlled trial. BMC Musculoskelet Disord. 2015;16:211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Zuk PA, Zhu M, Mizuno H, Huan J, Futrell JW, Katz AJ, Benhaim P, Lorenz HP, Hedrick MH. Multilineage cells from human adipose tissue: implications for cell-based therapies. Tissue Eng. 2001;7(2):211–228. [DOI] [PubMed] [Google Scholar]
- 35. Zuk PA, Zhu M, Ashjian P, De Ugarte DA, Huang JI, Mizuno H, Alfonso ZC, Fraser JK, Benhaim P, Hedrick MH. Human adipose tissue is a source of multipotent stem cells. Mol Biol Cell. 2002;13(12):4279–4295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Zuk PA. The adipose-derived stem cell: looking back and looking ahead. Mol Biol Cell. 2010;21(11):1783–1787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Gimble JM, Katz AJ, Bunnell BA. Adipose-derived stem cells for regenerative medicine. Circ Res. 2007;100(9):1249–1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Kuhbier JW, Weyand B, Radtke C, Vogt PM, Kasper C, Reimers K. Isolation, characterization, differentiation, and application of adipose-derived stem cells. Adv Biochem Eng Biotechnol. 2010;123:55–105. [DOI] [PubMed] [Google Scholar]
- 39. Rehman J, Traktuev D, Li J, Merfeld-Clauss S, Temm-Grove CJ, Bovenkerk JE, Pell CL, Johnstone BH, Considine RV, March KL. Secretion of angiogenic and antiapoptotic factors by human adipose stromal cells. Circulation. 2004;109(10):1292–1298. [DOI] [PubMed] [Google Scholar]
- 40. Fraser JK, Wulur I, Alfonso Z, Hedrick MH. Fat tissue: an underappreciated source of stem cells for biotechnology. Trends Biotechnol. 2006;24(4):150–154. [DOI] [PubMed] [Google Scholar]
- 41. Liao HT, Chen CT. Osteogenic potential: comparison between bone marrow and adipose-derived mesenchymal stem cells. World J Stem Cells. 2014;6(3):288–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Barba M, Cicione C, Bernardini C, Michetti F, Lattanzi W. Adipose-derived mesenchymal cells for bone regeneration: state of the art. Biomed Res Intl. 2013;2013:416391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Schubert T, Xhema D, Vériter S, Schubert M, Behets C, Delloye C, Gianello P, Dufrane D. The enhanced performance of bone allografts using osteogenic-differentiated adipose-derived mesenchymal stem cells. Biomaterials. 2011;32(34):8880–8891. [DOI] [PubMed] [Google Scholar]
- 44. Schubert T, Lafont S, Beaurin G, Grisay G, Behets C, Gianello P, Dufrane D. Critical size bone defect reconstruction by an autologous 3D osteogenic-like tissue derived from differentiated adipose MSCs. Biomaterials. 2013;34(18):4428–4438. [DOI] [PubMed] [Google Scholar]
- 45. Schubert T, Poilvache H, Galli C, Gianello P, Dufrane D. Galactosyl-knock-out engineered pig as a xenogenic donor source of adipose MSCs for bone regeneration. Biomaterials. 2013;34(13):3279–3289. [DOI] [PubMed] [Google Scholar]
- 46. Yoshimura K, Suga H, Eto H. Adipose-derived stem/progenitor cells: roles in adipose tissue remodeling and potential use for soft tissue augmentation. Regenerative Med. 2009;4(2):265–273. [DOI] [PubMed] [Google Scholar]
- 47. Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW., Jr Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest. 2003;112(12):1796–1808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Xu H, Barnes GT, Yang Q, Tan G, Yang D, Chou CJ, Sole J, Nichols A, Ross JS, Tartaglia LA, et al. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J Clin Invest. 2003;112(12):1821–1830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Prunet-Marcassus B, Cousin B, Caton D, André M, Pénicaud L, Casteilla L. From heterogeneity to plasticity in adipose tissues: site-specific differences. Exp Cell Res. 2006;312(6):727–736. [DOI] [PubMed] [Google Scholar]
- 50. Yang XF, He X, He J, Zhang LH, Su XJ, Dong ZY, Xu YJ, Li Y, Li YL. High efficient isolation and systematic identification of human adipose-derived mesenchymal stem cells. J Biomed Sci. 2011;18:59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Minamino T, Orimo M, Shimizu I, Kunieda T, Yokoyama M, Ito T, Nojima A, Nabetani A, Oike Y, Matsubara H, et al. A crucial role for adipose tissue p53 in the regulation of insulin resistance. Nat Med. 2009;15(9):1082–1087. [DOI] [PubMed] [Google Scholar]
- 52. Shimizu I, Yoshida Y, Suda M, Minamino T. DNA damage response and metabolic disease. Cell Metab. 2014;20(6):967–977. [DOI] [PubMed] [Google Scholar]
- 53. Wu W, Niklason L, Steinbacher DM. The effect of age on human adipose-derived stem cells. Plast Reconstr Surg. 2013;131(1):27–37. [DOI] [PubMed] [Google Scholar]
- 54. Kornicka K, Marycz K, Tomaszewski KA, Marędziak M, Śmieszek A. The effect of age on osteogenic and adipogenic differentiation potential of human adipose derived stromal stem cells (hASCs) and the impact of stress factors in the course of the differentiation process. Oxid Med Cell Longev. 2015;2015:309169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Choudhery MS, Badowski M, Muise A, Pierce J, Harris DT. Donor age negatively impacts adipose tissue-derived mesenchymal stem cell expansion and differentiation. J Transl Med. 2014;12:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Dufrane D, Docquier PL, Delloye C, Poirel HA, André W, Aouassar N. Scaffold-free three-dimensional graft from autologous adipose-derived stem cells for large bone defect reconstruction: clinical proof of concept. Medicine (Baltimore). 2015;94(50):e2220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Vériter S, André W, Aouassar N, Poirel HA, Lafosse A, Docquier PL, Dufrane D. Human adipose-derived mesenchymal stem cells in cell therapy: safety and feasibility in different “hospital exemption” clinical applications. PLoS One. 2015;10(10):e0139566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Prockop DJ, Brenner M, Fibbe WE, Horwitz E, Le Blanc K, Phinney DG, Simmons PJ, Sensebe L, Keating A. Defining the risks of mesenchymal stromal cell therapy. Cytotherapy. 2010;12(5):576–578. [DOI] [PubMed] [Google Scholar]
- 59. Bernardo ME, Zaffaroni N, Novara F, Cometa AM, Avanzini MA, Moretta A, Montagna D, Maccario R, Villa R, Daidone MG, et al. Human bone marrow derived mesenchymal stem cells do not undergo transformation after long-term in vitro culture and do not exhibit telomere maintenance mechanisms. Cancer Res. 2007;67(19):9142–9149. [DOI] [PubMed] [Google Scholar]
- 60. Meza-Zepeda LA, Noer A, Dahl JA, Micci F, Myklebost O, Collas P. High-resolution analysis of genetic stability of human adipose tissue stem cells cultured to senescence. J Cell Mol Med. 2008;12(2):553–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Chen HT, Lee MJ, Chen CH, Chuang SC, Chang LF, Ho ML, Hung SH, Fu YC, Wang YH, Wang HI, et al. Proliferation and differentiation potential of human adipose-derived mesenchymal stem cells isolated from elderly patients with osteoporotic fractures. J Cell Mol Med. 2012;16(3):582–593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Lafosse A, Desmet C, Aouassar N, André W, Hanet MS, Beauloye C, Vanwijck R, Poirel HA, Gallez B, Dufrane D. Autologous adipose stromal cells seeded onto a human collagen matrix for dermal regeneration in chronic wounds: clinical proof of concept. Plast Reconstr Surg. 2015;136(2):279–295. [DOI] [PubMed] [Google Scholar]
- 63. Tarte K, Gaillard J, Lataillade JJ, Fouillard L, Becker M, Mossafa H, Tchirkov A, Rouard H, Henry C, Splingard M, et al . Clinical-grade production of human mesenchymal stromal cells: occurrence of aneuploidy without transformation. Blood. 2010;115(8):1549–1553. [DOI] [PubMed] [Google Scholar]
- 64. Trobaugh-Lotrario AD, Kletzel M, Quinones RR, McGavran L, Proytcheva MA, Hunger SP, Malcolm J, Schissel D, Hild E, Giller RH. Monosomy 7 associated with pediatric acute myeloid leukemia (AML) and myelodysplastic syndrome (MDS): successful management by allogeneic hematopoietic stem cell transplant (HSCT). Bone Marrow Transplant. 2005;35(2):143–149. [DOI] [PubMed] [Google Scholar]
- 65. Nikitina VA, Osipova EY, Katosova LD, Rumyantsev SA, Skorobogatova EV, Shamanskaya TV, Bochkov NP. Study of genetic stability of human bone marrow multipotent mesenchymal stromal cells. Bull Exp Biol Med. 2011;150(5):627–631. [DOI] [PubMed] [Google Scholar]
- 66. Bochkov NP, Voronina ES, Kosyakova NV, Liehr T, Rzhaninova AA, Katosova LD, Platonova VI, Gol’dshtein DV. Chromosome variability of human multipotent mesenchymal stromal cells. Bull Exp Biol Med. 2007;143(1):122–126. [DOI] [PubMed] [Google Scholar]
- 67. Haniffa MA, Collin MP, Buckley CD, Dazzi F. Mesenchymal stem cells: the fibroblasts’ new clothes? Haematologica. 2009;94(2):258–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Ho AD, Wagner Franke W. Heterogeneity of mesenchymal stromal cell preparations. Cytotherapy. 2008;10(4):320–330. [DOI] [PubMed] [Google Scholar]
- 69. Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S, Marshak DR. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284(5411):143–147. [DOI] [PubMed] [Google Scholar]
- 70. Bourin P, Bunnell BA, Casteilla L, Dominici M, Katz AJ, March KL, Redl H, Rubin JP, Yoshimura K, Gimble JM. Stromal cells from the adipose tissue-derived stromal vascular fraction and culture expanded adipose tissue-derived stromal/stem cells: a joint statement of the International Federation for Adipose Therapeutics and Science (IFATS) and the International Society for Cellular Therapy (ISCT). Cytotherapy. 2013;15(6):641–648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Hematti P. Mesenchymal stromal cells and fibroblasts: a case of mistaken identity? Cytotherapy. 2012;14(5):516–521. [DOI] [PubMed] [Google Scholar]
- 72. Wagner W, Ho AD, Zenke M. Different facets of aging in human mesenchymal stem cells. Tissue Eng Part B Rev. 2010;16(4):445–453. [DOI] [PubMed] [Google Scholar]
- 73. Dufrane D, Lafosse A. A simple method to determine the purity of adipose-derived stem cell-based cell therapies. Stem Cells Transl Med. 2016;5(11):1575–1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Backly RM, Cancedda R. Bone marrow stem cells in clinical application: harnessing paracrine roles and niche mechanisms. Adv Biochem Eng Biotechnol. 2010;123:265–292. [DOI] [PubMed] [Google Scholar]
- 75. Salgado AJ, Reis RL, Sousa NJ, Gimble JM. Adipose tissue derived stem cells secretome: soluble factors and their roles in regenerative medicine. Curr Stem Cell Res Ther. 2010;5(2):103–110. [DOI] [PubMed] [Google Scholar]
- 76. Nakanishi C, Nagaya N, Ohnishi S, Yamahara K, Takabatake S, Konno T, Hayashi K, Kawashiri MA, Tsubokawa T, Yamagishi M. Gene and protein expression analysis of mesenchymal stem cells derived from rat adipose tissue and bone marrow. Circ J. 2011;75(9):2260–2268. [DOI] [PubMed] [Google Scholar]
- 77. Lee K, Kim H, Kim JM, Kim JR, Kim KJ, Kim YJ, Park SI, Jeong JH, Moon YM, Lim HS, et al. Systemic transplantation of human adipose-derived stem cells stimulates bone repair by promoting osteoblast and osteoclast function. J Cell Mol Med. 2011;15(10):2082–2094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Langenbach F, Naujoks C, Smeets R, Berr K, Depprich R, Kübler N, Handschel J. Scaffold-free microtissues: differences from monolayer cultures and their potential in bone tissue engineering. Clin Oral Investig. 2013;17(1):9–17. [DOI] [PMC free article] [PubMed] [Google Scholar]