Abstract
Retinal degenerative diseases, which include age-related macular degeneration, retinitis pigmentosa, diabetic retinopathy, and glaucoma, mostly affect the elderly population and are the most common cause of decreased quality of vision or even blindness. So far, there is no satisfactory treatment protocol to prevent, stop, or cure these disorders. A great hope and promise for patients suffering from retinal diseases is represented by stem cell–based therapy that could replace diseased or missing retinal cells and support regeneration. In this respect, mesenchymal stem cells (MSCs) that can be obtained from the particular patient and used as autologous cells have turned out to be a promising stem cell type for treatment. Here we show that MSCs can differentiate into cells expressing markers of retinal cells, inhibit production of pro-inflammatory cytokines by retinal tissue, and produce a number of growth and neuroprotective factors for retinal regeneration. All of these properties make MSCs a prospective cell type for cell-based therapy of age-related retinal degenerative diseases.
Keywords: age-related retinal degenerative diseases, mesenchymal stem cells, stem cell therapy
Introduction
Retinal degenerative diseases, such as age-related macular degeneration, retinitis pigmentosa, diabetic retinopathy, or glaucoma, represent the leading cause of a decreased quality of vision or even blindness among the elderly population worldwide. Irrespective of the primary cause and etiology, cumulative damage and loss of retinal pigment epithelium (RPE), choriocapillaris, and degeneration of photoreceptors or ganglion cells cause consequential visual impairment leading to a total loss of vision. The current treatment regimens are based on surgical and medical interventions to slow down the disease progression. Since the main cause of retinal degenerative diseases is an impairment and loss of specialized retinal cells, their support or replacement would represent a prospective treatment option. In this respect, stem cell–based therapy holds great promise.1,2
Among various stem cell types that have been suggested or already tested for treatment of retinal diseases, the mesenchymal stem cells (MSCs) turned out to be the most promising cells. These cells can be obtained relatively easily from bone marrow or adipose tissue, multiplied ex vivo, and used as autologous (patient’s own) stem cells. It has been shown that MSCs possess a number of useful properties3–6 that make them a promising candidate cell population for stem cell–based therapy of retinal degenerative diseases.
In this communication, we provide support for the above suggestion. Using highly purified mouse MSCs, we show that these cells are a potent source of various growth factors, inhibit expression of genes for pro-inflammatory molecules in stimulated retinal cells, and can differentiate into cells expressing markers of different retinal cell types.
Materials and Methods
Preparation of MSCs
The female mice of the inbred strain BALB/c at the age of 7 to 9 wk (20–25 g of weight) were purchased from the breeding unit of the Institute of Molecular Genetics, Prague. MSCs were prepared from the bone marrow as we have described previously.7 In brief, adherent bone marrow cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM) containing 10% fetal calf serum (FCS), antibiotics, and 10 mM 4-(2-hydroxyethyl)piperazine-1-ethanesulfonic acid (HEPES) buffer for 3 wk, and MSCs were purified by magnetic cell sorting to eliminate contaminating cells. The separated MSCs adhered to plastic, had a typical fibrocyte-like morphology, were positive for CD44 and CD73 markers and negative for CD11b and CD45, and were able to undergo adipogenic and osteogenic differentiation.7 The use of the animals was approved by the local Ethical Committee of the Institute of Experimental Medicine.
Targeted Differentiation of MSCs into Cells Expressing Markers of Retinal Cells
To differentiate MSCs into cells expressing markers of retinal cells, we attempted to mimic the inflammatory environment of the diseased retina. For this purpose, we prepared tissue extracts from the posterior segment of the mouse eye (100 μL of serum-free medium per eye). Control tissue extracts were prepared from the heart, muscle, or lung tissue. To further mimic the environment of the inflammatory site in diseased tissue, we prepared supernatants after a 48-h stimulation of mouse spleen cells with T-cell mitogen Concanavalin A (1 μg/mL, Sigma-Aldrich, St. Louis, MO, USA) for 48 h. The preparation of tissue extracts and cytokine-containing supernatants has been described elsewhere.8 Purified MSCs (6 × 104 cells in 1 mL of DMEM in 12-well tissue culture plates) were cultured for 7 d with the extract (30% of the culture volume) and supernatant (30% of the volume), and the expression of genes for retinal cell markers rhodopsin, S-antigen, retinaldehyde-binding protein (Rlbp), and calbindin 2 (Calb2; which are not, or only very weakly expressed in MSCs) was determined by real-time polymerrase chain reaction (PCR). The conditions of cell differentiation, RNA extraction, and real-time PCR are described in detail elsewhere.8 In brief, the total RNA was extracted using TRI Reagent and the first-strand cDNA was synthesized using random hexamers. Quantitative real-time PCR was performed in a StepOnePlus system. The PCR parameters and fluorescence data analysis have been described previously.8,9
Anti-Inflammatory Effects of MSCs
Small pieces (1 × 1 mm) of the posterior segment of the mouse eye bulb (containing the retina) were cultured in 500 μL of Roswell Park Memorial Institute (RPMI) 1640 medium (Sigma-Aldrich), containing 10% FCS, antibiotics, and 10 mM HEPES buffer in 48-well tissue culture plates (Nunc, Roskilde, the Netherlands) alone, in the presence of pro-inflammatory cytokines (10 ng/mL of interleukin [IL]-17 and 10 ng/mL of interferon [IFN]-γ), or in the presence of cytokines in wells containing 2 × 104 adherent MSCs. After a 48-h incubation period, the pieces of the eye tissue samples were harvested from the wells, and the expression of genes for pro-inflammatory molecules IL-1α, IL-6, tumor necrosis factor-α, and inducible nitric oxide synthase (iNOS) was determined by real-time PCR.
Production of Growth and Differentiation Factors by MSCs
MSCs (4 × 104 cells in 1 mL of culture medium) were cultured for 48 h in 48-well tissue culture plates (Nunc) unstimulated or in the presence of pro-inflammatory cytokines (10 ng/mL of IL-17 and 10 ng/mL of IFN-γ). The expression of genes for a panel of cytokines and growth factor (including IL-6, transforming growth factor-β [TGF-β], insulin-like growth factor-1 [IGF-1], insulin-like growth factor-2 [IGF-2], nerve growth factor [NGF], hepatocyte growth factor [HGF], plateled-derived growth factor [PEDF], and glial cell line-derived neurotrophic factor [GDNF]) was determined by real-time PCR.8
Statistical Analysis
The results are expressed as the mean (SD). Comparisons between the 2 groups were analyzed using Student’s t-test. A P value of <0.05 was considered statistically significant.
Results
Differentiation Potential, Immunosuppressive Properties, and Secretory Activity of MSCs
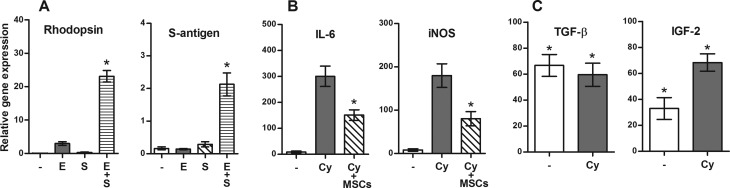
Purified MSCs were cultured for 7 d in a standard culture medium or in a medium containing retinal tissue extract, supernatant from activated lymphocytes or extract, and supernatant together (differentiation medium). The expression of genes for the retina-associated markers rhodopsin, S-antigen, Rlbp, and Calb2 was determined by real-time PCR. As demonstrated in Fig. 1A for rhodopsin and S-antigen, a very low expression of these genes was detected in undifferentiated MSCs, but a significant expression was induced in cells cultured in the differentiation medium. Similar effects of differentiation medium were observed on the expression of Rlbp and Calb2 genes (data not shown). No significant expression of retinal markers was found in MSC cultures containing supernatants from activated spleen cells and control tissue extracts (lung, liver, and muscle; data not shown).
Fig. 1.
The ability of mesenchymal stem cells (MSCs) to differentiate into cells expressing markers of retinal cells, to inhibit expression of genes for pro-inflammatory molecules, and to produce growth and differentiation factors. (A) MSCs were cultured for 7 d alone (-) or in the presence of retinal extract (E), in the presence of supernatant from activated T lymphocytes (S), or in the presence of E and S. The expression of genes for rhodopsin and S-antigen was determined by real-time PCR. The explants of the posterior segment of the eye were cultured for 48 h alone (-), with interleukin (IL)-17 and interferon (IFN)-γ (Cy), or were stimulated with cytokines in the presence of MSCs. The expression of genes for pro-inflammatory molecules IL-1β and inducible nitric oxide synthase was determined by PCR. Production of TGF-β and IGF-2 by MSCs. MSCs were cultured for 48 h unstimulated (-) or in the presence of IL-17 and IFN-γ (Cy). The expression of genes for TGF-β and IGF-2 was determined by real-time PCR. Each bar represents the mean (SD) from at least 3 independent determinations. Values with asterisk represent statistical significance (P < 0.05; A) gene expression, (B) inhibition of cytokine production, and (C) expression of genes for growth factors.
As demonstrated in Fig. 1B, organotypic tissue cultures of the posterior segment of the eye expressed very low levels of genes for pro-inflammatory molecules (such as IL-6 or iNOS). However, in the presence of pro-inflammatory cytokines IFN-γ and IL-17, a significant expression of genes for pro-inflammatory molecules was detected. This expression was significantly suppressed if the explants were stimulated with cytokines in the presence of MSCs (Fig. 1B).
To demonstrate the secretory activity of MSCs, the cells were cultured unstimulated or in the presence of IFN-γ and IL-17, and the expression of genes for a panel of cytokines and growth factors was determined by real-time PCR. As demonstrated in Fig. 1C for TGF-β and IGF-2, MSCs significantly expressed genes for the tested molecules either constitutively (such as TGF-β) or after stimulation with cytokines (such as IGF-2).
Discussion
In spite of great progress in medical research, there are still missing effective therapeutic protocols for the treatment of retinal degenerative diseases, and millions of people worldwide are waiting for a treatment option. In this respect, stem cell–based therapy offers a promising therapeutic approach, which could inhibit degenerative processes or even replace missing retinal cells. Age-related retinal disorders are caused mainly by a degeneration and loss of specialized retinal cells and therefore the support of their survival or even their replacement by descendants of stem cells may offer effective treatment approaches. We observed that MSCs are producers of numerous growth and differentiation factors that can support the survival of the remaining cells in the diseased retina. The damage of the retina is also associated with a local inflammatory reaction that impedes the healing process. We showed that MSCs, by their known immunosuppressive properties,3,5 inhibit the production of pro-inflammatory cytokines by the cells of the posterior ocular segment. These immunoregulatory properties of MSCs may represent an important mechanism to prevent a harmful local inflammatory reaction and to support the healing process. Finally, it has been shown that MSCs can differentiate into various cell types including cells expressing markers and characteristics of retinal cells. For example, it has been shown that cocultivation of MSCs with RPE cells induced expression of the RPE cell phenotype.6,10 In our experiments to differentiate MSCs, attempts were made to mimic the inflammatory environment of a diseased retina. We showed that incubation of MSCs with retinal tissue extracts and supernatant from cultures of stimulated spleen cells induced expression of genes for rhodopsin, S-antigen, Rlbp, and Calb2, which are the markers of cells of individual retinal layers.
We have previously shown that bone marrow–derived MSCs have comparable therapeutic properties for ocular surface regeneration as have tissue-specific limbal stem cells.9 The advantages of MSCs for the therapy of retinal dysfunctions have also been recently discussed by Park et al.11 Here we showed experimentally that MSCs possess at least 3 different types of properties (immunoregulation ability, secretory activity, and differentiation potential), making them a promising candidate for the cell-based therapy of retinal degenerative diseases. To speed up the transfer of experimental results into clinical practice, numerous MSC-based clinical trials for the treatment of retinal diseases have been initiated.12 However, further preclinical studies would be desirable.
Footnotes
Ethical Approval: The use of animals was approved by the local Ethical Committee of the Institute of Experimental Medicine.
Statement of Human and Animal Rights: Mice were purchased from the breeding unit of the Institute of Molecular Genetics, Prague. Their treatment was approved by the local Ethical Committee of the Institute of Experimental Medicine.
Statement of Informed Consent: There are no human subjects in this article and informed consent is not applicable.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by grant 17-04800S from the Grant Agency of the Czech Republic, project 80815 from the Grant Agency of the Charles University and by the projects SVV 260206, CZ.1.05/1.1.00/02.0109, CZ.2.16/3.1.00/21528, and NPUI: LO1309.
References
- 1. Huang Y, Enzmann V, Ildstad ST. Stem cell-based therapeutic applications in retinal degenerative diseases. Stem Cell Rev. 2011;7(2):434–445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Nazari H, Zhang L, Zhu D, Chader GJ, Falabella P, Stefanini F, Rowland T, Clegg DO, Kashani AH, Hinton DR, et al. Stem cell based therapies for age-related macular degeneration: the promises and the challenges. Prog Retin Eye Res. 2015;48:1–39. [DOI] [PubMed] [Google Scholar]
- 3. Abumaree M, Al Jumah M, Pace RA, Kalionis B. Immunosuppressive properties of mesenchymal stem cells. Stem Cell Rev. 2012;8(2):375–392. [DOI] [PubMed] [Google Scholar]
- 4. Zajicova A, Pokorna K, Lencova A, Krulova M, Svobodova E, Kubinova S, Sykova E, Pradny M, Michalek J, Svobodova J, et al. Treatment of ocular surface injuries by limbal and mesenchymal stem cells growing on nanofiber scaffolds. Cell Transplant. 2010;19(10):1281–1290. [DOI] [PubMed] [Google Scholar]
- 5. English K. Mechanisms of mesenchymal stromal cell immunomodulation. Immunol Cell Biol. 2013;91(1):19–26. [DOI] [PubMed] [Google Scholar]
- 6. Mathivanan I, Trepp C, Brunold C, Baerlocher G, Enzmann V. Retinal differentiation of human bone marrow-derived stem cells by co-culture with retinal pigment epithelium in vitro. Exp Cell Res. 2015;333(1):11–20. [DOI] [PubMed] [Google Scholar]
- 7. Javorkova E, Trosan P, Zajicova A, Krulova M, Hajkova M, Holan V. Modulation of the early inflammatory microenvironment in the alkali-burned eye by systemically administered interferon-γ-treated mesenchymal stromal cells. Stem Cells Dev. 2014;23(20):2490–2500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Chudickova M, Bruza P, Zajicova A, Trosan P, Svobodova L, Javorkova E, Kubinova S, Holan V. Targeted neural differentiation of murine mesenchymal stem cells by a protocol simulating the inflammatory site of neural injury. J Tissue Eng Regen Med. 2017;11(5):1588–1597. doi:10.1002/term.2059. [DOI] [PubMed] [Google Scholar]
- 9. Holan V, Trosan P, Cejka C, Javorkova E, Zajicova A, Hermankova B, Chudickova M, Cejkova J. A comparative study of the therapeutic potential of mesenchymal stem cells and limbal epithelial stem cells for ocular surface reconstruction. Stem Cells Transl Med. 2015;4(9):1052–1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Chiou SH, Kao CL, Peng CH, Chen SJ, Tarng YW, Ku HH, Chen YC, Shyr YM, Liu RS, Hsu CJ, et al. A novel in vitro retinal differentiation model by co-culturing adult human bone marrow stem cells with retinal pigmented epithelium cells. Biochem Biophys Res Commun. 2005;326(3):578–585. [DOI] [PubMed] [Google Scholar]
- 11. Park SS, Moisseiev E, Bauer G, Anderson JD, Grant MB, Zam A, Zawadzki RJ, Werner JS, Nolta JA. Advances in bone marrow stem cell therapy for retinal dysfunction. Prog Retin Eye Res. 2017;56:148–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ng TK, Yung JS. Research progress and human clinical trials of mesenchymal stem cells in ophthalmology: a mini review. SM Ophthalmol J. 2015;1(1):1003–1011. [Google Scholar]