Abstract
Molecular communications in the gut–brain axis, between the central nervous system and the gastrointestinal tract, are critical for maintaining healthy brain function, particularly in aging. Epidemiological analyses indicate type 2 diabetes mellitus (T2DM) is a risk factor for neurodegenerative disorders including Alzheimer's disease (AD) and Parkinson's diseases (PD) for which aging shows a major correlative association. Common pathophysiological features exist between T2DM, AD, and PD, including oxidative stress, inflammation, insulin resistance, abnormal protein processing, and cognitive decline, and suggest that effective drugs for T2DM that positively impact the gut–brain axis could provide an effective treatment option for neurodegenerative diseases. Glucagon-like peptide-1 (GLP-1)-based antidiabetic drugs have drawn particular attention as an effectual new strategy to not only regulate blood glucose but also decrease body weight by reducing appetite, which implies that GLP-1 could affect the gut–brain axis in normal and pathological conditions. The neurotrophic and neuroprotective effects of GLP-1 receptor (R) stimulation have been characterized in numerous in vitro and in vivo preclinical studies using GLP-1R agonists and dipeptidyl peptidase-4 inhibitors. Recently, the first open label clinical study of exenatide, a long-acting GLP-1 agonist, in the treatment of PD showed long-lasting improvements in motor and cognitive function. Several double-blind clinical trials of GLP-1R agonists including exenatide in PD and other neurodegenerative diseases are already underway or are about to be initiated. Herein, we review the physiological role of the GLP-1R pathway in the gut–brain axis and the therapeutic strategy of GLP-1R stimulation for the treatment of neurodegenerative diseases focused on PD, for which age is the major risk factor.
Keywords: Parkinson disease, glucagon-like peptide-1, exendin-4, exenatide, neuroinflammation, neuroprotection, neurogenesis, neurotrophic, gut–brain axis
Introduction
Neurodegenerative disorders generally show a mixture of abnormal motor and cognitive function and are characterized by a selective type of neurological pathology, derived from quite distinct areas of the brain.1,2 Although the exact causes of the neurodegenerative diseases are generally unknown and distinct from one another, common pathological features between the diseases exist on multiple levels. A good example is the accumulation of abnormal peptides and/or proteins in the disease-specific areas of the brain. In Alzheimer's disease (AD), there are 2 well-characterized abnormal peptide/proteins that include extraneuronal deposition of the amyloid β-protein (Aβ) in the form of plaques and intraneuronal deposition of the microtubule-associated protein τ in the form of filaments.3,4 In contrast, in Parkinson's disease (PD), neuronal deposits of α-synuclein are present and form the primary structural component of Lewy body fibrils that are characteristic of the disorder, whereas in Huntington's disease (HD), cytoplasmic and nuclear deposition of the huntingtin protein and fragments thereof are found.5,6 Such neurotoxic aggregates appear critical in the disease progression of each of these disorders3–6 and can occur across them. For example, Alzheimer's-type Aβ plaques and τ neurofibrillary tangles can coexist in PD,7–9 particularly in patients with PD-related dementia. Indeed, a significant linear relationship between cortical Aβ and α-synuclein has been described in a subgroup of PD,7,9 in line with experimental studies demonstrating that these proteins may promote and cross-seed one another’s aggregation.10
In addition, mitochondrial dysfunction and the resulting energy failure have been repeatedly implicated as the cause of death of dopaminergic (DAergic) neurons in PD as well as major causes of age-related neural dysfunction.11–16 Toxins used to model DA loss in PD, such as 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) and rotenone, impair respiratory chain function by inhibiting complex I.17–22 In further support of this “mitochondrial genetics” hypothesis for PD pathophysiology, Bender et al. reported higher levels of mitochondrial DNA deletions in nigral neurons from PD patients.23 Moreover, both Bender et al.23 and Kraytsberg et al.24 reported higher levels of mitochondrial DNA deletions in nigral neurons in aged humans with sharp elevations starting shortly before age 70. This correlates with the known risk factor of age in PD development.
Genetic variation and/or protein or peptide-mediated neurotoxic signaling is also common in the neurodegenerative process. Inflammation, oxidative stress, and deficits in neurotransmitters are the common features of the biochemical switch that ultimately results in the activation of the apoptotic pathways that lead to neuronal cell dysfunction and death and eventually to the neurological disorder.2,25 Interestingly, these pathological events are also manifested in the processes that lead to type 2 diabetes mellitus (T2DM) that has commonalities with AD and other neurodegenerative diseases.4,6,26 Indeed, AD is sometimes termed as a “type 3 diabetes,” describing the similarity of the 2 diseases in the metabolic and physiologic status of brain, particularly in relation to the development of insulin resistance.27,28
The similarities between neurodegenerative diseases and diabetes imply the homeostasis in the gut–brain axis is crucially important for the maintenance of health in both the central nervous system (CNS) and peripheral system, which can closely influence each other in multiple pathways. Most importantly, insulin-mediated glucose control is critical for the gut–brain axis because glucose is the irreplaceable source of energy in brain.29 Generally, insulin secretion is regulated by blood glucose. When the blood glucose level is high, pancreatic β cells secrete insulin to reduce blood glucose levels, which is facilitated by incretins secreted by the gastrointestinal tract.1,30–32
Incretins are small hormonal peptides that can stimulate pancreatic β cells and/or gastric cells to regulate insulin release and gastric emptying after eating. They additionally have important roles in the interplaying of the gut–brain axis to modulate the needs of energy, food uptake, and blood glucose.33,34 In this regard, glucagon-like peptide-1 (GLP-1) is one of the incretins with a pivotal role in many aspects of glucose regulation in the gut–brain axis because its receptor, the GLP-1 receptor (GLP-1R), is expressed not only in the pancreas but also widely in the periphery (such as in the lung, stomach, intestine, kidney, and heart) as well as most regions of the brain.35–38 Several GLP-1R agonists are already developed as therapeutics for diabetes and have demonstrated various beneficial effects in the clinic in addition to action on blood glucose control, such as weight loss and even cardiovascular benefits.39–41
Additional roles for GLP-1 agonists in the brain have recently been evaluated in numerous preclinical and clinical studies from a basic biochemical approach to their repurposing as therapeutics for neurodegenerative diseases.1,38,42–45 In an open label trial led by Prof. Thomas Foltynie and colleagues at the Institute of Neurology, University College London, a GLP-1R agonist, exenatide, showed clinical benefits in the treatment of moderate PD.43,46 This trial appraised key motor functions, as assessed by blinded ratings of the Movement Disorders Society Unified Parkinson’s Disease Rating Scale (MDS-UPDRS) part 3, together with several nonmotor tests using the Mattis dementia rating scale—2 (DRS-2) at baseline, 6 mo, and 12 mo and following a further 2-mo washout period to compare exenatide-treated subjects on conventional PD medication to those on conventional PD medication alone.46 A successive randomized, double-blind, placebo controlled clinical trial following largely the same format has just been completed with once weekly formulation of exenatide in patients with PD (NCT01971242), and the results largely cross-validated the former open label study.47
In this review, we will discuss the role of GLP-1 agonists in PD model systems, the mechanism of action, and future perspectives of GLP-1 agonists in the treatment of neurological disorders, chiefly focused on PD.
PD, Diabetes, and the Gut–Brain Axis
PD and AD are associated with a higher incidence rate in patients with T2DM, suggesting that shared pathological processes in the gut–brain axis, such as insulin dysregulation, may underlie these conditions.4,6,27 Although associated with diverse cell types in different tissues (e.g., the involvement of DAergic neurons within the substantia nigra and midbrain DAergic neurons in PD, vs. pancreatic β cells in T2DM), parallel biochemical signaling leads to the cellular dysfunction and death characteristic of both disorders. For example, increased apoptosis induced by cellular stress leads to a loss of function and mass of pancreatic β cells in T2DM.32,47 The key factors increasing β cell death include endoplasmic reticulum stress (leading to accumulation of unfolded and misfolded proteins within this key subcellular compartment critical in the biosynthesis of secretory and structural proteins as well as steroids, cholesterol, and other lipids), oxidative stress, mitochondrial dysfunction, inflammatory stress, and inclusions of aggregated peptides that reside within a similar intracellular milieu as do neuronal cells in PD.48,49 Consequently, 1 effective treatment strategy for both diseases would be ameliorating the common toxic factors in the gut–brain axis to protect the mass and function of β cells and/or neurons. GLP-1R agonists have been thought to be an excellent candidate due to their pleiotropic effects on the gut–brain axis.
Recently, several GLP-1R agonists (Table 1) have been approved by the US Food and Drug Administration for the treatment of T2DM and studied extensively to elucidate the exact mechanisms of action not only for their insulin-mediated glucose regulation but also for their beneficial effect on β cell function. GLP-1R agonists can stimulate β cell proliferation in vitro by activation of the phosphoinositide 3-kinase (PI3K)/protein kinase B (Akt) and 5′ adenosine monophosphate–activated protein kinase/mechanistic target of rapamycin signaling pathways and the inactivation of Forkhead box protein O1 (FOXO1) in pancreatic cell lines as well as in β cells in human islets of Langerhans.32,50 GLP-1R agonists are also able to increase β cell mass in several diabetic animal models by increasing proliferation and decreasing apoptosis in pancreatic β cells.51 Antiapoptotic effects of GLP-1R agonists are associated with activation of PI3K/Akt signaling pathways and downregulation of apoptotic pathways including caspase-3 (CASP3) and poly(adenosine diphosphate ribose) polymerase.52 GLP-1R agonists also regulate gene expression related to β cell maturation (pancreatic and duodenal homeobox 1, MAF bZIP transcription factor A, and neuronal differentiation 1, upregulated) and to apoptosis (thioredoxin interacting protein, CASP3, and B-cell lymphoma 2 (Bcl-2) associated X, apoptosis regulator, downregulated).53
Table 1.
GLP-1 Agonists Approved by the US FDA.
| Drug | Generic | Dosing Regimen | Dosing | FDA Approval | Indication |
|---|---|---|---|---|---|
| Byetta | Exenatide | BID | 5, 10 mcg | 2005 | T2DM |
| Bydureon | Exenatide | QW | 2 mg | 2012 | T2DM |
| Victoza (Saxenda) | Liraglutide | Daily | 0.6, 1.2, 1.8 mg | 2010 (2014) | T2DM (obesity) |
| Adlyxin | Lixisenatide | Daily | 10, 20 mcg | 2016 | T2DM |
| Tanzeum | Albiglutide | QW | 30, 50 mg | 2014 | T2DM |
| Trulicity | Dulaglutide | QW | 0.75, 1.5 mg | 2014 | T2DM |
Abbreviations: FDA, Food and Drug Administration; BID, twice a day; QW, once weekly; T2DM, type 2 diabetes mellitus; GLP-1, glucagon-like peptide-1.
As the described GLP-1R agonist protective actions on β cell mass from a variety of toxic insults are relevant to the actions of GLP-1R agonists in the CNS, the other partner of the gut–brain axis, the therapeutic potential of this drug class is an area of intense current research in neurodegenerative diseases.
GLP-1R Activation in the Brain: Neurotrophic Effects, Neuroprotection, Anti-Inflammation, Neurogenesis, and Synaptic Plasticity
As expected with the similar mechanisms of disease progression in the gut–brain axis and the broad expression profile of GLP-1Rs in this region, GLP-1R agonists are able to show neuroprotective and neurotrophic effects in various in vitro and in vivo model systems.1,43–45,54 In primary mouse hypothalamic cultures, exenatide induced ciliary neurotrophic factor (CNTF)-mediated cell proliferation. GLP-1R-dependent signaling is essential for the CNTF-induced cell proliferation, evidenced by the increased expression of GLP-1 in the hypothalamus of mice and the lack of efficacy in GLP-1R knockout mice.1,55
Chronic treatment with exenatide induced cell proliferation in the rat dentate gyrus of the hippocampus with elevated Ki-67 gene expression (a cellular marker of proliferation used to determine the growth fraction of a cell population, as it is present during all active phases of the cell cycle [G1, S, G2, and mitosis] but is absent from resting cells [G0]). Long-term administration (21 d) of exenatide in mice also increased proliferation in the subgranular zone of the dentate gyrus. Additionally, in mouse models of diabetes, chronic treatment with GLP-1R agonists for 4 to 10 wk significantly increased the number of progenitor cells or doublecortin (DCX)-positive young neurons in the dentate gyrus.1,56 (DCX is a microtubule-associated protein that is expressed by neuronal precursor cells and immature neurons in embryonic and adult cortical structures while actively dividing, with neuronal daughter cells continuing to express DCX for 2–3 wk as the cells mature into neurons.)
Oxidative stress, a common feature in several neurodegenerative conditions, plays a key contributory role in the progressive nature of diseases like PD and AD as well as in acute disorders such as stroke and traumatic brain injury (TBI).57–59 Principal sources of reactive factors responsible for oxidative cell damage are mitochondria that generate reactive oxygen species (ROS) as a function of normal cellular processes; however, problems can occur due to an imbalance between the processes that generate and those that eliminate ROS.58 A further source of oxidative damage can originate from activated peripheral macrophages or brain resident glial cells in response to microenvironmental activators, such as Aβ and α-synuclein protein as well as circulating cytokines, all of which are observed in the setting of neurodegenerative disease.25,60 GLP-1R stimulation has been shown to attenuate the synthesis of the pro-inflammatory cytokine interleukin-1β (IL-1β) in activated astrocytes.61 A classic in vitro model of oxidative stress involves the use of hydrogen peroxide (H2O2) added to culture media. It has been shown that GLP-1R stimulation is able to ameliorate the detrimental cellular changes induced by this form of oxidative stress. In this regard, GLP-1 and exenatide dose-dependently protected SH-SY5Y cells from H2O2 induced cell death.35,62 It is likewise known that the DAergic cell toxin 6-hydroxydopamine (6-OHDA) exerts its toxicity through oxidative stress, as do Aβ and iron (Fe2+). As mentioned before, GLP-1R stimulation is able to reduce 6-OHDA-induced cell death in SH-SY5Y cells62 as well as primary ventral mesencephalic (DAergic) neurons.35 GLP-1 and exendin-4 protect cultured hippocampal neurons against death induced by Aβ and Fe2+.63 Likewise, the incretin mimetic geniposide has been reported to mitigate H2O2-mediated death in PC12 cells.64,65
Similar to measurement of levels of cell division, assessments of cell differentiation can be chiefly determined by immunohistochemistry methods. These methods allow the identification of the different maturation stages of neuronal cells and can also be used to determine the phenotype of any given cell. As indicated above, GLP-1R activators can induce the differentiation of neural stem cells into neurons. Treatment of adult mice with exenatide showed a 1.7-fold increase in DCX-positive cells in the medial striatum and doubled the number of bromodeoxyuridine (BrdU)-positive cells in the subventricular zone, a known pool of neuronal precursor cells.66 Isacson et al.56 demonstrated that the administration of exenatide (2 wk) to adult rodents induced an elevation in DCX and mammalian achaete scute homolog-1 (Mash-1) gene transcripts, both markers of neurogenesis in the hippocampus. Li et al.’s67 study also showed an enhanced level of neurogenesis and neuroblast differentiation in the mouse dentate gyrus, as indicated by double staining with BrdU and DCX. It is important to note that such processes can occur in the adult and even aging mammalian brain.68,69
Another beneficial feature of the incretin signaling pathway lies in neurite outgrowth. Neurites are essential components in the formation of functional synapses between neurons and their surrounding microenvironment. The treatment of human SH-SY5Y cells with exenatide has been shown to increase the numbers of neurite-bearing cells, in addition to the actual number of neurites per cell.70 Notably, when the morphology of exenatide-induced neurites was evaluated alongside neurites generated by exposure to retinoic acid (RA), a well-characterized promoter of neurite outgrowth known to induce nuclear factor erythroid-derived 2 (Nrf2) upregulation, the overall morphology was similar to that of RA neurites; although the exenatide-stimulated neurites were shorter in length.70 Interestingly, GLP-1 agonists have been shown to augment the expression of Nrf2 when providing cytoprotective actions in cardiomyocytes71 as well as pancreatic β cells.72 These SH-SY5Y studies cross-validate another widely used model of neuronal differentiation using PC12 cells, in which GLP-1R stimulation elicited neurite outgrowth in a manner similar to nerve growth factor (NGF).73 As with the findings in SH-SY5Y cells, exenatide-induced PC12 cell neurites displayed comparable morphology, although they were slightly shorter in length and smaller in number, with less branching when compared with NGF untreated cells.73
Therapeutic Potential of GLP-1R Agonists in Various Animal PD Models
PD is characterized by a loss of DAergic neurons and degeneration of the DAergic pathway to the striatum. It is associated with deficits in motor function, which are often the primary indicators of the disease in humans. It should be noted, however, that there are peripheral autonomic dysfunctions in the gastrointestinal and cardiovascular system that often predate motor deficits.74,75 To study PD and possible therapeutics that may ameliorate the disease symptoms and progression, investigators typically utilize toxins that selectively kill DAergic cells or use transgenic animal models that possess mutations in genes associated with the human disease.76,77
A toxin widely used as a basic research tool for PD in humans and other mammals is MPTP. This agent, following its metabolism to 1-methyl-4-phenylpyridinium, is then selectively transported into the DAergic neurons causing cell death by inhibiting mitochondrial complex I.78–81 Complex I is the largest and most complicated enzyme of the respiratory chain; catalyzing the first step of the mitochondrial electron transport chain, it oxidizes nicotinamide adenine dinucleotide transferring electrons to ubiquinone (Coenzyme Q), a lipid soluble electron carrier embedded in the lipid bilayer of the inner mitochondrial membrane. MPTP/rotenone-mediated toxicity is thought to be a consequence of formation of ROS.80,82 When MPTP was administered to rats or mice, substantial losses of DAergic neurons occurred, which were associated with a heightened inflammatory response. The use of GLP-1R agonists has been shown to protect animals against MPTP toxic insults. The MPTP toxicity was fully reversed by exenatide, which increased the numbers of viable DAergic neurons and modulated the level of inflammation.35,83 Tyrosine hydroxylase (TH) is a key enzyme that is important for the production of DA; it converts tyrosine into l-dihydroxyphenylalanine (l-DOPA), a direct precursor of DA. Cell culture studies have demonstrated that exenatide elevates endogenous TH levels in primary DAergic neurons,35 leading to the augmentation of the phenotype under resting conditions. Studies in TH expressing catecholamine neurons in the area postrema have, likewise, demonstrated that exenatide significantly elevates TH levels and suggest this is mediated by exenatide induction of TH gene expression through the TH promoter.84 In contrast, MPTP treatment reduces TH-positive neurons, decreases concentrations of dopamine as well as its metabolites 3,4-dihydroxyphenylacetic acid and homovanillic acid, and increases the ratio of dopamine metabolites to dopamine. These effects were fully prevented by treatment with exenatide.35 Similarly, MPTP administration in mice resulted in severe impairments in motor function, yet treatment with exenatide restored the observed deficits.35
6-OHDA is a neurotoxin that, similar to MPTP, kills DAergic neurons; likewise, GLP-1R stimulation has been shown to protect neurons from exposure to 6-OHDA. GLP-1 and exenatide dose-dependently protected SH-SY5Y cells against 6-OHDA-induced cell death.35,62 In ventral mesencephalic neuronal cultures that are rich in DAergic neurons, 6-OHDA lowered the number of TH-positive cells, indicating DAergic neuronal toxicity. Treatment with exenatide was not only shown to rescue DAergic neurons but induced a 60% increase in TH-positive cells over control values,35 additionally, lowering levels of proapoptotic proteins. In rats injected with 6-OHDA or lipopolysaccharide (LPS), nigrostriatal dopamine levels and the l-DOPA synthesizing capabilities of these cells were markedly diminished, in line with reduced levels of TH-positive neurons. These deficits were reversed by exenatide treatment.85 Similarly, in 6-OHDA brain-lesioned rats, lower levels of TH-positive and vesicular monoamine transporter 2 (VMAT2)-positive cells were observed, and these changes were halted by exenatide treatment.66 VMAT2 has regulatory functions involved in the storage and processing of dopamine into axonal storage vesicles.
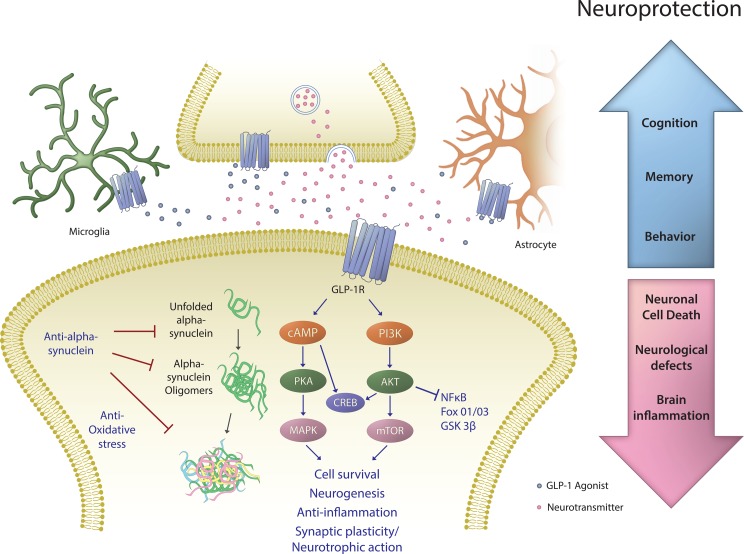
PD is associated with neuronal DAergic cell degeneration and consequently disturbances in motor function. Apomorphine, a DAergic drug, has been used to induce circling behavior in PD animals (often involving rodents administered 6-OHDA unilaterally into the left medial forebrain bundle to induce a hemi-Parkinsonian state); the degree of circling behavior correlates with the severity of PD-like damage in the striatum.86 In 6-OHDA/LPS-challenged rodents, the apomorphine circling behavior was alleviated by exenatide in a dose-dependent manner.85 Additional experiments using 6-OHDA to induce lesions and abnormal behavior have supported these findings. The measurement of an animal’s circling behavior before and after exenatide treatment indicated a near complete normalization in behavior.66 Taken together, these data illustrate GLP-1R stimulation benefits in the setting of neurotoxin-derived models of PD in terms of dopamine cell survival, cell functionality, and the resolution of abnormal behavior. Furthermore, these findings strengthen the hypothesis that GLP-1R stimulation may have therapeutic value in the setting of human PD (Fig. 1).
Fig. 1.
Proposed mechanisms underpinning the beneficial neurological action of glucagon-like peptide-1 (GLP-1) receptor (R) agonists. The GLP-1R, a class B1 G-protein-coupled receptor, is present on numerous cells within the nervous system—including throughout the brain on multiple types of neurons as well as on astrocytes and microglia. GLP-1, chiefly generated by L cells within the gastrointestinal tract, is also produced by select preproglucagon neurons that are primarily localized to the nucleus of the solitary tract within the hind brain87—providing projections to extensive brain areas. Notably, GLP-1 appears also be generated by M2 microglia88 to potentially provide reparative/anti-inflammatory actions. Endogenous GLP-1 and/or long-acting GLP-1 agonists gaining access to the brain can provide neurotrophic/protective actions that are mediated via GLP-1R binding and activation (as such actions are lost in the presence of GLP-1R antagonists and in GLP-1R knockout studies35,89,90). Stimulation of the GLP-1R results to a rapid rise in intracellular cAMP levels, which then activates protein kinase A and phosphoinositide 3-kinase (PI3K); phosphorylating and activating a variety of downstream signalling pathways. These can be broadly subdivided into 2 divisons: the mitogen-associated protein kinase/extracellular signal–regulated kinase and PI3K/protein kinase B pathways. These modulate multiple intracellular events including augmenting protein synthesis, cellular proliferation (neurogenesis), mitochondrial biogenesis and inhibiting apoptosis, inflammation, and protein aggregation. Both singly and in combination these pathways can lead to improved cell survival and a more robust cellular phenoype. cAMP, cyclic AMP; CREB, cAMP response element-binding protein; FoxO1/O3, forkhead box O1/O3; GSK-3β, glycogen synthase 3 β; mTOR, mammalian target of rapamycin; NF-κB, nuclear factor kappa-light-chain-enhancer of activated B cells.
Molecular Mechanism: The GLP-1R Signaling Pathway
The expression of GLP-1R in the brain is not limited to neurons in specific brain regions including the frontal cortex, hypothalamus, thalamus, hippocampus, cerebellum, and substantia nigra but also includes microglial cells involved in neuroinflammation. This suggests multiple mechanisms of action for the therapeutic effects of GLP-1R agonists for the treatment of PD.
The GLP-1R is a 7-transmembrane spanning class B G-protein-coupled receptor, and when stimulated on neuronal, cellist elevates intracellular adenosine monophosphate (cAMP) levels leading to activation of protein kinase A (PKA). It also activates the PI3K/AKT signaling pathway. These signaling pathways regulate many downstream targets such as glycogen synthase kinase 3 β (GSK3-B) and FOXO1, which are involved in pathological processes of PD, promoting an antiapoptotic cell survival pathway.62
In a neuroinflammatory environment, PI3K/AKT signaling upon GLP-1R activation can regulate nuclear factor-κB (NFκB) which, in turn, controls microglial cell activation and the expression of proinflammatory cytokines including tumor necrosis factor-α (TNF-α) except change the a to the alpha symbol and IL-1β. This results in reduced neuroinflammation, and as neuroinflammation is a hallmark in the pathogenesis of PD, such a GLP-1R-mediated action is potentially beneficial.
Whereas the GLP-1R has been broadly found across most types of neuronal cells within the brain, spinal cord, and ganglion and peripheral nerve1,89,91, with GLP-1 agonsits enhancing neuronal phenotypic markers (e.g., augmenting choline acetyltransferase levels in cholinergic cells and TH levels in DAergic neurons 35,91), there is increasing evidence that astrocytes likewise express GLP-1Rs and that injury and neuroinflammation elevate their expression.61,92,93 Similarly, microglia, CNS-resident myeloid cells of embryonic origin that comprise ∼15% of all cells in brain, express GLP-1Rs.61,94 These cells are known to actively survey the CNS environment to orchestrate changes to maintain brain homeostasis, meet changing physiological needs, and respond to pathological events by serving as brain immune cells to coordinate innate immune responses.95 The physical association of microglia with neuronal cell synapses implicates microglia with synaptic refinement, which is achieved by continuous sampling of specific signals derived from neuronal and astrocyte-derived factors that are sensed by the presence of numerous microglial surface receptors/ion channels. Signaling via these induces changes in membrane potential, intracellular Ca2+, cellular motility, and cytokine release, which are accompanied by potential changes in microglial phenotype from a relatively quiescent (surveillance) state to an activated (reactive) one. Microglia are adept at exhibiting an activated proinflammatory phenotype, as arises after stimulation with LPS, or an anti-inflammatory phenotype can be induced by IL-4, typified by the release of trophic factors such as insulin like growth factor-1 (IGF-1), anti-inflammatory IL-10,96 and, notably, the incretin GLP-1.88,94 These micoglial states have been termed “M1 and M2 phenotypes” (adapted from T-helper cell 1 and 2), although these 2 polarized states are too constricting in relation to micoglia, as they have the plasticity to heterogeniously change phenotype to optimize function in response to an almost endless variety of envioronmental challenges.95,97 Hence, GLP-1 and its agonists cannot only bind to glial cells to modify them into a more quiescent state, but microglial cells additionally generate GLP-1 in their M2 anti-inflammatory state both as a potential trophic and as a anti-inflammatory factor. The administration of exogenous sources of GLP-1R agonists, such as exenatide or liraglutide, can be considered to augment such endogenous actions and appears to be mediated via the same signaling pathways present for the endogenous ligand mediated by the activation of the cAMP/PKA/cAMP responsive element binding protein.62,98
Clinical Studies in PD
In a proof of concept open label clinical trial, 45 patients with moderate PD, on convential PD therapy, were randomly assigned to receive subcutaneous exenatide injection (in the form Byetta: 5 μg administered subcutaneously twice daily for the first month followed by a 10 μg dose twice daily thereafter) or were controls for 12 mo, followed by a 2-mo washout period to allow comparison between the exenatide and control groups in the absence of drug (to potentially avoid any potential symptomatic effects of exenatide). After 14 mo, the results showed significant and clinically meaningful differences in both motor and cognitive symptoms: improvements in both the MDS-UPDRS Part 3 (7.2 points difference in exenatide vs. control group; 95% CI, P = 0.006) and the Mattis DRS (6.3 points difference in exenatide vs. control group; 95% CI, P = 0.001).46 Exenatide proved to be well tolerated in PD patients, with weight loss being the most common noted adverse effect—which did not impact study outcome. Despite the limitations of the single-blind design in this clinical study, the advantages in exenatide-treated group in both cognitive and motor functions persisted in the follow-up study after a 12-mo “wash-out period.”99 This suggests disease-modifying effects of exenatide, and the results are encouraging enough that a subsequent double-blind clinical study (NCT01971242) was initiated by the same investigators using a sustained release formulation of exenatide (Bydureon: 2 mg administered subcutaneously once weekly) to evaluate in a similar group of PD subjects over a similar duration of time.47
In this recent double-blind clinical trial,47 60 “mid-stage” PD subjects who were already on dopaminergic replacement therapy were randomized to self-administer exenatide (2 mg Bydureon - slow release exenatide) or a matching placebo once weekly for a duration of 48 weeks. The primary outcome of the study, as in the prior open clinical trial,46 was the severity of PD motor symptoms by using the MDS-UPDRS part 3 in the “Practically defined OFF medication state” at the 60-week time-point; specifically, following a 12-week washout period (occurring directly after the 48 weeks of Bydureon/placebo dosing). This primary outcome was met, as patients using exenatide displayed better motor function compared to the placebo group. The difference in the MDS-UPDRS part 3 scale, following adjustment for base-line scores, was 4.3 points following 48 weeks of exenatide treatment. Notably, this advantage remained (3.5 points (statistically significant)) following the 12-week washout period, when concentrations of exenatide were no longer detectable in serum.47 In this regard at 60 weeks, those on exenatide presented with a 1.0 point improvement in their off-medication scores on part 3 of the MDS-UPDRS as compared to those on placebo that worsened by 2.1 points over the same duration (to provide an adjusted mean difference of −3.5 points). A broad spectrum of secondary measures was additionally evaluated in this double-blind exenatide PD clinical trial,47 and although none demonstrated statistical significance following correction for multiple comparisons, the majority were in the direction favoring an exenatide advantage. In synopsis, although small studies, the results of the open and double-blind exenatide PD trials cross-validate one another and are suggestive that exenatide provides more than just acute symptomatic effects; as the typical rate of progression in PD is approximately 3 UPDRS points per year – which was not evident in the exenatide exposed group (albeit a longer duration and larger patient number clinical trial is needed). Hence, initial clinical studies of exenatide in PD can be viewed as highly encouraging, supportive of further larger studies, and the analysis of available biomarkers in the time-dependently collected serum and CSF samples obtained from the double-blind study could well provide insight into the molecular mechanisms that underpin exenatide’s positive actions in PD.
The selected formulations and doses of exenatide used in the PD clinical studies are licensed and widely used for the treatment of T2DM.46,47,99,100 Data available from the open label clinical trial in PD involving exenatide in the form of Byetta99,100 and Bydureon in the double-blind trial47 suggests that the agent proved to be well tolerated in PD subjects. Weight loss proved to be the most common concern, preventing the trial completion of 1 patient, and proved fully reversible on cessation of the drug in the open trial.99,100 Likewise, weight loss was noted in the double-blind trial.47 Importantly, a direct comparison was made between the degree of weight loss and change in MDS UPDRS part 3 OFF scores and revealed no correlation between these two parameters, and the primary analysis result remained statistically significant when adjusted for the degree of weight loss. Gastrointestinal symptoms represent a not uncommon side effect of exenatide use in T2DM and also were evident in the PD cohorts of both open and double-blind clinical studies but, importantly, did not impact trial participation of the subjects. Indeed, the frequency of the adverse events in the exenatide group appeared to be analogous to that reported in prior clinical trials of exenatide in T2DM.46,47,100
Although the effects of GLP-1R agonists in PD have been cross validated in many preclinical studies (Table 2), data to compare each GLP-1R agonist under the same conditions are still limited. In relation to the clinical use of GLP-1R agonists for the treatment of PD, pharmacokinetic–pharmacodynamic correlations of each GLP-1R agonist, from relatively short acting exenatide (in the form of Byetta [twice daily] and sustained release Bydureon [once weekly]) to longer acting dulaglutide, should be closely examined as to their potency, selectivity, blood–brain barrier (BBB) penetration, and tolerability, which may be very different from one another and ultimately impact their efficacy in PD. There are only few studies trying to answer these questions. In a recent study comparing exenatide, lixisenatide, and liraglutide in a mouse model of PD, exenatide failed to mitigate the MPTP-induced neuronal defects, in contrast to the protective effect of lixisenatide, and liraglutide.101 However, considering the previous positive findings with exenatide in the same PD model, differences in dosing regimen could be the main cause of failure rather than the drug characteristic itself. In many of these studies, drug doses selected for evaluation are not necessarily related to clinical studies and plasma drug levels (a useful measure to compare whether preclinical studies have direct clinical relevance) are not reported and thus cannot be compared to those achievable in humans. BBB penetration of GLP-1 agonsits has also been tested in several studies using mice. In separate studies, exenatide, liraglutide, and lixisenatide were all able to cross the BBB.102,103 A recent study showed that lixisenatide was able to cross the BBB and increase cAMP more than liraglutide with a low dose (2.5 nmol/kg body weight).102 However, it is still difficult to interpret these results, as chemical or pharmacokinetic properties of the drugs could not be unequivocally determined with the methods described. Nevertheless, these initial studies have provided valuable information. Albeit, in this regard, further quantitative studies determining drug concentrations in plasma and brain are required, and evaluation of plasma and cerebrospinal fluid levels in human studies from ongoing clinical trials would prove valuable.
Table 2.
GLP-1 Agonists Examined in Preclinical Animal Models of Parkinson Diseases.
| Animal PD models | Glp-1 agonists | Treatment | Dosing Regimen | Neuroprotection | References |
|---|---|---|---|---|---|
| 6-OHDA (rats) | Exenatide | 7-d postlesion | BID for 7 d | ✓ | 62 |
| LPS (rats) | Exenatide | 7-d postlesion | BID for 7 d | ✓ | 62 |
| 6-OHDA (rats) | Exenatide | 5-wk postlesion | BID for 21 d | ✓ | 43 |
| MPTP (mice) | Exenatide | 2-h pretreatment | 7 d | ✓ | 23 |
| MPTP (mice) | Exenatide | 30-min pretreatment | 4 times in a day | ✓ | 60 |
| MPTP (mice) | Exenatide | Posttreatment | QD for 7 d | x | 75 |
| MPTP (mice) | Liraglutide | Posttreatment | QD for 7 d | ✓ | 75 |
| MPTP (mice) | Lixisenatide | Posttreatment | QD for 7 d | ✓ | 75 |
Abbreviations: 6-OHDA, 6-hydroxydopamine; LPS, lipopolysaccharide; MPTP, 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine; BID, twice a day; QD, once daily; GLP-1, glucagon-like peptide-1.
Future Perspectives
A combination of activating the GLP-1R with other receptor agonists (or antagonists) should be considered if there are additional benefits or superior efficacy compared to the individual use of each drug. Dual or triple agonists, for example, GLP-1/GIP/glucagon, have great potential as they have already shown a better therapeutic window in T2DM, as compared to a GLP-1 agonist alone.104–107 Some of these agents are already being evaluated in preclinical models of neurodegenerative disorders.101,108–112 As an example, a natural dual agonist, oxyntomodulin could be a good lead compound for this class of molecules targeting the gut–brain axis.101,107,109 Improving drug delivery across the BBB can be another important direction, other than comparing potency of the various GLP-1 agonists to differentiate within this drug class. Regardless of their characteristics, it might be reasonable to employ long-acting, sustained release formulations to maintain optimal steady-state therapeutic drug levels in light of difficulties in cognitive and motor function of patients with PD and other neurodegenerative diseases to best achieve compliance and efficacy.
Footnotes
Authors’ Note: DSK and H-I Choi are employees of Peptron Inc. The Intramural Research Program of the National Institute on Aging, NIH, and Peptron Inc. have a Cooperative Research and Development Agreement to develop exendin-4 (exenatide) as a treatment strategy for neurodegenerative disorders for which NIA and Peptron Inc. hold patent rights via the work of DSK and NHG.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This research was supported in part by (i) the Technological Innovation R&D Program (S2174574) funded by the Small and Medium Business Administration (Republic of Korea); (ii) Peptron Inc., Daejeon, Republic of Korea; (iii) the Intramural Research Program of the National Institute on Aging, National Institutes of Health, USA; and (iv) the National Institutes of Health NINDS grant RO1NS094152.
References
- 1. Salcedo I, Tweedie D, Li Y, Greig NH. Neuroprotective and neurotrophic actions of glucagon-like peptide-1: an emerging opportunity to treat neurodegenerative and cerebrovascular disorders. Br J Pharmacol. 2012;166(5):1586–1599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ransohoff RM. How neuroinflammation contributes to neurodegeneration. Science. 2016;353(6301):777–783. [DOI] [PubMed] [Google Scholar]
- 3. Irvine GB, El-Agnaf OM, Shankar GM, Walsh DM. Protein aggregation in the brain: the molecular basis for Alzheimer’s and Parkinson’s diseases. Mol Med. 2008;14(7-8):451–464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ahmad K, Baig MH, Mushtaq G, Kamal MA, Greig NH, Choi I. Commonalities in biological pathways, genetics, and cellular mechanism between Alzheimer Disease and other neurodegenerative diseases: an in silico-updated overview. Curr Alzheimer Res. 2017. https://www.ncbi.nlm.nih.gov/pubmed/28164765. doi: 10.2174/1567205014666170203141151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Polymeropoulos MH, Lavedan C, Leroy E, Ide SE, Dehejia A, Dutra A, Pike B, Root H, Rubenstein J, Boyer R, et al. Mutation in the alpha-synuclein gene identified in families with Parkinson’s disease. Science. 1997;276(5321):2045–2047. [DOI] [PubMed] [Google Scholar]
- 6. Ashraf GM, Greig NH, Khan TA, Hassan I, Tabrez S, Shakil S, Sheikh IA, Zaidi SK, Akram M, Jabir NR, et al. Protein misfolding and aggregation in Alzheimer’s disease and type 2 diabetes mellitus. CNS Neurol Disord Drug Targets. 2014;13(7):1280–1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Compta Y, Parkkinen L, Kempster P, Selikhova M, Lashley T, Holton JL, Lees AJ, Revesz T. The significance of alpha-synuclein, amyloid-beta and tau pathologies in Parkinson’s disease progression and related dementia. Neurodegener Dis. 2014;13(2-3):154–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Pletnikova O, West N, Lee MK, Rudow GL, Skolasky RL, Dawson TM, Marsh L, Troncoso JC. Abeta deposition is associated with enhanced cortical alpha-synuclein lesions in Lewy body diseases. Neurobiol Aging. 2005;26(8):1183–1192. [DOI] [PubMed] [Google Scholar]
- 9. Lashley T, Holton JL, Gray E, Kirkham K, O’Sullivan SS, Hilbig A, Wood NW, Lees AJ, Revesz T. Cortical alpha-synuclein load is associated with amyloid-beta plaque burden in a subset of Parkinson’s disease patients. Acta Neuropathol. 2008;115(4):417–425. [DOI] [PubMed] [Google Scholar]
- 10. Clinton LK, Blurton-Jones M, Myczek K, Trojanowski JQ, LaFerla FM. Synergistic interactions between Abeta, tau, and alpha-synuclein: acceleration of neuropathology and cognitive decline. J Neurosci. 2010;30(21):7281–7289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Dawson TM, Dawson VL. Molecular pathways of neurodegeneration in Parkinson’s disease. Science. 2003;302(5646):819–822. [DOI] [PubMed] [Google Scholar]
- 12. Ellis CE, Murphy EJ, Mitchell DC, Golovko MY, Scaglia F, Barcelo-Coblijn GC, Nussbaum RL. Mitochondrial lipid abnormality and electron transport chain impairment in mice lacking alpha-synuclein. Mol Cell Biol. 2005;25(22):10190–10201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ved R, Saha S, Westlund B, Perier C, Burnam L, Sluder A, Hoener M, Rodrigues CM, Alfonso A, Steer C, et al. Similar patterns of mitochondrial vulnerability and rescue induced by genetic modification of alpha-synuclein, parkin, and DJ-1 in caenorhabditis elegans. J Biol Chem. 2005;280(52):42655–42668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Dauer W, Przedborski S. Parkinson’s disease: mechanisms and models. Neuron. 2003;39(6):889–909. [DOI] [PubMed] [Google Scholar]
- 15. Shen J, Cookson MR. Mitochondria and dopamine: new insights into recessive parkinsonism. Neuron. 2004;43(3):301–304. [DOI] [PubMed] [Google Scholar]
- 16. Mizuno Y, Sone N, Saitoh T. Effects of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine and 1-methyl-4-phenylpyridinium ion on activities of the enzymes in the electron transport system in mouse brain. J Neurochem. 1987;48(6):1787–1793. [DOI] [PubMed] [Google Scholar]
- 17. Langston JW, Ballard P, Tetrud JW, Irwin I. Chronic Parkinsonism in humans due to a product of meperidine-analog synthesis. Science. 1983;219(4587):979–980. [DOI] [PubMed] [Google Scholar]
- 18. Mizuno Y, Ohta S, Tanaka M, Takamiya S, Suzuki K, Sato T, Oya H, Ozawa T, Kagawa Y. Deficiencies in complex I subunits of the respiratory chain in Parkinson’s disease. Biochem Biophys Res Commun. 1989;163(3):1450–1455. [DOI] [PubMed] [Google Scholar]
- 19. Betarbet R, Sherer TB, MacKenzie G, Garcia-Osuna M, Panov AV, Greenamyre JT. Chronic systemic pesticide exposure reproduces features of Parkinson’s disease. Nat Neurosci. 2000;3(12):1301–1306. [DOI] [PubMed] [Google Scholar]
- 20. Sherer TB, Betarbet R, Testa CM, Seo BB, Richardson JR, Kim JH, Miller GW, Yagi T, Matsuno-Yagi A, Greenamyre JT. Mechanism of toxicity in rotenone models of Parkinson’s disease. J Neurosci. 2003;23(34):10756–10764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Jackson-Lewis V, Smeyne RJ. MPTP and SNpc DA neuronal vulnerability: role of dopamine, superoxide and nitric oxide in neurotoxicity. Minireview. Neurotox Res. 2005;7(3):193–202. [DOI] [PubMed] [Google Scholar]
- 22. Smeyne RJ, Jackson-Lewis V. The MPTP model of Parkinson’s disease. Brain Res Mol Brain Res. 2005;134(1):57–66. [DOI] [PubMed] [Google Scholar]
- 23. Bender A, Krishnan KJ, Morris CM, Taylor GA, Reeve AK, Perry RH, Jaros E, Hersheson JS, Betts J, Klopstock T, et al. High levels of mitochondrial DNA deletions in substantia nigra neurons in aging and Parkinson disease. Nat Genet. 2006;38(5):515–517. [DOI] [PubMed] [Google Scholar]
- 24. Kraytsberg Y, Kudryavtseva E, McKee AC, Geula C, Kowall NW, Khrapko K. Mitochondrial DNA deletions are abundant and cause functional impairment in aged human substantia nigra neurons. Nat Genet. 2006;38(5):518–520. [DOI] [PubMed] [Google Scholar]
- 25. Frankola KA, Greig NH, Luo W, Tweedie D. Targeting TNF-alpha to elucidate and ameliorate neuroinflammation in neurodegenerative diseases. CNS Neurol Disord Drug Targets. 2011;10(3):391–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Verdile G, Keane KN, Cruzat VF, Medic S, Sabale M, Rowles J, Wijesekara N, Martins RN, Fraser PE, Newsholme P. Inflammation and oxidative stress: the molecular connectivity between insulin resistance, obesity, and Alzheimer’s disease. Mediators Inflamm. 2015;2015:105828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. de la Monte SM, Wands JR. Alzheimer’s disease is type 3 diabetes-evidence reviewed. J Diabetes Sci Technol. 2008;2(6):1101–1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kandimalla R, Thirumala V, Reddy PH. Is Alzheimer’s disease a Type 3 Diabetes? a critical appraisal. Biochim Biophys Acta. 2017;1863(5):1078–1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Weber C. Neurogastroenterology: improving glucose tolerance via the gut-brain axis. Nat Rev Gastroenterol Hepatol. 2016;13(1):4. [DOI] [PubMed] [Google Scholar]
- 30. Edholm T, Degerblad M, Gryback P, Hilsted L, Holst JJ, Jacobsson H, Efendic S, Schmidt PT, Hellstrom PM. Differential incretin effects of GIP and GLP-1 on gastric emptying, appetite, and insulin-glucose homeostasis. Neurogastroenterol Motil. 2010;22(11):1191–1200, e315. [DOI] [PubMed] [Google Scholar]
- 31. Duarte AI, Candeias E, Correia SC, Santos RX, Carvalho C, Cardoso S, Placido A, Santos MS, Oliveira CR, Moreira PI. Crosstalk between diabetes and brain: glucagon-like peptide-1 mimetics as a promising therapy against neurodegeneration. Biochim Biophys Acta. 2013;1832(4):527–541. [DOI] [PubMed] [Google Scholar]
- 32. Campbell JE, Drucker DJ. Pharmacology, physiology, and mechanisms of incretin hormone action. Cell Metab. 2013;17(6):819–837. [DOI] [PubMed] [Google Scholar]
- 33. Kim W, Egan JM. The role of incretins in glucose homeostasis and diabetes treatment. Pharmacol Rev. 2008;60(4):470–512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Campbell JE, Drucker DJ. Islet alpha cells and glucagon—critical regulators of energy homeostasis. Nat Rev Endocrinol. 2015;11(6):329–338. [DOI] [PubMed] [Google Scholar]
- 35. Li Y, Perry T, Kindy MS, Harvey BK, Tweedie D, Holloway HW, Powers K, Shen H, Egan JM, Sambamurti K, et al. GLP-1 receptor stimulation preserves primary cortical and dopaminergic neurons in cellular and rodent models of stroke and Parkinsonism. Proc Natl Acad Sci U S A. 2009;106(4):1285–1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Muscogiuri G, Cignarelli A, Giorgino F, Prodam F, Santi D, Tirabassi G, Balercia G, Modica R, Faggiano A, Colao A. GLP-1: benefits beyond pancreas. J Endocrinol Invest. 2014;37(12):1143–1153. [DOI] [PubMed] [Google Scholar]
- 37. Heppner KM, Kirigiti M, Secher A, Paulsen SJ, Buckingham R, Pyke C, Knudsen LB, Vrang N, Grove KL. Expression and distribution of glucagon-like peptide-1 receptor mRNA, protein and binding in the male nonhuman primate (Macaca mulatta) brain. Endocrinology. 2015;156(1):255–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Holscher C. Central effects of GLP-1: new opportunities for treatments of neurodegenerative diseases. J Endocrinol. 2014;221(1):T31–T41. [DOI] [PubMed] [Google Scholar]
- 39. Mora PF, Johnson EL. Cardiovascular outcome trials of the incretin-based therapies: what do we know so far? Endocr Pract. 2017;23(1):89–99. [DOI] [PubMed] [Google Scholar]
- 40. Manigault KR, Thurston MM. Liraglutide: a glucagon-like peptide-1 agonist for chronic weight management. Consult Pharm. 2016;31(12):685–697. [DOI] [PubMed] [Google Scholar]
- 41. Drucker DJ. The cardiovascular biology of glucagon-like peptide-1. Cell Metab. 2016;24(1):15–30. [DOI] [PubMed] [Google Scholar]
- 42. Greig NH, Tweedie D, Rachmany L, Li Y, Rubovitch V, Schreiber S, Chiang YH, Hoffer BJ, Miller J, Lahiri DK, et al. Incretin mimetics as pharmacologic tools to elucidate and as a new drug strategy to treat traumatic brain injury. Alzheimers Dement. 2014;10(Suppl 1):S62–S75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Athauda D, Foltynie T. The glucagon-like peptide 1 (GLP) receptor as a therapeutic target in Parkinson’s disease: mechanisms of action. Drug Discov Today. 2016;21(5):802–818. [DOI] [PubMed] [Google Scholar]
- 44. Holscher C. Insulin, incretins and other growth factors as potential novel treatments for Alzheimer’s and Parkinson’s diseases. Biochem Soc Trans. 2014;42(2):593–599. [DOI] [PubMed] [Google Scholar]
- 45. Bassil F, Fernagut PO, Bezard E, Meissner WG. Insulin, IGF-1 and GLP-1 signaling in neurodegenerative disorders: targets for disease modification? Prog Neurobiol. 2014;118:1–18. [DOI] [PubMed] [Google Scholar]
- 46. Foltynie T, Aviles-Olmos I. Exenatide as a potential treatment for patients with Parkinson’s disease: first steps into the clinic. Alzheimers Dement. 2014;10(Suppl 1):S38–S46. [DOI] [PubMed] [Google Scholar]
- 47. Athauda D, Maclagan K, Skene SS, Bajwa-Joseph M, Letchford D, Chowdhury K, Hibbert S, Budnik N, Zampedri L, Dickson J, Li Y, Aviles-Olmos I, Warner TT, Limousin P, Lees AJ, Greig NH, Tebbs S, Foltynie T. Exenatide once weekly versus placebo in Parkinson’s disease: a randomised, double-blind, placebo-controlled trial. Lancet. 2017. August 3 doi: 10.1016/S0140-6736(17)31585-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Dias V, Junn E, Mouradian MM. The role of oxidative stress in Parkinson’s disease. J Parkinsons Dis. 2013;3(4):461–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Niranjan R. The role of inflammatory and oxidative stress mechanisms in the pathogenesis of Parkinson’s disease: focus on astrocytes. Mol Neurobiol. 2014;49(1):28–38. [DOI] [PubMed] [Google Scholar]
- 50. Alismail H, Jin S. Microenvironmental stimuli for proliferation of functional islet beta-cells. Cell Biosci. 2014;4(1):12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Tamura K, Minami K, Kudo M, Iemoto K, Takahashi H, Seino S. Liraglutide improves pancreatic Beta cell mass and function in alloxan-induced diabetic mice. PLoS One. 2015;10(5):e0126003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Zhu H, Zhang Y, Shi Z, Lu D, Li T, Ding Y, Ruan Y, Xu A. The neuroprotection of liraglutide against ischaemia-induced apoptosis through the activation of the PI3K/AKT and MAPK pathways. Sci Rep. 2016;6:26859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Tuduri E, Lopez M, Dieguez C, Nadal A, Nogueiras R. Glucagon-like peptide 1 analogs and their effects on pancreatic islets. Trends Endocrinol Metab. 2016;27(5):304–318. [DOI] [PubMed] [Google Scholar]
- 54. Perry T, Greig NH. The glucagon-like peptides: a double-edged therapeutic sword? Trends Pharmacol Sci. 2003;24(7):377–383. [DOI] [PubMed] [Google Scholar]
- 55. Belsham DD, Fick LJ, Dalvi PS, Centeno ML, Chalmers JA, Lee PK, Wang Y, Drucker DJ, Koletar MM. Ciliary neurotrophic factor recruitment of glucagon-like peptide-1 mediates neurogenesis, allowing immortalization of adult murine hypothalamic neurons. FASEB J. 2009;23(12):4256–4265. [DOI] [PubMed] [Google Scholar]
- 56. Isacson R, Nielsen E, Dannaeus K, Bertilsson G, Patrone C, Zachrisson O, Wikstrom L. The glucagon-like peptide 1 receptor agonist exendin-4 improves reference memory performance and decreases immobility in the forced swim test. Eur J Pharmacol. 2011;650(1):249–255. [DOI] [PubMed] [Google Scholar]
- 57. Toklu HZ, Tumer N. Oxidative stress, brain edema, blood-brain barrier permeability, and autonomic dysfunction from traumatic brain injury In: Kobeissy FH, editor. Brain neurotrauma: molecular, neuropsychological, and rehabilitation aspects, frontiers in neuroengineering. Boca Raton (FL; ): CRC Press/Taylor & Francis; 2015. [Google Scholar]
- 58. Yan MH, Wang X, Zhu X. Mitochondrial defects and oxidative stress in Alzheimer disease and Parkinson disease. Free Radic Biol Med. 2013;62:90–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Jena I, Nayak SR, Behera S, Singh B, Ray S, Jena D, Singh S, Sahoo SK. Evaluation of ischemia-modified albumin, oxidative stress, and antioxidant status in acute ischemic stroke patients. J Nat Sci Biol Med. 2017;8(1):110–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Penkowa M, Giralt M, Carrasco J, Hadberg H, Hidalgo J. Impaired inflammatory response and increased oxidative stress and neurodegeneration after brain injury in interleukin-6-deficient mice. Glia. 2000;32(3):271–285. [DOI] [PubMed] [Google Scholar]
- 61. Iwai T, Ito S, Tanimitsu K, Udagawa S, Oka J. Glucagon-like peptide-1 inhibits LPS-induced IL-1beta production in cultured rat astrocytes. Neurosci Res. 2006;55(4):352–360. [DOI] [PubMed] [Google Scholar]
- 62. Li Y, Tweedie D, Mattson MP, Holloway HW, Greig NH. Enhancing the GLP-1 receptor signaling pathway leads to proliferation and neuroprotection in human neuroblastoma cells. J Neurochem. 2010;113(6):1621–1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Perry T, Lahiri DK, Sambamurti K, Chen D, Mattson MP, Egan JM, Greig NH. Glucagon-like peptide-1 decreases endogenous amyloid-beta peptide (Abeta) levels and protects hippocampal neurons from death induced by Abeta and iron. J Neurosci Res. 2003;72(5):603–612. [DOI] [PubMed] [Google Scholar]
- 64. Guo LX, Liu JH, Xia ZN. Geniposide inhibits CoCl2-induced PC12 cells death via the mitochondrial pathway. Chin Med J (Engl). 2009;122(23):2886–2892. [PubMed] [Google Scholar]
- 65. Liu J, Yin F, Zheng X, Jing J, Hu Y. Geniposide, a novel agonist for GLP-1 receptor, prevents PC12 cells from oxidative damage via MAP kinase pathway. Neurochem Int. 2007;51(6-7):361–369. [DOI] [PubMed] [Google Scholar]
- 66. Bertilsson G, Patrone C, Zachrisson O, Andersson A, Dannaeus K, Heidrich J, Kortesmaa J, Mercer A, Nielsen E, Ronnholm H, et al. Peptide hormone exendin-4 stimulates subventricular zone neurogenesis in the adult rodent brain and induces recovery in an animal model of Parkinson’s disease. J Neurosci Res. 2008;86(2):326–338. [DOI] [PubMed] [Google Scholar]
- 67. Li Y, Duffy KB, Ottinger MA, Ray B, Bailey JA, Holloway HW, Tweedie D, Perry T, Mattson MP, Kapogiannis D, et al. GLP-1 receptor stimulation reduces amyloid-beta peptide accumulation and cytotoxicity in cellular and animal models of Alzheimer’s disease. J Alzheimers Dis. 2010;19(4):1205–1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Goncalves JT, Schafer ST, Gage FH. Adult Neurogenesis in the Hippocampus: from stem cells to behavior. Cell. 2016;167(4):897–914. [DOI] [PubMed] [Google Scholar]
- 69. Conover JC, Todd KL. Development and aging of a brain neural stem cell niche. Exp Gerontol. 2017;94:9–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Luciani P, Deledda C, Benvenuti S, Cellai I, Squecco R, Monici M, Cialdai F, Luciani G, Danza G, Di Stefano C, et al. Differentiating effects of the glucagon-like peptide-1 analogue exendin-4 in a human neuronal cell model. Cell Mol Life Sci. 2010;67(21):3711–3723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Noyan-Ashraf MH, Momen MA, Ban K, Sadi AM, Zhou YQ, Riazi AM, Baggio LL, Henkelman RM, Husain M, Drucker DJ. GLP-1R agonist liraglutide activates cytoprotective pathways and improves outcomes after experimental myocardial infarction in mice. Diabetes. 2009;58(4):975–983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Puddu A, Sanguineti R, Durante A, Nencioni A, Mach F, Montecucco F, Viviani GL. Glucagon-like peptide-1 triggers protective pathways in pancreatic beta-cells exposed to glycated serum. Mediators Inflamm. 2013;2013:317120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Perry T, Lahiri DK, Chen D, Zhou J, Shaw KT, Egan JM, Greig NH. A novel neurotrophic property of glucagon-like peptide 1: a promoter of nerve growth factor-mediated differentiation in PC12 cells. J Pharmacol Exp Ther. 2002;300(3):958–966. [DOI] [PubMed] [Google Scholar]
- 74. Palma JA, Kaufmann H. Autonomic disorders predicting Parkinson’s disease. Parkinsonism Relat Disord. 2014;20(Suppl 1):S94–S98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Iodice V, Low DA, Vichayanrat E, Mathias CJ. Cardiovascular autonomic dysfunction in MSA and Parkinson’s disease: similarities and differences. J Neurol Sci. 2011;310(1-2):133–138. [DOI] [PubMed] [Google Scholar]
- 76. Jackson-Lewis V, Blesa J, Przedborski S. Animal models of Parkinson’s disease. Parkinsonism Relat Disord. 2012;18(Suppl 1):S183–S185. [DOI] [PubMed] [Google Scholar]
- 77. Chesselet MF, Carmichael ST. Animal models of neurological disorders. Neurotherapeutics. 2012;9(2):241–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Denton T, Howard BD. A dopaminergic cell line variant resistant to the neurotoxin 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine. J Neurochem. 1987;49(2):622–630. [DOI] [PubMed] [Google Scholar]
- 79. Kindt MV, Heikkila RE, Nicklas WJ. Mitochondrial and metabolic toxicity of 1-methyl-4-(2′-methylphenyl)-1,2,3,6-tetrahydropyridine. J Pharmacol Exp Ther. 1987;242(3):858–863. [PubMed] [Google Scholar]
- 80. Nicklas WJ, Youngster SK, Kindt MV, Heikkila RE. MPTP, MPP+ and mitochondrial function. Life Sci. 1987;40(8):721–729. [DOI] [PubMed] [Google Scholar]
- 81. Heikkila RE, Nicklas WJ, Duvoisin RC. Studies on the mechanism of the dopaminergic neurotoxicity of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine. Adv Neurol. 1987;45:149–152. [PubMed] [Google Scholar]
- 82. Kovacic P, Edwards WD, Ming G. Theoretical studies on mechanism of MPTP action: ET interference by MPP+ (1-methyl-4-phenylpyridinium) with mitochondrial respiration vs. oxidative stress. Free Radic Res Commun. 1991;14(1):25–32. [DOI] [PubMed] [Google Scholar]
- 83. Kim S, Moon M, Park S. Exendin-4 protects dopaminergic neurons by inhibition of microglial activation and matrix metalloproteinase-3 expression in an animal model of Parkinson’s disease. J Endocrinol. 2009;202(3):431–439. [DOI] [PubMed] [Google Scholar]
- 84. Yamamoto H, Kishi T, Lee CE, Choi BJ, Fang H, Hollenberg AN, Drucker DJ, Elmquist JK. Glucagon-like peptide-1-responsive catecholamine neurons in the area postrema link peripheral glucagon-like peptide-1 with central autonomic control sites. J Neurosci. 2003;23(7):2939–2946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Harkavyi A, Abuirmeileh A, Lever R, Kingsbury AE, Biggs CS, Whitton PS. Glucagon-like peptide 1 receptor stimulation reverses key deficits in distinct rodent models of Parkinson’s disease. J Neuroinflammation. 2008;5:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Arnt J. Hyperactivity induced by stimulation of separate dopamine D-1 and D-2 receptors in rats with bilateral 6-OHDA lesions. Life Sci. 1985;37(8):717–723. [DOI] [PubMed] [Google Scholar]
- 87. Trapp S, Richards JE. The gut hormone glucagon-like peptide-1 produced in brain: is this physiologically relevant? Curr Opin Pharmacol. 2013;13(6):964–969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Kappe C, Tracy LM, Patrone C, Iverfeldt K, Sjoholm A. GLP-1 secretion by microglial cells and decreased CNS expression in obesity. J Neuroinflammation. 2012;9:276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Perry T, Holloway HW, Weerasuriya A, Mouton PR, Duffy K, Mattison JA, Greig NH. Evidence of GLP-1-mediated neuroprotection in an animal model of pyridoxine-induced peripheral sensory neuropathy. Exp Neurol. 2007;203(2):293–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. During MJ, Cao L, Zuzga DS, Francis JS, Fitzsimons HL, Jiao X, Bland RJ, Klugmann M, Banks WA, Drucker DJ, et al. Glucagon-like peptide-1 receptor is involved in learning and neuroprotection. Nat Med. 2003;9(9):1173–1179. [DOI] [PubMed] [Google Scholar]
- 91. Li Y, Chigurupati S, Holloway HW, Mughal M, Tweedie D, Bruestle DA, Mattson MP, Wang Y, Harvey BK, Ray B, et al. Exendin-4 ameliorates motor neuron degeneration in cellular and animal models of amyotrophic lateral sclerosis. PLoS One. 2012;7(2):e32008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Lee CH, Yan B, Yoo KY, Choi JH, Kwon SH, Her S, Sohn Y, Hwang IK, Cho JH, Kim YM, et al. Ischemia-induced changes in glucagon-like peptide-1 receptor and neuroprotective effect of its agonist, exendin-4, in experimental transient cerebral ischemia. J Neurosci Res. 2011;89(7):1103–1113. [DOI] [PubMed] [Google Scholar]
- 93. Chowen JA, de Fonseca FR, Alvarez E, Navarro M, Garcia-Segura LM, Blazquez E. Increased glucagon-like peptide-1 receptor expression in glia after mechanical lesion of the rat brain. Neuropeptides. 1999;33(3):212–215. [DOI] [PubMed] [Google Scholar]
- 94. Jia Y, Gong N, Li TF, Zhu B, Wang YX. Peptidic exenatide and herbal catalpol mediate neuroprotection via the hippocampal GLP-1 receptor/beta-endorphin pathway. Pharmacol Res. 2015;102:276–285. [DOI] [PubMed] [Google Scholar]
- 95. Harry GJ. Microglia during development and aging. Pharmacol Ther. 2013;139(3):313–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Suh HS, Zhao ML, Derico L, Choi N, Lee SC. Insulin-like growth factor 1 and 2 (IGF1, IGF2) expression in human microglia: differential regulation by inflammatory mediators. J Neuroinflammation. 2013;10:37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Heppner FL, Ransohoff RM, Becher B. Immune attack: the role of inflammation in Alzheimer disease. Nat Rev Neurosci. 2015;16(6):358–372. [DOI] [PubMed] [Google Scholar]
- 98. Bao Y, Jiang L, Chen H, Zou J, Liu Z, Shi Y. The Neuroprotective effect of liraglutide is mediated by glucagon-like peptide 1 receptor-mediated activation of cAMP/PKA/CREB pathway. Cell Physiol Biochem. 2015;36(6):2366–2378. [DOI] [PubMed] [Google Scholar]
- 99. Aviles-Olmos I, Dickson J, Kefalopoulou Z, Djamshidian A, Kahan J, Ell P, Whitton P, Wyse R, Isaacs T, Lees A, et al. Motor and cognitive advantages persist 12 months after exenatide exposure in Parkinson’s disease. J Parkinsons Dis. 2014;4(3):337–344. [DOI] [PubMed] [Google Scholar]
- 100. Aviles-Olmos I, Dickson J, Kefalopoulou Z, Djamshidian A, Ell P, Soderlund T, Whitton P, Wyse R, Isaacs T, Lees A, et al. Exenatide and the treatment of patients with Parkinson’s disease. J Clin Invest. 2013;123(6):2730–2736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Liu W, Jalewa J, Sharma M, Li G, Li L, Holscher C. Neuroprotective effects of lixisenatide and liraglutide in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine mouse model of Parkinson’s disease. Neuroscience. 2015;303:42–50. [DOI] [PubMed] [Google Scholar]
- 102. Hunter K, Holscher C. Drugs developed to treat diabetes, liraglutide and lixisenatide, cross the blood brain barrier and enhance neurogenesis. BMC Neurosci. 2012;13:33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Kastin AJ, Akerstrom V. Entry of exendin-4 into brain is rapid but may be limited at high doses. Int J Obes Relat Metab Disord. 2003;27(3):313–318. [DOI] [PubMed] [Google Scholar]
- 104. Sadry SA, Drucker DJ. Emerging combinatorial hormone therapies for the treatment of obesity and T2DM. Nat Rev Endocrinol. 2013;9(7):425–433. [DOI] [PubMed] [Google Scholar]
- 105. Soni H. Peptide-based GLP-1/glucagon co-agonists: a double-edged sword to combat diabesity. Med Hypotheses. 2016;95:5–9. [DOI] [PubMed] [Google Scholar]
- 106. Finan B, Ma T, Ottaway N, Muller TD, Habegger KM, Heppner KM, Kirchner H, Holland J, Hembree J, Raver C, et al. Unimolecular dual incretins maximize metabolic benefits in rodents, monkeys, and humans. Sci Transl Med. 2013;5(209):209ra151. [DOI] [PubMed] [Google Scholar]
- 107. Finan B, Yang B, Ottaway N, Smiley DL, Ma T, Clemmensen C, Chabenne J, Zhang L, Habegger KM, Fischer K, et al. A rationally designed monomeric peptide triagonist corrects obesity and diabetes in rodents. Nat Med. 2015;21(1):27–36. [DOI] [PubMed] [Google Scholar]
- 108. Tamargo IA, Bader M, Li Y, Yu SJ, Wang Y, Talbot K, DiMarchi RD, Pick CG, Greig NH. Novel GLP-1R/GIPR co-agonist “twincretin” is neuroprotective in cell and rodent models of mild traumatic brain injury. Exp Neurol. 2017;288:176–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Li Y, Wu KJ, Yu SJ, Tamargo IA, Wang Y, Greig NH. Neurotrophic and neuroprotective effects of oxyntomodulin in neuronal cells and a rat model of stroke. Exp Neurol. 2017;288:104–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Cao L, Li D, Feng P, Li L, Xue GF, Li G, Holscher C. A novel dual GLP-1 and GIP incretin receptor agonist is neuroprotective in a mouse model of Parkinson’s disease by reducing chronic inflammation in the brain. Neuroreport. 2016;27(6):384–391. [DOI] [PubMed] [Google Scholar]
- 111. Jalewa J, Sharma MK, Gengler S, Holscher C. A novel GLP-1/GIP dual receptor agonist protects from 6-OHDA lesion in a rat model of Parkinson’s disease. Neuropharmacology. 2017;117:238–248. [DOI] [PubMed] [Google Scholar]
- 112. Ji C, Xue GF, Lijun C, Feng P, Li D, Li L, Li G, Holscher C. A novel dual GLP-1 and GIP receptor agonist is neuroprotective in the MPTP mouse model of Parkinson’s disease by increasing expression of BNDF. Brain Res. 2016;1634:1–11. [DOI] [PubMed] [Google Scholar]