Abstract
Cardiovascular disease is a major cause of morbidity, disability, and mortality in kidney transplant patients. Cumulative reports indicate that the excessive risk of cardiovascular events is not entirely explained by the increased prevalence of traditional cardiovascular risk factors. Atherosclerosis is a chronic inflammatory disease, and it has been postulated that posttransplant immune disturbances may explain the gap between the predicted and observed risks of cardiovascular events. Although concordant data suggest that innate immunity contributes to the posttransplant accelerated atherosclerosis, only few arguments plead for a role of adaptive immunity. We report and discuss here consistent data demonstrating that CD8+ T cell activation is a frequent posttransplant immune feature that may have pro-atherogenic effects. Expansion of exhausted/activated CD8+ T cells in kidney transplant recipients is stimulated by several factors including cytomegalovirus infections, lymphodepletive therapy (e.g., antithymocyte globulins), chronic allogeneic stimulation, and a past history of renal insufficiency. This is observed in the setting of decreased thymic activity, a process also found in elderly individuals and reflecting accelerated immune senescence.
Keywords: CD8+ T cells, atherosclerosis, cardiovascular diseases, kidney transplantation, immune senescence, aging
Introduction
Kidney transplant patients experience accelerated atherosclerosis. An increased prevalence of posttransplant traditional cardiovascular risk factors and the consequences of pretransplant uremia-associated cardiovascular changes are the main explanations for an excess of atherosclerotic events.1,2 Whether specific transplant-related immunological factors also participate in posttransplant atherosclerosis is a matter of debate. However, atherosclerosis is an inflammatory disease and some immunological factors have been associated with cardiovascular events in both the general and transplant populations.3–7 Nevertheless, most of them concern innate immunity and scarce data support an association between posttransplant adaptive immune responses and atherosclerosis.8,9 We present in this review consistent published and unpublished data suggesting that CD8+ T cell activation is frequent posttransplant and might contribute to the so-called posttransplant accelerated atherosclerosis. We discuss also the different factors involved in CD8+ T cell activation of renal transplantation.
Role of CD8+ T Cells in Experimental Atherosclerosis
Both innate and adaptive immune responses are critical determinants of atherosclerotic lesion formation and growth.10–13 Whereas both CD4+ and CD8+ T cells are present in human atherosclerotic lesions, most studies have focused on CD4+ T cells. Nevertheless, the role of cytotoxic CD8+ T cells in atherosclerotic lesion development is increasingly recognized.14
First, some studies linked increased CD8+ T cell infiltration in atherosclerotic plaques with atherosclerosis progression in animal models,15,16 but animal models failed initially to demonstrate direct evidence of their involvement in atherosclerosis.17,18 It was admitted that the absence of CD8+ T cells in atherosclerotic plaques was compensated by other pro-inflammatory T cell types. More recently, Kyaw et al.19 shed new light on the role of CD8+ T lymphocytes in experimental atherosclerosis. They reported that CD8+ T-lymphocyte depletion by anti-CD8α or CD8β monoclonal antibody (mAb) in apolipoprotein E-deficient (ApoE–/–) mice (i.e., mice prone to develop atherosclerosis) reduced atherosclerosis. Accordingly, transfer of CD8+ T cells into lymphocyte-deficient ApoE–/– mice was associated with a CD8+ T-cell infiltration and increased lipid and macrophage accumulation in atherosclerotic lesions. Transfer of CD8+ T cells deficient in perforin, granzyme B, or tumor necrosis factor α (TNF-α)—but not the transfer of CD8+ T cells deficient in interferon γ (IFN-γ)—failed to increase atherosclerotic lesions.19 These results suggest that the inflammatory cytokine TNF-α plays a major role in CD8+ T-cell-dependent atherosclerosis. Despite the absence of CD8+ T-cell-derived IFN-γ involvement in the atherosclerosis progression in these ApoE–/– mice,19 the importance of T-cell-derived IFN-γ in the pathogenesis of posttransplant accelerated atherosclerosis has been repeatedly shown after mouse heart transplantation.20–22 Indeed, coronary atherosclerosis, a complication observed after allogeneic heart transplantation, is prevented by the persistent treatment with an anti-IFN-γ mAb.20 Similar results have been obtained when IFN-γ-deficient mice are used as heart recipients.21,22 In addition, the cytotoxic functions of CD8+ T cells mediated by perforin and granzyme B are implicated in atherosclerosis progression perhaps through the generation of apoptotic cells and necrotic cores within atherosclerotic lesions/plaques.
Cochain et al.23 suggested that CD8+ T cells promote atherosclerosis by controlling monopoiesis and circulating monocyte levels, which ultimately contributes to plaque macrophage burden without affecting direct monocyte recruitment. CD8+ T cells could, in addition, potentiate systemic and vascular inflammation through the secretion of pro-atherogenic cytokines. For instance, CD8+ T cells were shown to highly secrete IFN-γ in aortic root-draining lymph nodes of hypercholesterolemic mice24 and TNF-α secretion after CD8+ T-cell transfer is required for atherosclerotic lesions in lymphocyte-deficient ApoE–/– mice.19 However, the role of CD8+ T cells is not unambiguous. Clement et al.25 recently showed increased frequencies of follicular helper T cells and germinal center B cells in ApoE–/– mice, whereas CD8+ regulatory T cell (Treg) levels were reduced. Genetic disruption of CD8+ Treg functions was associated with an expansion of the follicular helper T-cell and germinal center B cell compartments, enhanced immunoglobulin G deposition in atherosclerotic plaques, and larger atherosclerotic lesions.25 These results suggest that CD8+ Tregs may offset the pro-atherogenic germinal center B-cell activation by follicular helper T cells during atherogenesis. Moreover, the pro- versus antiatherogenic properties of CD8+ T cells may depend on their activation stage (naive versus activated CD8+ T cells) or on particular CD8+ T-cell subsets (e.g., CD8+ Treg).
Role of CD8+ T Cells in Human Atherosclerosis
This section focuses on data supporting the role of CD8+ T-cell activation in human atherosclerosis, excluding data obtained in renal transplantation settings (please see next section). Several large studies have demonstrated that HIV-infected patients have a 1.5- to 2-fold higher risk of developing cardiovascular diseases, such as acute myocardial infarction (AMI), cerebral ischemia, and peripheral artery disease.26–29 An increase in T-cell activation is observed in HIV-infected patients, even in those with a controlled/suppressed viral load.30 Cardiovascular disease is likely to be, at least in part, related to the increased proportion of activated T cells and the resulting systemic and local inflammation.30 Indeed, concordant studies have reported an association between activated CD8+ T cells (exhibiting either a CD38+ or CD28−CD57+ phenotype) and subclinical atherosclerosis.31–34 Additionally, CD8+ T-cell count has been recently associated with the risk of AMI in HIV-infected patients.35 In this study including 73,398 patients, high baseline CD8+ T-cell counts (>1,065 cells/mm3) doubled the risk of AMI.35 Similarly, a low CD4/CD8 ratio has been associated with coronary artery disease in HIV-infected patients.36
The potential role of CD8+ T-cell expansion in atherosclerosis has been also described in some studies concerning non-HIV-infected patients. Senescent CD8+ T cells are predictors of overall cardiovascular mortality as well as death from myocardial infarction and stroke in octogenarians.37 Kolbus et al.38 reported an association between IFN-γ+ CD56−CD8+ T cells and carotid stenosis in the general population. However, higher frequencies of circulating interleukin (IL)-6Rαlow effector memory CD8+ T cells were observed in patients with acute coronary syndrome. This subset has high cytotoxic capacities and correlates with terminally differentiated CD57+CD8+ T cells.39 The high cytotoxic capacities of these CD8+ T cells may confirm the significant reduction of atherosclerotic lesions in CD8+ T-lymphocyte-deficient ApoE–/– mice after adoptive transfer of CD8+ T cells deficient in perforin or granzyme A,19 as discussed above.
The discrepancies between the large number of studies reporting an association between immune activation and atherosclerosis in HIV-infected patients and the scarce reported data in other patients may be explained by the requirement of a significant immune activation to promote atherosclerosis. For readers who are not familiar with CD8+ T-cell activation phenotypes, we recommend the following review dealing classical markers used to delineate T-cell subsets.40 Briefly, progression from a naive stage to an effector memory phenotype is denoted by the extinction of CD28 expression, and expression of the CD57 marker on T cells reflects a terminal differentiation phase. Another alternative explanation for these discrepancies is the lack of searching for this association between immune activation status and accelerated atherosclerosis. Of note, hypertension, the most frequent risk factor for cardiovascular disease, has been associated with an increased frequency of senescent pro-inflammatory CD8+ T cells.41 Future research should investigate whether immune activation plays a role in atherogenesis in the general population and in specific groups of patients. A recent publication studies this association between CD8+ T-cell activation and cardiovascular diseases in rheumatoid arthritis (RA) patients.42 The authors show that RA patients with coronary artery calcification (CAC) (i.e., those with an increased risk of subclinical coronary artery disease) had higher percentages of the CD8+ T-cell subsets with an activation status (human leukocyte antigen–antigen D related [HLA-DR+]) or exhibiting a differentiation to effector memory (CD28−) or exhausted CD57+ CD8+ T cells compared to RA patients without CAC. Thus, development of subclinical atherosclerosis, defined by the presence of CAC, in RA patients is significantly and separately associated with an elevated percentage of circulating activated/exhausted CD8+ T cells. These associations are independent of traditional cardiovascular risk factors and other RA clinical characteristics and treatments.42 Increased levels of CD8+ T-cell subsets characterized by the expression of CD57 and loss of CD28 have been similarly associated with atherosclerosis and coronary artery disease in the general population.43 This provides an additional argument for searching for this association between CD8+ T-cell activation and accelerated atherosclerosis. Now, we will evoke how data associating CD8+ T-cell activation and atherosclerosis in HIV patients, in patients suffering from autoimmune/chronic inflammatory diseases, or in the general population could be transposed in renal transplant recipients.
Chronic T-Cell Activation After Kidney Transplantation as a Factor Propagating Atherosclerosis
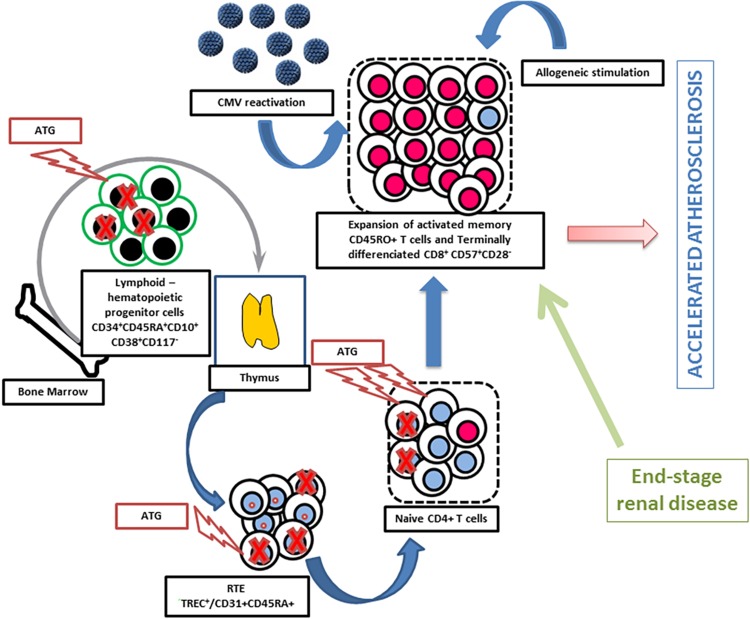
As suggested above, a significant level of CD8+ T lymphocyte activation is probably required to induce vascular damage. A key point is to establish whether this condition is observed in renal transplant patients. In fact, different factors contribute to chronic/persistent T-cell activation after transplantation. These factors will be evoked below. This includes at least cytomegalovirus (CMV) infection, lymphodepletive therapies (particularly in the setting of thymic involution and aging which impairs naive T-cell reconstitution), allogeneic stimulation (by the grafted kidney), and a past history of renal insufficiency (Fig. 1).
Figure 1.
Factors involved in the expansion of the pool of memory T cells and terminally differentiated effector memory T cells (TEMRA) in kidney transplant recipients. Antithymocyte globulins (ATG) mainly deplete naive T cells and lymphoid hematopoietic progenitor cells.44 This results in a decreased number of recent thymic emigrants (RTE) and favors homeostatic proliferation of memory T cells and TEMRA. The latter phenomenon is amplified by cytomegalovirus (CMV) reactivation and allogeneic stimulation. The patient thymic activity is critical for T-cell reconstitution.44,45
CMV Infection, Chronic T-Cell Activation, and Atherogenic Effects
Compelling data suggest that the pro-atherogenic effects of CMV are due to the cellular immune response directed against CMV and not to CMV infection per se. Hsue et al.46 reported that CMV-specific T-cell responses were independently associated with carotid intima–media thickness in patients with HIV infection. The same group recently reported that the progression of atherosclerosis in HIV-infected patients is associated with a high frequency of CMV-specific CX3CR1+CD4+ T cells.47 Furthermore, these cells may induce endothelial cells to secrete CX3CL1, which itself drives the progressive infiltration of the arterial wall by pro-inflammatory cells and promotes atherosclerosis. Betjes et al.48,49 also reported a strong association between CMV seropositivity, terminally differentiated CD4+ T cells, and atherosclerotic disease in patients with end-stage renal disease (ESRD). Altogether, these studies demonstrate that cellular immune responses directed against CMV, at least CD4+ T-cell responses, may contribute to atherosclerosis.
We recently reported that both CMV exposure and posttransplant CMV replication predicted cardiovascular events after kidney transplantation.50 CMV infection is known to be associated with an accumulation of CD57+CD28− T cells.51 In our study, the frequency of terminally differentiated CD57+CD28−CD8+ T cells among all CD8+ T cells gradually increased among the 3 groups, that is, patients who were CMV negative during the follow-up (6.1%), CMV-positive patients without replication after transplantation (13.8%), and those with CMV replication after transplantation (23.7%).50 CMV-exposed patients also exhibited a significant systemic inflammation compared to CMV-naive patients. The cumulative incidence of atherosclerotic events increased gradually in the 3 groups described above.50 All these data suggest that CMV-driven CD8+ T-lymphocyte activation contributes to the posttransplant accelerated atherosclerosis.
Lymphodepletive Therapies as Factors Amplifying Chronic T-Cell Activation and Atherogenesis
Broad T-cell depletion by polyclonal antithymocyte globulins (ATG) has been used for many years as a part of immunosuppressive treatment in transplantation. These polyclonal antibodies are a complex mixture of antibodies with multiple specificities directed against both T and non-T cells, including thymic stromal cells.52–54 They produce profound T-cell depletion52,53,55 and induce persistent changes in T-cell subsets characterized by a low CD4+ T-cell count and a CD8+ T-cell expansion.55 It has been reported that CD4+ T cells are more sensitive to ATG-induced depletion than CD8+ T cells.56 The kinetics of reconstitution after lymphopenia are dependent on the considered T-cell subsets, with memory T cells expanding more rapidly than naive T cells and naive CD8+ T cells undergoing faster proliferation rates than naive CD4+ T cells.56,57 Havenith et al.58 confirmed that CD8+ T cells repopulate rapidly after lymphocyte-depleting treatment, whereas CD4+ T-cell reconstitution is significantly delayed. The repopulating CD8+ T-cell pool consists mainly of highly differentiated effector T cells. They observed a fast CD8+ T-cell repopulation only in the CMV-positive but not in the CMV-negative patients. This rapid repopulation was even more pronounced in patients who developed CMV reactivation.58 Thus, CMV infection appears to be a driving factor for T-cell repopulation following ATG treatment. Indeed, when analyzing CMV-specific CD8+ T cells, they noticed a fast reemergence and ultimately accumulation of these cells.58
T-cell reconstitution following a massive depletion—as observed after ATG—is based on 2 main pathways: thymopoiesis (i.e., the capacity of producing new T cells from hematopoietic stem cells in the thymus) and homeostatic proliferation expansion of residual host T lymphocytes that resist depletion.59 Thus, the thymic function is critical for T-cell reconstitution following ATG. It is well-known that thymus involutes with age. Over the age of 45 to 50, thymic activity is reduced and naive T-cell recovery may take until 5 y after severe iatrogenic lymphopenia.60 We previously identified the thymic activity at the time of kidney transplantation as a major factor predicting CD4+ T-cell immune reconstitution after ATG administration.9,45 We did not study the role of thymic function on CD8+ T cells. However, CD8+ T cells expand faster than CD4+ T cells after homeostatic proliferation with the acquisition of a memory phenotype.61 We recently reported that late-stage differentiated CD8+ T cells increased 1 y posttransplant in ATG-treated patients, while naive CD8+ T cells significantly decreased.44 These modifications were amplified in CMV-exposed patients.44 All CD8+ T-cell subsets remained unchanged in patients having received nondepleting anti-CD25 mAb induction therapy.44 In this study, we also report that ATG and CMV stimulate immune senescence in kidney transplant recipients, as attested by different T-cell aging parameters, including T-cell receptor excision circle content, CD31+ recent thymic emigrants (RTE), and decreased T-cell relative telomere length.44 We also demonstrated that ATG is associated with an increased incidence of atherosclerotic events.62 To take into account the absence of randomization, we performed a propensity score analysis to address potential confounding variables by indication. Cox regression analysis revealed that ATG use was an independent risk factor for cardiovascular events. Results obtained in the propensity score-matched analysis recapitulated those obtained from the overall cohort. Because CMV infection amplifies CD8+ T-cell activation in ATG-treated patients, we studied the effect of ATG on atherosclerotic events in CMV-naive and CMV-exposed patients. ATG was not associated with cardiovascular events in CMV-naive patients. By contrast, CMV-exposed patients who received ATG had an increased risk of cardiovascular events compared with those who did not receive ATG.62 Interestingly, an increased incidence of cardiovascular mortality has been previously reported in ATG-treated renal transplant recipients.63 Thus, lymphodepletive therapies, at least ATG, participate in amplified CD8+ T-cell activation in part together with CMV-induced immune responses.
A Potential Role for Persistent Allogeneic Stimulation in Chronic T-Cell Activation and Atherogenesis
As described for viral antigens, repeated stimulation by graft-derived alloantigens could generate T-cell activation, chronic inflammation, and progression of atherosclerotic disease (Fig. 1). We recently tested this hypothesis and observed a significant correlation between human leukocyte antigen mismatch numbers and circulating terminally differentiated CD57+CD28−CD8+ T cells.64 Moreover, we also observed in a large cohort of renal transplant recipients (n = 577) that the cumulative incidence of atherosclerotic events increased with the number of HLA mismatches (18%, 10%, and 5% in patients with 5-6, 3-4, and 0-2 HLA mismatches, respectively; P = 0.012). HLA mismatch number was found to be an independent risk factor for atherosclerotic events in multivariate analysis.64 This study suggests that HLA mismatches may cause chronic antigenic stimulation and favor both immune hyperactivation/exhaustion and atherosclerosis. Concordant with this hypothesis, a recent report by Opelz and Dohler65 suggests a higher rate of cardiovascular death in renal transplant recipients receiving a kidney graft with more HLA mismatches.
Impact of a Past History of Renal Insufficiency on Chronic T-Cell Activation and Atherogenesis
Terminally differentiated CD28−CD57+CD8+ T cells are increased in patients with ESRD,49 suggesting a premature aging of T cells in the setting of ESRD. We also observed that patients with stage IV chronic kidney disease (CKD) exhibit similar changes in T-cell phenotypes (Fig. 2, unpublished data). This expansion is again mainly dependent on CMV seropositivity but is exacerbated in patients with CKD. Report by our group showed that CD8+ T-cell expansion is not modified after successful kidney transplantation even with a long period and in patients receiving nondepleting anti-CD25 mAb.44 Thus, ESRD-related T-lymphocyte activation persists after transplantation and may contribute to posttransplant inflammation.
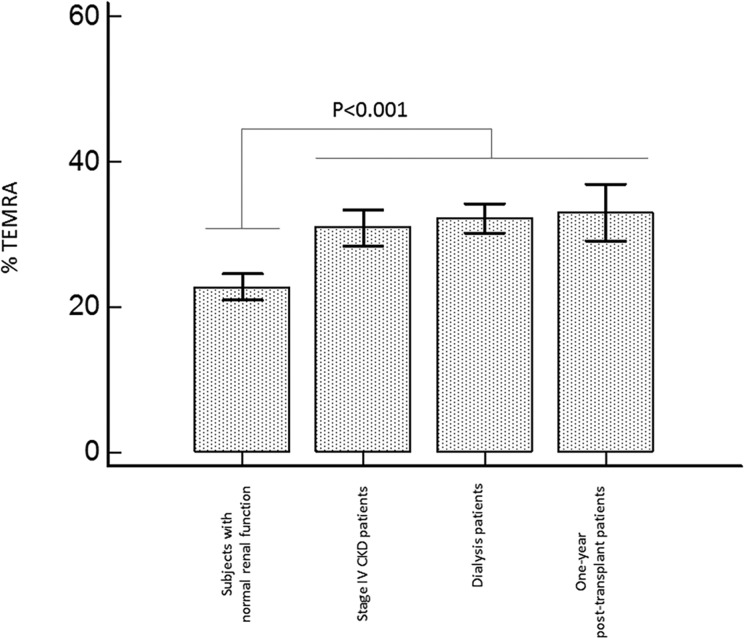
Figure 2.
The frequencies of circulating terminally differentiated effector memory CD57+CD28−CD8+ T cells (TEMRA) were compared in patients with end-stage renal disease (ESRD), those with stage IV chronic kidney disease (CKD), kidney transplant recipients, and controls with normal kidney function. We observed a significant increase in TEMRA in transplant patients as compared with controls with normal renal function. The frequencies of TEMRA were similar in patients with ESRD, those with stage IV CKD, and kidney transplant patients. CD8+ T-cell subsets were analyzed on peripheral blood samples by flow cytometry as previously reported.44
Even if many factors may contribute to T-lymphocyte activation in kidney transplant patients, few data indicate that these patients exhibit increased levels of circulating activated CD8+ T cells. The ratio of effector memory CD8+ T cell/central memory CD8+ T cell is increased in kidney transplant recipients as compared with patients on the waiting list.66 Yap et al.67 also observed a significant expansion of terminally differentiated effector memory CD8+ T cells (TEMRA) in a significant subset of kidney transplant patients. Finally, we compared the frequencies of TEMRA in patients with ESRD, patients with stage IV CKD, kidney transplant recipients, and controls with normal kidney function. We observed a significant increase in TEMRA percentages in renal transplant patients as compared with controls with normal renal function (Fig. 2). The frequencies of TEMRA were similar in patients with ESRD, those with stage IV CKD, and kidney transplant patients (Fig. 2). Although these different studies do not establish a direct association between T-lymphocyte activation and atherosclerosis, it is remarkable to note that all factors associated with CD8+ T-cell activation are themselves associated with atherosclerosis.
Perspectives and Future Areas of Research
Compelling data suggest that CD8+ T-cell activation contributes to the so-called posttransplant accelerated atherosclerosis. Future studies should investigate precisely the direct relationships between CD8+ T-cell expansion and cardiovascular outcomes. Moreover, as different CD8+ T-cell subsets seem to have opposite effects, better definitions of the clinical consequences of different CD8+ T-cell phenotypes are required.
Therapeutic strategies limiting T-cell activation (avoidance of systematic use of lymphodepletive therapies, viral prophylaxis) should be tested, as well as their impacts on posttransplant outcomes. Considering the central role of CMV in T-lymphocyte activation, systematic prophylaxis in all CMV-seropositive patients has a central role to prevent posttransplant atherosclerosis. Alternatively, in HIV-infected patients, treatment with statins or aspirin resulted in a decrease in T-cell activation.68,69
An alternative elegant strategy could be to prevent T-lymphocyte activation occurring during reconstitution following T-lymphocyte depletion. It has been reported that administration of recombinant IL-7 expands RTE, whereas the proportion of senescent effector CD8+ T cells decreases.70 However, some doubts about a potentiation of alloimmune responses (i.e., directed against the grafted organ) may limit such therapeutic options. Indeed, we recently reported that higher RTE frequency may be predictive of acute kidney graft rejection.45 Blocking effector T cell and/or enhancing Treg responses are new potential research directions. CD31 engagement decreases T-cell activation, and ApoE–/– mice treated with a CD31 receptor globulin had both reduced circulating activated T cells and atherosclerotic lesions.71 Cell-based therapies, such as human monocyte-derived suppressive cells72 or apoptotic cells,73 may be an additional option to limit CD8+ T-cell activation and favor the expansion of atheroprotective CD4+ or CD8+ Tregs.72,73 Such therapeutic approaches may offer the opportunity to control the immune part of the posttransplant accelerated atherosclerosis in addition to prevent graft rejection.
Footnotes
Authors’ Note: This work is a part of the RIALTO (Research in Immunology of AtheroscLerosis after TransplantatiOn) program.
Author Contributions: JB, CC, DD, TC, JMR, and PS designed the studies reported in this review and drafted the manuscript. JB, TC, CC, and DD participated in acquisition of data and patient follow-up. CC, JB, CT and PS participated in T-cell subset analysis in patients. All authors saw and approved the final version of the manuscript.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research and/or authorship of this article: This work is supported by the Fédération hospitalo-universitaire INCREASE (INtegrated Centre for Research in Inflammatory DisEASEs) and by the Agence Nationale de la Recherche (ANR-11-LBX-0021 to LabEx LipSTIC).
References
- 1. Kasiske BL, Chakkera HA, Roel J. Explained and unexplained ischemic heart disease risk after renal transplantation. J Am Soc Nephrol. 2000;11(9):1735–1743. [DOI] [PubMed] [Google Scholar]
- 2. Stoumpos S, Jardine AG, Mark PB. Cardiovascular morbidity and mortality after kidney transplantation. Transpl Int. 2015;28(1):10–21. [DOI] [PubMed] [Google Scholar]
- 3. Ridker PM. From C-reactive protein to interleukin-6 to interleukin-1: moving ppstream to identify novel targets for atheroprotection. Circ Res. 2016;118(1):145–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ducloux D, Deschamps M, Yannaraki M, Ferrand C, Bamoulid J, Saas P, Kazory A, Chalopin JM, Tiberghien P. Relevance of Toll-like receptor-4 polymorphisms in renal transplantation. Kidney Int. 2005;67(6):2454–2461. [DOI] [PubMed] [Google Scholar]
- 5. Ducloux D, Kazory A, Chalopin JM. Predicting coronary heart disease in renal transplant recipients: a prospective study. Kidney Int. 2004;66(1):441–447. [DOI] [PubMed] [Google Scholar]
- 6. Bamoulid J, Courivaud C, Deschamps M, Mercier P, Ferrand C, Penfornis A, Tiberghien P, Chalopin JM, Saas P, Ducloux D. IL-6 promoter polymorphism -174 is associated with new-onset diabetes after transplantation. J Am Soc Nephrol. 2006;17(8):2333–2340. [DOI] [PubMed] [Google Scholar]
- 7. Bamoulid J, Courivaud C, Deschamps M, Gaugler B, Tiberghien P, Chalopin JM, Saas P, Ducloux D. The interleukin-6 gene promoter polymorphism -174 and atherosclerotic events in overweight transplanted patients. J Transplant. 2011;2011:803429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ducloux D, Challier B, Saas P, Tiberghien P, Chalopin JM. CD4 cell lymphopenia and atherosclerosis in renal transplant recipients. J Am Soc Nephrol. 2003;14(3):767–772. [DOI] [PubMed] [Google Scholar]
- 9. Ducloux D, Courivaud C, Bamoulid J, Vivet B, Chabroux A, Deschamps M, Rebibou JM, Ferrand C, Chalopin JM, Tiberghien P, et al. Prolonged CD4 T cell lymphopenia increases morbidity and mortality after renal transplantation. J Am Soc Nephrol. 2010;21(5):868–875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Tse K, Tse H, Sidney J, Sette A, Ley K. T cells in atherosclerosis. Int Immunol. 2013;25(11):615–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Weber C, Noels H. Atherosclerosis: current pathogenesis and therapeutic options. Nat Med. 2011;17(11):1410–1422. [DOI] [PubMed] [Google Scholar]
- 12. Legein B, Temmerman L, Biessen EA, Lutgens E. Inflammation and immune system interactions in atherosclerosis. Cell Mol Life Sci. 2013;70(20):3847–3869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ait-Oufella H, Sage AP, Mallat Z, Tedgui A. Adaptive (T and B cells) immunity and control by dendritic cells in atherosclerosis. Circ Res. 2014;114(10):1640–1660. [DOI] [PubMed] [Google Scholar]
- 14. Cochain C, Zernecke A. Macrophages and immune cells in atherosclerosis: recent advances and novel concepts. Basic Res Cardiol. 2015;110(4):34. [DOI] [PubMed] [Google Scholar]
- 15. Ludewig B, Freigang S, Jaggi M, Kurrer MO, Pei YC, Vlk L, Odermatt B, Zinkernagel RM, Hengartner H. Linking immune-mediated arterial inflammation and cholesterol-induced atherosclerosis in a transgenic mouse model. Proc Natl Acad Sci U S A. 2000;97(23):12752–12757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Cochain C, Chaudhari SM, Koch M, Wiendl H, Eckstein HH, Zernecke A. Programmed cell death-1 deficiency exacerbates T cell activation and atherogenesis despite expansion of regulatory T cells in atherosclerosis-prone mice. PLoS One. 2014;9(4):e93280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Elhage R, Gourdy P, Brouchet L, Jawien J, Fouque MJ, Fievet C, Huc X, Barreira Y, Couloumiers JC, Arnal JF, et al. Deleting TCR alpha beta+ or CD4+ T lymphocytes leads to opposite effects on site-specific atherosclerosis in female apolipoprotein E-deficient mice. Am J Pathol. 2004;165(6):2013–2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kolbus D, Ljungcrantz I, Soderberg I, Alm R, Bjorkbacka H, Nilsson J, Fredrikson GN. TAP1-deficiency does not alter atherosclerosis development in Apoe-/- mice. PLoS One. 2012;7(3):e33932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kyaw T, Winship A, Tay C, Kanellakis P, Hosseini H, Cao A, Li P, Tipping P, Bobik A, Toh BH. Cytotoxic and proinflammatory CD8+ T lymphocytes promote development of vulnerable atherosclerotic plaques in apoE-deficient mice. Circulation. 2013;127(9):1028–1039. [DOI] [PubMed] [Google Scholar]
- 20. Russell PS, Chase CM, Winn HJ, Colvin RB. Coronary atherosclerosis in transplanted mouse hearts. III. Effects of recipient treatment with a monoclonal antibody to interferon-gamma. Transplantation. 1994;57(9):1367–1371. [PubMed] [Google Scholar]
- 21. Nagano H, Mitchell RN, Taylor MK, Hasegawa S, Tilney NL, Libby P. Interferon-gamma deficiency prevents coronary arteriosclerosis but not myocardial rejection in transplanted mouse hearts. J Clin Invest. 1997;100(3):550–557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Furukawa Y, Cole SE, Shah RV, Fukumoto Y, Libby P, Mitchell RN. Wild-type but not interferon-gamma-deficient T cells induce graft arterial disease in the absence of B cells. Cardiovasc Res. 2004;63(2):347–356. [DOI] [PubMed] [Google Scholar]
- 23. Cochain C, Koch M, Chaudhari SM, Busch M, Pelisek J, Boon L, Zernecke A. CD8+ T cells regulate monopoiesis and circulating Ly6C-high monocyte levels in atherosclerosis in mice. Circ Res. 2015;117(3):244–253. [DOI] [PubMed] [Google Scholar]
- 24. Kolbus D, Ramos OH, Berg KE, Persson J, Wigren M, Bjorkbacka H, Fredrikson GN, Nilsson J. CD8+ T cell activation predominate early immune responses to hypercholesterolemia in Apoe-(/)- mice. BMC Immunol. 2010;11:58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Clement M, Guedj K, Andreata F, Morvan M, Bey L, Khallou-Laschet J, Gaston AT, Delbosc S, Alsac JM, Bruneval P, et al. Control of the T follicular helper-germinal center B-cell axis by CD8(+) regulatory T cells limits atherosclerosis and tertiary lymphoid organ development. Circulation. 2015;131(6):560–570. [DOI] [PubMed] [Google Scholar]
- 26. Currier JS, Taylor A, Boyd F, Dezii CM, Kawabata H, Burtcel B, Maa JF, Hodder S. Coronary heart disease in HIV-infected individuals. J Acquir Immune Defic Syndr. 2003;33(4):506–512. [DOI] [PubMed] [Google Scholar]
- 27. Triant VA, Lee H, Hadigan C, Grinspoon SK. Increased acute myocardial infarction rates and cardiovascular risk factors among patients with human immunodeficiency virus disease. J Clin Endocrinol Metab. 2007;92(7):2506–2512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ho JE, Hsue PY. Cardiovascular manifestations of HIV infection. Heart. 2009;95(14):1193–1202. [DOI] [PubMed] [Google Scholar]
- 29. Cruse B, Cysique LA, Markus R, Brew BJ. Cerebrovascular disease in HIV-infected individuals in the era of highly active antiretroviral therapy. J Neurovirol. 2012;18(4):264–276. [DOI] [PubMed] [Google Scholar]
- 30. Krikke M, van Lelyveld SF, Tesselaar K, Arends JE, Hoepelman IM, Visseren FL. The role of T cells in the development of cardiovascular disease in HIV-infected patients. Atherosclerosis. 2014;237(1):92–98. [DOI] [PubMed] [Google Scholar]
- 31. Kaplan RC, Sinclair E, Landay AL, Lurain N, Sharrett AR, Gange SJ, Xue X, Parrinello CM, Hunt P, Deeks SG, et al. T cell activation predicts carotid artery stiffness among HIV-infected women. Atherosclerosis. 2011;217(1):207–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Longenecker CT, Funderburg NT, Jiang Y, Debanne S, Storer N, Labbato DE, Lederman MM, McComsey GA. Markers of inflammation and CD8 T-cell activation, but not monocyte activation, are associated with subclinical carotid artery disease in HIV-infected individuals. HIV Med. 2013;14(6):385–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. D’Abramo A, Zingaropoli MA, Oliva A, D’Agostino C, Al Moghazi S, De Luca G, Iannetta M, Mastroianni CM, Vullo V. Immune activation, immunosenescence, and osteoprotegerin as markers of endothelial dysfunction in subclinical HIV-associated atherosclerosis. Mediators Inflamm. 2014;2014:192594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Karim R, Mack WJ, Kono N, Tien PC, Anastos K, Lazar J, Young M, Desai S, Golub ET, Kaplan RC, et al. T-cell activation, both pre- and post-HAART levels, correlates with carotid artery stiffness over 6.5 years among HIV-infected women in the WIHS. J Acquir Immune Defic Syndr. 2014;67(3):349–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Badejo OA, Chang CC, So-Armah KA, Tracy RP, Baker JV, Rimland D, Butt AA, Gordon AJ, Rinaldo CR, Jr, Kraemer K, et al. CD8+ T-cells count in acute myocardial infarction in HIV disease in a predominantly male cohort. Biomed Res Int. 2015;2015:246870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Castilho JL, Shepherd BE, Koethe J, Turner M, Bebawy S, Logan J, Rogers WB, Raffanti S, Sterling TR. CD4+/CD8+ ratio, age, and risk of serious noncommunicable diseases in HIV-infected adults on antiretroviral therapy. AIDS. 2016;30(6):899–908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Spyridopoulos I, Martin-Ruiz C, Hilkens C, Yadegarfar ME, Isaacs J, Jagger C, Kirkwood T, von Zglinicki T. CMV seropositivity and T-cell senescence predict increased cardiovascular mortality in octogenarians: results from the Newcastle 85+ study. Aging Cell. 2016;15(2):389–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Kolbus D, Ljungcrantz I, Andersson L, Hedblad B, Fredrikson GN, Bjorkbacka H, Nilsson J. Association between CD8+ T-cell subsets and cardiovascular disease. J Intern Med. 2013;274(1):41–51. [DOI] [PubMed] [Google Scholar]
- 39. Hwang Y, Yu HT, Kim DH, Jang J, Kim HY, Kang I, Kim HC, Park S, Lee WW. Expansion of CD8(+) T cells lacking the IL-6 receptor alpha chain in patients with coronary artery diseases (CAD). Atherosclerosis. 2016;249:44–51. [DOI] [PubMed] [Google Scholar]
- 40. Larbi A, Fulop T. From “truly naive” to “exhausted senescent” T cells: when markers predict functionality. Cytometry A. 2014;85(1):25–35. [DOI] [PubMed] [Google Scholar]
- 41. Youn JC, Yu HT, Lim BJ, Koh MJ, Lee J, Chang DY, Choi YS, Lee SH, Kang SM, Jang Y, et al. Immunosenescent CD8+ T cells and C-X-C chemokine receptor type 3 chemokines are increased in human hypertension. Hypertension. 2013;62(1):126–133. [DOI] [PubMed] [Google Scholar]
- 42. Winchester R, Giles JT, Nativ S, Downer K, Zhang HZ, Bag-Ozbek A, Zartoshti A, Bokhari S, Bathon JM. Association of elevations of specific T Cell and monocyte subpopulations in rheumatoid arthritis with subclinical coronary artery atherosclerosis. Arthritis Rheumatol. 2016;68(1):92–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Strioga M, Pasukoniene V, Characiejus D. CD8+ CD28- and CD8+ CD57+ T cells and their role in health and disease. Immunology. 2011;134(1):17–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Crepin T, Carron C, Roubiou C, Gaugler B, Gaiffe E, Simula-Faivre D, Ferrand C, Tiberghien P, Chalopin JM, Moulin B, et al. ATG-induced accelerated immune senescence: clinical implications in renal transplant recipients. Am J Transplant. 2015;15(4):1028–1038. [DOI] [PubMed] [Google Scholar]
- 45. Bamoulid J, Courivaud C, Crepin T, Carron C, Gaiffe E, Roubiou C, Laheurte C, Moulin B, Frimat L, Rieu P, et al. Pretransplant thymic function predicts acute rejection in antithymocyte globulin-treated renal transplant recipients. Kidney Int. 2016;89(5):1136–1143. [DOI] [PubMed] [Google Scholar]
- 46. Hsue PY, Hunt PW, Sinclair E, Bredt B, Franklin A, Killian M, Hoh R, Martin JN, McCune JM, Waters DD, et al. Increased carotid intima-media thickness in HIV patients is associated with increased cytomegalovirus-specific T-cell responses. AIDS. 2006;20(18):2275–2283. [DOI] [PubMed] [Google Scholar]
- 47. Sacre K, Hunt PW, Hsue PY, Maidji E, Martin JN, Deeks SG, Autran B, McCune JM. A role for cytomegalovirus-specific CD4+CX3CR1+ T cells and cytomegalovirus-induced T-cell immunopathology in HIV-associated atherosclerosis. AIDS. 2012;26(7):805–814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Betjes MG, Litjens NH, Zietse R. Seropositivity for cytomegalovirus in patients with end-stage renal disease is strongly associated with atherosclerotic disease. Nephrol Dial Transplant. 2007;22(11):3298–3303. [DOI] [PubMed] [Google Scholar]
- 49. Betjes MG, Langerak AW, van der Spek A, de Wit EA, Litjens NH. Premature aging of circulating T cells in patients with end-stage renal disease. Kidney Int. 2011;80(2):208–217. [DOI] [PubMed] [Google Scholar]
- 50. Courivaud C, Bamoulid J, Chalopin JM, Gaiffe E, Tiberghien P, Saas P, Ducloux D. Cytomegalovirus exposure and cardiovascular disease in kidney transplant recipients. J Infect Dis. 2013;207(10):1569–1575. [DOI] [PubMed] [Google Scholar]
- 51. Meijers RW, Litjens NH, Hesselink DA, Langerak AW, Baan CC, Betjes MG. Primary cytomegalovirus infection significantly impacts circulating T cells in kidney transplant recipients. Am J Transplant. 2015;15(12):3143–3156. [DOI] [PubMed] [Google Scholar]
- 52. Bonnefoy-Berard N, Vincent C, Revillard JP. Antibodies against functional leukocyte surface molecules in polyclonal antilymphocyte and antithymocyte globulins. Transplantation. 1991;51(3):669–673. [DOI] [PubMed] [Google Scholar]
- 53. Rebellato LM, Gross U, Verbanac KM, Thomas JM. A comprehensive definition of the major antibody specificities in polyclonal rabbit antithymocyte globulin. Transplantation. 1994;57(5):685–694. [DOI] [PubMed] [Google Scholar]
- 54. Mohty M. Mechanisms of action of antithymocyte globulin: T-cell depletion and beyond. Leukemia. 2007;21(7):1387–1394. [DOI] [PubMed] [Google Scholar]
- 55. Muller TF, Grebe SO, Neumann MC, Heymanns J, Radsak K, Sprenger H, Lange H. Persistent long-term changes in lymphocyte subsets induced by polyclonal antibodies. Transplantation. 1997;64(10):1432–1437. [DOI] [PubMed] [Google Scholar]
- 56. Cherkassky L, Lanning M, Lalli PN, Czerr J, Siegel H, Danziger-Isakov L, Srinivas T, Valujskikh A, Shoskes DA, Baldwin W, et al. Evaluation of alloreactivity in kidney transplant recipients treated with antithymocyte globulin versus IL-2 receptor blocker. Am J Transplant. 2011;11(7):1388–1396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Tchao NK, Turka LA. Lymphodepletion and homeostatic proliferation: implications for transplantation. Am J Transplant. 2012;12(5):1079–1090. [DOI] [PubMed] [Google Scholar]
- 58. Havenith SH, Remmerswaal EB, Bemelman FJ, Yong SL, van Donselaar-van der Pant KA, van Lie RA, Ten Berg IJ. Rapid T cell repopulation after rabbit anti-thymocyte globulin (rATG) treatment is driven mainly by cytomegalovirus. Clin Exp Immunol. 2012;169(3):292–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Mackall CL, Hakim FT, Gress RE. T-cell regeneration: all repertoires are not created equal. Immunol Today. 1997;18(5):245–251. [DOI] [PubMed] [Google Scholar]
- 60. Williams KM, Hakim FT, Gress RE. T cell immune reconstitution following lymphodepletion. Semin Immunol. 2007;19(5):318–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Stock P, Kirk AD. The risk and opportunity of homeostatic repopulation. Am J Transplant. 2011;11(7):1349–1350. [DOI] [PubMed] [Google Scholar]
- 62. Ducloux D, Courivaud C, Bamoulid J, Crepin T, Chalopin JM, Tiberghien P, Saas P. Polyclonal antithymocyte globulin and cardiovascular disease in kidney transplant recipients. J Am Soc Nephrol. 2014;25(6):1349–1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Meier-Kriesche HU, Arndorfer JA, Kaplan B. Association of antibody induction with short- and long-term cause-specific mortality in renal transplant recipients. J Am Soc Nephrol. 2002;13(3):769–772. [DOI] [PubMed] [Google Scholar]
- 64. Ducloux D, Courivaud C, Bamoulid J, Bisaccia V, Roubiou C, Crepin T, Gaugler B, Laheurte C, Rebibou JM, Chalopin JM, et al. Alloimmune responses and atherosclerotic disease after kidney transplantation. Transplantation. 2015;99(1):220–225. [DOI] [PubMed] [Google Scholar]
- 65. Opelz G, Dohler B. Association of HLA mismatch with death with a functioning graft after kidney transplantation: a collaborative transplant study report. Am J Transplant. 2012;12(11):3031–3038. [DOI] [PubMed] [Google Scholar]
- 66. Segundo DS, Fernandez-Fresnedo G, Gago M, Beares I, Ruiz-Criado J, Gonzalez M, Ruiz JC, Gomez-Alamillo C, Lopez-Hoyos M, Arias M. Kidney transplant recipients show an increase in the ratio of T-cell effector memory/central memory as compared to nontransplant recipients on the waiting list. Transplant Proc. 2010;42(8):2877–2879. [DOI] [PubMed] [Google Scholar]
- 67. Yap M, Boeffard F, Clave E, Pallier A, Danger R, Giral M, Dantal J, Foucher Y, Guillot-Gueguen C, Toubert A, et al. Expansion of highly differentiated cytotoxic terminally differentiated effector memory CD8+ T cells in a subset of clinically stable kidney transplant recipients: a potential marker for late graft dysfunction. J Am Soc Nephrol. 2014;25(8):1856–1868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Ganesan A, Crum-Cianflone N, Higgins J, Qin J, Rehm C, Metcalf J, Brandt C, Vita J, Decker CF, Sklar P, et al. High dose atorvastatin decreases cellular markers of immune activation without affecting HIV-1 RNA levels: results of a double-blind randomized placebo controlled clinical trial. J Infect Dis. 2011;203(6):756–764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. O’Brien M, Montenont E, Hu L, Nardi MA, Valdes V, Merolla M, Gettenberg G, Cavanagh K, Aberg JA, Bhardwaj N, Berger JS. Aspirin attenuates platelet activation and immune activation in HIV-1-infected subjects on antiretroviral therapy: a pilot study. J Acquir Immune Defic Syndr. 2013;63(3):280–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Sportes C, Hakim FT, Memon SA, Zhang H, Chua KS, Brown MR, Fleisher TA, Krumlauf MC, Babb RR, Chow CK, et al. Administration of rhIL-7 in humans increases in vivo TCR repertoire diversity by preferential expansion of naive T cell subsets. J Exp Med. 2008;205(7):1701–1714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Groyer E, Nicoletti A, Ait-Oufella H, Khallou-Laschet J, Varthaman A, Gaston AT, Thaunat O, Kaveri SV, Blatny R, Stockinger H, et al. Atheroprotective effect of CD31 receptor globulin through enrichment of circulating regulatory T-cells. J Am Coll Cardiol. 2007;50(4):344–350. [DOI] [PubMed] [Google Scholar]
- 72. Janikashvili N, Trad M, Gautheron A, Samson M, Lamarthee B, Bonnefoy F, Lemaire-Ewing S, Ciudad M, Rekhviashvili K, Seaphanh F, et al. Human monocyte-derived suppressor cells control graft-versus-host disease by inducing regulatory forkhead box protein 3-positive CD8+ T lymphocytes. J Allergy Clin Immunol. 2015;135(6):1614–1624.e4. [DOI] [PubMed] [Google Scholar]
- 73. Saas P, Daguindau E, Perruche S. Concise Review: Apoptotic cell-based therapies-rationale, preclinical results and future clinical developments. Stem Cells. 2016;34(6):1464–1473. [DOI] [PubMed] [Google Scholar]